Abstract
Insulin resistance is associated with subclinical vascular disease that is not justified by conventional cardiovascular risk factors, such as smoking or hypercholesterolemia. Vascular injury associated to insulin resistance involves functional and structural damage to the arterial wall that includes impaired vasodilation in response to chemical mediators, reduced distensibility of the arterial wall (arterial stiffness), vascular calcification, and increased thickness of the arterial wall. Vascular dysfunction associated to insulin resistance is present in asymptomatic subjects and predisposes to cardiovascular diseases, such as heart failure, ischemic heart disease, stroke, and peripheral vascular disease. Structural and functional vascular disease associated to insulin resistance is highly predictive of cardiovascular morbidity and mortality. Its pathogenic mechanisms remain undefined. Prospective studies have demonstrated that animal protein consumption increases the risk of developing cardiovascular disease and predisposes to type 2 diabetes (T2D) whereas vegetable protein intake has the opposite effect. Vascular disease linked to insulin resistance begins to occur early in life. Children and adolescents with insulin resistance show an injured arterial system compared with youth free of insulin resistance, suggesting that insulin resistance plays a crucial role in the development of initial vascular damage. Prevention of the vascular dysfunction related to insulin resistance should begin early in life. Before the clinical onset of T2D, asymptomatic subjects endure a long period of time characterized by insulin resistance. Latent vascular dysfunction begins to develop during this phase, so that patients with T2D are at increased cardiovascular risk long before the diagnosis of the disease.
Keywords: Diabetes, Cardiovascular risk, Arterial stiffness, Arterial elasticity, Intima-media thickness, Vascular calcification, Insulin resistance, Animal protein, Vegetable protein
Core tip: Vascular injury associated to insulin resistance includes impaired vasodilation in response to chemical mediators, reduced distensibility of the arterial wall (arterial stiffness), vascular calcification, and increased thickness of the arterial wall. Vascular dysfunction associated to insulin resistance is present in asymptomatic subjects and predisposes to cardiovascular diseases, such as heart failure, ischemic heart disease, stroke, and peripheral vascular disease. Structural and functional vascular disease associated to insulin resistance is highly predictive of cardiovascular morbidity and mortality.
INTRODUCTION
Cardiovascular disease is a major cause of morbidity and mortality particularly in patients with diabetes. Cardiovascular risk in this population group begins decades prior the clinical diagnosis of the disease and is not fully explained by traditional risk factors such as hypercholesterolemia and smoking. Multiple investigations provide compelling evidence of an association between insulin resistance by itself and cardiovascular risk in the general population and patients with diabetes. More insulin-resistant subjects endure higher cardiovascular risk compared to those who are more insulin-sensitive[1]. A causative link between insulin resistance by itself and vascular disease is very likely to exist, but the pathogenic mechanisms that explain the vascular dysfunction related to insulin resistance remain elusive. There is conclusive evidence that dietary habits that include animal protein increase the risk of type 2 diabetes (T2D) and cardiovascular disease whereas dietary patterns with elevated content of vegetable protein reduce the risk of both disorders[2]. Population groups that change their dietary routine to augment animal protein intake experience a dramatic increase in the rate of T2D and cardiovascular events[3]. Animal protein consumption activates glucagon secretion. Glucagon is the primary hormone that opposes insulin action. Animal protein ingestion may predispose to T2D and cardiovascular events by intensifying insulin resistance via glucagon secretion (Figure 1)[4].
Figure 1.
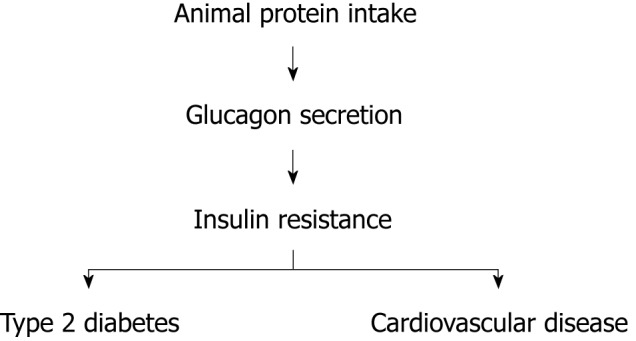
A simplified proposed mechanism underlying vascular disease associated with insulin resistance.
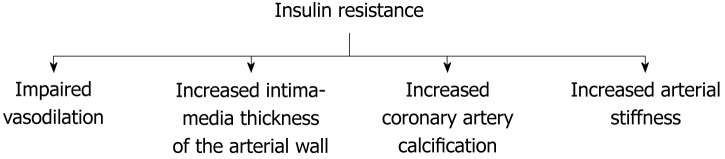
Asymptomatic individuals with insulin resistance experience striking vascular damage that is not justified by traditional cardiovascular risk factors, such as hypercholesterolemia or smoking. Vascular injury related to insulin resistance develops progressively in asymptomatic subjects during a period of time that may begin during childhood. A long phase of insulin resistance and latent vascular injury precedes the clinical onset of T2D increasing cardiovascular risk before the diagnosis of the disease[5-7]. Accordingly, subclinical vascular dysfunction is evident in patients with screen-detected T2D[8]. Vascular damage associated with insulin resistance includes functional and structural vascular injury, such as impaired vasodilation, loss of elasticity of the arterial wall (arterial stiffness), increased intima-media thickness of the arterial wall, and vascular calcification. (Figure 2) The presence of subclinical vascular disease associated with insulin resistance is highly predictive of future cardiovascular events[9-12].
Figure 2.
Pathophysiological changes associated with insulin resistance-mediated vascular disease.
INSULIN RESISTANCE IS INDEPENDENTLY ASSOCIATED WITH SUBCLINICAL IMPAIRMENT OF VASCULAR REACTIVITY
Vascular smooth muscle cells normally undergo contraction or relaxation to regulate the magnitude of the blood flow according to physiological conditions. Normal endothelial cells generate vasoactive substances that modulate the reactivity of vascular smooth muscle cells. Among them, nitric oxide is a short-lived gas that induces vasodilation. Acetylcholine is an endogenous transmitter that activates endothelial nitric oxide production by acting on muscarinic receptors. Acetylcholine induces endothelium-dependent vasodilation while exogenous sources of nitric oxide (such as nitroglycerin and sodium nitroprusside) induce endothelium-independent vasodilation. In response to increased blood flow, vascular smooth muscle cells normally relax to produce vasodilation and accommodate the elevated blood flow. Flow-mediated vasodilation is attributed to nitric oxide release by endothelial cells. The degree of flow-mediated vasodilation is considered a measure of endothelium-dependent vasodilation and can be determined by ultrasonography performed at the brachial artery[13-15].
A number of investigations show that insulin resistance is independently associated with blunted flow-mediated arterial vasodilation in asymptomatic healthy individuals compared to control subjects[5,16,17].
Similarly, insulin resistance is associated with limited vasodilation in response to metacholine chloride, a muscarinic agent. The increment in blood flow in response to metacholine is lower in insulin-resistant subjects compared to insulin-sensitive controls[18].
Likewise, arterial response to exogenous sources of nitric oxide, such as nitroglycerin, sodium nitroprusside, and nitrates is impaired in subjects with insulin resistance compared to control subjects[10,16,18].
Similarly to healthy subjects, flow-mediated vasodilation is defective in nondiabetic patients with coronary heart disease, compared to control subjects. On multivariate analysis, the extent of flow-mediated vasodilation is correlated with serum high-density lipoprotein (HDL)-c, but not with low-density lipoprotein (LDL)-c or total cholesterol levels[10].
