Abstract
Over the last decade, endoscopic ultrasound-guided biliary drainage (EUS-BD) has evolved into a widely accepted alternative to the percutaneous approach in cases of biliary obstruction with failed endoscopic retrograde cholangiopancreaticography (ERCP). The available evidence suggests that, in experienced hands, EUS-BD might even replace ERCP as the first-line procedure in specific situations such as malignant distal bile duct obstruction. The aim of this review is to summarize the available data on EUS-BD and propose an evidence-based algorithm clarifies the role of the different EUS-BD techniques in the management of benign and malignant biliary obstructive disease.
Keywords: Endoscopic ultrasound, Endoscopic retrograde cholangiopancreaticography, Biliary drainage, Rendez-vous, Hepaticogastrostomy, Choledochoduodenostomy
Core tip: Endoscopic ultrasound-guided biliary drainage (EUS-BD) has recently been introduced as a valuable approach case in patients with failed endoscopic retrograde cholangiopancreaticography (ERCP). Evidence suggests that EUS-BD is equally effective and safer than the percutaneous approach. EUS-BD has even been proposed as a first-line procedure (replacing ERCP) in selected indications. Various approaches for EUS-BD exist, depending on the type (malignant or benign) and location (distal or proximal) of the biliary obstruction, and the anatomy of the upper gastrointestinal tract (surgically altered or not). This review gives an overview of the technique and the available data of EUS-BD in several indications.
INTRODUCTION
Endoscopic biliary drainage, achieved by endoscopic retrograde cholangiopancreaticography (ERCP), has been the established first-line therapy for both benign and malignant biliary obstruction since the beginning of the 1990s. However, even in experienced hands, ERCP fails in 5%-10% of cases[1] because of impossible cannulation or inaccessibility of the major papilla (for example due to tumoral invasion of the ampullary region or surgically altered anatomy). Moreover, ERCP can be complicated by pancreatitis, cholangitis, bleeding, perforation or stent dysfunction requiring reintervention[1,2]. Until recently, percutaneous transhepatic biliary drainage (PTBD) was the only non-surgical alternative to achieve biliary drainage in cases of failed ERCP. However, reported adverse rates of PTBD are high with 1 out of 4 patients suffering from bleeding, bile leak or acute cholangitis after the procedure[3].
Endosonographic-guided biliary drainage (EUS-BD) techniques have recently been introduced as an alternative to PTBD. Over the last decade, increasing operator experience reduced the number of adverse events and augmented technical and clinical success rates of EUS-guided biliary drainage. Many retrospective comparative analyses have concluded that EUS-BD is associated with fewer adverse events as compared to PTBD and should be the treatment of choice in cases of failed ERCP[4].
EUS-BD might even be considered a first-line approach in patients with distal malignant bile duct obstruction. Three randomized controlled trials that compared EUS-BD with ERCP have been published within the last year suggesting that the success-rate of both techniques is similar, but adverse events and reintervention rates might be lower for EUS-BD[5-7]. In other words, the “ERCP-first” paradigm is not sacrosanct, at least for specific indications.
In this review, we describe the different EUS techniques for biliary drainage. Contemporary evidence regarding the efficacy and safety of EUS-BD in benign as well as malignant biliary obstructive diseases is discussed. We highlight the comparison between EUS-BD, PTBD and ERCP. Finally, we provide a practical flowchart that positions EUS-BD in the current therapeutic algorithm of biliary obstruction and conclude with some future perspectives.
SEARCH STRATEGY
We searched for relevant publications using PubMed, EMBASE, and the Cochrane Library, from their inception until Dec 1, 2018. Our search algorithm included the following terms: Endoscopic ultrasound, biliary drainage, ERCP, bile duct, percutaneous, rendez-vous, hepaticogastrostomy, choledochobulbostomy, choledochoduodenostomy, hepatico-enterostomy, choledocho-enterostomy in various combinations. We critically reviewed articles published in English and gave priority to randomized controlled trials and meta-analyses.
TECHNIQUES
General technique
EUS-guided biliary drainage involves the visualization of dilated extra- and or intrahepatic bile ducts and the puncture of these ducts with a needle or a direct access device (LAMS). Puncture of dilated left intrahepatic bile ducts is usually performed from the upper part of the stomach whereas the common bile duct is best accessed from the bulbar portion of the duodenum. Aspiration of bile confirms the position within the bile duct. If necessary contrast injection provides cholangiography to plan the desired intervention. Subsequently, several procedures can be performed, depending on the clinical scenario and the puncture site.
Rendez-vous technique: This technique is mainly used for benign indications when retrograde cannulation of the bile duct fails. A prerequisite for the technique is an endoscopically accessible ampulla or anastomosis. After puncture of the bile duct, a guidewire is advanced via the needle through the ampullary orifice into the duodenum or the surgical anastomosis. While this might be easy in some cases, several challenges may occur. Firstly, with the trans-bulbar approach, the wire may find its way into the intrahepatic bile ducts rather than the ampulla. This can usually be overcome by moving to a long scope position, by deflecting the endoscope tip towards the ampulla or by using a guidewire with an angled tip. Secondly, the wire may not be able to pass the ampullary orifice (due to a distal stricture, impacted stone, ampullary stenosis, etc.). In that case, it might be necessary to insert a papillotome over the wire into the bile duct to further steer and support the wire. Advancement of a papillotome requires a prior cystogastrotomy using a 6 Fr cystogastrotome (preferred) or 4 mm balloon dilatation. Once transpapillary passage of the wire is achieved, the wire should be introduced deeply in the duodenum. The needle and the endoscope are removed leaving the wire in place. Next, a duodenoscope is introduced to visualize the wire protruding from the ampullary orifice. In most cases it is possible to cannulate the bile duct next to the wire. Occasionally, the wire needs to be retrieved using a snare into the endoscope instrument channel. In this way a papillotome can be introduced over the wire directly in the bile duct.
