Abstract
Soy-based formula contains high concentrations of the isoflavone genistein. Genistein possesses estrogenic and tyrosine kinase inhibitory activity and interferes with cellular proliferation and development. To date, the acute and chronic effects of genistein on ovarian and uterine development have not been fully elucidated. In this study, mice at postnatal day 1 were subcutaneously injected with 100 mg/kg genistein for 10 consecutive days, and then their ovaries and uteri were collected on days 10, 21, and 90. Histological evaluation was performed after hematoxylin and eosin staining. The proliferating activity was indicated by the proliferating indicator protein Ki67. Results showed that the subcutaneous injection of genistein to neonatal mice induced the formation of multi-oocyte follicles and delayed the primordial follicle assembly in the ovaries. Genistein significantly enlarged the cross-sectional area of the uterine cavity and wall and disrupted the regularity between the uterine stroma and myometrium. Genistein exposure inhibited proliferative activity because fewer Ki67-positive nuclei were detected in ovarian and uterine cell populations than in the control. Furthermore, most ovaries from adult mice given neonatal genistein were without corpora lutea, and there appeared to be cystic follicles and hypertrophy of the theca, and cortical and medullary layers. Considering the high concentration of isoflavone in soy-based infant formulas and livestock feed, we suggest that the use of isoflavone-rich diets in humans and livestock receive closer examination.
Keywords: Genistein, Ki67, Morphology, Ovary, Uterus
Genistein is an isoflavone phytoestrogen commonly found in plants and plant-based products and is especially abundant in soy and soy-derived products. Human exposure to genistein occurs primarily via the consumption of soy and soy-based dietary products, and livestock are also commonly exposed to genistein via soybean-rich diets. Many beneficial effects of genistein supplements have been reported, including relieving menopausal symptoms, protecting the cardiovascular system, and preventing breast cancer [1,2,3]. However, it can also act as an endocrine-disrupting chemical due to its estrogenic activity [4], and therefore questions are currently being raised about genistein. Many adverse effects have been observed after genistein exposure at distinct stages during the lifespan of animals [5]. Of greater concern is what might happen during the early postnatal developmental period, which is a particularly vulnerable stage of life. Endogenous estrogen production at this stage is low, allowing genistein to more freely bind to estrogen receptors (ERs) in estrogen-sensitive tissues, and thus to exert its maximal ER-mediated effects. Additionally, infants consuming soy-based formulas have plasma concentrations of total genistein of approximately 1–5 µM and even up to 10 µM, which is several orders of magnitude greater than that of breast-fed or cow milk formula-fed infants [6,7,8]. Therefore, exposure to genistein earlier in life may produce more serious pathologies and lead to permanent structural and/or functional changes.
Animal studies suggest that neonatal treatment with genistein causes a series of adverse consequences on the development of the female reproductive system. Moreover, alterations in ovarian differentiation have been observed in mice treated neonatally with genistein, including formation of multi-oocyte follicles (MOFs) and oocyte nest breakdown [9]. The time of vaginal opening was advanced in female rats after neonatal exposure to genistein [10]. Additionally, fertility in adult rodents was reduced after neonatal genistein exposure, which was closely related to the disruption of estrous cyclicity [11], ovulation failure [12, 13], poor oocyte quality [14], and microenvironmental alterations of the reproductive tract [15, 16]. However, these studies showed limited data that described the state of the corresponding cells for genistein-caused pathologies.
The primary mechanism for the action of genistein is interference with estrogen signaling because the structural similarity of genistein to estradiol allows it to bind to nuclear ERs (ERα and ERβ) [17]. Once it binds to these ERs, it can initiate transcription classically by interacting with estrogen-response elements or binding early immediate genes, and ultimately function as an estrogen agonist or antagonist to regulate cellular growth, proliferation, and development in the target tissue [4]. The distribution of ERα and ERβ varies across tissues and cell types [18]. In the ovary, ERα is generally localized in the interstitium and theca cells (TCs), and ERβ is highly expressed in granulosa cells (GCs), whereas in the uterus, all cells (luminal and glandular epithelial, and stromal and myometrial cells) express only ERα. Furthermore, genistein possesses distinct binding affinity for ERα (4%) and ERβ (87%) [19]. The ability of genistein to inhibit tyrosine-specific kinases can act on the process of cell proliferation and differentiation [20], which is independent of its ER-mediated estrogenic activity [21, 22]. Based on this specificity and complexity, we hypothesized that there would be different genistein-mediated cell vitality during the development of various target tissues.
Therefore, the aim of the present study was to explore the relationship between the genistein-mediated cell activity and the genistein-caused histomorphological changes in reproductive tissues. Considering the susceptibility of the neonatal period, genistein exposure was undertaken on postnatal day 1 (PND 1) mice and lasted for 10 days. The serum circulating level of genistein following subcutaneous injection of 50 mg/kg·day is 6.8 ± 1.4 µM [23, 24], which is approximately half the level in an infant fed exclusively on soy-based formula [8]. In addition, genistein over a physiologically relevant concentration range has been proven to have cell growth-inhibitory actions in human breast cancer cells [25, 26]. Therefore, experiments were performed under a higher dosage (100 mg/kg) to ensure the plasma genistein concentration would reach up to 10 µM.
Materials and Methods
Animal care and experimental design
Forty female Institute of Cancer Research mice (Qinglongshan Experimental Animal, Nanjing, China) were fed in an animal room with moderate light (12 h light:12 h dark) and suitable temperature (23 ± 2°C). To ensure consistent estrous cyclicity, we placed all female mice in the same cage during the adaptation phase. After 2 weeks, estrous females were identified by vaginal observation, and then cohabited for 24 h with mature male mice in a 2:1 ratio. After 18–20 days of gestation, the pregnant dams delivered naturally. A total of 80 female pups of similar weight were randomly selected and pooled together, and then we randomly allocated 10 pups per litter. The experimental mice (50 individuals in total) were subcutaneously injected with 100 mg genistein/kg body weight for 10 days from PND 1 to PND 10, and the control mice (30 individuals in total) were treated with an equal amount of vehicle (Dimethyl Sulfoxide + peanut oil). The genistein was purchased from Sigma-Aldrich (≥ 98% pure; G6649; Sigma Chemical, St Louis, MO, USA). To ensure adequate dissolution and dilution of genistein, we followed previously published methods [23]. All experiments were performed according to the guidelines of the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Nanjing Agricultural University, China.