Impairment of flow-mediated vasodilation associated with insulin resistance is already apparent in childhood. Obese children show impaired arterial vasodilation compared to control children. Further, regular exercise over 6 mo restores abnormal vascular dysfunction in obese children. The improvement in flow-mediated vasodilation after 6-mo exercise program correlates with enhanced insulin sensitivity, reflected by reduced body mass index (BMI), waist-to-hip ratio, systolic blood pressure, fasting insulin, triglycerides, and LDL/HDL ratio[19].
In normal weight and overweight adolescents, there is a gradual deterioration of flow-mediated vasodilation with worsening of insulin resistance evaluated by the euglycemic hyperinsulinemic clamp[20].
INSULIN RESISTANCE IS INDEPENDENTLY ASSOCIATED WITH SUBCLINICAL ARTERIAL STIFFNESS
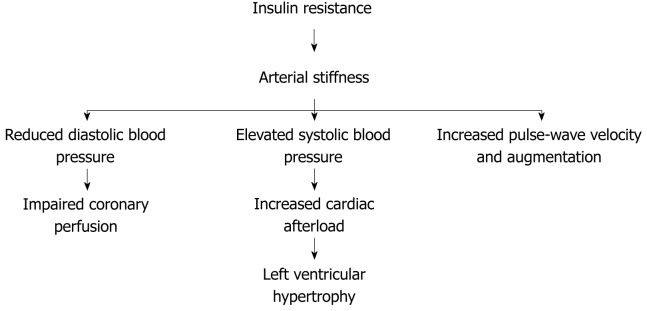
Loss of distensibility of the arterial wall (arterial stiffness) leads to elevated systolic blood pressure and consequently increases cardiac afterload resulting in left ventricular hypertrophy that contributes to the development of congestive heart failure. In addition, arterial stiffness leads to reduced diastolic blood pressure, which may deteriorate diastolic coronary blood flow contributing to ischemic heart disease[21,22] (Figure 3). Arterial stiffness is associated with wide pulse pressure (systolic blood pressure minus diastolic blood pressure)[7,23].
Figure 3.
Cardiovascular disease associated to arterial stiffness.
Parameters that estimate arterial stiffness include blood pressure, pulse pressure, pulse-wave velocity, augmentation index, coefficients of distensibility and compliance, and the Young’s elastic modulus, which includes intima-media thickness and estimates arterial stiffness controlling for arterial wall thickness[6]. Pulse-wave velocity is the speed of the pressure wave generated by left ventricular contraction. Arterial stiffness impairs the ability of the arterial wall to cushion the pressure wave and increases pulse-wave velocity[21]. Augmentation is the pressure difference between the second and first systolic peaks of the central arterial pressure waveform. Increased augmentation reflects arterial stiffness[24,25]. The augmentation index has been defined as augmentation divided by pulse pressure, being a measure of peripheral wave reflection. A higher augmentation index reflects increased arterial stiffness[26,27].
Age is consistently associated with arterial stiffness, but the loss of arterial elasticity related with age is not justified by conventional cardiovascular risk factors. Insulin resistance becomes deeper with age and may be a major pathophysiological determinant of arterial stiffness in the elderly population[12,28,29].
Numerous investigations document an association between insulin resistance and subclinical arterial stiffness in nondiabetic individuals across all ages. Arterial stiffness related to insulin resistance begins early in life and progresses in asymptomatic subjects during a latent period of time before the diagnosis of cardiovascular disease. Subclinical arterial stiffness associated with insulin resistance strongly predicts future cardiovascular events. Conventional cardiovascular risk factors do not explain the loss of arterial elasticity related to insulin resistance[7,22].
Arterial stiffness is apparent in asymptomatic subjects with insulin resistance ascertained either by its clinical expression, the metabolic syndrome, or by estimates of insulin sensitivity.
Estimates of insulin resistance are associated with subclinical arterial stiffness
In a variety of population groups, insulin resistance identified by different estimates is consistently associated with measures of arterial stiffness independently of classic cardiovascular risk factors (Table 1).
Table 1.
Studies that find an independent association between insulin resistance and subclinical arterial stiffness unexplained by classic cardiovascular risk factors
| Ref. | Population group | Insulin resistance | Arterial stiffness |
| Salomaa et al[6] | African American and Caucasian | IGT | Arterial compliance, Young’s elastic modulus |
| Henry et al[22] | General population | IGT | Arterial compliance |
| Shin et al[30] | Healthy Chinese subjects | IGT | Brachial-ankle PWV |
| Liye et al[17] | IGT versus normal glucose tolerance | IGT, serum adiponectin levels | Brachial artery PWV |
| Giltay et al[21] | Healthy subjects | Hyperinsulinemic euglycemic clamp | Carotid-femoral PWV |
| Vyssoulis et al[32] | Patients with hypertension | IGT | Carotid-femoral PWV |
| Sengstock et al[31] | Patients with hypertension | Frequently sampled IV tolerance test | Aortic PWV, pulse pressure |
| Kasayama et al[33] | Healthy adults | HOMA | Brachial-ankle PWV |
| Park et al[34] | Postmenopausal women | HOMA-IR | Aortic and peripheral PWV |
| Maple-Brown et al[4] | Indigenous Australians | HOMA-IR | Augmentation index |
| Scuteri et al[35] | Family history of diabetes | HOMA-IR | Carotid-femoral PWV |
| Sakuragi et al[36] | Prepubescent children | HOMA-IR | Carotid-femoral PWV |
| Whincup et al[11] | British children | HOMA-IR | Brachial artery distensibility |
| Gungor et al[38] | Children and adolescents | HOMA-IR | Aortic PWV |
| Iannuzzi et al[39] | Children and adolescents | HOMA-IR | Aortic PWV |
| Tomsa et al[20] | Adolescents | Hyperinsulinemic euglycemic clamp | Augmentation index |
IGT: Impaired glucose tolerance; PWV: Pulse-wave velocity; HOMA: Homeostasis model assessment; HOMA-IR: Homeostasis model assessment-insulin resistance.
The Atherosclerosis Risk in Communities study is a prospective population-based trial with African American and Caucasian participants. A cross-sectional analysis showed an independent association between insulin resistance (assessed by glucose tolerance tests) and arterial stiffness. Subjects with insulin resistance had stiffer arteries compared to those with normal glucose tolerance after adjustment for confounding factors[6].
Similarly, insulin resistance (glucose tolerance tests) in individuals from the general population was independently associated with arterial stiffness estimated by distensibility and compliance of the carotid, femoral and brachial arteries, compared to normal glucose tolerance. Arterial stiffness worsened with deteriorating glucose tolerance[22].
Comparable findings were obtained in healthy Chinese subjects. Insulin resistance (impaired glucose tolerance) was independently associated with arterial stiffness (estimated by brachial-ankle pulse-wave velocity) compared to normal glucose tolerance. Normoglycemic subjects with altered glucose metabolism have increased arterial stiffness[30].
Likewise, arterial stiffness (brachial artery pulse-wave velocity) is positively correlated with postprandial glucose and negatively correlated with plasma adiponectin level, suggesting that arterial stiffness is greater in patients with insulin resistance compared to those with normal glucose tolerance[17].
Assessment of insulin resistance with the euglycemic hyperinsulinemic clamp is also independently associated with subclinical arterial stiffness of the common carotid and femoral arteries evaluated by pulse-wave velocity in asymptomatic healthy adults[21]. In patients with hypertension, insulin resistance (glucose tolerance tests) is independently associated with arterial stiffness (carotid-femoral pulse-wave velocity and pulse pressure) as well[31,32].