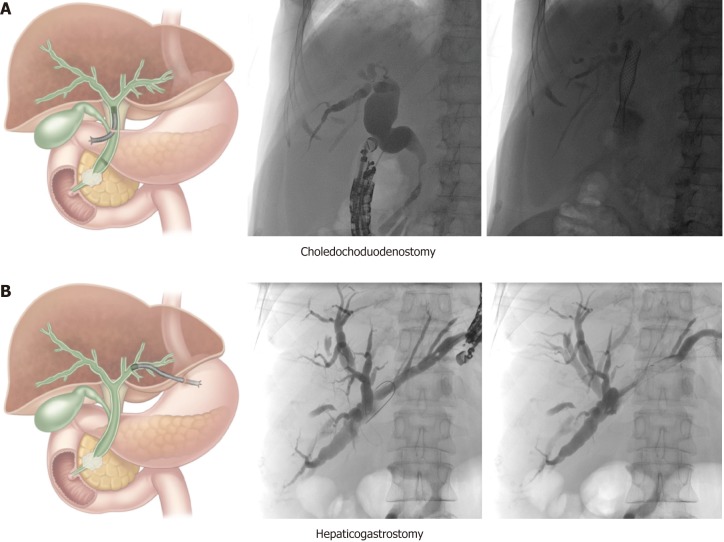
Choledochoduodenostomy (CDS): This technique (Figure 1A) is mainly used for malignant distal bile duct obstruction when the ampulla is not accessible or when retrograde cannulation fails. It is important to verify duodenal patency beforehand, or to place a duodenal stent or an endoscopic gastrojejunostomy if indicated. The conventional technique involves trans-bulbar puncture of the dilated common bile duct, then a guidewire is advanced upstream into an intrahepatic bile duct and the puncture tract is dilated with a cystogastrotome (6 Fr) or a dilation balloon (4 mm). Thereafter, a fully covered metallic stent can be left in place to achieve biliary drainage. Stent migration can be an issue and can be overcome in different ways: By using a long covered metal stent, a LAMS (AxiosR, Boston Scientific, USA; NagiR stent, Taewoong Medical, South Korea) or by placing a partially covered stent with the uncovered portion within the bile duct. However, no short partially covered biliary stents are available at the current time (minimal length is currently 8cm with an uncovered part of 3 cm or 4 cm).
Figure 1.
Schematic and case illustration of the choledochoduodenostomy (A) and the hepaticogastrostomy (B). Patient A had a distal bile duct obstruction due to a locally advanced pancreatic head carcinoma. Patient B had a large perihilar metastasis of a small cell lung carcinoma with a complete obstruction of the proximal common bile duct but preserved left-right intrahepatic bile duct communication. The choledochoduodenostomy can be combined with a duodenal stent or an endoscopic gastrojejunostomy if indicated. Adapted from Paik et al[5].
The novel approach involves the use of a LAMS, with direct puncture of the dilated common bile duct using pure cut current, optional placement of a guidewire and delivery of the LAMS without a further dilation step. This technique is now favored in most centers. An 8 mm or 10 mm diameter stent is usually used, and for safe LAMS placement the diameter of the CBD should exceed 10 mm, to avoid misplacement
Hepaticogastrostomy (HGS): This technique (Figure 1B) can be used for proximal (perihilar) bile duct obstruction when the ampulla is not accessible, when retrograde cannulation fails, or when the left lobe cannot be drained by ERCP. It can also be used for malignant distal bile duct obstruction if the common bile duct is not accessible due to surgically altered anatomy (e.g., after Whipple procedure or roux-en-Y gastric bypass). It is the preferred technique by some experts in any distal malignant obstruction. In cases of perihilar bile duct obstruction, this route of drainage can only drain the left hepatic ducts in case of total hilar obstruction, or both liver lobes in case of left-right biliary communication. After puncture, guidewire introduction and dilatation of the puncture tract (using a 6 Fr cystogastrotome or a 4 mm dilatation balloon), a partially covered stent can be placed, with the uncovered part in the bile duct to prevent migration and the covered part bridging the bile duct and the gastric lumen (Giobor stent, Taewoong Medical, South Korea).
In benign diseases, the HGS can be created with a plastic stent to allow for removal, dilation and sequential repeat access to the bile ducts either for stricture dilatation, or stone lithotripsy.
EUS-guided antegrade transpapillary stent placement: This technique involves the same initial steps as described above for the rendez-vous technique but after placement of the guidewire, a metallic stent is advanced through the ampullary orifice in an antegrade fashion. This is technically more challenging than EUS-guided transmural drainage and does not eliminate the risk of pancreatitis. As such, the technique should be reserved for patients with benign distal bile duct strictures (e.g., in the context of chronic pancreatitis) in whom both the retrograde and rendez-vous approaches have failed. HGS and antegrade stent placement may be combined.
INDICATIONS
Malignant biliary obstructive disease
In 2001, Giovannini et al[8] first reported a successful EUS-guided CDS procedure in a patient with pancreatic carcinoma and distal malignant biliary obstruction after failed ERCP. Two years later Burmester et al[9] published a one-step method without the need for switching from the ERCP to the EUS scope. This was followed by the publication of several small case series and studies demonstrating technical and clinical feasibility of EUS-guided BD for malignant indications after ERCP failure with an acceptable safety profile[10-21]. Due to the small size of the individual studies, the overall efficacy and adverse event profile of EUS-BD had not yet been established. A meta-analysis of Moole et al[22] pooled 16 studies (until January 2016, n = 528)[13,15,18-20,23-30] and reported a 90.9% success rate for rescue EUS-guided BD with an overall procedure related adverse event rate of 16.5%. Khan et al[31] showed very similar results in their meta-analysis that pooled 20 studies (until 2015, n = 1186, 6 studies overlap with Moole et al[22])[21,32-35]. The technical success and post-procedure adverse event rate were 90% and 17%, respectively. Both meta-analyses included studies that evaluated EUS-BD both in benign and malignant indications. In Table 1 all published case series or studies investigating exclusively malignant biliary obstruction are listed (case reports with less than 10 cases are not considered). From this table it is evident that inclusion criteria were not homogenous, different techniques and materials were used and the definition of technical and clinical success was diverse. Some studies examined subpopulations such as patients with altered biliary anatomy[36] or ascites[37]. More recent publications have larger patient cohorts, but the majority are retrospective and single center[29,37-44]. Khashab et al[45] published a larger (n = 96), prospective, multicenter study and demonstrated excellent efficacy and safety of EUS-BD for malignant distal biliary obstruction. It is generally advised that the procedure should be performed by experts in biliopancreatic endoscopy and advanced endoscopic ultrasound.
Table 1.
