Abstract
Purpose of review:
To review the recent advances in understanding how primary brain tumors affect vision in children.
Recent findings:
Children with primary brain tumors may have vision loss due to involvement of their afferent visual pathways or from papilledema. These vision deficits may go unrecognized until later in life, years after treatment of the primary lesion. Strabismus and cranial nerve palsies may occur as a result of brain tumors. Ophthalmologists can monitor and treat young children at risk for vision loss from amblyopia as a result of effects from their underlying lesion. Advances in imaging techniques have made it possible to quantify damage to the visual pathways with objective tests.
Summary:
Systematic referrals for evaluation by an ophthalmologist should occur early in the course of treatment of primary brain tumors as these evaluations may improve visual outcomes and quality of life.
Keywords: pediatric brain tumors, vision, quality of life, visual pathways
Introduction
Having a child diagnosed with a primary pediatric brain tumor is often a devastating event for the child and for their family. Pediatric brain rumors are relatively unusual occurrence, and survivorship of these lesions is improving, however they can have long-term sequelae for the survivors of such lesions.1 A significant portion of the brain is involved in some way in vision. These tumors can affect vision by distorting, damaging, and destroying both the afferent and efferent visual pathways. Intrinsic tumors of the optic pathways (optic pathway gliomas) will not be specifically discussed in this review as they are extensively discussed in other reviews.
Demographics
Currently cancer is the second most common cause of death in the United States.2 In the pediatric and adolescent population cancer is the most common cause of non-accidental death.2 Leukemia is the most common cause of death from cancer in this population, and central nervous system lesions are the second most common cause of death.2 In children under 14 years old, brain and central nervous system tumors are the most common solid malignancy.3,4 For the year 2018 an estimated 4,610 children and young adults will be diagnosed with a new brain or central nervous system neoplasm.4
Survivorship of pediatric central nervous system neoplasms has been improving over time. This is likely due to improvements in our treatment modalities, the introduction of new and risk-adapted chemotherapies and immunotherapies, improved neuroimaging techniques for monitoring for disease recurrence or progression, and improved radiation techniques.5, 6 Children are more often having their care transitioned to long-term survivorship clinics as they are surviving their primary disease, and in such settings outcome measures tend to be geared more toward quality of life measures than survival alone.7,8
Effects on afferent visual system
Brain tumors in children can lead to decreased visual acuity, contrast sensitivity, color vision loss, and visual field loss, from involvement of the optic nerves directly (through compression or infiltration), or from involvement of the primary visual cortex, optic tracts, optic radiations, and lateral geniculate nuclei. Obstructive hydrocephalus, mass effect, invasion or obstruction of the venous sinus system, or leptomeningeal disease can lead to elevated intracranial pressure and papilledema, causing loss of vision as well.
Evaluating children with brain tumors that may be affecting the afferent visual system can be challenging as obtaining the subjective visual examination may be nearly impossible, and may only give a gross estimation of function. For example in a young child one may only be able to perform confrontational visual fields with a toy to achieve a gross response of the child looking at a novel stimulus, whearas for a cooperative adult one is able to perform formal automated perimetry. Young children with brain tumors have the additional risk of developing vision loss from amblyopia due to deprivation or strabismus, in addition to the risk of vision loss from the tumor itself.7 In normal maturation, the occipital cortex continues to develop through approximately 8 years of age. If normal vision is not restored to the amblyopic eye before the end of this period, that potential vision is permanently lost. Ophthalmologists can treat amblyopia through correcting refractive errors, occlusion therapy, and strabismus surgery when necessary. Many articles have been published on outcomes following treatment for pediatric brain tumors, and we will examine some of the most recent visual system outcomes.