Several studies document an association between insulin resistance evaluated by the homeostasis model assessment (HOMA) index and arterial stiffness in asymptomatic individuals from different population groups. In healthy subjects and in Korean post-menopausal women, insulin resistance is independently associated with increased arterial stiffness (evaluated by brachial-ankle, aortic and peripheral pulse-wave velocity). Arterial stiffness increases sequentially with the degree of insulin resistance[33,34]. Analogous findings are observed in normotensive normoglycemic first-degree relatives of patients with diabetes. Arterial stiffness (carotid-femoral pulse-wave velocity) is increased in the relatives with insulin resistance compared to those more insulin-sensitive[35]. Insulin resistance and arterial stiffness (augmentation index and pulse-wave velocity) were compared in Indigenous Australians (a population group with elevated rate of T2D) and European Australians. The Indigenous population group had higher HOMA-IR values and increased arterial stiffness compared to their European counterparts, suggesting that intensified insulin resistance among Indigenous participants contributes to explain increased arterial stiffness in this group[4].
Subclinical arterial stiffness is already present in children and adolescents with insulin resistance, compared to insulin-sensitive control subjects. In healthy children and adolescents from the general population of different countries, insulin resistance (HOMA-IR values) is independently associated with increased arterial stiffness evaluated by carotid-femoral pulse-wave velocity or brachial artery distensibility compared to control subjects[11,36,37]. In obese children and adolescents, a profound independent effect of insulin resistance on vascular compliance has been observed. Insulin-resistant subjects (HOMA-IR) experience increased vascular stiffness (aortic pulse-wave velocity) compared to control individuals[38-40]. In normal weight and overweight adolescents, insulin resistance assessed by euglycemic hyperinsulinemic clamp is associated with higher augmentation index, indicating that insulin resistance in adolescents is related to increased arterial stiffness[20].
Clinical manifestations of insulin resistance are associated with subclinical arterial stiffness
The metabolic syndrome is a cluster of clinical features that reflects insulin resistance, including obesity, systolic hypertension, dyslipemia (hypertriglyceridemia and low HDL-c), and hyperinsulinemia. The metabolic syndrome and its individual components have been independently associated with arterial stiffness. Patients with any clinical expression of insulin resistance experience subclinical arterial stiffness that is not explained by conventional cardiovascular risk factors. Arterial stiffness has been considered a further clinical manifestation of insulin resistance[7] (Table 2).
Table 2.
Studies that find an independent association between the clinical expression of insulin resistance and subclinical arterial stiffness unexplained by classic cardiovascular risk factors
| Ref. | Population group | Insulin resistance | Arterial stiffness |
| Mackey et al[28] | Elderly | Metabolic syndrome | Aortic pulse-wave velocity |
| Salomaa et al[6] | General population | Metabolic syndrome | Arterial compliance, Young’s elastic modulus |
| Hyperinsulinemia | |||
| Scuteri et al[41] | Healthy subjects | Metabolic syndrome | Carotid artery stiffness |
| Van-Popele et al[9] | Women | Metabolic syndrome | Carotid artery stiffness |
| Obesity | |||
| Dyslipemia | |||
| Tomiyama et al[29] | Healthy subjects | Metabolic syndrome | Brachial-ankle pulse-wave velocity |
| Systolic hypertension | |||
| Maple-Brown et al[4] | Indigenous versus European Australians | Metabolic syndrome | Augmentation index, pulse-wave velocity |
| Whincup et al[11] | British children | Metabolic syndrome | Brachial artery distensibility |
| Obesity | |||
| Hyperinsulinemia | |||
| Xi et al[37] | Chinese children | Metabolic syndrome | Brachial artery distensibility |
| Ianuzzi et al[42] | Obese children | Metabolic syndrome | Carotid artery stiffness |
| Hopkins et al[43] | Relatives of patients with type 2 diabetes | Metabolic syndrome | Aortic pulse-wave velocity |
| Juonala et al[44] | Children | Metabolic syndrome | Carotid artery stiffness |
| Hyperinsulinemia | |||
| Zebekakis et al[45] | General population | Obesity | Carotid, femoral, and brachial arteries stiffness |
| Maple-Brown et al[46] | Indigenous versus European Australians | Obesity | Augmentation index |
| Greenfield et al[47] | Female twins | Abdominal obesity | Augmentation index |
| Sakuragi et al[35] | Children | Obesity | Brachial artery distensibility |
| Dyslipemia | |||
| Hyperinsulinemia | |||
| Gungor et al[38] | Adolescents and young adults | Obesity | Aortic pulse-wave velocity |
| Jourdan et al[47] | Dyslipemia | ||
| Urbina et al[49] | |||
| Kappus et al[50] | |||
| Wildman et al[51] | Young and older adults | Obesity | Aortic pulse-wave velocity |
| Iannuzzi et al[39] | Systolic hypertension | Aortic pulse-wave velocity | |
| Kasayama et al[33] | Dyslipemia | ||
| Ceceija et al[12] | Hyperinsulinemia | ||
| Urbina et al[52] | Triglyceride/HDL-c | Aortic pulse-wave velocity |
The metabolic syndrome is associated with arterial stiffness: The longitudinal association between the metabolic syndrome and arterial stiffness was investigated in the Cardiovascular Health Study. Metabolic syndrome at baseline (obesity, systolic hypertension, hyperinsulinemia and hypertriglyceridemia) independently predicted increased arterial stiffness (aortic pulse-wave velocity) at follow-up[28].
In the Atherosclerosis Risk in Communities study, the joint effect of elevated glucose, hyperinsulinemia and hypertriglyceridemia (reflecting insulin resistance) is independently associated with arterial stiffness in subjects from the general population[6]. Similarly, the clustering of at least three components of the metabolic syndrome is related with increased carotid artery stiffness among healthy participants across all age groups in the Baltimore Longitudinal Study on Aging independently of other cardiovascular risk factors[41].
Likewise, the metabolic syndrome is strongly and independently associated with reduced distensibility of the common carotid artery in healthy women from the general population[9]. In 12517 subjects with no history of cardiovascular disease, systolic hypertension, obesity, hypertriglyceridemia, and hyperuricemia are independent determinants for arterial stiffness (brachial-ankle pulse-wave velocity) on multiple regression analysis[29]. Arterial stiffness (augmentation index and pulse-wave velocity) was compared in Indigenous and European Australians. Factor analysis revealed that metabolic syndrome components clustered with Indigenous Australian participants. Arterial stiffness was more pronounced among Indigenous compared to European Australians[4].
Subclinical arterial stiffness is already present in children and adolescents with the metabolic syndrome, suggesting that insulin resistance plays an important role in the early pathogenesis of vascular disease. British and Chinese children and adolescents with the metabolic syndrome have increased arterial stiffness compared to control children after adjustment for covariates. There is a strong graded inverse relationship between the number of metabolic syndrome components and brachial artery distensibility[11,37]. In obese children, common carotid artery stiffness is more prominent in the group with the metabolic syndrome compared to the control group[42]. Normoglycemic young adults (mean age 20 years) with a positive family history of T2D have higher BMI and fasting insulin and increased arterial stiffness (aortic pulse-wave velocity) than their counterparts without T2D relatives[43]. The longitudinal relationship between the metabolic syndrome identified in childhood and arterial elasticity assessed in adulthood was investigated in a prospective population-based cohort study with 21 years of follow-up, the Cardiovascular Risk in Young Finns Study. Childhood metabolic syndrome (obesity, systolic hypertension, hypertriglyceridemia and hyperinsulinemia) predicts independently carotid artery stiffness in adulthood[44].
Obesity is associated with arterial stiffness: Longitudinal and cross-sectional studies consistently show that measures of adiposity (BMI, waist circumference, waist-to-hip ratio, body fat, and abdominal fat) are independently associated with estimates of arterial stiffness in diverse population groups. This association is already apparent during childhood and cannot be explained by traditional cardiovascular risk factors. In a population-based setting, adulthood obesity (BMI and waist-to-hip ratio) is associated with increased stiffness of carotid, femoral, and brachial arteries after adjusting for cardiovascular risk factors. Arterial distensibility consistently decreased with higher BMI[9,45]. Similarly, obesity (BMI and waist circumference) is independently related to increased arterial stiffness (augmentation index) in Indigenous Australians free of T2D compared to European Australians[46]. In female twins, abdominal adiposity is a determinant of arterial stiffness (augmentation index) independent of genetic effects and other confounding factors[47].