Outcome of endoscopic ultrasound-guided biliary drainage
| First Author, Yr | Type of study | Type of malignant obstruction | Number patients | Technical Success rate | Clinical Success rate | Adverse events |
| Kanno et al[40], 2018 | (1) Retrospective, single center; and (2) Failed ERCP/inaccessible papilla | Unresectable | 99 | 98% | 93% | Overall: 10% |
| Rai et al[38], 2018 | (1) Retrospective, single center; and (2) Failed ERCP or duodenal obstruction | (1) Unresectable; and (2) Distal | 30 | 93.3% | 93.3% | (1) Overall: 10%; and (2) 83% stent patency (3 mo) |
| Alvarez-Sánchez et al[37], 2018 | (1) Retrospective, single center; and (2) Failed ERCP | (1) With/out ascites; and (2) Distal or proximal | 31; Ascites: 11 | 100% | (1) No ascites: 95%; and (2) Ascites: 64% | (1) No ascites: 20%; and (2) Ascites: 9% |
| Iwashita et al[36], 2017 | (1) Prospective, single center; and (2) Altered anatomy | Unresectable | 20 | 95% | 95% | 20% |
| Minaga et al[52], 2017 | (1) Retrospective, single center; and (2) Failed ERCP | (1) Unresectable; and (2) Hilar obstruction | 30 | 96.7% | 75.9% | (1) Early: 10%; and (2) Late: 23.3% |
| Makmun et al[41], 2017 | (1) Retrospective, single center; and (2) Failed ERCP | Distal and proximal | 24 | 100% | 79.1% | 16.7% |
| Ogura et al[53], 2017 | (1) Retrospective, single center; Failed ERCP | Hilar obstruction | 10 | 100% | 90% | 0% |
| Lu et al[42], 2017 | (1) Retrospective, single center; and (2) Failed ERCP | Distal and proximal | 24 | 95.8% | 100% | 13% |
| Cho et al[51], 2017 | (1) Prospective; and (2) Failed ERCP | 54 | 100% | 94.4% | 16.6% | |
| Amano et al[48], 2017 | Prospective | 20 | 100% | 15% | ||
| Kunda et al[43], 2016 | (1) Retrospective, single center; and (2) Failed ERCP | (1) Unresectable; and (2) Distal | 57 | 98.2% | 94.7% | 7% |
| Nakai et al[61], 2016 | (1) Retrospective, multicenter; and (2) Primary EUS | (1) Unresectable Distal and proximal | 33 | 100% | 100% | 9% |
| Guo et al[44], 2016 | (1) Retrospective, single center; and (2) Failed ERCP | 21 | 100% | 100% | 19% | |
| Khashab et al[45], 2016 | (1) Prospective, multicenter; and (2) Failed ERCP | Distal | 96 | 95.8% | 89.5% | (1) 10.5%; and (2) 86% stent patency (1 yr) |
| Ogura et al[49], 2016 | Retrospective, single center | 39 | (1) CDS: 6%; and (2) HGS: 2% | |||
| Dhir et al[34], 2015 | (1) Retrospective, multicenter; and (2) Failed ERCP | 104 | 95.% | 90.9% | 6.8% | |
| Park et al[47], 2015 | (1) Prospective, randomized; and (2) After failed ERCP | Distal and proximal | 22 | (1) CDS: 92%; and (2) HGS: 100% | (1) CDS: 92%; and (2) HGS: 100% | (1) Early CDS: 25% vs HGS: 0%; and (2) Late CDS: 8.3% vs HGS: 25% |
| Artifon et al[50], 2015 | (1) Prospective, randomized, single center; Failed ERCP | Distal | 49 | (1) CDS: 91%; HGS: 96% | (1) CDS: 77%; HGS: 91% | (1) CDS: 12.5%; and (2) HGS: 20% |
| Dhir et al[33], 2014 | (1) Retrospective, multicenter; and (2) Failed ERCP | Distal and proximal | 68 | 95.6% | 20.6% | |
| Kawakubo et al[32], 2014 | (1) Retrospective, multicenter; and (2) Failed ERCP | Unresectable Proximal and distal | 64 | 95% | 19% | |
| Song et al[21], 2014 | (1) Prospective, single center; and (2) Failed ERCP | Proximal and distal | 27 | 100% | 96.3% | 18.5% |
| Prachayakul et al[35], 2013 | (1) Retrospective, single center; and (2) Failed ERCP | Proximal and distal | 22 | 95.2% | 90.5% | 9.5% |
| Hara et al[62], 2013 | (1) Prospective , single center; and (2) First line | Distal | 18 | 95% | 95% | 11% |
| Khashab et al[45], 2013 | (1) Retrospective, multicenter; and (2) Failed ERCP | Distal | 35 | 97% | 94% | 12% |
| Kim et al[27], 2012 | (1) Retrospective, single center; and (2) Failed ERCP | Proximal and distal | 13 | 92.3% | 91.7% | |
| Iwashita et al[30], 2012 | (1) Retrospective, single center; and (2) Failed ERCP | 40 | 73% | 13% | ||
| Song et al[21], 2012 | (1) Prospective, single center; and (2) Failed ERCP | Distal | 15 | 86.7% | 100% | 23.1% |
| Hara et al[19], 2011 | (1) Prospective, single center; and (2) Failed ERCP | Distal | 18 | 94% | 100% | 17% |
| Ramírez-Luna et al[18], 2011 | (1) Prospective, single center; and (2) Failed ERCP or PTC | 11 | 91% | 90% | n = 2 | |
| Fabbri et al[16], 2011 | (1) Prospective, single center; and (2) Failed ERCP | Proximal and distal | 16 | 100% | 75% | 6.3% |
| Park et al[11], 2009 | (1) Prospective, single center; and (2) Failed ERCP | Distal | 14 | 100% | 100% |
EH: Extrahepatic; IH: Intrahepatic; AG: Antegrade; CDS: Choledochoduodenostomy; HGS: Hepaticogastrostomy; RV: Rendezvous; GG: Gastro-gallbladder; HES: Hepaticoesophageostomy; SEMS: Self-expandable metal.
CDS vs HGS: The meta-analysis by Uemura et al[46] in 2018 (10 studies until April 2017, n = 434 patients)[21,32,35,44,45,47-51] did not demonstrate superiority in terms of technical success (CDS: 94.1% vs HGS: 93.7%) and clinical success (CDS: 88.5% vs HGS: 84.5%) comparing EUS-CDS and EUS-HGS in patients with malignant biliary obstruction (only 2 studies included distal obstruction). They also found both procedures to be equivalent in terms of safety. This is contrary to previously published studies that concluded EUS-HGS was associated with more adverse events[31,33]. The authors proposed that the choice of approach may be selected based on patient anatomy and the presence of bile duct dilatation. For example, EUS-CDS is not suitable for proximal (hilar) biliary obstruction, where an intrahepatic EUS-BD approach is required. In the specific situation of hilar malignancy EUS-guided HGS was found to be safe and effective[52,53], although the duration of efficacy was limited[40] and lower clinical success rates were demonstrated than for distal obstruction[41].