A study of 10 patients who underwent neurosurgical procedures for brain tumors found that four of these children had significant vision loss in the perioperative setting, however this study did include optic nerve gliomas.9 Seven of the eight of the children who had preoperative ophthalmologic examinations had decreased vision prior to their surgery.9
Craniopharyngiomas often cause vision loss as a result of the tumor origin’s close relationship with the optic nerves, optic chiasm, and optic tracts [Figure]. A recent retrospective study of 59 children with craniopharyngiomas evaluated their long term visual outcomes, and found that over a median follow-up time of 5.2 years, 29% of patients had worsening of visual function.10 Approximately half of the patients who experienced worsening of vision after diagnosis lost vision within four months of diagnosis, the remainder lost vision with no particular pattern to the timing of vision loss throughout their follow-up.10 Younger age at diagnosis and tumor recurrence were associated with worsening of vision.10 At final follow-up only 42% of patients had normal vision, 24% were visually impaired, and 34% were legally blind.10 A retrospective evaluation of 61 children with sellar or parasellar lesions who underwent neurosurgical procedures found that the majority had no change in vision (58.3%), and 10% had an improvement in vision, but 25% had worsening of vision.11 Four patients (6.7%) had complete vision loss in one eye, eight (13.3%) developed new visual field defects.11
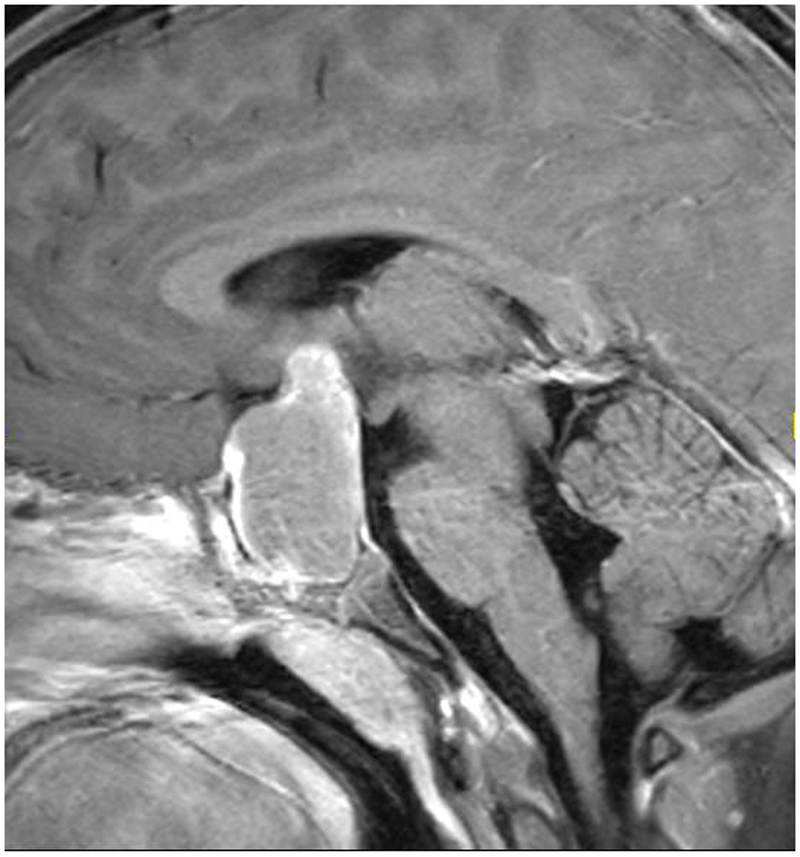
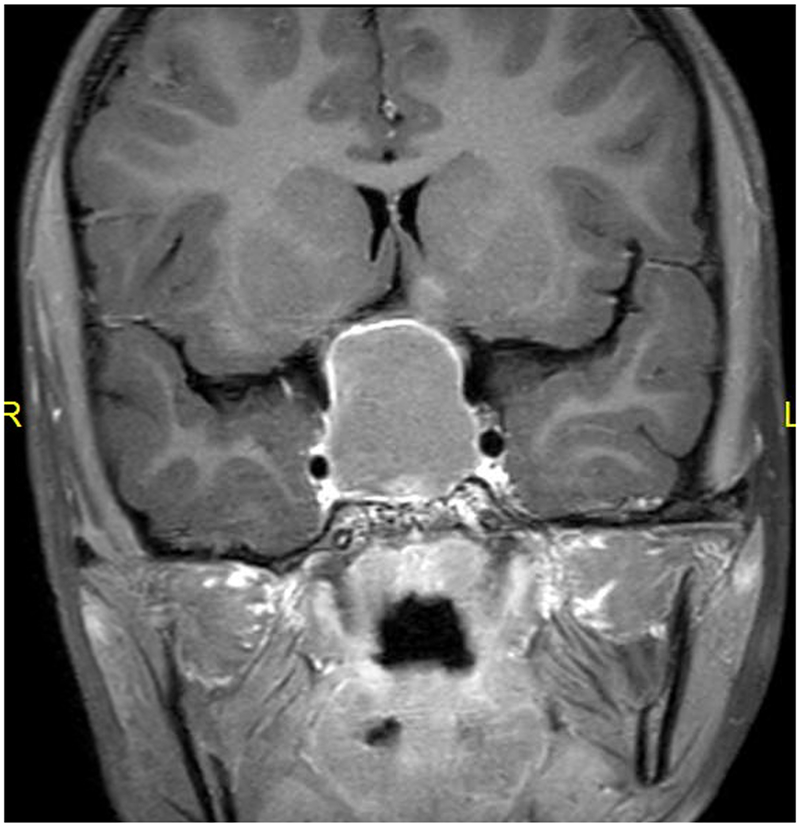
Figure: Craniopharyngioma leading to vision loss with bitemporal hemianopia.
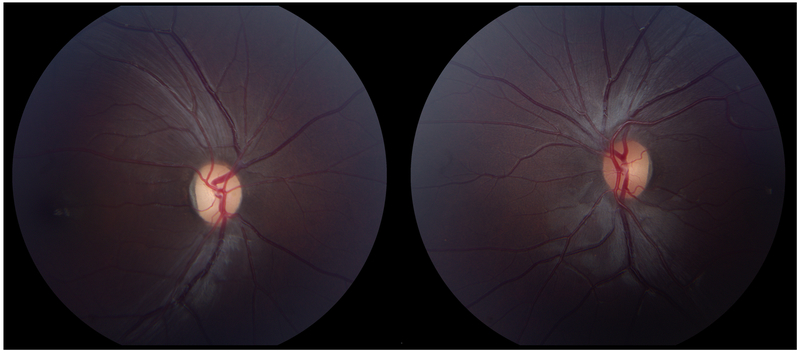