Sample collection
A total of 10 genistein-treated and 6 control mice at each age (across a total of 4 ages: PND 3, PND 10, PND 21, and PND 90) were randomly selected and sacrificed to collect ovaries and uteri. Subsequently, these tissues were fixed in 4% paraformaldehyde at room temperature (25–30°C) for 18 h, and then embedded in paraffin after dehydration and permeabilization for histopathologic observations and immunohistochemical staining. The remaining 10 genistein-treated mice and 6 control mice at PND 60 were placed for 2 months with mature male mice in a 2:1 ratio to measure the fertility of mice after neonatal genistein exposure.
Histopathologic observations
Paraffin-embedded ovaries and uteri were sectioned serially at 4 µm with a microtome and were dried using a chip drying device after the sections had been adhered to slides. The slices from different depths were stained with hematoxylin and eosin after deparaffinization and rehydration. After staining, the slices were sealed with neutral resin and mounted with cover slips. Histomorphology was assessed using a virtual light microscope (model BX51TF; Olympus, Tokyo, Japan). The cross-sectional areas of the uterine cavity and uterine wall were established by evaluating 15 slices (3 slices/uterus × 5 uteri) in each group. The structural characteristics of the ovary and uterus were observed from at least five mice in each treatment group.
Immunohistochemical staining
The slices were deparaffinized and rehydrated in a graded series of xylene and ethanol. Antigen retrieval was performed by heating slides to 100°C for 4 min in 10 mM phosphate-buffered saline (PBS) with 100 mM trisodium citrate dihydrate (9:1, vol/vol). The endogenous peroxidase activity was abolished by 0.3% H2O2 treatment, and then the non-specific binding was blocked by incubation with 5% bovine serum albumin (A4737; Sigma-Aldrich, St. Louis, MO, USA) for 1.5 h at room temperature. The slices were incubated overnight at 4°C with specific primary antibodies: rabbit monoclonal antibody Ki67 (1:250 dilution; D3B5; Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal antibody HSD17B (17β-hydroxysteroid dehydrogenase; 1:150 dilution; sc-32872; Santa Cruz, California, USA), and goat polyclonal antibody HSD3B (3β-hydroxysteroid dehydrogenase; 1:150 dilution; sc-30820; Santa Cruz). The immunoreactivity of these specific proteins was then detected using rabbit IgG or goat IgG-SABC kits (SA2002/SA1023; Boster Biological Technology, Wuhan, China), and visualized by 0.05% 3, 3-diaminobenzidine tetrachloride (D8001; Sigma Chemical) in PBS buffer for 40 sec. As a negative control, the slices were incubated with PBS instead of the primary antibody. Finally, the reacted sections were counter-stained with hematoxylin and eosin and mounted with cover slips. Images were captured under a virtual light microscope (model BX51TF; Olympus). Expression patterns and staining intensities of the proteins were established following the evaluation of 15 slices (3 slices/ovary and uterus × 5 ovaries and uteri). The staining intensities of HSD17B and HSD3B were measured based on methods established in previous studies [27, 28].
Cell culture and cell cycle progression analysis
Primary GCs were obtained from the ovarian follicles of similar size from mice at PND 23. Cultures were maintained in Hyclone Modified Eagle’s Medium Ham’s F12 medium supplemented with 10% fetal bovine serum (SA102.02; CellMax, Beijing, China) and 100 IU/ml penicillin-streptomycin (SV30010; Hyclone, Logan, UT, USA) plus 50 µg/ml amphotericin B (A9528; Sigma Chemical) at 37°C with 5% CO2. Two days after inoculation, 10 µM genistein was added into fresh culture medium. After 24 h incubation, cells were removed from the culture dishes by trypsinization and centrifugation. After washing with PBS, they were suspended in 0.5 ml PI/RNase staining buffer (BD Pharmingen™, SA, 550825), and incubated for 15 min in the dark at room temperature. The suspension of 1 × 106 cells was analyzed for each DNA histogram using flow cytometer with a FACS Calibur (BD Biosciences, Bedford, MA) in 1 h and the percentages of G0/G1, S, G2/M were analysed by Flowjo software. Each treatment was repeated three times in each independent experiment (n = 6); “n” represents the number of independent experiments.
Data analysis
Statistical analyses were performed using GraphPad Prism software version 5.0 (San Diego, CA, USA) and determined with analysis of variance (ANOVA). The results depicting cross-sectional areas of the uterine cavity, uterine wall, and cell cycle progression are presented as means ± standard error of the mean (SEM). Significant differences between the genistein-treated and control groups were considered at the P < 0.05 level and are marked with an “*.”