EUS vs PTBD: Two meta-analyses compared EUS-BD and PTBD after failed ERCP or an inaccessible papilla for malignant biliary obstruction (Table 2). In the meta-analysis by Moole et al[22], 3 studies were included[34,54,55]. The pooled odds ratio for successful biliary drainage was higher in EUS-PD versus the PTBD group and the difference for overall procedure related complications was lower. Other studies found EUS-BD to be superior[55] or have comparable efficacy[54] with lower[54] or comparable[54] adverse event rates, need for reintervention and costs. Sharaiha et al[56] included 6 studies[34,54-59] in their meta-analysis (2 studies were published only in abstract form). There was no difference in technical success rates between the two procedures but EUS-BD was associated with better clinical outcomes, fewer post-procedural adverse events and a lower rate of reintervention. They found no difference in length of hospital stay after the procedures, but EUS-BD was more cost-effective[4]. In 2018, a retrospective showed similar results with the additional finding of a shorter hospital stay for EUS-BD[60].
Table 2.
Studies comparing endoscopic ultrasound-guided biliary drainage and percutaneous transhepatic cholangiography
| Author, Yr | Type of study | Type malignant obstruction | Number patients | Technical Succes rate | Clinical Succes rate | Complications, EUS vs PTC |
| Téllez-Ávila et al[60], 2018 | (1) Retrospective; and (2) Failed ERCP | (1) Malignant 56.4%; and (2) Distal | (1) Total: 62; (2) EUS: 30; and (3) PTC: 32 | (1) EUS: 90%; and (2) PTC: 78.1% | (1) EUS: 96%; and (2) PTC: 63% | Overall: 6% vs 28.1% |
| Sportes et al[57], 2017 | (1) Retrospective, multicenter; and (2) Failed ERCP or altered anatomy | (1) Unresectable; and (2) Distal | (1) Total: 51; (2) EUS: 31; and (3) PTC: 20 | (1) EUS: 100%; and (2) PTC: 100% | (1) EUS: 86%; and (2) PTC: 83% | (1) Overall: 16% vs 10%; and (2) Reintervention: 6.5% vs 105% |
| Lee et al[58], 2016 | (1) Randomized, multicenter; and (2) Inaccessible papilla | (1) Unresectable; and (2) Distal | (1) Total: 66; (2) EUS: 34; and (3) PTC: 32 | (1) EUS: 94.1%; and (2) PTC: 96.9% | (1) EUS: 87.5%; and (2) PTC: 87.1% | (1) Overall: 8.8% vs 31.2%; and (2) Reintervention: 25% vs 54.8% |
| Torres-Ruiz, 2016; Abstract | Failed ERCP | Distal and proximal | (1) Total: 66; (2) EUS: 35; and (3) PTC: 31 | (1) EUS: 81%; and (2) PTC: 90.3% | (1) EUS: 90%; and (2) PTC: 68.7% | (1) Early: 10.8% vs 9%; (2) Late: 16.6% vs 54%; and (3) Reintervention: 8.5% vs 45.1% |
| Sharaiha et al[56], 2016 | (1) Retrospective, single center; and (2) Failed ERCP | Malignant: 83.3% | (1) Total: 60; (2) EUS: 47; and (3) PTC: 13 | (1) EUS: 93.3%; and (2) PTC: 91.6% | (1) EUS: 62.2%; and (2) PTC: 25% | (1) Late: 6.6% vs 53.8%; and (2) Reintervention: 6.6% vs 53.8% |
| Bill et al[59], 2015 | (1) Retrospective, single center; and (2) Failed ERCP | Distal | (1) Total: 50; (2) EUS: 25; and (3) PTC: 25 | (1) EUS: 76%; and (2) PTC: 100% | (1) EUS: 96%; and (2) PTC: 80% | (1) Early: 16% vs 12%; (2) Late: 12% vs 5%; and (3) Reintervention: 15.8% vs 60% |
| Giovannini, 2015; Abstract | (1) Randomized, multicenter; and (2) Failed ERCP or impossible | Malignant: 90.2% | (1) Total: 41; (2) EUS: 20; and (3) PTC: 21 | (1) EUS: 95%; and (2) PTC: 100% | (1) EUS: 95%; and (2) PTC: 85% | Overall: 35% vs 60% |
| Khashab et al[45], 2015 | (1) Retrospective, multicenter; and (2) Failed ERCP | Distal | (1) Total: 73; (2) EUS: 22; and (3) PTC: 51 | (1) EUS: 86.4%; and (2) PTC: 100% | (1) EUS: 86.4%; and (2) PTC: 92.2% | (1) Overall: 18.2% vs 39.2%; and (2) Reintervention: 15.7% vs 80.4% |
| Bapaye et al[55], 2013 | (1) Retrospective, single center; and (2) Inaccessible papil | Unresectable | (1) Total: 51; (2) EUS: 25; and (3) PTC: 26 | (1) EUS: 92%; and (2) PTC: 46% | (1) EUS: 92%; and (2) PTC: 46% | Overall: 20% vs 46% |
| Artifon et al[54], 2012 | (1) Prospective, randomized; and (2) Failed ERCP | Unresectable | (1) Total: 25; (2) EUS: 13; and (3) PTC: 12 | (1) CDS: 100%; and (2) PTC: 100% | (1) CDS: 100%; and (2) PTC: 100% | Overall: 15.3% vs 25% |
EH: Extrahepatic; IH: Intrahepatic; AG: Antegrade; CDS: Choledochoduodenostomy; HGS: Hepaticogastrostomy; RV: Rendezvous; GG: Gastro-gallbladder; HES: Hepaticoesophageostomy; SEMS: Self-expandable metal stent.
When ERCP fails to achieve biliary drainage, EUS-guided BD seems preferable over PTBD if the required expertise and logistics are available. The additional advantages are the avoidance of external drainage catheters and the option of performing the procedure under the same sedation as the attempted ERCP.
EUS vs ERCP: A limited number of studies reported results for primary EUS-guided BD without prior ERCP (Table 3). Nakai et al[61] performed EUS-HGS in 33 patients with gastric outlet obstruction, surgically altered anatomy or history of ERCP-related adverse events. The procedure appeared safe and effective. These findings have also been confirmed for primary EUS-CDS[62]. Kawakubo et al[63] found comparable technical success rates with ERCP for EUS-CDS as a first-line treatment for patients with distal malignant biliary obstruction, and a significantly decreased rate of acute pancreatitis in the CDS group.
Table 3.