The association between adiposity parameters and increased arterial stiffness begins during childhood. In obese children, there is a marked effect of insulin resistance associated with obesity on vascular compliance. Obese children are more insulin-resistant and have stiffer arteries compared with lean controls[39,40]. In a population-based setting, childhood obesity (BMI and waist circumference) is associated with increased arterial stiffness after adjustment for confounding factors. There is a strong graded inverse relationship between BMI and brachial artery distensibility, This association is apparent even at BMI levels below those considered to represent obesity[11,36]. Similar results are observed in adolescents and young adults. Obesity is associated with subclinical arterial stiffness independently of cardiovascular risk factors[38,48-50].
The association between obesity and arterial stiffness (aortic pulse-wave velocity) was evaluated in young adults (20 to 40 years, 50% African American) and older adults (41 to 70 years, 33% African American). Obesity parameters (BMI, waist circumference, hip circumference, and waist-to-hip ratio) were strongly correlated with higher aortic pulse-wave velocity, independently of risk factors. Obesity is an independent and strong predictor of aortic stiffness for both races and age groups[51].
Systolic hypertension, dyslipemia, and hyperinsulinemia are associated with arterial stiffness: Other clinical manifestations of insulin resistance, including systolic hypertension[12,29,39,40], dyslipemia[9,33,36,38-40,52], and hyperinsulinemia[6,11,36,40,44] are also consistently associated with different measures of arterial stiffness independently of other cardiovascular risk factors, in diverse population groups, across all ages. Longitudinal studies such as the Atherosclerosis Risk in Communities study and the Multi-Ethnic Study of Atherosclerosis have shown that arterial stiffness predicts the development of systolic hypertension[53,54]. In healthy subjects 10 to 26 years old, triglyceride-to-HDL-c ratio is an independent predictor of arterial stiffness after adjustment for cardiovascular risk factors, particularly in the obese. Arterial stiffness rose progressively across tertiles of triglyceride-to-HDL-c ratio[52].
INSULIN RESISTANCE IS INDEPENDENTLY ASSOCIATED WITH SUBCLINICAL STRUCTURAL CHANGES OF THE ARTERIAL WALL
Similarly to arterial stiffness, a gradual increase in carotid intima-media thickness occurs with age. A systematic review documents a strong association between age and carotid intima-media thickness in healthy subjects and individuals with cardiovascular disease. This relationship is not affected by cardiovascular risk factors. Ageing is associated with magnification of insulin resistance that may explain the increase in intima-media thickness[55].
Insulin resistance either ascertained by estimates or by its clinical expression is associated with increased intima-media thickness and increased calcification of the arterial wall in asymptomatic subjects. This association is not mediated by classic cardiovascular risk factors, suggesting that insulin resistance plays a crucial role in the development of initial vascular damage (Table 3).
Table 3.
Studies that find an independent association between insulin resistance and subclinical vascular calcification or increased intima-media thickness of the arterial wall unexplained by traditional cardiovascular risk factors
| Ref. | Population group | Insulin resistance | Vascular disease |
| Laakso et al[56] | Healthy subjects | Euglycemic hyperinsulinemic clamp | Increased carotid IMT |
| Agewall et al[57] | Healthy men | Euglycemic hyperinsulinemic clamp | Increased carotid wall thickness |
| Howard et al[58] | Healthy Caucasians | Frequently sampled IV glucose tolerance test | Increased carotid IMT |
| Bertoni et al[59] | Multiethnic healthy subjects | HOMA-IR | Increased carotid IMT, elevated coronary calcium |
| Rajala et al[60] | Healthy subjects | Insulin sensitivity check index | Increased carotid IMT |
| Iannuzzi et al[39] | Obese children | HOMA-IR | Increased carotid IMT |
| Ryder et al[61] | Healthy children | Euglycemic hyperinsulinemic clamp | Increased carotid IMT |
| Arad et al[62] | Healthy subjects | HOMA-IR | Elevated coronary calcium score |
| Ong et al[63] | Healthy subjects | HOMA-IR | Elevated coronary calcium score |
| Meigs et al[64] | Healthy subjects | Glucose tolerance tests | Coronary artery calcification |
| Dabelea et al[65] | Healthy and type 1 diabetes children | Glucose disposal rate | Coronary artery calcification |
| Reilly et al[66] | Family history of cardiovascular disease | HOMA-IR | Coronary artery calcification |
| Qasim et al[67] | Family history of cardiovascular disease | HOMA-IR | Coronary artery calcification |
| Young et al[68] | Patients with coronary artery disease | Glucose tolerance test | Coronary artery calcification |
| Shinozaki et al[69] | Family history of cardiovascular disease | Glucose tolerance test | Coronary artery calcification |
HOMA-IR: Homeostasis model assessment-insulin resistance; IMT: Intima-media thickness.
Estimates of insulin resistance are associated with increased thickness of the arterial wall and increased coronary calcification
Increased thickness of the arterial wall: In healthy subjects from the Kuopio Ischemic Heart Disease Risk Factor study, insulin resistance was determined by the euglycemic hyperinsulinemic clamp technique and the presence of subclinical vascular disease in the femoral and carotid arteries was evaluated by ultrasonography. Subjects with asymptomatic vascular disease were more insulin-resistant compared to control subjects[56].
The association between insulin resistance and subclinical vascular disease was confirmed in healthy Swedish men. Insulin resistance was determined by the hyperinsulinemic euglycemic clamp in subjects with high cardiovascular risk (hypercholesterolemia, smoking) and subjects with no cardiovascular risk factors. Asymptomatic vascular disease was evaluated by B-mode ultrasound of the common carotid artery. A negative correlation between insulin sensitivity and carotid intima-media thickness was observed in both population groups (high and low cardiovascular risk). Participants with insulin resistance had greater carotid wall thickness compared to insulin-sensitive subjects[57].
A similar association between insulin resistance and subclinical vascular disease (increased intima-media thickness of the arterial wall) was observed in healthy Caucasian participants of the Insulin Resistance Atherosclerosis Study. Insulin sensitivity was evaluated by the frequently sampled intravenous glucose tolerance test with analysis by the minimal model of Bergman. Asymptomatic vascular disease was assessed by the measurement of intima-media thickness of the carotid artery by B-mode ultrasonography. In Caucasian men, insulin resistance is associated with a subclinical increase in carotid intima-media thickness, after adjustment for traditional cardiovascular risk factors[58].
The independent association between insulin resistance (HOMA-IR) and subclinical vascular disease (increased carotid intima-media thickness) has been confirmed in healthy subjects of four ethnic groups (non-Hispanic Whites, African-Americans, Hispanic Americans, and Chinese Americans) from the Multi-Ethnic Study of Atherosclerosis[59].
In asymptomatic patients with impaired glucose tolerance, insulin resistance (calculated by the insulin sensitivity check index) is strongly associated with severe carotid atherosclerosis (assessed by ultrasonography) on multiple regression analysis after adjustment for confounders. Carotid intima-media thickness correlated inversely with insulin sensitivity[60].
The association between insulin resistance and asymptomatic increased intima-media thickness is apparent in childhood. In healthy children, insulin resistance measured with the euglycemic hyperinsulinemic clamp is associated with higher carotid intima-media thickness[61]. Likewise, obese children aged 6-14 years with higher HOMA-IR had increased carotid intima-media thickness compared to control children[39].