Results
Genistein-induced alterations in ovarian and uterine differentiation
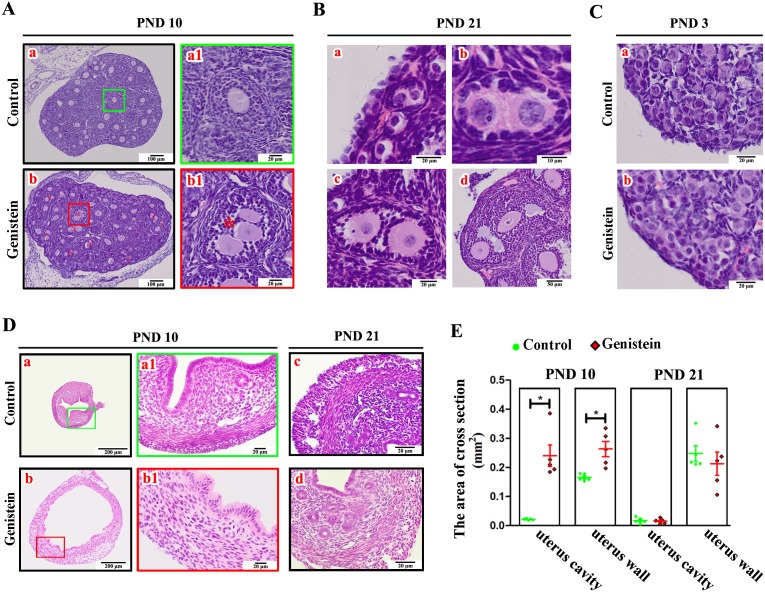
Body weight was lower in the group that received genistein than that in the control group; however, there were no significant differences (Supplementary Fig. 1: online only, P = 0.3032). Histological observations of ovaries from mice after consecutive subcutaneous genistein injections for 10 days showed no obvious abnormalities among somatic cell populations, including GCs, TCs, and stromal cells. However, there was an abundant appearance of MOFs, i.e., a single follicle with two or more oocytes (Fig. 1A-b–b1) in the genistein treatment group than that in the control mice (Fig. 1A-a-a1). Furthermore, these MOFs at different stages persisted to PND 21, including primordial MOFs (Fig. 1B-a), primary MOFs (Fig. 1B-b), secondary small MOFs (Fig. 1B-c), and secondary large MOFs (Fig. 1B-d). Additionally, oocytes were assembled with squamous GCs and formed a single primordial follicle in the control group at PND 3 (Fig. 1C-a). However, there were many oocytes not surrounded by squamous GCs that retained the features of oocyte nests after 100 mg/kg genistein exposure for 3 days (Fig. 1C-b). With respect to the uteri, genistein treatment for 10 days markedly enlarged the cross-sectional area of the uterine cavity (P = 0.0004) and uterine wall (P = 0.0063) (Fig. 1D-b and Fig. 1E) compared to the control mice (Fig. 1D-a and Fig. 1E). In the control mice, the arrangement among different uterine cellular layers showed apparent regularity (Fig. 1D-a–a1). However, the arrangement of cells between stromal layers and myometrial layers after genistein treatment was disorganized, and the compactness among uterine cells decreased (Fig. 1D-b–b1). In addition, numerous epithelial cells aggregated in the uterine endothelium of genistein-treated mice (Fig. 1D-b1). To confirm whether the genistein-induced adverse effects on uteri could be abolished after genistein removal, we compared the morphology of uteri at PND 21. We observed that the regularity of the interface between the myometrium and stroma after neonatal genistein exposure (Fig. 1D-d) was restored to that of the control mice (Fig. 1D-c), as was the cross-sectional area of the uterine cavity and uterine wall (Fig. 1E). However, the compactness among uterine cells (Fig. 1D-d) with neonatal genistein treatment was lower than that of the control mice (Fig. 1D-c).
Fig. 1.
Effects of exposure to 100 mg/kg genistein on ovarian and uterine development. A) Ovarian histological organization after consecutive subcutaneous genistein injections for 10 days. a and b are the representative sections of ovaries from control mice and neonatal genistein-treated mice, respectively; a1 and b1 are the partially magnified drawings of a and b, respectively. The red “*” in b represents MOFs. B) Different stages of MOFs in ovaries from mice at PND 21. a is primordial MOFs; b is primary MOFs; c is secondary MOFs; and d is antral MOFs. C) Ovarian histological organization after consecutive subcutaneous genistein injections for 3 days. a and b are the representative sections of ovaries from the control mice and neonatal genistein-treated mice, respectively. D) Uterine histological organization in mice at PND 10 and PND 21. a–a1 and b–b1 are the representative sections of uteri from control mice and neonatal genistein-treated mice at PND 10, respectively; c and d are the representative sections of uteri from controls and neonatal genistein-treated mice at PND 21. E) The cross-sectional area of the uterine cavity and uterine wall in mice at PND 10 (n = 5) and PND 21 (n = 5). Results are shown as mean ± SEM and statistical differences were determined by ANOVA. The green spot indicates the control, red prismatic points indicate genistein treatment, and “*” indicates a significant difference between controls and genistein treatment (P < 0.05).
Genistein-induced inhibition of cell proliferation
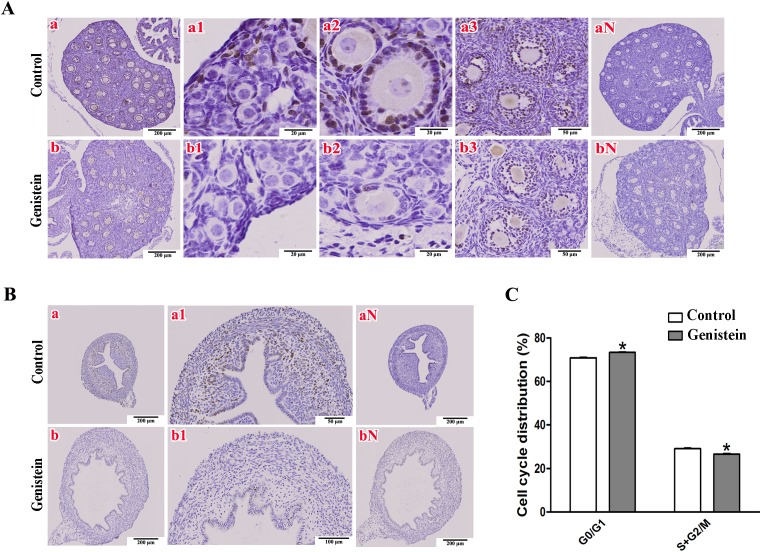
Cellular proliferation is the basis of organizational growth and differentiation; therefore, we further explored the proliferative activity in ovarian and uterine cells by Ki67 immunohistochemical staining in mice at PND 10. Ovaries from the control mice showed Ki67-positive nuclei widely distributed in the ovarian somatic cells (Fig. 2A-a), especially in follicular GCs (Fig. 2A-a1–a3). With respect to the uteri, cells with Ki67-positive nuclei were generalized to the myometrium (Fig. 2B-a) and were mainly concentrated in the vertical myometrial cell population (Fig. 2A-a1). However, we observed fewer cells with Ki67-positive nuclei in ovaries (Fig. 2A-b) or uteri (Fig. 2B-b-b1) after genistein exposure by consecutive subcutaneous injections. Furthermore, the extent of genistein-induced deprivation of Ki67-positive cells decreased following the development of ovarian follicles, i.e., fewer Ki67-positive cells were found in primordial follicles (Fig. 2A-b1) and primary follicles (Fig. 2A-b2) than that in secondary follicles (Fig. 2A-b3). These phenomena suggest that genistein inhibits cellular proliferation in both ovaries and uteri.
Fig. 2.