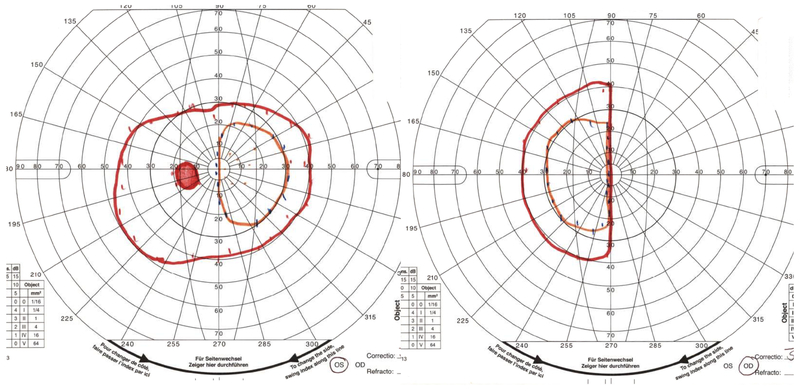
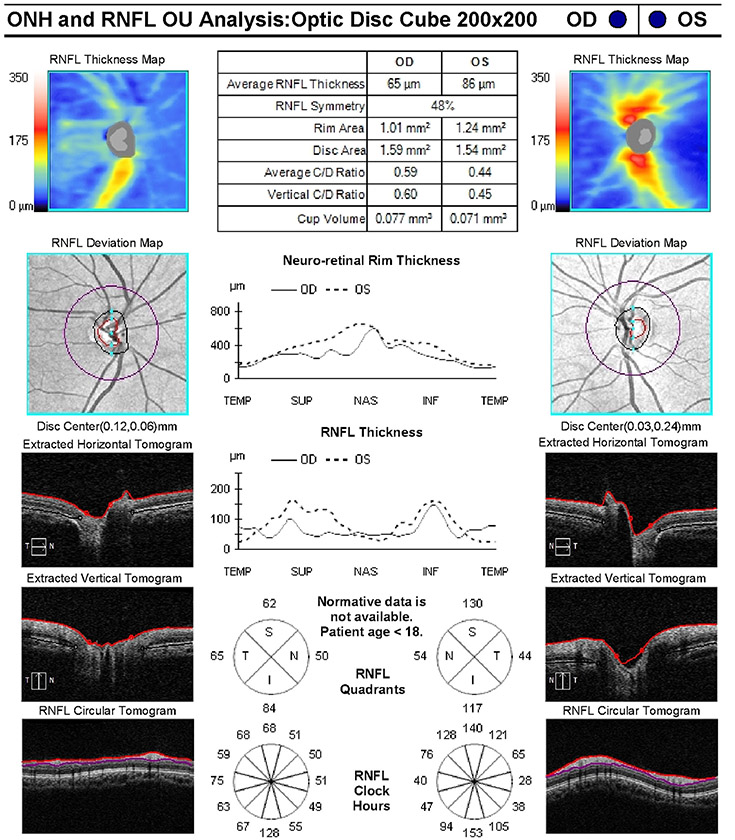
An 8-year old girl presented to her ophthalmologist after her parents suspected she had a visual field defect. She was found to have severe vision loss in the right eye and a temporal visual field defect in the left eye. Sagittal T1-weighted with fat suppression and FLAIR post-gadolinium image (A) and coronal T1-weighted with fat suppression and FLAIR post-gadolinium image (B) demonstrated a large predominantly cystic sellar and suprasellar mass with nodular enhancement and severe compression of the optic chiasm. Surgical resection revealed craniopharyngioma. Neuro-ophthalmic examination one year following resection and proton-beam radiation demonstrated visual acuities of 20/40 OD, 20/20 OS, a 0.9 log unit right relative afferent pupillary defect, diffuse pallor of the right optic nerve and temporal pallor of the left optic nerve (C). Compression of the optic chiasm let to a bitemporal hemianopia, worse in the right eye, as demonstrated on Goldmann visual fields (D). Optical coherence tomography demonstrated thinning of the retinal nerve fiber layer OD worse than OS (E).
Pineal tumors can lead to vision loss as well, and a retrospective chart review of 20 children describing optic atrophy in 5%, and homonymous hemianopia in 5%.12 Hankinson et al. reviewed the charts of 29 children with pineal tumors and found that 69% had papilledema, but only 4% had visual field defects, and no child had decreased vision at their last visit.13