Studies comparing primary endoscopic ultrasound-guided biliary drainage and endoscopic retrograde cholangiopancreaticography
| First Author, Yr | Type of study | Type malignant obstruction | Number patients | Technical Success rate | Clinical Sucess rate | Adverse events; EUS vs ERCP |
| Paik et al[5], 2018 | Prospective randomized multicenter | Unresectable; Distal | Total: 125; CDS: 32; HGS: 32; ERCP: 61 | EUS: 93.8%; CDS: 90.6%; HGS: 96.9%; ERCP: 90.2% | EUS: 90.0%; ERCP: 94.5% | Overall: 6.3% vs 19.7%; Pancreatitis: 0% vs 14.8%; Reintervention: 15.6% vs 42.6%; Stent patency: 85.1% vs 48.9% |
| Bang et al[6], 2018 | Prospective randomized single center | Pancreatic cancer; Distal | Total: 67; CDS: 33; ERCP: 34 | CDS: 90.9%; ERCP: 94.1% | CDS: 97%; ERCP: 91.2% | Overall: 21.2% vs 14.7%; Reintervention: 3.0% vs 2.9% |
| Park et al[7], 2018 | Prospective randomized single center | Unresectable; Extrahepatic; Distal | Total: 30; CDS: 15; ERCP: 15 | CDS: 92.8%; ERCP: 100% | CDS: 100%; ERCP: 92.8% | Overall: 0% vs 0%; Stent dysfunction: 15.4% vs 30.8% |
| Kawakubo et al[63], 2016 | Retrospective single center | Distal | Total: 82; CDS: 26; ERCP: 56 | CDS: 96.2%; ERCP: 98.2% | Overall: 26.9% vs 35.7%; Pancreatitis: 0% vs 16.1%; Reintervention (1 yr): 16.6% vs 13.6% |
CDS: Choledochoduodenostomy; HGS: Hepaticogastrostomy; SEMS: Self-expandable metal stent.
In 2018, the results of 3 prospective, randomized trials comparing primary EUS-guided BD with ERCP were published. All of them described similar technical success rates and clinical outcomes. Paik et al[5] found lower rate of adverse events in the EUS-guided BD group, including post-procedural pancreatitis. This study did not exclude patients with duodenal obstruction or altered anatomy and also performed EUS-HGS. The study demonstrated a lower need for reintervention and higher rate of stent patency in the EUS-guided BD group. The latter finding was attributed to lower risk of tumor ingrowth and/or overgrowth with transmural stenting bypassing the site of malignancy[5]. Bang et al[6] and Park et al[7] reported similar rates of adverse events, reinterventions and stent patency. In the EUS-guided BD group stent occlusion was commonly caused by migration[63] or food impaction[6]. Paik et al[5] reported that the median procedure time and length of hospital stay was shorter with EUS-BD. Park et al[7] found no difference in procedure time between the techniques.
Taking these studies together it would be reasonable to consider EUS-BD as the primary biliary drainage approach in certain situations where the risk of ERCP failure or adverse events is substantial.
Benign biliary obstructive disease
The first report on EUS-BD for benign biliary obstructive disease was published in 2005[64]. In this report, a “neopapilla” was created under endoscopic ultrasound guidance near to the original papilla to extract bile duct stones. After this report, several case series describing the EUS-ERCP rendez-vous technique included patients with benign diseases such as bile duct stones or ampullary stenosis[26,65,66]. A hepaticogastrostomy has been proposed as a technique to obtain biliary access for antegrade interventions (stone extraction, dilatation of the bilioenteric anastomosis, etc.) in patients with surgically altered anatomy (roux-en-y gastric bypass, Whipple intervention, etc.)[67,68].
COMPLICATIONS
Despite the fact that procedure-related complications of EUS-BD appear to be lower than for PTBD and potentially also than for ERCP in selected indications (see above), it remains an invasive procedure with potentially serious adverse events. These may include a pneumoperitoneum (always perform the procedure under CO2 insufflation), bile peritonitis, biliary gastritis, haemorrhage, cholangitis, stent obstruction and (life-treatening) stent migration. Procedure-related deaths have been reported[22,69]. The adverse event rate tends to decrease with the learning curve[22]. For this reason, we believe that EUS-BD should only be performed in referral centres with high volume experience in EUS and ERCP.
CONCLUSION
In patients with malignant bile duct obstruction, EUS-BD is a viable option in cases where ERCP has failed, in the context of surgically altered anatomy or in patients with an inaccessible papilla due to tumoral invasion. Based on the results of three randomized studies, EUS-BD might be a reasonable alternative to ERCP as the first-line procedure in patients with distal malignant bile duct obstruction.
The role of EUS in establishing biliary drainage where obstruction is due to a benign aetiology is rather limited (less than 5% in most case series)[22]. A rendez-vous approach can be particularly useful in patients with an accessible duodenum in whom the papilla cannot be identified or cannulated (e.g., in the case of a large duodenal diverticulum). Temporary transmural drainage with a choledochoduodenostomy or hepaticogastrostomy and subsequent antegrade treatment after the fistula tract has matured has been described (especially in patients with altered anatomy due to previous surgery) but should be reserved for cases in which less invasive alternatives have failed.
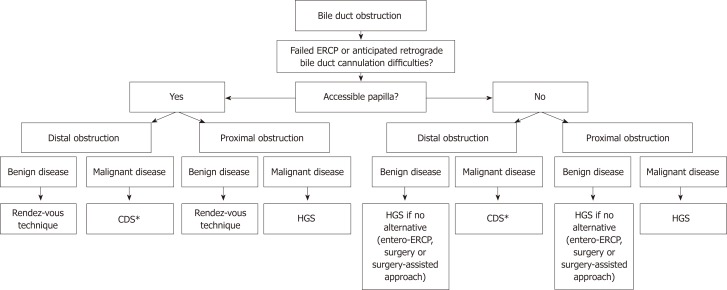
In Figure 2, we propose a practical flowchart that suggests roles for EUS-BD within the current management algorithm of benign and malignant biliary obstruction.
Figure 2.
Proposed algorithm that positions endoscopic ultrasound-guided biliary drainage in the current management of biliary obstructive disease. *The choledochoduodenostomy can be combined with a duodenal stent or an endoscopic gastrojejunostomy if indicated. CDS: Choledochoduodenostomy; HGS: Hepaticogastrostomy; ERCP: Endoscopic retrograde cholangiopancreaticography.