Increased coronary artery calcification: Insulin resistance is also associated with subclinical coronary artery calcification. Asymptomatic subjects with insulin resistance (HOMA-IR) have increased coronary calcification score (derived from electron-beam computed tomography) that is not explained by traditional cardiovascular risk factors[59,62,63].
In the Framingham Offspring Study, there is a graded increase in subclinical coronary artery calcification with worsening insulin resistance (impaired glucose tolerance) among asymptomatic subjects[64].
The association between insulin resistance (estimated glucose disposal rate) and coronary artery calcification was examined among patients with type 1 diabetes and healthy subjects in the Coronary Artery Calcification in Type 1 Diabetes study. Insulin resistance was independently associated with coronary artery calcification (electron-beam computed tomography) in both population groups[65].
In the Study of Inherited Risk of Coronary Atherosclerosis, insulin resistance (HOMA-IR) is associated with coronary artery calcification after adjustment for confounding factors in asymptomatic subjects with a family history of premature cardiovascular disease. The HOMA-IR index predicts coronary artery calcification scores beyond other cardiovascular risk factors in this population group[66,67].
In normoglycemic patients with coronary artery disease, insulin resistance (glucose tolerance tests) is associated with severity of the coronary disease documented by coronary arteriography compared to control subjects. Nondiabetic patients with coronary artery disease are insulin-resistant compared to control subjects[68,69].
Clinical manifestations of insulin resistance are associated with subclinical structural damage to the arterial wall
The metabolic syndrome and its individual components are associated with subclinical structural vascular disease that is not explained by conventional cardiovascular risk factors.
The metabolic syndrome: In healthy participants of several studies, including the Atherosclerosis Risk in Communities study, the Baltimore Longitudinal Study on Aging study, and the Multi-Ethnic Study of Atherosclerosis, the metabolic syndrome is independently associated with asymptomatic increased carotid intima-media thickness across all age groups and ethnicities[6,41,59,70]. Likewise, the metabolic syndrome is associated with coronary artery calcification independently of other cardiovascular risk factors in asymptomatic subjects with a family history of premature cardiovascular disease participants of the Study of Inherited Risk of Coronary Atherosclerosis[66].
Subclinical vascular damage is detectable at young age in the presence of metabolic syndrome. Asymptomatic carotid intima-media thickness is increased in children with metabolic syndrome as compared with healthy control children, after adjustment for confounders[71]. Regular exercise over 6 mo improves the metabolic syndrome and reduces carotid intima-media thickness in obese children compared to control subjects[19].
In analyses from four cohort studies (Cardiovascular Risk in Young Finns study, Bogalusa Heart study, Princeton Lipid Research study, Insulin study) with a mean follow-up of 22.3 years, the presence of the metabolic syndrome during childhood is associated with higher carotid intima-media thickness in adulthood[72].
In the Bogalusa Heart study, postmortem examinations performed in children and adolescents from a biracial (African American and Caucasian) community showed that the antemorten presence of the metabolic syndrome (obesity, dyslipemia, and hypertension) strongly predicted the extent of vascular disease in the aorta and coronary arteries[73].
In the Pathobiological Determinants of Atherosclerosis in Youth study, arteries collected from autopsies aged 15-34 years whose deaths were accidental showed that vascular disease in the aorta and right coronary artery is associated with the presence of impaired glucose tolerance, obesity, hypertension, and low HDL-c level. This association is not explained by hypercholesterolemia or smoking[74].
Obesity: In healthy asymptomatic adults, greater BMI and waist-to-hip ratio are independently associated with increased carotid intima-media thickness[70,75]. Increased diameter of the arterial wall associated with obesity is present in several areas of the arterial system, including carotid, femoral and brachial arteries. Across a wide age range, intima-media thickness of several arteries increased with higher BMI in a population-based sample of participants[45].
The independent relationship between obesity and subclinical increased intima-media thickness of carotid and femoral arteries is present in children and adolescents. Obese children have increased carotid and femoral intima-media thickness compared to control children[39,48,50]. In a prospective cohort of children and adolescents, BMI assessed at 11, 15, and 18 years was associated with higher carotid intima-media thickness after controlling for confounders. Overweight/obese subjects had higher carotid intima-media thickness compared to subjects with normal BMI[76].
In analyses from four cohort studies (Cardiovascular Risk in Young Finns study, Bogalusa Heart study, Princeton Lipid Research study, Insulin study) with a mean follow-up of 22.3 years, childhood BMI was associated with higher carotid intima-media thickness in adulthood[72].
Systolic hypertension, dyslipemia, and hyperinsulinemia: Fasting hyperinsulinemia is independently associated with greater carotid intima-media thickness and coronary artery calcification in asymptomatic healthy subjects[6,62,64,70]. The association between hyperinsulinemia and increased carotid intima-media thickness is similar in African American and Caucasian subjects[6,70]. The Mexico City Diabetes study investigated the longitudinal relationship between systolic hypertension and vascular damage in a population-based prospective trial. In normotensive subjects who progress to hypertension (prehypertensive subjects), baseline carotid intima-media thickness increased in comparison with subjects who remained normotensive. After adjusting for multiple cardiovascular risk factors, converter status was independently associated with a higher carotid intima-media thickness[77].
Autopsy examinations from the Pathobiological Determinants of Atherosclerosis in Youth study show that systolic hypertension is associated with greater vascular injury in both the aorta and right coronary artery (particularly fibrous plaques) in subjects throughout the 15-34 year age span. The association of hypertension with vascular damage remained after adjusting for BMI and glycohemoglobin[74].
Longitudinal autopsy studies conducted in children and adults show that low HDL-c is independently associated with vascular disease. The degree of vascular lesions in both the aorta and right coronary artery is negatively associated with serum HDL-c on multiple regression analysis[73,74,78,79].
SUBCLINICAL VASCULAR DISEASE ASSOCIATED WITH INSULIN RESISTANCE PREDICTS CARDIOVASCULAR DISEASE
Subclinical structural and functional vascular dysfunction associated with insulin resistance in otherwise healthy subjects is highly predictive of future cardiovascular events. Reduced vasodilation, loss of arterial distensibility, and increased arterial intima-media thickness in asymptomatic subjects are all associated with future cardiovascular disease.
In a systematic review and meta-analysis of prospective studies, impaired brachial flow-mediated vasodilatation was associated with future cardiovascular events both in asymptomatic and diseased population groups[15]. Impaired nitroglycerin-mediated vasodilatation of the brachial artery has been independently associated with coronary artery calcification in a population-based study[80]. In a prospective study, impaired coronary vasoreactivity was independently associated with a higher incidence of cardiovascular events. Baseline coronary vasoreactivity in response to several stimuli (acetylcholine, sympathetic activation, increased blood flow, and nitroglycerin) predicted incident cardiovascular events at follow-up, after adjustment for traditional cardiovascular risk factors[81].
The ability of arterial stiffness to predict cardiovascular events independently of other cardiovascular risk factors has been documented in cross-sectional and prospective studies, systemic reviews and meta-analyses.
Prospective studies show that increased arterial stiffness (estimated by wide pulse pressure, carotid-femoral pulse-wave velocity, and common carotid distensibility) is a powerful predictor of incident cardiovascular events in asymptomatic individuals from the general population, patients with hypertension, subjects with impaired glucose tolerance, and patients with T2D beyond classic cardiovascular risk factors[82-84]. A systematic review of cross-sectional studies concludes that arterial stiffness is highly predictive of cardiovascular events[12]. A systematic review and meta-analysis of longitudinal studies that followed-up 15877 subjects for a mean of 7.7 years concludes that aortic stiffness (expressed as aortic pulse-wave velocity) is a strong predictor of future cardiovascular events, cardiovascular mortality, and all-cause mortality, independently of classic cardiovascular risk factors. The predictive value of increased arterial stiffness is larger in patients with higher baseline cardiovascular risk states, such as renal disease, coronary artery disease, or hypertension compared with low-risk subjects (general population)[85].