Obtaining visual fields in young children can be extremely challenging. For some of the youngest children, confrontation visual fields are the best test that can be performed, with questionable reliability. Visual field defects may go unnoticed at the time of brain tumor diagnosis due to lack of formal testing, normal or near normal visual acuity, and lack of complaints from the patient. A review of 81 children presenting to a tertiary eye center who were found to have a homonymous hemianopia were found to have a traumatic etiology for their visual field defect in 34%, and intracranial neoplasms caused 27%.14 Improvement in the hemianopia occurred at final follow-up in 39% of the patients.14 An evaluation of children diagnosed with central nervous system tumors who had not been previously diagnosed with visual field defects found that 15.2% had unrecognized visual field defects.15 These children were diagnosed with visual field defects an average delay of 3.7 years after their initial tumor diagnosis.15 Juvenile pilocytic astrocytomas were the most frequently associated neoplasm with unrecognized visual field defects, and the most frequent locations were in the temporal lobes and hemispheric lesions.15 Hemianopias were present in 79% of those patients who were discovered to have visual field defects.15 This is extremely important, especially from a quality of life standpoint, as the presence of a hemianopia prevents these children and adolescents from driving for their entire lives, which can affect access to employment opportunities in the future. Interestingly the authors found no association between the presence of an unrecognized visual field defect and age.15 If detection of a visual field defect was not reliable due to limited testing ability in younger children, one would expect more frequent unrecognized visual field defects going missed in younger children.