Given the development of EUS-BD over the last few years, it is anticipated that novel dedicated endoscopic devices and tools will be released. New LAMS allowing puncture, tract dilatation and stent delivery in one step, provide significant advantages over needle/guidewire/dilation and stent delivery techniques. A steerable wire specifically designed for EUS-BD is being developed (oral communication, Boston Scientific, Massachusetts, USA). A 4 cm partially covered stent for CDS is expected mid 2019 (oral communication, Taewoong Medical, South Korea). A one-step dedicated stent introducer with a push-type dilator without the need for pre-dilatation or use of electrocautery has recently been described and will, when it becomes available, further facilitate EUS-guided transmural biliary drainage[5].
Future studies should address whether EUS-BD should be the first-line therapy (rather than ERCP or PTBD) in patients with malignant bile duct obstruction with preserved left-right intrahepatic bile duct communication.
Footnotes
Conflict-of-interest statement: None of the authors report conflicts of interest with regard to this manuscript.
Manuscript source: Invited manuscript
Peer-review started: December 21, 2019
First decision: January 6, 2019
Article in press: February 13, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gong JS, Tan HJ S- Editor: Wang JL L- Editor: A E- Editor: Tan WW
Contributor Information
Pieter Hindryckx, Department of Gastroenterology, University Hospital of Ghent, Ghent 9000, Belgium. pieter.hindryckx@uzgent.be.
Helena Degroote, Department of Gastroenterology, University Hospital of Ghent, Ghent 9000, Belgium.
David J Tate, Department of Gastroenterology, University Hospital of Ghent, Ghent 9000, Belgium.
Pierre H Deprez, Hepato-Gastroenterology Department, Cliniques universitaires Saint-Luc, Brussels 1200, Belgium.
References
- 1.Enochsson L, Swahn F, Arnelo U, Nilsson M, Löhr M, Persson G. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest Endosc. 2010;72:1175–1184, 1184.e1-1184.e3. doi: 10.1016/j.gie.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–1788. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 3.Nennstiel S, Weber A, Frick G, Haller B, Meining A, Schmid RM, Neu B. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J Clin Gastroenterol. 2015;49:764–770. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 4.Sharaiha RZ, Khan MA, Kamal F, Tyberg A, Tombazzi CR, Ali B, Tombazzi C, Kahaleh M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–914. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Paik WH, Lee TH, Park DH, Choi JH, Kim SO, Jang S, Kim DU, Shim JH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol. 2018;113:987–997. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 6.Bang JY, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos) Gastrointest Endosc. 2018;88:9–17. doi: 10.1016/j.gie.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Park JK, Woo YS, Noh DH, Yang JI, Bae SY, Yun HS, Lee JK, Lee KT, Lee KH. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc. 2018;88:277–282. doi: 10.1016/j.gie.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 9.Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246–251. doi: 10.1067/mge.2003.85. [DOI] [PubMed] [Google Scholar]
- 10.Yamao K, Bhatia V, Mizuno N, Sawaki A, Ishikawa H, Tajika M, Hoki N, Shimizu Y, Ashida R, Fukami N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40:340–342. doi: 10.1055/s-2007-995485. [DOI] [PubMed] [Google Scholar]
- 11.Park DH, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168–2174. doi: 10.1038/ajg.2009.254. [DOI] [PubMed] [Google Scholar]
- 12.Hanada K, Iiboshi T, Ishii Y. Endoscopic ultrasound-guided choledochoduodenostomy for palliative biliary drainage in cases with inoperable pancreas head carcinoma. Dig Endosc. 2009;21 Suppl 1:S75–S78. doi: 10.1111/j.1443-1661.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232–236. doi: 10.1055/s-0029-1243858. [DOI] [PubMed] [Google Scholar]
- 14.Artifon EL, Takada J, Okawa L, Moura EG, Sakai P. EUS-guided choledochoduodenostomy for biliary drainage in unresectable pancreatic cancer: a case series. JOP. 2010;11:597–600. [PubMed] [Google Scholar]
- 15.Park DH, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos) Gastrointest Endosc. 2010;71:413–419. doi: 10.1016/j.gie.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Fabbri C, Luigiano C, Fuccio L, Polifemo AM, Ferrara F, Ghersi S, Bassi M, Billi P, Maimone A, Cennamo V, Masetti M, Jovine E, D'Imperio N. EUS-guided biliary drainage with placement of a new partially covered biliary stent for palliation of malignant biliary obstruction: a case series. Endoscopy. 2011;43:438–441. doi: 10.1055/s-0030-1256097. [DOI] [PubMed] [Google Scholar]
- 17.Belletrutti PJ, DiMaio CJ, Gerdes H, Schattner MA. Endoscopic ultrasound guided biliary drainage in patients with unapproachable ampullae due to malignant duodenal obstruction. J Gastrointest Cancer. 2011;42:137–142. doi: 10.1007/s12029-010-9175-7. [DOI] [PubMed] [Google Scholar]
- 18.Ramírez-Luna MA, Téllez-Ávila FI, Giovannini M, Valdovinos-Andraca F, Guerrero-Hernández I, Herrera-Esquivel J. Endoscopic ultrasound-guided biliodigestive drainage is a good alternative in patients with unresectable cancer. Endoscopy. 2011;43:826–830. doi: 10.1055/s-0030-1256406. [DOI] [PubMed] [Google Scholar]
- 19.Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y, Matumoto K, Bhatia V, Shimizu Y, Ito A, Hirooka Y, Goto H. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239–1245. doi: 10.1038/ajg.2011.84. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui AA, Sreenarasimhaiah J, Lara LF, Harford W, Lee C, Eloubeidi MA. Endoscopic ultrasound-guided transduodenal placement of a fully covered metal stent for palliative biliary drainage in patients with malignant biliary obstruction. Surg Endosc. 2011;25:549–555. doi: 10.1007/s00464-010-1216-6. [DOI] [PubMed] [Google Scholar]