Immunohistochemical staining of Ki67 proteins in ovaries and uteri from mice at PND 10 following 100 mg/kg genistein exposure for 10 consecutive days. A) The representative sections of Ki67 location in ovaries. a and b are the global distribution of Ki67-positive nuclei, a1 and b1 are the Ki67 locations in primordial follicles, a2 and b2 are the Ki67 locations in primary follicles, a3 and b3 are the Ki67 locations in secondary follicles, and aN and bN are negative controls for the elimination of non-specificity. B) The representative sections of Ki67 locations in uteri. a and b are the global distributions of Ki67-positive nuclei, a1 and b1 are the Ki67 locations in myometrium, and aN and bN are negative controls for non-specificity. C) Effect of genistein on ovarian follicle granulosa cell cycle progression. The percentages of G0/G1, S, G2/M were obtained by flow-cytometric analysis (*P < 0.05, n = 6, vs. control).
To further verify the proliferation inhibition effects of genistein, cell cycle progression analysis for ovarian GCs were performed in vitro. A significant increase in the cell ratio during the G0/G1 phase (P < 0.0001) and an obvious decline in the S + G2/M phase (P < 0.0001) were observed in genistein-treated GCs compared with the control group (Fig. 2C).
Genistein-induced decline of ovarian function in adulthood
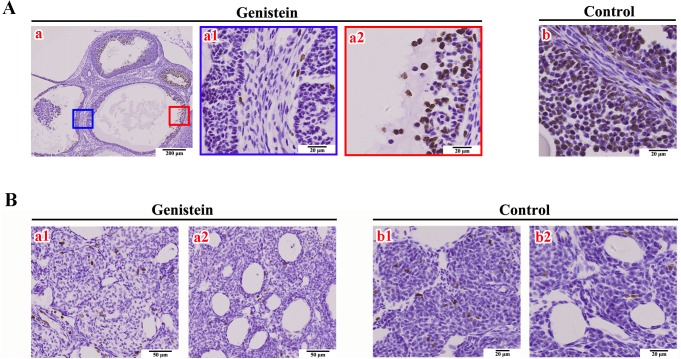
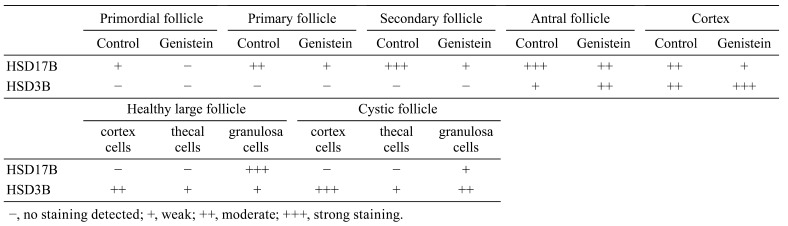
The basic function of ovaries is to maintain folliculogenesis and then to ovulate eggs. Histopathologic observations of ovaries in female mice at PND 90 (adulthood) showed a significantly lower ratio of ovaries with corpora lutea in the neonatal genistein-treated group (2/10; 20%) compared to the control group (7/10; 70%). The number of corpora lutea in the ovaries was obviously different between the control group (5 individuals, numbers of corpora lutea were 8, 18, 12, 6, and 13; representative sections are shown in Fig. 3A-a) and the genistein-treated mice (2 individuals, number of corpora lutea were 1 and 3; representative sections are shown in Fig. 3A-b). To explore the potential mechanism(s) underlying delayed corpora lutea formation after neonatal genistein exposure, we focused on folliculogenesis in mouse ovaries. Histopathologic observations showed that most ovaries (5/8; 63%) from genistein-treated mice had cystic follicles (representative characteristics are shown in Fig. 3B-b1). Furthermore, these ovaries showed different degrees of hypertrophy in follicular TCs (Fig. 3B-b2), ovarian cortex (Fig. 3B-b2), and medullary cells (Fig. 3B-b3), and the blood vessels in these ovaries also increased (Fig. 3B-b4). Immunohistochemical staining further showed that the number of Ki67-positive nuclei in GCs and TCs visibly decreased in these cystic follicles (Fig. 4A-a1) compared to the control group (Fig. 4A-b), and that Ki67-positive nuclei were only visible in a few regions (Fig. 4A-a2). The number of Ki67-positive nuclei in ovarian medullary cells (Fig. 4B-a1) and vascular epithelial cells (Fig. 4B-a2) had no obvious differences compared to the control group (Fig. 4B-b1 and b2). No genistein-treated adult achieved a successful pregnancy after 2 months of mating with males, whereas all control females became pregnant.
Fig. 3.
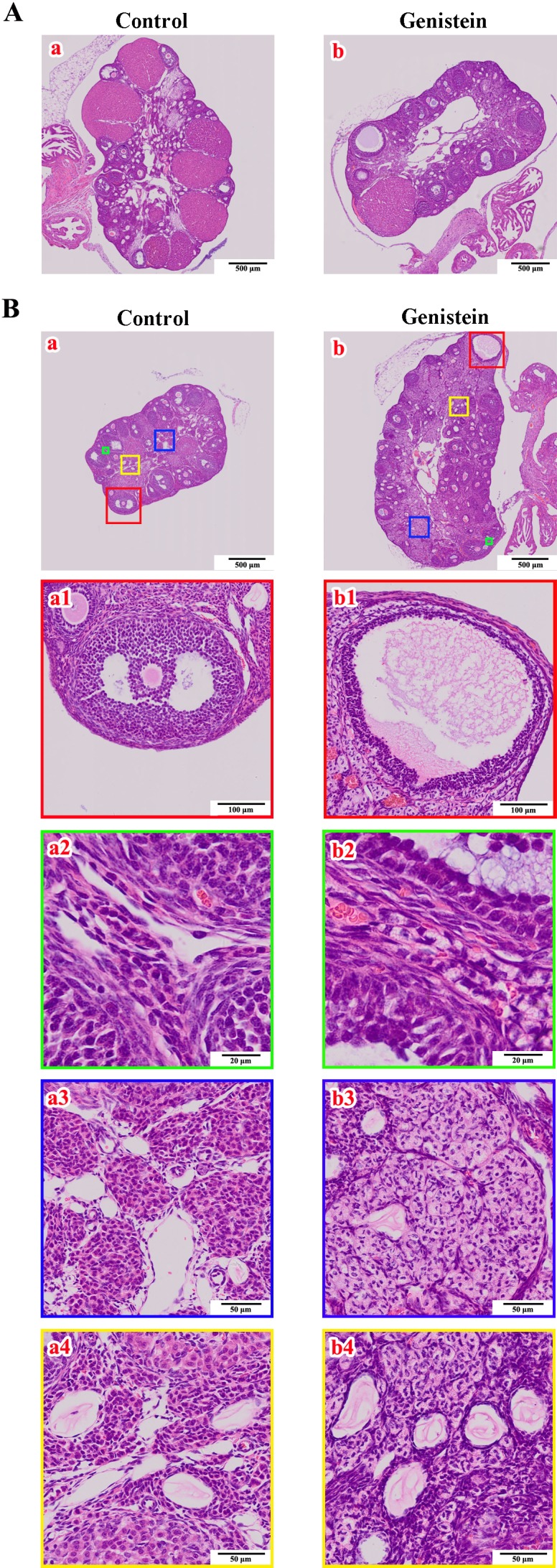
Effects of neonatal genistein exposure on ovarian ovulation and folliculogenesis at adulthood. A) The histology of ovaries with corpora lutea from control mice (a) and neonatal genistein-treated mice (b). B) The histology of ovaries with many growing follicles and few regressing corpora lutea from control mice (a) and neonatal genistein-treated mice (b). a1–a4 and b1–b4 are the partially magnified drawings of a and b, respectively.