Effects on Efferent visual system
Intracranial neoplasms can lead to strabismus and cranial nerve palsies. This can be particularly difficult to manage in the pediatric population as strabismus, and ptosis, from a third nerve palsy in young children can lead to amblyopia and permanent vision loss due to lack of proper development of the visual pathways. Once vision is lost in an eye from amblyopia or damage to the visual pathways, restoring binocular visual function becomes more difficult.
Children with pineal tumors have efferent visual system derangements more often than afferent visual system, with all patients having some element of Parinaud syndrome, horizontal strabismus in 25%, and skew deviation in 15%.12 In Unsinn’s retrospective evaluation of neurosurgery patients with sellar or parasellar lesions, 10 (16.7%) patients developed diplopia following their surgical intervention.11 Posterior fossa tumors more frequently lead to strabismus or cranial nerve palsies than more anterior lesions.16 Following treatment of a posterior fossa lesion 23% of children developed nystagmus and 42% of children developed strabismus.17 At some point strabismus surgery was performed for 41% of those children who had developed strabismus.17
One study of 23 children with brain tumors who developed strabismus evaluated the ocular motility outcomes in this population.18 A total of 60% of these patients were returned to orthotropia either after treatment of their underlying lesion, or after strabismus surgery (9/23), suggesting that strabismus surgery is a reasonable option for these patients.18 For patients with brain tumors improvements in quality of life should be balanced with the risk for clinical deterioration in the short-term and should be discussed prior to undergoing a surgical intervention.
Presenting signs of an intracranial tumor in the pediatric population
Many of the signs and symptoms of harboring an intracranial tumor are non-specific in the pediatric population. These children may have multiple visits to the emergency room or to their pediatrician for non-specific complaints prior to having a diagnosis of an intracranial tumor made. A meta-analysis of 74 papers on central nervous system tumors identified the most frequent signs and symptoms of intracranial tumors, including headache (33%), nausea and vomiting (32%), gait and coordination abnormalities (27%), and papilledema (13%).19 Children under 4 years of age had a rate of macrocephaly of 41%.19 For posterior fossa tumors specificially, nausea and vomiting were more frequent (75%), as well as headache (67%), abnormalities of gait and coordination (60%), and papilledema (34%).19 Cranial nerve palsies were present in 52% of patients with brainstem tumors.19 Overall symptoms of increased intracranial pressure were present in about 40% of patients, and could be present in affected children regardless of the location of the underlying tumor.19 The authors stressed the importance of a thorough ophthalmologic examination, as visual system abnormalities were present frequently in these patients.19 A recent analysis of pediatric craniopharyngioma patients in Toronto similarly found that 76% of patients presented with symptoms of headaches, 32% with nausea and vomiting, 31% with vision loss, and 12% with diplopia.10 Signs on examination included 63% with hydrocephalus, 42% with optic nerve edema, and 41% with optic nerve pallor.10
A single center retrospective chart review evaluated the presenting signs and symptoms of children in the emergency department who were eventually diagnosed with intracranial tumors. This study of 87 pediatric brain tumor patients found that some of the most frequent signs and symptoms were headache (67%), nausea and vomiting (49%), gait disturbance (43%), cranial nerve deficits (23%), vision changes (21%), papilledema (13%), ptosis (6%), and anisocoria (1%).20 However, the authors note that only 58% of these children had any funduscopic evaluation documented, and a large percentage of children had no documentation regarding the presence or absence of papilledema.20 The authors acknowledged that fundoscopic examination is an essential part of the neurologic examination, and is essential to perform for children who are suspected of harboring an intracranial central nervous system lesion.20
A retrospective chart review of pediatric brain tumor patients looked specifically at children who were diagnosed with brain tumors as a result of an evaluation by an ophthalmologist. This review of 26 children found that 46% of children had optic nerve atrophy, 46% had vision loss, 24% had papilledema, 24% had nystagmus, 19% had sixth nerve palsies, and 12% had a third nerve palsy.21 Multiple ophthalmic abnormalities could be present in the same patient, and this again highlights the importance of an ophthalmologic examination in making the diagnosis of a brain tumor, and the importance of an eye examination for assessing vision status following the initial diagnosis of a brain tumor.