- 21.Song TJ, Hyun YS, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided choledochoduodenostomies with fully covered self-expandable metallic stents. World J Gastroenterol. 2012;18:4435–4440. doi: 10.3748/wjg.v18.i32.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moole H, Bechtold ML, Forcione D, Puli SR. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine (Baltimore) 2017;96:e5154. doi: 10.1097/MD.0000000000005154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poincloux L, Rouquette O, Buc E, Privat J, Pezet D, Dapoigny M, Bommelaer G, Abergel A. Endoscopic ultrasound-guided biliary drainage after failed ERCP: cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794–801. doi: 10.1055/s-0034-1391988. [DOI] [PubMed] [Google Scholar]
- 24.Will U, Fueldner F, Kern C, Meyer F. EUS-Guided Bile Duct Drainage (EUBD) in 95 Patients. Ultraschall Med. 2015;36:276–283. doi: 10.1055/s-0034-1366557. [DOI] [PubMed] [Google Scholar]
- 25.Will U, Thieme A, Fueldner F, Gerlach R, Wanzar I, Meyer F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39:292–295. doi: 10.1055/s-2007-966215. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson JA, Johnstone M, Raraty MG, Evans JC. Endoscopic ultrasound-guided choledoco-duodenostomy as an alternative to percutaneous trans-hepatic cholangiography. HPB (Oxford) 2012;14:483–486. doi: 10.1111/j.1477-2574.2012.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010;42:496–502. doi: 10.1055/s-0029-1244082. [DOI] [PubMed] [Google Scholar]
- 28.Park DH, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–1284. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 29.Bories E, Pesenti C, Caillol F, Lopes C, Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007;39:287–291. doi: 10.1055/s-2007-966212. [DOI] [PubMed] [Google Scholar]
- 30.Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60–65. doi: 10.1055/s-0030-1256871. [DOI] [PubMed] [Google Scholar]
- 31.Khan MA, Akbar A, Baron TH, Khan S, Kocak M, Alastal Y, Hammad T, Lee WM, Sofi A, Artifon EL, Nawras A, Ismail MK. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:684–703. doi: 10.1007/s10620-015-3933-0. [DOI] [PubMed] [Google Scholar]
- 32.Kawakubo K, Isayama H, Kato H, Itoi T, Kawakami H, Hanada K, Ishiwatari H, Yasuda I, Kawamoto H, Itokawa F, Kuwatani M, Iiboshi T, Hayashi T, Doi S, Nakai Y. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328–334. doi: 10.1002/jhbp.27. [DOI] [PubMed] [Google Scholar]
- 33.Dhir V, Artifon EL, Gupta K, Vila JJ, Maselli R, Frazao M, Maydeo A. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430–435. doi: 10.1111/den.12153. [DOI] [PubMed] [Google Scholar]
- 34.Dhir V, Itoi T, Khashab MA, Park DH, Yuen Bun Teoh A, Attam R, Messallam A, Varadarajulu S, Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913–923. doi: 10.1016/j.gie.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 35.Prachayakul V, Aswakul P. A novel technique for endoscopic ultrasound-guided biliary drainage. World J Gastroenterol. 2013;19:4758–4763. doi: 10.3748/wjg.v19.i29.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwashita T, Yasuda I, Mukai T, Iwata K, Doi S, Uemura S, Mabuchi M, Okuno M, Shimizu M. Endoscopic ultrasound-guided antegrade biliary stenting for unresectable malignant biliary obstruction in patients with surgically altered anatomy: Single-center prospective pilot study. Dig Endosc. 2017;29:362–368. doi: 10.1111/den.12800. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Sánchez MV, Luna OB, Oria I, Marchut K, Fumex F, Singier G, Salgado A, Napoléon B. Feasibility and Safety of Endoscopic Ultrasound-Guided Biliary Drainage (EUS-BD) for Malignant Biliary Obstruction Associated with Ascites: Results of a Pilot Study. J Gastrointest Surg. 2018;22:1213–1220. doi: 10.1007/s11605-018-3731-z. [DOI] [PubMed] [Google Scholar]
- 38.Rai P, Lokesh CR, Goel A, Aggarwal R. Endoscopic ultrasound-guided choledochoduodenostomy using partially-covered self-expandable metal stent in patients with malignant distal biliary obstruction and unsuccessful ERCP. Endosc Int Open. 2018;6:E67–E72. doi: 10.1055/s-0043-120664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mora Soler AM, Álvarez Delgado A, Piñero Pérez MC, Velasco-Guardado A, Marcos Prieto H, Rodríguez Pérez A. Endoscopic ultrasound-guided choledochoduodenostomy after a failed or impossible ERCP. Rev Esp Enferm Dig. 2018;110:299–305. doi: 10.17235/reed.2018.5040/2017. [DOI] [PubMed] [Google Scholar]
- 40.Kanno Y, Ito K, Koshita S, Ogawa T, Masu K, Kusunose H, Sakai T, Masaki Y, Murabayashi T, Hasegawa S, Kozakai F, Horaguchi J, Matsuo H, Noda Y. EUS-guided Biliary Drainage for Malignant Perihilar Biliary Strictures after Further Transpapillary Intervention Has Been Judged to Be Impossible or Ineffective. Intern Med. 2017;56:3145–3151. doi: 10.2169/internalmedicine.9001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makmun D, Fauzi A, Abdullah M, Syam AF. The Role of EUS-BD in the Management of Malignant Biliary Obstruction: The Indonesian Perspective. Diagn Ther Endosc. 2017;2017:4856276. doi: 10.1155/2017/4856276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Tang X, Jin H, Yang J, Zhang X. Endoscopic Ultrasound-Guided Biliary Drainage Using Self-Expandable Metal Stent for Malignant Biliary Obstruction. Gastroenterol Res Pract. 2017;2017:6284094. doi: 10.1155/2017/6284094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunda R, Pérez-Miranda M, Will U, Ullrich S, Brenke D, Dollhopf M, Meier M, Larghi A. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc. 2016;30:5002–5008. doi: 10.1007/s00464-016-4845-6. [DOI] [PubMed] [Google Scholar]
- 44.Guo J, Sun S, Liu X, Wang S, Ge N, Wang G. Endoscopic Ultrasound-Guided Biliary Drainage Using a Fully Covered Metallic Stent after Failed Endoscopic Retrograde Cholangiopancreatography. Gastroenterol Res Pract. 2016;2016:9469472. doi: 10.1155/2016/9469472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khashab MA, Van der Merwe S, Kunda R, El Zein MH, Teoh AY, Marson FP, Fabbri C, Tarantino I, Varadarajulu S, Modayil RJ, Stavropoulos SN, Peñas I, Ngamruengphong S, Kumbhari V, Romagnuolo J, Shah R, Kalloo AN, Perez-Miranda M, Artifon EL. Prospective international multicenter study on endoscopic ultrasound-guided biliary drainage for patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Endosc Int Open. 2016;4:E487–E496. doi: 10.1055/s-0042-102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uemura RS, Khan MA, Otoch JP, Kahaleh M, Montero EF, Artifon ELA. EUS-guided Choledochoduodenostomy Versus Hepaticogastrostomy: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2018;52:123–130. doi: 10.1097/MCG.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 47.Park DH, Lee TH, Paik WH, Choi JH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. Feasibility and safety of a novel dedicated device for one-step EUS-guided biliary drainage: A randomized trial. J Gastroenterol Hepatol. 2015;30:1461–1466. doi: 10.1111/jgh.13027. [DOI] [PubMed] [Google Scholar]