Fig. 4.
Immunohistochemical staining of Ki67 proteins on ovaries. A) Ki67 staining in cystic follicles from neonatal genistein-treated ovaries (a, a1–a2) and large follicles from control ovaries (b). B) Ki67 staining in ovarian medullary cells (a1: genistein group; b1: control group) and vascular epithelial cells (a2: genistein group; b2: control group).
Genistein-induced alteration in HSD17B and HSD3B expression
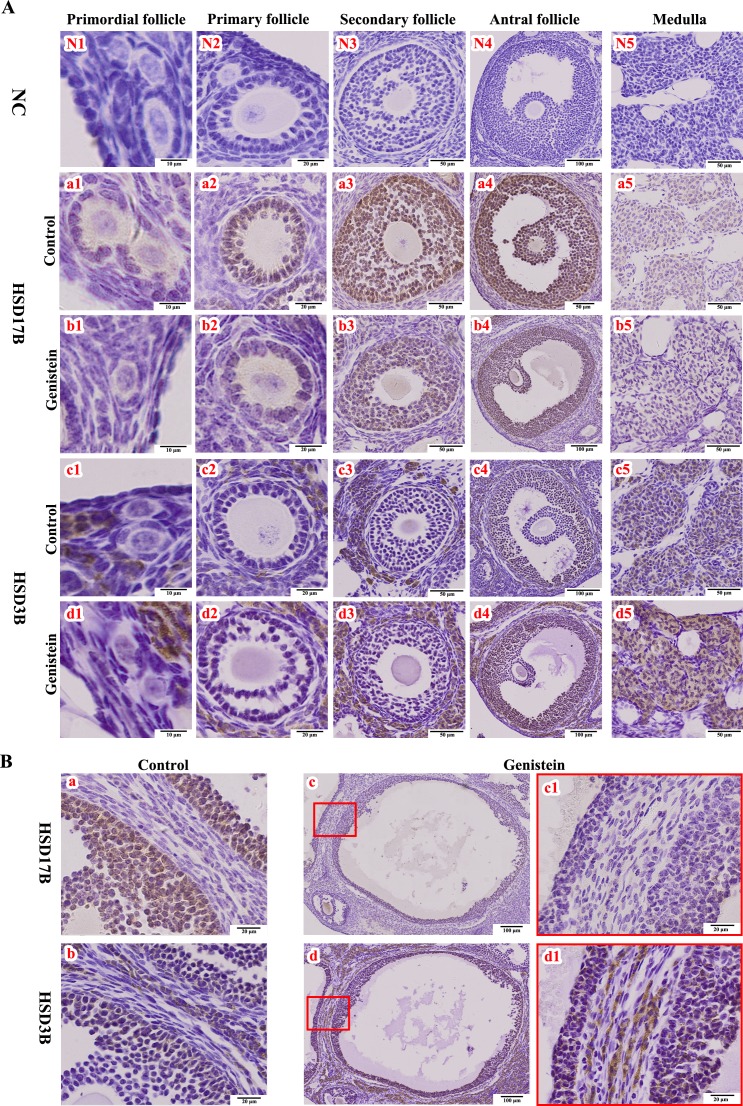
In control individuals, HSD17B was mainly located in the GCs and was expressed differentially at different follicle stages (Fig. 5A-a1–a5 and Table 1), whereas HSD3B was highly expressed in thecal, cortical, and medullary cells (Fig. 5A-c1–c5 and Table 1). However, in genistein-treated neonatal mice, we noted a diminution in the HSD17B expression level (shown as the lighter color in the follicles of different sizes; Fig. 5A-b1–b5 and Table 1). Additionally, the HSD3B expression level was very low in control individuals (Fig. 5A-c1–c5 and Table 1), although the relative staining for HSD3B was visibly increased in ovaries from genistein-treated individuals (Fig. 5A-d1–d5 and Table 1). In the cystic follicles from ovaries of genistein-treated mice, the relative expression levels of HSD17B and HSD3B were both significantly different from that of the control group (Fig. 5B and Table 1).
Fig. 5.
Immunohistochemical staining of HSD17B and HSD3B proteins in ovaries. A) HSD17B and HSD3B staining at different stages of follicular development, and in cortex from controls (a1–a5 and c1–c5) and neonatal genistein-treated ovaries (b1–b5 and d1–d5). B) HSD17B and HSD3B staining in cystic follicles from neonatal genistein-treated ovaries (c–c1 and d–d1) and in normal large follicles from control ovaries (a and b). NC (N1–N5), negative control; HSD17B, 17β-hydroxysteroid dehydrogenase; HSD3B, 3β-hydroxysteroid dehydrogenase.
Table 1. Relative levels of immunostaining of HSD17B and HSD3B in mouse ovaries.