The prospective association between arterial stiffness and postmortem vascular damage was investigated among elderly subjects. There was a weak correlation between baseline arterial stiffness (pulse-wave velocity) and the degree of vascular damage observed at autopsy[86].
A large cross-sectional study with 10828 participants investigated the ability of brachial-ankle pulse-wave velocity for screening cardiovascular risk in the general population. On multivariate analysis, brachial-ankle pulse-wave velocity was associated with cardiovascular risk independently from conventional risk factors[87].
In a population-based cohort study in the elderly (Rotterdam study), arterial stiffness had a strong positive association with structural vascular disease. Aortic and carotid stiffness (assessed by carotid-femoral pulse-wave velocity and common carotid distensibility) was associated with carotid intima-media thickness after adjustment for cardiovascular risk factors[88].
Subclinical carotid intima-media thickness predicts cardiovascular events in healthy subjects and patients with coronary artery disease. A systematic review and meta-analysis concluded that carotid intima-media thickness is a strong independent predictor of future vascular events, although data for younger individuals are limited[89]. A prospective cohort study of women shows that increased carotid intima-media thickness predicts cardiovascular events during 7-year follow-up regardless of glucose tolerance and other cardiovascular risk factors[90]. In a systematic review, population groups with cardiovascular disease had a higher carotid intima-media thickness compared to population groups free of cardiovascular disease[55].
CONCLUSION
Numerous studies provide compelling evidence of an association between insulin resistance and subclinical cardiovascular disease that is not explained by traditional cardiovascular risk factors, such as hypercholesterolemia or smoking. Pathogenic mechanisms underlying vascular damage linked to insulin resistance are undefined. Vascular injury associated with insulin resistance begins early in life and includes impaired vasodilation, loss of arterial distensibility, increased intima-media thickness of the arterial wall and increased arterial calcification. Subclinical vascular dysfunction associated with insulin resistance in otherwise healthy subjects is highly predictive of future cardiovascular events. Reduced vasodilation, loss of arterial distensibility, increased arterial intima-media thickness and vascular calcification in asymptomatic subjects are associated with future cardiovascular disease.
ACKNOWLEDGEMENTS
We wish to thank Ms. Gema Souto for her help during the writing of this manuscript.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: January 17, 2019
First decision: January 25, 2019
Article in press: February 12, 2019
Specialty type: Endocrinology and metabolism
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Koch TR S- Editor: Ji FF L- Editor: A E- Editor: Song H
Contributor Information
María M Adeva-Andany, Internal Medicine Department, Hospital General Juan Cardona, Ferrol 15406, Spain. madevaa@yahoo.com.
Eva Ameneiros-Rodríguez, Internal Medicine Department, Hospital General Juan Cardona, Ferrol 15406, Spain.
Carlos Fernández-Fernández, Internal Medicine Department, Hospital General Juan Cardona, Ferrol 15406, Spain.
Alberto Domínguez-Montero, Internal Medicine Department, Hospital General Juan Cardona, Ferrol 15406, Spain.
Raquel Funcasta-Calderón, Internal Medicine Department, Hospital General Juan Cardona, Ferrol 15406, Spain.
References
- 1.Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32:361–366. doi: 10.2337/dc08-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016;13:e1002039. doi: 10.1371/journal.pmed.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 4.Maple-Brown LJ, Piers LS, O'Rourke MF, Celermajer DS, O'Dea K. Increased arterial stiffness in remote Indigenous Australians with high risk of cardiovascular disease. J Hypertens. 2007;25:585–591. doi: 10.1097/HJH.0b013e328011f766. [DOI] [PubMed] [Google Scholar]
- 5.Jaap AJ, Hammersley MS, Shore AC, Tooke JE. Reduced microvascular hyperaemia in subjects at risk of developing type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1994;37:214–216. doi: 10.1007/s001250050096. [DOI] [PubMed] [Google Scholar]
- 6.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 7.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 8.Johansen NB, Charles M, Vistisen D, Rasmussen SS, Wiinberg N, Borch-Johnsen K, Lauritzen T, Sandbæk A, Witte DR. Effect of intensive multifactorial treatment compared with routine care on aortic stiffness and central blood pressure among individuals with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2012;35:2207–2214. doi: 10.2337/dc12-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Popele NM, Westendorp IC, Bots ML, Reneman RS, Hoeks AP, Hofman A, Grobbee DE, Witteman JC. Variables of the insulin resistance syndrome are associated with reduced arterial distensibility in healthy non-diabetic middle-aged women. Diabetologia. 2000;43:665–672. doi: 10.1007/s001250051356. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Zhao SP, Li XP, Gao M, Zhou QC. Endothelium-dependent and -independent functions are impaired in patients with coronary heart disease. Atherosclerosis. 2000;149:19–24. doi: 10.1016/s0021-9150(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 11.Whincup PH, Gilg JA, Donald AE, Katterhorn M, Oliver C, Cook DG, Deanfield JE. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112:1789–1797. doi: 10.1161/CIRCULATIONAHA.104.532663. [DOI] [PubMed] [Google Scholar]
- 12.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 13.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 14.Adams MR, Robinson J, McCredie R, Seale JP, Sorensen KE, Deanfield JE, Celermajer DS. Smooth muscle dysfunction occurs independently of impaired endothelium-dependent dilation in adults at risk of atherosclerosis. J Am Coll Cardiol. 1998;32:123–127. doi: 10.1016/s0735-1097(98)00206-x. [DOI] [PubMed] [Google Scholar]
- 15.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 16.Su Y, Liu XM, Sun YM, Wang YY, Luan Y, Wu Y. Endothelial dysfunction in impaired fasting glycemia, impaired glucose tolerance, and type 2 diabetes mellitus. Am J Cardiol. 2008;102:497–498. doi: 10.1016/j.amjcard.2008.03.087. [DOI] [PubMed] [Google Scholar]
- 17.Liye H, Lvyun Z, Guangyao S, Luping R. Investigation of early change of endothelial function and related factors in individuals with hyperglycemia. Diabetes Res Clin Pract. 2011;92:194–197. doi: 10.1016/j.diabres.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol. 2006;48:1865–1870. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Tomsa A, Klinepeter Bartz S, Krishnamurthy R, Krishnamurthy R, Bacha F. Endothelial Function in Youth: A Biomarker Modulated by Adiposity-Related Insulin Resistance. J Pediatr. 2016;178:171–177. doi: 10.1016/j.jpeds.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Giltay EJ, Lambert J, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Arterial compliance and distensibility are modulated by body composition in both men and women but by insulin sensitivity only in women. Diabetologia. 1999;42:214–221. doi: 10.1007/s001250051141. [DOI] [PubMed] [Google Scholar]
- 22.Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, Kamp O, Westerhof N, Bouter LM, Stehouwer CD Hoorn Study. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation. 2003;107:2089–2095. doi: 10.1161/01.CIR.0000065222.34933.FC. [DOI] [PubMed] [Google Scholar]