Late effects of intracranial neoplasms and their treatment on vision
Following treatment and survival of a pediatric brain tumor, these children remain at risk for vision loss. In the Childhood Cancer Survivor Study, children had an 11.9 relative risk of developing cataracts when compared to their siblings.1 They were also had a 14.8 relative risk for unilateral or bilateral blindness, and a 8.8 relative risk of diplopia.1 Radiation therapy may lead to radiation necrosis and radiation optic neuropathy, leading to vision loss occurring months to years after treatment. One study found that 82% of patients with central nervous system tumors developed a late effect of vision loss following radiotherapy, compared with 50% among those who did not receive radiation.22
Advances in monitoring vision in pediatric brain tumor patients
Visual field techniques have not changed significantly in recent years, but several proposed methods for screening and evaluating for visual field defects have been developed. These include advanced neuroimaging combined with functional MRI,23 behavioral kinetic perimetry,24 static perimetry with eye tracking (saccadic vector optokinetic perimetry),25 static perimetry with video tracking,26 and a game based visual field assessor.27
Optical coherence tomography has given ophthalmologists another advanced method for monitoring visual pathway status in patients with brain lesions. Retinal nerve fiber layer thickness has been found to correlate with visual acuity and visual field defects in pediatric craniopharyngioma patients.28 Avery has demonstrated that hand-held optical coherence tomography devices can be used in pediatric optic pathway glioma patients, and that retinal nerve fiber layer defects measured with this device correlate with visual field defects and visual acuity loss.29,30 Ganglion cell layer complex thickness can also be used to monitor optic neuropathies, with ganglion cell layer defects correlating with visual field defects.31 It has been found that patients with compressive chiasmal lesions with visual defects without ganglion cell complex layer thinning have better chances of visual recovery after relief of compression.32 Additionally, adult patients with pituitary lesions who have radiologic compression of the optic chiasm but normal vision with normal visual fields may demonstrate ganglion cell layer thinning as a subtle sign of early compressive optic neuropathy that otherwise would go undetected due to their normal visual parameters, and they may even have a normal retinal nerve fiber layer measurement.33
Quality of life
Quality of life can be adversely affected as a result of a brain tumor’s effects on vision. Pediatric brain tumor survivors have lower health-related quality of life scores than other cancer survivors as well as healthy counterparts.7,34,35 Vision related quality of life has been assessed in pediatric optic pathway glioma patients, and patients with vision loss were found to have lower scores than patients with normal vision,36 but this has not been assessed in pediatric patients with primary brain tumors.
Conclusions
Primary brain tumors can have significant effects on vision and quality of life. These effects may not be identified at presentation, and may not be identified until a formal ophthalmologic evaluation is performed. Systematic referral to ophthalmologists for evaluation of children with brain tumors may help identify visual pathway abnormalities, and lead to better visual outcomes and quality of life.
Key Points.
Children with primary brain tumors are at risk for vision loss, optic neuropathy, strabismus, and cranial nerve palsies, and should have ophthalmologic evaluations systematically.
Despite treatment of the underlying primary brain tumor, children may have residual visual function abnormalities that can continue to affect quality of life.
Papilledema is frequently not documented due to lack of a funduscopic examination in children with primary brain tumors, and visual field defects often go unrecognized until later in life.
Acknowledgments
Financial support:
Supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology). This work was not industry supported.