Genistein-induced disruptions in uterine morphology
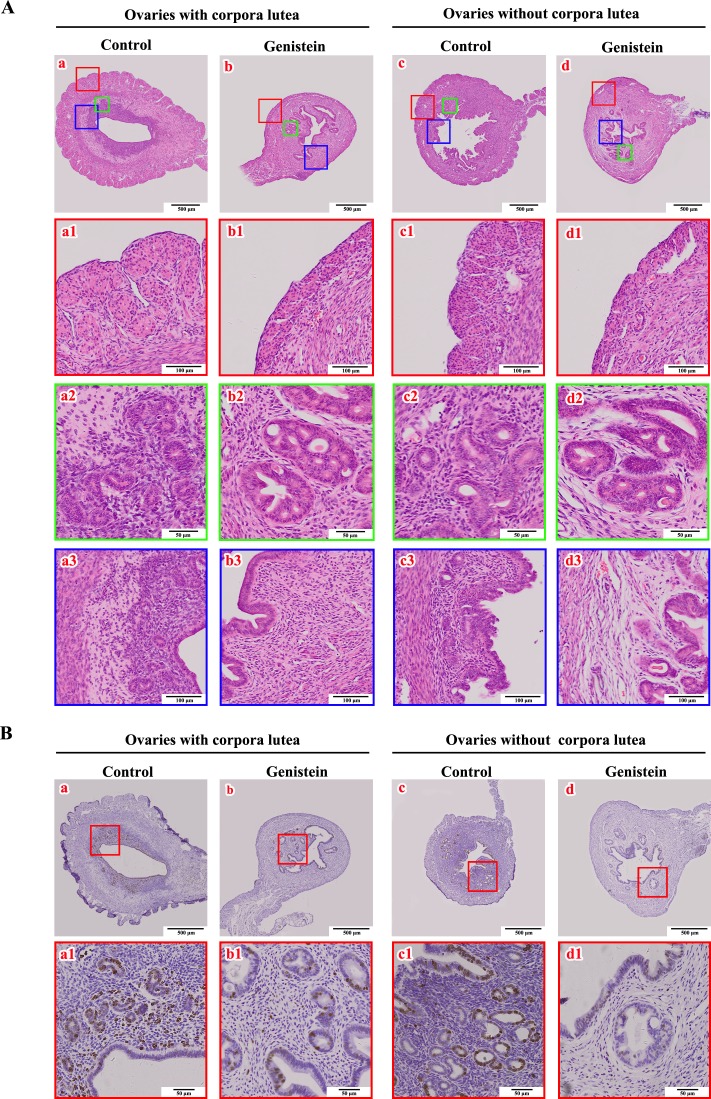
Based on the status of the ovary (with or without corpora lutea), we further compared the uterine histology between control and experimental mice. The neonatally exposed mice (Fig. 6A-b1 and d1) could not form a normal uterine serosal layer (Fig. 6A-a1 and c1). Epithelial cells of the uterine glands proliferated and adhered to each other, resulting in the formation of irregularly shaped glands (Fig. 6A-b2 and d2), and the density of uterine stroma cells also decreased (Fig. 6A-b3 and d3) compared to the control group. In addition, the number of Ki67-positive nuclei in neonatally treated uteri (Fig. 6B-b1 and d1) was lower than that in the control group (Fig. 6B-a1 and c1), including in the uterine stroma, endometrium, and glandular epithelium.
Fig. 6.
Effects of neonatal genistein exposure on uterine morphologic migration and Ki67 expression in mice at PND 90 corresponding to the status of the ovary (with or without corpora lutea). A) Uterine histology. a and c represent the development of uteri from control mice. b and d represent the development of uteri from neonatal genistein-exposed mice. a1–a3, b1–b3, c1–c3, and d1–d3 are the partially magnified drawings of a, b, c, and d, respectively; B) Immunohistochemical staining of Ki67 protein in uteri from control (a–a1 and c–c1) and neonatal genistein-exposed mice (b–b1 and d–d1).
Discussion
Our study clearly showed that neonatal genistein exposure directly altered the normal differentiation of ovaries and uteri of nepionic mice and decreased the proliferation indicator Ki67 protein expression. Genistein exposure during early postnatal development also caused the appearance of many pathological characteristics in the ovaries and uteri of adult mice. We hypothesized that neonatal genistein exposure disrupted development in the mouse ovary and uterus by inhibiting cellular proliferation. The effect of genistein on ovarian differentiation in the present study was consistent with earlier findings by Jefferson et al. [9]. In their study, ovaries from genistein-treated mice produced MOFs and inhibition of oocyte nest breakdown at a dose of 50 mg/kg of genistein on PNDs 1–5. We also observed numerous follicles with two or more oocytes and a large number of unassembled oocytes in ovaries from PND 10 and PND 3 mice, respectively, after genistein administration (100 mg/kg). Furthermore, these MOFs developed continuously because MOFs at different stages persisted in the ovaries of mice at PND 21. In rodents and wildlife, MOFs are correlated with reduced fertility and decreased embryonic survival rates [14, 29]. Previous studies utilizing neonatal genistein injections and oral genistein administration have shown increased MOFs associated with decreased fertility as well as premature reproductive senescence [13, 30, 31]. Therefore, we speculated that exposure to high levels of genistein induced MOFs and disordered oocyte nest breakdown, and subsequently lowered female fertility.
The uterus appears to be another sensitive organ for genistein targeting. In the present study, we observed the enlargement of uterine volume that was supported by an increase in the cross-sectional areas of the uterine cavity. This effect was probably caused by excessive secretion and accumulation of genistein-mediated luminal fluid because previous studies have shown a dose-dependent increase in luminal fluid secretion rate and luminal fluid volume in the uteri of ovariectomized rats under subcutaneous genistein treatment [26, 32]. Another phenomenon in genistein-treated mice was the disorganized arrangement among different uterine cellular layers. The augmented cross-sectional area of the uterine cavity and the (ir)-regularity of the uterine structure gradually returned to normal after genistein removal as shown in mouse uteri at PND 21. It is still unclear how the augmented cross-sectional areas of these mice uteri were recovered. However, we suspect that the effect of genistein on uterine structure may depend upon its timeliness.