- 48.Amano M, Ogura T, Onda S, Takagi W, Sano T, Okuda A, Miyano A, Masuda D, Higuchi K. Prospective clinical study of endoscopic ultrasound-guided biliary drainage using novel balloon catheter (with video) J Gastroenterol Hepatol. 2017;32:716–720. doi: 10.1111/jgh.13489. [DOI] [PubMed] [Google Scholar]
- 49.Ogura T, Chiba Y, Masuda D, Kitano M, Sano T, Saori O, Yamamoto K, Imaoka H, Imoto A, Takeuchi T, Fukunishi S, Higuchi K. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy. 2016;48:156–163. doi: 10.1055/s-0034-1392859. [DOI] [PubMed] [Google Scholar]
- 50.Artifon EL, Marson FP, Gaidhane M, Kahaleh M, Otoch JP. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: is there any difference? Gastrointest Endosc. 2015;81:950–959. doi: 10.1016/j.gie.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 51.Cho DH, Lee SS, Oh D, Song TJ, Park DH, Seo DW, Lee SK, Kim MH. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos) Gastrointest Endosc. 2017;85:1067–1075. doi: 10.1016/j.gie.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Minaga K, Takenaka M, Kitano M, Chiba Y, Imai H, Yamao K, Kamata K, Miyata T, Omoto S, Sakurai T, Watanabe T, Nishida N, Kudo M. Rescue EUS-guided intrahepatic biliary drainage for malignant hilar biliary stricture after failed transpapillary re-intervention. Surg Endosc. 2017;31:4764–4772. doi: 10.1007/s00464-017-5553-6. [DOI] [PubMed] [Google Scholar]
- 53.Ogura T, Onda S, Takagi W, Sano T, Okuda A, Masuda D, Yamamoto K, Miyano A, Kitano M, Takeuchi T, Fukunishi S, Higuchi K. Clinical utility of endoscopic ultrasound-guided biliary drainage as a rescue of re-intervention procedure for high-grade hilar stricture. J Gastroenterol Hepatol. 2017;32:163–168. doi: 10.1111/jgh.13437. [DOI] [PubMed] [Google Scholar]
- 54.Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–774. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 55.Bapaye A, Dubale N, Aher A. Comparison of endosonography-guided vs. percutaneous biliary stenting when papilla is inaccessible for ERCP. United European Gastroenterol J. 2013;1:285–293. doi: 10.1177/2050640613490928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharaiha RZ, Kumta NA, Desai AP, DeFilippis EM, Gabr M, Sarkisian AM, Salgado S, Millman J, Benvenuto A, Cohen M, Tyberg A, Gaidhane M, Kahaleh M. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc. 2016;30:5500–5505. doi: 10.1007/s00464-016-4913-y. [DOI] [PubMed] [Google Scholar]
- 57.Sportes A, Camus M, Greget M, Leblanc S, Coriat R, Hochberger J, Chaussade S, Grabar S, Prat F. Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography: a retrospective expertise-based study from two centers. Therap Adv Gastroenterol. 2017;10:483–493. doi: 10.1177/1756283X17702096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee TH, Choi JH, Park do H, Song TJ, Kim DU, Paik WH, Hwangbo Y, Lee SS, Seo DW, Lee SK, Kim MH. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–1019.e3. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 59.Bill JG, Darcy M, Fujii-Lau LL, Mullady DK, Gaddam S, Murad FM, Early DS, Edmundowicz SA, Kushnir VM. A comparison between endoscopic ultrasound-guided rendezvous and percutaneous biliary drainage after failed ERCP for malignant distal biliary obstruction. Endosc Int Open. 2016;4:E980–E985. doi: 10.1055/s-0042-112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Téllez-Ávila FI, Herrera-Mora D, Duarte-Medrano G, Lopez-Arce G, Lindoro-Barraza D, Casanova I, Elizondo-Rivera J, Ramírez-Luna M, Valdovinos-Andraca F. Biliary Drainage in Patients With Failed ERCP: Percutaneous Versus EUS-guided Drainage. Surg Laparosc Endosc Percutan Tech. 2018;28:183–187. doi: 10.1097/SLE.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 61.Nakai Y, Isayama H, Yamamoto N, Matsubara S, Ito Y, Sasahira N, Hakuta R, Umefune G, Takahara N, Hamada T, Mizuno S, Kogure H, Tada M, Koike K. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy. 2016;48:1125–1128. doi: 10.1055/s-0042-116595. [DOI] [PubMed] [Google Scholar]
- 62.Hara K, Yamao K, Hijioka S, Mizuno N, Imaoka H, Tajika M, Kondo S, Tanaka T, Haba S, Takeshi O, Nagashio Y, Obayashi T, Shinagawa A, Bhatia V, Shimizu Y, Goto H, Niwa Y. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013;45:392–396. doi: 10.1055/s-0032-1326076. [DOI] [PubMed] [Google Scholar]
- 63.Kawakubo K, Kawakami H, Kuwatani M, Kubota Y, Kawahata S, Kubo K, Sakamoto N. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy. 2016;48:164–169. doi: 10.1055/s-0034-1393179. [DOI] [PubMed] [Google Scholar]
- 64.Püspök A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005;100:1743–1747. doi: 10.1111/j.1572-0241.2005.41806.x. [DOI] [PubMed] [Google Scholar]
- 65.Park DH, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video) Gastrointest Endosc. 2013;78:91–101. doi: 10.1016/j.gie.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 66.Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos) Gastrointest Endosc. 2012;75:354–359. doi: 10.1016/j.gie.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 67.Iwashita T, Yasuda I, Mukai T, Doi S, Uemura S, Mabuchi M, Shimizu M. Successful management of biliary stones in the hepatic duct after a Whipple procedure by using an EUS-guided antegrade approach and temporary metal stent placement. Gastrointest Endosc. 2014;80:337. doi: 10.1016/j.gie.2014.05.317. [DOI] [PubMed] [Google Scholar]
- 68.Weilert F. Prospective evaluation of simplified algorithm for EUS-guided intra-hepatic biliary access and anterograde interventions for failed ERCP. Surg Endosc. 2014;28:3193–3199. doi: 10.1007/s00464-014-3588-5. [DOI] [PubMed] [Google Scholar]
- 69.Martins FP, Rossini LG, Ferrari AP. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: fatal complication. Endoscopy. 2010;42 Suppl 2:E126–E127. doi: 10.1055/s-0029-1243911. [DOI] [PubMed] [Google Scholar]