Footnotes
Disclosures: The author has no financial disclosures or conflicts of interest.
Conflicts of interest:
None
References:
- 1).Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood cancer survivor study. J Clin Oncol 2003;21:3255–3261. [DOI] [PubMed] [Google Scholar]
- 2).Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–10. [DOI] [PubMed] [Google Scholar]
- 3).Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Ostrom QT, de Blank PM, Kruchko C, et al. Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous tumors diagnosed in the United States in 2007–2011. Neuro Oncol 2015; 16(Suppl 10):x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Pollack IF. Multidisciplinary management of childhood brain tumors: a review of outcomes, recent advances, and challenges. J Neurosurg Pediatrics 2011;8:135–148. [DOI] [PubMed] [Google Scholar]
- 6).Pollack IF, Jakacki RI. Childhood brain tumors: epidemiology, current management and future directions. Nat Rev Neurol 2011;7:495–506. [DOI] [PubMed] [Google Scholar]
- 7).Jariyakosol S, Peragallo JH. The effects of primary brain tumors on vision and quality of life in pediatric patients. Semin Neurol 2015;35:587–598. [DOI] [PubMed] [Google Scholar]
- 8).Pollack IF. Diagnosis and treatment of childhood brain tumors: Current perspectives. J Child Neurol 2009;24:1464–1465. [DOI] [PubMed] [Google Scholar]
- 9).Goldenberg-Cohen N, Ehrenberg M, Toledano H, et al. Preoperative visual loss is the main cause of irreversible poor vision in children with a brain tumor. Front Neurol 2011;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10)*.Wan MJ, Zapotocky M, Bouffet E, et al. Long-term visual outcomes of craniopharyngioma in children. J Neurooncol 2018;137:645–651. [DOI] [PubMed] [Google Scholar]; This article summarizes findings regarding visual outcomes of pediatric craniopharyngioma patients, including timing of vision loss.
- 11).Unsinn C, Neidert NC, Burkhardt JK, et al. Sellar and parasellar lesions – clinical outcome in 61 children. Clin Neurol Neurosurg 2014;123:102–108. [DOI] [PubMed] [Google Scholar]
- 12).Hart MG, Sarkies NJ, Santarius T, Kirollos RW. Ophthalmological outcome after resection of tumors based on the pineal gland. J Neurosurg 2013;119:420–426. [DOI] [PubMed] [Google Scholar]
- 13).Hankinson EB, Lyons CJ, Hukin J, Cochrane DD. Ophthalmological outcomes of patients treated for pineal region tumors. J Neurosurg Pediatr; 2016;17:558–563. [DOI] [PubMed] [Google Scholar]
- 14).Kedar S, Zhang X, Lynn MJ, et al. Pediatric homonymous hemianopia. J AAPOS 2006;10:249–252. [DOI] [PubMed] [Google Scholar]
- 15).Harbert MJ, Yeh-Nayre LA, O’Halloran HS, et al. Unrecognized visual field deficits in children with primary central nervous system brain tumors. J Neurooncol 2012;107:545–549. [DOI] [PubMed] [Google Scholar]
- 16).Peeler CE. A review of visual and oculomotor outcomes in children with posterior fossa tumors. Semin Pediatr Neurol 2017;24:100–103. [DOI] [PubMed] [Google Scholar]
- 17)*.Peeler CE, Edmond JC, Hollander J, et al. Visual and ocular motor outcomes in children with posterior fossa tumors. J AAPOS 2017;21:375–379. [DOI] [PubMed] [Google Scholar]; This article summarizes afferent and efferent visual system outcomes of patients specifically with posterior fossa tumors.