- 23.Dart AM, Kingwell BA. Pulse pressure--a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 24.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Fukui M, Kitagawa Y, Nakamura N, Mogami S, Ohnishi M, Hirata C, Ichio N, Wada K, Kamiuchi K, Shigeta M, Sawada M, Hasegawa G, Yoshikawa T. Augmentation of central arterial pressure as a marker of atherosclerosis in patients with type 2 diabetes. Diabetes Res Clin Pract. 2003;59:153–161. doi: 10.1016/s0168-8227(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 26.Brooks B, Molyneaux L, Yue DK. Augmentation of central arterial pressure in type 1 diabetes. Diabetes Care. 1999;22:1722–1727. doi: 10.2337/diacare.22.10.1722. [DOI] [PubMed] [Google Scholar]
- 27.Shah AS, Wadwa RP, Dabelea D, Hamman RF, D'Agostino R, Jr, Marcovina S, Daniels SR, Dolan LM, Fino NF, Urbina EM. Arterial stiffness in adolescents and young adults with and without type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes. 2015;16:367–374. doi: 10.1111/pedi.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens. 2002;15:16–23. doi: 10.1016/s0895-7061(01)02228-2. [DOI] [PubMed] [Google Scholar]
- 29.Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis. 2003;166:303–309. doi: 10.1016/s0021-9150(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 30.Shin JY, Lee HR, Lee DC. Increased arterial stiffness in healthy subjects with high-normal glucose levels and in subjects with pre-diabetes. Cardiovasc Diabetol. 2011;10:30. doi: 10.1186/1475-2840-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengstock DM, Vaitkevicius PV, Supiano MA. Arterial stiffness is related to insulin resistance in nondiabetic hypertensive older adults. J Clin Endocrinol Metab. 2005;90:2823–2827. doi: 10.1210/jc.2004-1686. [DOI] [PubMed] [Google Scholar]
- 32.Vyssoulis G, Pietri P, Vlachopoulos C, Alexopoulos N, Kyvelou SM, Terentes-Printzios D, Stefanadis C. Early adverse effect of abnormal glucose metabolism on arterial stiffness in drug naive hypertensive patients. Diab Vasc Dis Res. 2012;9:18–24. doi: 10.1177/1479164111422827. [DOI] [PubMed] [Google Scholar]
- 33.Kasayama S, Saito H, Mukai M, Koga M. Insulin sensitivity independently influences brachial-ankle pulse-wave velocity in non-diabetic subjects. Diabet Med. 2005;22:1701–1706. doi: 10.1111/j.1464-5491.2005.01718.x. [DOI] [PubMed] [Google Scholar]
- 34.Park JS, Nam JS, Cho MH, Yoo JS, Ahn CW, Jee SH, Lee HS, Cha BS, Kim KR, Lee HC. Insulin resistance independently influences arterial stiffness in normoglycemic normotensive postmenopausal women. Menopause. 2010;17:779–784. doi: 10.1097/gme.0b013e3181cd3d60. [DOI] [PubMed] [Google Scholar]
- 35.Scuteri A, Tesauro M, Rizza S, Iantorno M, Federici M, Lauro D, Campia U, Turriziani M, Fusco A, Cocciolillo G, Lauro R. Endothelial function and arterial stiffness in normotensive normoglycemic first-degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2008;18:349–356. doi: 10.1016/j.numecd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Sakuragi S, Abhayaratna K, Gravenmaker KJ, O'Reilly C, Srikusalanukul W, Budge MM, Telford RD, Abhayaratna WP. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53:611–616. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 37.Xi B, Zhang L, Mi J. Reduced arterial compliance associated with metabolic syndrome in Chinese children and adolescents. Biomed Environ Sci. 2010;23:102–107. doi: 10.1016/S0895-3988(10)60038-4. [DOI] [PubMed] [Google Scholar]
- 38.Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005;28:1219–1221. doi: 10.2337/diacare.28.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, Romano ML, Panico S, Rubba P, Trevisan M. Increased carotid intima-media thickness and stiffness in obese children. Diabetes Care. 2004;27:2506–2508. doi: 10.2337/diacare.27.10.2506. [DOI] [PubMed] [Google Scholar]
- 40.Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, De Marco D, Mayer MC, De Michele M, Russo V. Preclinical changes in the mechanical properties of abdominal aorta in obese children. Metabolism. 2004;53:1243–1246. doi: 10.1016/j.metabol.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 42.Iannuzzi A, Licenziati MR, Acampora C, Renis M, Agrusta M, Romano L, Valerio G, Panico S, Trevisan M. Carotid artery stiffness in obese children with the metabolic syndrome. Am J Cardiol. 2006;97:528–531. doi: 10.1016/j.amjcard.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 43.Hopkins KD, Lehmann ED, Jones RL, Turay RC, Gosling RG. A family history of NIDDM is associated with decreased aortic distensibility in normal healthy young adult subjects. Diabetes Care. 1996;19:501–503. doi: 10.2337/diacare.19.5.501. [DOI] [PubMed] [Google Scholar]
- 44.Juonala M, Järvisalo MJ, Mäki-Torkko N, Kähönen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–1493. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- 45.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23:1839–1846. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 46.Maple-Brown LJ, Piers LS, O'Rourke MF, Celermajer DS, O'Dea K. Central obesity is associated with reduced peripheral wave reflection in Indigenous Australians irrespective of diabetes status. J Hypertens. 2005;23:1403–1407. doi: 10.1097/01.hjh.0000173524.80802.5a. [DOI] [PubMed] [Google Scholar]
- 47.Greenfield JR, Samaras K, Campbell LV, Jenkins AB, Kelly PJ, Spector TD, Hayward CS. Physical activity reduces genetic susceptibility to increased central systolic pressure augmentation: a study of female twins. J Am Coll Cardiol. 2003;42:264–270. doi: 10.1016/s0735-1097(03)00631-4. [DOI] [PubMed] [Google Scholar]
- 48.Jourdan C, Wühl E, Litwin M, Fahr K, Trelewicz J, Jobs K, Schenk JP, Grenda R, Mehls O, Tröger J, Schaefer F. Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens. 2005;23:1707–1715. doi: 10.1097/01.hjh.0000178834.26353.d5. [DOI] [PubMed] [Google Scholar]
- 49.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kappus RM, Fahs CA, Smith D, Horn GP, Agiovlasitis S, Rossow L, Jae SY, Heffernan KS, Fernhall B. Obesity and overweight associated with increased carotid diameter and decreased arterial function in young otherwise healthy men. Am J Hypertens. 2014;27:628–634. doi: 10.1093/ajh/hpt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42:468–473. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 52.Urbina EM, Khoury PR, McCoy CE, Dolan LM, Daniels SR, Kimball TR. Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics. 2013;131:e1082–e1090. doi: 10.1542/peds.2012-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 54.Peralta CA, Adeney KL, Shlipak MG, Jacobs D, Jr, Duprez D, Bluemke D, Polak J, Psaty B, Kestenbaum BR. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2010;171:63–71. doi: 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Munckhof ICL, Jones H, Hopman MTE, de Graaf J, Nyakayiru J, van Dijk B, Eijsvogels TMH, Thijssen DHJ. Relation between age and carotid artery intima-medial thickness: a systematic review. Clin Cardiol. 2018;41:698–704. doi: 10.1002/clc.22934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laakso M, Sarlund H, Salonen R, Suhonen M, Pyörälä K, Salonen JT, Karhapää P. Asymptomatic atherosclerosis and insulin resistance. Arterioscler Thromb. 1991;11:1068–1076. doi: 10.1161/01.atv.11.4.1068. [DOI] [PubMed] [Google Scholar]
- 57.Agewall S, Fagerberg B, Attvall S, Wendelhag I, Urbanavicius V, Wikstrand J. Carotid artery wall intima-media thickness is associated with insulin-mediated glucose disposal in men at high and low coronary risk. Stroke. 1995;26:956–960. doi: 10.1161/01.str.26.6.956. [DOI] [PubMed] [Google Scholar]