To determine the cause of genistein-altered ovarian and uterine differentiation, we examined the protein expression of proliferation indicator Ki67 in these two major reproductive organs and found that the number of Ki67-positive cells was obviously decreased in both ovaries and uteri from genistein-treated mice. Previous literature has shown that the nuclear antigen Ki67 is only present in proliferating cells [33]. Therefore, the fewer Ki67-positive cells suggest that genistein inhibited cellular proliferation in the ovary and uterus. This inhibitory property is supported by a study that reported a significant decrease in the relative mRNA expression level of the proliferation markers PCNA and Ki67 genes in uteri from pre-pubertal rats given a genistein-rich diet [34]. In addition, the cycle progression by flow cytometry in vitro showed that genistein caused a significant increase in ovarian primary GCs at G0/G1-phase cells. It is well known that the Ki67 protein is present during active cell cycle phases (G1, S, G2, and M phase); however, it is absent during the quiescent state (G0 phase). Therefore, genistein probably arrests cell cycle progression and leads cells into the G0 phase [34]. These results suggest that genistein could dramatically inhibit cell viability, which might be the cellular mechanism for the timely alteration of reproductive tissues influenced by genistein.
It is also worth mentioning the inhibitory effect on proliferation of somatic pregranulosa cells. The proliferation and differentiation of ovarian GCs are dependent upon ERβ-mediated estrogen action [35], and ERβ has also been proven to be responsible for the formation of MOFs [36]. This inhibited proliferative effect of genistein on somatic pregranulosa cells might then delay the process of assembly by somatic pregranulosa cells of individual oocytes to form primordial follicles. Consequently, genistein appeared to limit the number of pregranulosa cells surrounding multi-oocytes, and this might be another possibility governing MOF origination.
The present study clearly demonstrated that ovaries and uteri from adult mice were adversely affected by neonatal genistein exposure. Only a few ovaries successfully ovulated and formed corpora lutea, and their corresponding uteri failed to transition morphologically in terms of pregnancy preparation. Moreover, none of the treated mice became pregnant despite cohabitation with a male for 2 months. These phenomena indicate that adult fertility was impaired or abolished following developmental exposure to high doses of genistein, which has also been reported in previous studies for mice (50 mg/kg) [30] and rats (100 mg/kg) [37]. Our microscopic examination further showed that most of the ovaries from genistein-treated mice manifested cystic follicles, with differing degrees of hypertrophy in thecal, cortical, and medullary cells. The altered expression levels of HSD17B and HSD3B (the two enzymes that are responsible for estradiol and progesterone synthesis, respectively), indirectly reflected the disorder of intercellular steroidogenesis in the various functional cells of the ovary. Therefore, we hypothesized that the morphological alterations in ovaries from neonatal genistein-treated mice caused dysfunction of distinct cells, and ultimately caused a failure in folliculogenesis and ovulation. Furthermore, we observed that the uteri morphology of neonatally-treated mice failed to accomplish the corresponding transformation to allow for embryonic implantation after ovulation, which was mainly reflected in the uterine serosa that was unable to thicken compared to the control mice. This effect was possibly caused by an abnormality of the uterine gland because the glands from the genistein-treated mice were hyperplastic and the glandular epithelial cells adhered to one another. The fewer Ki67-positive staining cells in these uteri further proved that the proliferative activity was affected, reflecting the failure of proper uterine transformation essential for sperm transport and embryo implantation [38]. Therefore, the uterine pathologies induced by neonatal genistein exposure might also contribute to female infertility. One explanation given for the morphological alteration of these organs was the disruption of the hypothalamic-pituitary-gonadal axis function. Investigators have previously reported that neonatal genistein exposure in rats decreased the responsiveness of the pituitary to the gonadotropin releasing hormone [39]. Lasa et al. [40] showed that genistein exposure during the neonatal period adversely affected the ontogeny of hypothalamic kisspeptin signaling pathways, resulting in premature anestrous and ovarian development disruption in peripubertal rats. Alternatively, the proliferative inhibition of genistein during neonatal exposure could also contribute to the morphological alteration of these organs in adults by disrupting the timing of ovarian and uterine development. Therefore, both the disruption of the hypothalamic-pituitary-gonadal axis and the inhibition of ovarian and uterine development by neonatal genistein exposure might contribute to the later infertility in mice; however, which factor mainly induced this phenomenon still requires further investigation.
The plasma genistein concentration in infants fed exclusively on soy-based formula has been reported to be up to 10 µM [8], which is comparable to two times of the neonatal female mice treated by subcutaneous injection with 50 mg/kg genistein (a serum genistein circulating level of 6.8 ± 1.4 µM) [24]. Soy-derived isoflavone supplements that contain higher amounts of genistein than that in soy-based infant formula are also widely consumed by the peri- and post-menopausal population as a treatment for menopause symptoms [41]. Besides, isoflavones exposure is constantly increasing in both humans and livestock due to the industrialization of the soybean process [42]. Therefore, there is a possibility that the genistein exposure level in humans and livestock could be far greater than the physiologically concentration. Our study serves as a caution with respect to the progressive internationalization of soybean use in human and livestock feed.
In conclusion, the present study clearly showed that ovarian and uterine morphological development of adult mice can be disrupted following neonatal genistein exposure. The resulting pathological structures possibly play a direct role in the disruption of multiple reproductive functions, including abnormalities in ovulation and failure of uterine morphological transformation. We also suspect that genistein induces MOFs in ovaries, possibly by inhibiting somatic pregranulosa cell proliferation. However, the molecular mechanisms underlying these adverse genistein-induced effects require further investigation.
Conflicts of interest
None declared.
Supplementary
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 31572403 and 31402075). We express our gratitude to Dr Reinhold J Hutz of the Department of Biological Sciences, University of Wisconsin-Milwaukee, USA, for reading the original manuscript and offering valuable suggestions.