- 18).Shalev B, Repka MX. Restoration of fusion in children with intracranial tumors and incomitant strabismus. Ophthalmology 2000;107:1880–1883. [DOI] [PubMed] [Google Scholar]
- 19).Wilne S, Collier J, Kennedy C, et al. Presentation of childhood CNS tumours: a systematic review. Lancet Oncol 2007;8:685–695. [DOI] [PubMed] [Google Scholar]
- 20).Lanphear J, Sarnaik S. Presenting symptoms of pediatric brain tumors diagnosed in the emergency department. Pediatr Emer Care 2014;30:77–80. [DOI] [PubMed] [Google Scholar]
- 21).Alswaina N, Elkhamary SM, Shammari MA, Khan AO. Ophthalmic features of outpatient children diagnosed with intracranial space-occupying lesions by ophthalmologists. Middle East Afr J Ophthalmol 2015;22:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Saha A, Salley CG, Saigal P, et al. Late effects in survivors of childhood CNS tumors treated on Head Start I and II protocols. Pediatr Blood Cancer 2014; 61:1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Merabet LB, Devaney KJ, Bauer CM, et al. Characterizing visual field deficits in cerebral/cortical visual impairment (CVI) using combined diffusion based imaging and functional retinotopic mapping: A case study. Front Syst Neurosci 2016;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Koenraads Y, Braun KP, van der Lindern DC, et al. Perimetry in young and neurologically impaired children: the Behavioral Visual Field (BEFIE) Screening Test revisited. JAMA Ophthalmol 2015;133:319–325. [DOI] [PubMed] [Google Scholar]
- 25).Murray IC, Fleck BW, Brash HM, et al. Feasibility of saccadic vector optokinetic perimetry: a method of automated static perimetry for children using eye tracking. Ophthalmology 2009;116:2017–2026. [DOI] [PubMed] [Google Scholar]
- 26).Satgunam P, Datta S, Chillakala K, et al. Pediatric perimeter – A novel device to measure visual fields in infants and pediatric patients with special needs. Transl Vis Sci Technol 2017;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Aslam TM, Rahman W, Henson D, Khaw PT. A novel paediatric game-based visual-fields assessor. Br J Ophthalmol 2011;95:921–924. [DOI] [PubMed] [Google Scholar]
- 28).Bialer OY, Goldenberg-Cohen N, Toledano H, et al. Retinal RNFL thinning on OCT correlates with visual field loss in pediatric craniopharyngioma. Can J Ophthalmol 2013;48:494–499. [DOI] [PubMed] [Google Scholar]
- 29).Avery RA, Hwang EI, Ishikawa H, et al. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol 2014;132:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Avery RA, Cnaan A, Schuman JS, et al. Longitudingal change of circumpapillary retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol 2015;160:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Vuong LN, Hedges TR. Ganglion cell layer complex measurements in compressive optic neuropathy. Curr Opin Ophthalmol 2017;28:573–578. [DOI] [PubMed] [Google Scholar]
- 32).Ohkubo S, Higashide T, Takeda H, et al. Relationship between macular ganglion cell complex parameters and visual field parameters after tumor resection in chiasmal compression. Jpn J Ophthalmol 2012;56:68–75. [DOI] [PubMed] [Google Scholar]
- 33)*.Blanch RJ, Micieli JA, Oyesiku NM, et al. Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression. Pituitary 2018;21:515–523. [DOI] [PubMed] [Google Scholar]; This article demonstrates the importance of analyzing both the retinal nerve fiber layer and ganglion cell complex in the context of visual field testing for potential chiasmal compression. Ganglion cell complex analysis can be more sensitive for chiasmal compression than visual fields or retinal nerve fiber layer analysis.
- 34).Macartney G, Harrison MB, VanDenKerkhof E, et al. Quality of life and symptoms in pediatric brain tumor survivors: A systematic review. J Pediatr Oncol Nurs 2014;31:65–77. [DOI] [PubMed] [Google Scholar]
- 35).Gupta P, Jalali R. Long-term survivors of childhood brain tumors: Impact on general health and quality of life. Curr Neurol Neurosci Rep 2017;17:99. [DOI] [PubMed] [Google Scholar]
- 36).Avery RA, Hardy KK. Vision specific quality of life in children with optic pathway gliomas. J Neurooncol 2014;116:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]