- 58.Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV, Saad MF, Savage P, Bergman R. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93:1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 59.Bertoni AG, Wong ND, Shea S, Ma S, Liu K, Preethi S, Jacobs DR, Jr, Wu C, Saad MF, Szklo M. Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2007;30:2951–2956. doi: 10.2337/dc07-1042. [DOI] [PubMed] [Google Scholar]
- 60.Rajala U, Laakso M, Päivänsalo M, Pelkonen O, Suramo I, Keinänen-Kiukaanniemi S. Low insulin sensitivity measured by both quantitative insulin sensitivity check index and homeostasis model assessment method as a risk factor of increased intima-media thickness of the carotid artery. J Clin Endocrinol Metab. 2002;87:5092–5097. doi: 10.1210/jc.2002-020703. [DOI] [PubMed] [Google Scholar]
- 61.Ryder JR, Dengel DR, Jacobs DR, Jr, Sinaiko AR, Kelly AS, Steinberger J. Relations among Adiposity and Insulin Resistance with Flow-Mediated Dilation, Carotid Intima-Media Thickness, and Arterial Stiffness in Children. J Pediatr. 2016;168:205–211. doi: 10.1016/j.jpeds.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arad Y, Newstein D, Cadet F, Roth M, Guerci AD. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron-beam computed tomographic study. Arterioscler Thromb Vasc Biol. 2001;21:2051–2058. doi: 10.1161/hq1201.100257. [DOI] [PubMed] [Google Scholar]
- 63.Ong KL, McClelland RL, Rye KA, Cheung BM, Post WS, Vaidya D, Criqui MH, Cushman M, Barter PJ, Allison MA. The relationship between insulin resistance and vascular calcification in coronary arteries, and the thoracic and abdominal aorta: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2014;236:257–262. doi: 10.1016/j.atherosclerosis.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meigs JB, Larson MG, D'Agostino RB, Levy D, Clouse ME, Nathan DM, Wilson PW, O'Donnell CJ. Coronary artery calcification in type 2 diabetes and insulin resistance: the framingham offspring study. Diabetes Care. 2002;25:1313–1319. doi: 10.2337/diacare.25.8.1313. [DOI] [PubMed] [Google Scholar]
- 65.Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M Coronary Artery Calcification in Type 1 Diabetes Study. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 66.Reilly MP, Wolfe ML, Rhodes T, Girman C, Mehta N, Rader DJ. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110:803–809. doi: 10.1161/01.CIR.0000138740.84883.9C. [DOI] [PubMed] [Google Scholar]
- 67.Qasim A, Mehta NN, Tadesse MG, Wolfe ML, Rhodes T, Girman C, Reilly MP. Adipokines, insulin resistance, and coronary artery calcification. J Am Coll Cardiol. 2008;52:231–236. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young MH, Jeng CY, Sheu WH, Shieh SM, Fuh MM, Chen YD, Reaven GM. Insulin resistance, glucose intolerance, hyperinsulinemia and dyslipidemia in patients with angiographically demonstrated coronary artery disease. Am J Cardiol. 1993;72:458–460. doi: 10.1016/0002-9149(93)91141-4. [DOI] [PubMed] [Google Scholar]
- 69.Shinozaki K, Suzuki M, Ikebuchi M, Hara Y, Harano Y. Demonstration of insulin resistance in coronary artery disease documented with angiography. Diabetes Care. 1996;19:1–7. doi: 10.2337/diacare.19.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
- 71.Iannuzzi A, Licenziati MR, Acampora C, De Michele M, Iannuzzo G, Chiariello G, Covetti G, Bresciani A, Romano L, Panico S, Rubba P. Carotid artery wall hypertrophy in children with metabolic syndrome. J Hum Hypertens. 2008;22:83–88. doi: 10.1038/sj.jhh.1002289. [DOI] [PubMed] [Google Scholar]
- 72.Koskinen J, Magnussen CG, Sinaiko A, Woo J, Urbina E, Jacobs DR, Jr, Steinberger J, Prineas R, Sabin MA, Burns T, Berenson G, Bazzano L, Venn A, Viikari JSA, Hutri-Kähönen N, Raitakari O, Dwyer T, Juonala M. Childhood Age and Associations Between Childhood Metabolic Syndrome and Adult Risk for Metabolic Syndrome, Type 2 Diabetes Mellitus and Carotid Intima Media Thickness: The International Childhood Cardiovascular Cohort Consortium. J Am Heart Assoc. 2017;6:e005632. doi: 10.1161/JAHA.117.005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 74.McGill HC, Jr, Herderick EE, McMahan CA, Zieske AW, Malcolm GT, Tracy RE, Strong JP. Atherosclerosis in youth. Minerva Pediatr. 2002;54:437–447. [PubMed] [Google Scholar]
- 75.Bonora E, Tessari R, Micciolo R, Zenere M, Targher G, Padovani R, Falezza G, Muggeo M. Intimal-medial thickness of the carotid artery in nondiabetic and NIDDM patients. Relationship with insulin resistance. Diabetes Care. 1997;20:627–631. doi: 10.2337/diacare.20.4.627. [DOI] [PubMed] [Google Scholar]
- 76.Menezes AMB, da Silva CTB, Wehrmeister FC, Oliveira PD, Oliveira IO, Gonçalves H, Assunção MCF, de Castro Justo F, Barros FC. Adiposity during adolescence and carotid intima-media thickness in adulthood: Results from the 1993 Pelotas Birth Cohort. Atherosclerosis. 2016;255:25–30. doi: 10.1016/j.atherosclerosis.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Femia R, Kozakova M, Nannipieri M, Gonzales-Villalpando C, Stern MP, Haffner SM, Ferrannini E. Carotid intima-media thickness in confirmed prehypertensive subjects: predictors and progression. Arterioscler Thromb Vasc Biol. 2007;27:2244–2249. doi: 10.1161/ATVBAHA.107.149641. [DOI] [PubMed] [Google Scholar]
- 78.Solberg LA, Strong JP. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arteriosclerosis. 1983;3:187–198. doi: 10.1161/01.atv.3.3.187. [DOI] [PubMed] [Google Scholar]
- 79.Holme I, Solberg LA, Weissfeld L, Helgeland A, Hjermann I, Leren P, Strong JP, Williams OD. Coronary risk factors and their pathway of action through coronary raised lesions, coronary stenoses and coronary death. Multivariate statistical analysis of an autopsy series: the Oslo Study. Am J Cardiol. 1985;55:40–47. doi: 10.1016/0002-9149(85)90296-6. [DOI] [PubMed] [Google Scholar]
- 80.Kullo IJ, Malik AR, Bielak LF, Sheedy PF 2nd, Turner ST, Peyser PA. Brachial artery diameter and vasodilator response to nitroglycerine, but not flow-mediated dilatation, are associated with the presence and quantity of coronary artery calcium in asymptomatic adults. Clin Sci (Lond) 2007;112:175–182. doi: 10.1042/CS20060131. [DOI] [PubMed] [Google Scholar]
- 81.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 82.Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieère P, Guize L. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. doi: 10.1161/01.hyp.30.6.1410. [DOI] [PubMed] [Google Scholar]
- 83.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 84.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 85.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 86.Sawabe M, Takahashi R, Matsushita S, Ozawa T, Arai T, Hamamatsu A, Nakahara K, Chida K, Yamanouchi H, Murayama S, Tanaka N. Aortic pulse wave velocity and the degree of atherosclerosis in the elderly: a pathological study based on 304 autopsy cases. Atherosclerosis. 2005;179:345–351. doi: 10.1016/j.atherosclerosis.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 87.Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, Yamamoto Y, Hori S. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–622. doi: 10.1291/hypres.26.615. [DOI] [PubMed] [Google Scholar]
- 88.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 89.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt C, Bergström G. Carotid Artery Intima-Media Thickness Predicts Major Cardiovascular Events During 7-Year Follow-Up in 64-Year-Old Women Irrespective of Other Glucometabolic Factors. Angiology. 2017;68:553–558. doi: 10.1177/0003319716672526. [DOI] [PubMed] [Google Scholar]