References
- 1.De Gregorio C, Marini H, Alibrandi A, Di Benedetto A, Bitto A, Adamo EB, Altavilla D, Irace C, Di Vieste G, Pancaldo D, Granese R, Atteritano M, Corrao S, Licata G, Squadrito F, Arcoraci V. Genistein supplementation and cardiac function in postmenopausal women with metabolic syndrome: results from a pilot strain-echo study. Nutrients 2017; 9: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaheer K, Humayoun Akhtar M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr 2017; 57: 1280–1293. [DOI] [PubMed] [Google Scholar]
- 3.Gencel VB, Benjamin MM, Bahou SN, Khalil RA. Vascular effects of phytoestrogens and alternative menopausal hormone therapy in cardiovascular disease. Mini Rev Med Chem 2012; 12: 149–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patisaul HB. Endocrine disruption by dietary phyto-oestrogens: impact on dimorphic sexual systems and behaviours. Proc Nutr Soc 2017; 76: 130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarver G, Bhatia J, Chambers C, Clarke R, Etzel R, Foster W, Hoyer P, Leeder JS, Peters JM, Rissman E, Rybak M, Sherman C, Toppari J, Turner K. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res B Dev Reprod Toxicol 2011; 92: 421–468. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 1997; 350: 23–27. [DOI] [PubMed] [Google Scholar]
- 7.Badger TM, Ronis MJ, Hakkak R, Rowlands JC, Korourian S. The health consequences of early soy consumption. J Nutr 2002; 132: 559S–565S. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol 2009; 19: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod 2006; 74: 161–168. [DOI] [PubMed] [Google Scholar]
- 10.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 2008; 29: 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awobajo FO, Nandedkar TD, Balasinor NH. Genistein alters oestrous cyclicity, oocyte fertilization and implantation process in rats. Nig Q J Hosp Med 2013; 23: 188–193. [Google Scholar]
- 12.Jefferson WN, Padilla-Banks E, Newbold RR. Studies of the effects of neonatal exposure to genistein on the developing female reproductive system. J AOAC Int 2006; 89: 1189–1196. [PubMed] [Google Scholar]
- 13.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod 2005; 73: 798–806. [DOI] [PubMed] [Google Scholar]
- 14.Chan WH. Impact of genistein on maturation of mouse oocytes, fertilization, and fetal development. Reprod Toxicol 2009; 28: 52–58. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, Williams CJ. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol Reprod 2009; 80: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferson WN, Padilla-Banks E, Phelps JY, Gerrish KE, Williams CJ. Permanent oviduct posteriorization after neonatal exposure to the phytoestrogen genistein. Environ Health Perspect 2011; 119: 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris HA, Bapat AR, Gonder DS, Frail DE. The ligand binding profiles of estrogen receptors alpha and beta are species dependent. Steroids 2002; 67: 379–384. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton KJ, Hewitt SC, Arao Y, Korach KS. Estrogen hormone biology. Curr Top Dev Biol 2017; 125: 109–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology 2001; 166: 79–89. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol 2014; 140: 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makarevich A, Sirotkin A, Taradajnik T, Chrenek P. Effects of genistein and lavendustin on reproductive processes in domestic animals in vitro. J Steroid Biochem Mol Biol 1997; 63: 329–337. [DOI] [PubMed] [Google Scholar]
- 22.Peterson G, Barnes S. Genistein inhibition of the growth of human breast cancer cells: independence from estrogen receptors and the multi-drug resistance gene. Biochem Biophys Res Commun 1991; 179: 661–667. [DOI] [PubMed] [Google Scholar]
- 23.Salleh N, Helmy MM, Fadila KN, Yeong SO. Isoflavone genistein induces fluid secretion and morphological changes in the uteri of post-pubertal rats. Int J Med Sci 2013; 10: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorski J, Hou Q. Embryonic estrogen receptors: do they have a physiological function? Environ Health Perspect 1995; 103(Suppl 7): 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr Cancer 1997; 27: 31–40. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res 1998; 58: 3833–3838. [PubMed] [Google Scholar]
- 27.Wei Q, Ding W, Shi F. Roles of poly (ADP-ribose) polymerase (PARP1) cleavage in the ovaries of fetal, neonatal, and adult pigs. Reproduction 2013; 146: 593–602. [DOI] [PubMed] [Google Scholar]
- 28.Wei Q, Shi F. Cleavage of poly (ADP-ribose) polymerase-1 is involved in the process of porcine ovarian follicular atresia. Anim Reprod Sci 2013; 138: 282–291. [DOI] [PubMed] [Google Scholar]
- 29.Guillette LJ, Jr, Moore BC. Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Semin Reprod Med 2006; 24: 134–141. [DOI] [PubMed] [Google Scholar]
- 30.Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol 2007; 23: 308–316. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect 2009; 117: 1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chinigarzadeh A, Kassim NM, Muniandy S, Salleh N. Genistein-induced fluid accumulation in ovariectomised rats’ uteri is associated with increased cystic fibrosis transmembrane regulator expression. Clinics (Sao Paulo) 2014; 69: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000; 182: 311–322. [DOI] [PubMed] [Google Scholar]
- 34.Möller FJ, Zierau O, Hertrampf T, Bliedtner A, Diel P, Vollmer G. Long-term effects of dietary isoflavones on uterine gene expression profiles. J Steroid Biochem Mol Biol 2009; 113: 296–303. [DOI] [PubMed] [Google Scholar]
- 35.Diel P, Hertrampf T, Seibel J, Laudenbach-Leschowsky U, Kolba S, Vollmer G. Combinatorial effects of the phytoestrogen genistein and of estradiol in uterus and liver of female Wistar rats. J Steroid Biochem Mol Biol 2006; 102: 60–70. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod 2002; 67: 1285–1296. [DOI] [PubMed] [Google Scholar]
- 37.Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol 2001; 15: 399–411. [DOI] [PubMed] [Google Scholar]
- 38.Giudice LC. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab 2006; 20: 235–244. [DOI] [PubMed] [Google Scholar]
- 39.Faber KA, Hughes CL., Jr Dose-response characteristics of neonatal exposure to genistein on pituitary responsiveness to gonadotropin releasing hormone and volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) in postpubertal castrated female rats. Reprod Toxicol 1993; 7: 35–39. [DOI] [PubMed] [Google Scholar]
- 40.Losa SM, Todd KL, Sullivan AW, Cao J, Mickens JA, Patisaul HB. Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptin signaling pathways and ovarian development in the peripubertal female rat. Reprod Toxicol 2011; 31: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Additives EPOF. Risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J 2016; 13: 4246–4246. [Google Scholar]
- 42.Bennetau-Pelissero C. Risks and benefits of phytoestrogens: where are we now? Curr Opin Clin Nutr Metab Care 2016; 19: 477–483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.