Abstract
Diet and nutrition are important factors in the promotion and maintenance of good health throughout the entire life course. A plant-based diet may be able to prevent and treat chronic diseases such as diabetes, heart disease and hypertension, obesity, chronic inflammation and cancer. Phytonutrient rich foods are found in traditional African diet which is mostly vegetarian, and most of these food plants are often used for medicinal purposes. This review focuses on a peculiar plant Moringa oleifera, called the “Miracle Tree”, considered to be one of nature’s healthiest and most nutritious foods. Countless studies describe the benefits of Moringa leaves, pods, seeds and flowers. Its well-documented role in prevention and treatment of chronic diseases is hypothesized here as a result of possible of cross-kingdom regulation by exogenous vegetal microRNAs and synergistic action of plant bioactive components on endogenous human microRNA regulation. The potential health impact of phytocomplexes from African dietary plants within the context of cross-kingdom and endogenous microRNA regulation on health improvement and the overall economic well-being of the continent is estimated to be enormous.
Key words: Moringa oleifera, chronic diseases, African diet, cross-kingdom, medicinal plants
Vegetarian dietary habits in African people
Africa, the world’s second-largest and second most populous continent, with an area of approximately 30 million square kilometers and a population of just over 1.0 billion people, is considered the birthplace of Homo sapiens and the cradle of human civilization with the oldest history of known human habitation.1 Characterized by cultural diversity due to presence of countless ancient tribes, languages, and traditions, the food and dietary habits in the different regions of Africa represent a significant nutritional legacy for the people throughout the world.
In the beginning of Africa’s history, Africa’s edibles represented some of humankind’s earliest food production. For many thousands of years, hundreds of wild and cultivated native species complemented each other to comprise the core of the continental food supply.2 A significant plant migration began with many tribes and people migrated or traded out of Africa, bringing on their journeys new foods and spices from other’s culture into their own. Some Asian foods (most notably rice, bananas and sugarcane) progressed westward to become a part of the African food chain - due to the increasing trade between India and Africa. Nevertheless, African food remained largely dependent on traditional plants up until five centuries ago when adventurers and slavers from the American seaboard introduced a collection of foreign crops such as maize (corn), cassava (manioc), peanut (groundnut), sweet potato, tomato, common bean, chili peppers, and pumpkin. These historical events conveyed the switch from Africa’s ancient vegetables in form of leaves, roots, tubers, rhizomes, bulbs, seeds, buds, shoots, stems, pods, or flowers to the main Africa’s food of today such as sweet potato (typical of Rwanda, Ethiopia, and Kenya), cooking banana (common in Rwanda), cassava, peanut, common bean (typical of Ethiopia), peppers, eggplant, and cucumber, almost all of foreign extraction. In fact, out of the continent’s top vegetables today, only cowpea, yam, and okra are African.2
The variety in traditional African diet is underlined by significant geographic differences across the African continent. But even though each region of Africa has its own distinctive dishes, preparation techniques and consumption mores, African food has some common basic features.3 Lunch is the main meal and typically consists of different kinds of vegetables, legumes, and sometimes meat. Due to economic restraints, meat is not easily attainable by many Africans: in the inland savannah, the traditional cuisine is distinct in that meat products are generally absent - beef, goat, and sheep (mutton) are regarded as a form of wealth and not generally consumed as food. Moreover, the countries of North Africa that border the Mediterranean Sea are largely Muslim thus their diet reflects Islamic traditions which do not permit eating pork while other animal meat has to be processed in accordance with the traditions of the faith. Like other regions of Africa, much of the North African diet is based on grains and cooking with olive oil, onions, and garlic is very common, with spices including cumin, caraway, clove, and cinnamon. Rice is predominant in the area between the Sahara and the southern savannas, while couscous (made from hard wheat and millet) - often the main dish at lunch, is prevalent in the Sahara. Along the Ivory Coast root crops, (primarily yam and cassava - imported from Brazil by the Portuguese) are very common. Okra - a vegetable native to the rainforests of Africa, is characteristic of the West African area together with beans, sweet potato leaves, cassava, eggplant, cabbage, carrots, French beans, lettuce, cherry tomatoes - all heavily spiced, often with chilies.3,4 East African cuisine is heavily influenced by migrations and continuous trades with Arabic and South Asian countries: in addition to fish -abundant in lakes, coastal regions and the Nile Valley, main meals generally include potatoes, rice, beans, matake (mashed plantains), and a meal that is usually cooked up into a thick porridge or a stew.3-5 A distinct eating pattern has been recorded in the two herding tribes (Maasai and Fulbe) who do not eat much meat, except for special occasions but instead, they endure on fresh and soured milk and butter based diet (this is considered an uncommon dietary pattern since most Africans are primarily lactose intolerant).6
Africa’s peculiar geographic conditions and variety of climates underlie the enormous biodiversity in its ecosystems (tropical forests, savannahs, the veldts, and the unique environments of sub-Sahara).7 Many food plants thrive in the harsh conditions thus accumulate important secondary metabolites as a natural means of survival in a hostile environment. Moreover, because of its tropical conditions, Africa has strong UV irradiation from tropical sunlight and numerous micro-pathogens (including several species of bacteria, fungi and viruses) so African plants could in response accumulate more chemo-protective substances than plants in the northern hemisphere. Consequently, many plants, apart from being exceptionally nutritious, have been used historically for therapeutic purposes as well. Some estimations count up to 45.000 different species of plants, of which 5.000 are used for medical purposes.8 Ancient and recent African healers have a rich tradition of medicinal plant use in mixtures of various herbs, animal parts, minerals, and clays. In contrast to Western medicine, in which drugs are only used in low doses and in a prescribed manner for curing diseases, in traditional African medicine it is hard to distinguish when a plant shifts from being a health food to being a remedy (Table 1) 2,9-20. Many medicinal food plants are not viewed as “necessary poisons”, instead “every disease to which men are liable is occasioned by the substances whereon they feed”.21,22
Table 1.
Commonly used African plants.
| Plant | Geographical distribution | Description and morphology | Medicinal properties | Nutritional properties | Food preparations | Ref. |
|---|---|---|---|---|---|---|
| Okra (Hibiscus esculentus) | Savanna, full sun areas | Upright herb (2 metres) | Chronic kidney disease | Low in calories, and high in fibre. Pod contains healthy quantities of vitamins C, A and flavonoid antioxidants | Salad, soups, stews | 9 |
| Yam (Dioscorea spp.) | Tropical and subtropical regions | Herbaceous, climbing, twining, perennial monocots | Antioxidant, anti-inflammatory, lipid metabolism Estrogenic activities | High in calories Source of vitamin B, C, D and minerals | In West Africa, yam is traditionally prepared by pounding of cooked yam to obtain a dough-like paste known as pounded yam or ‘foutou’. | 10-14 |
| Cowpea (Vigna unguiculata L. Walp) | Africa | Legume crop | Digestive Health Heart Protection Detoxification Sleep Problems Diabetes Management Circulatory Health Weight Loss | Vitamin A and C thiamin, riboflavin, niacin, vitamin B6 and pantothenic acid | From bean salads and soups to cakes and stand-alone vegetarian dishes, cowpeas are easy to prepare and provide far more nutrition than many other legume species. | 15 |
| Pearl Millet (Pennisetum glaucum) | Intertropical Africa | Herbaceous plant | Stomach ulcers, hearth health, diabetes, cholesterol | Calcium, iron, zinc, proteins, lipids | Used to prepare cous cous, porridge, beer | 16, 17 |
| Cassava (Manihot esculenta) | Tropical and subtropical regions | Perennial plant | Hearth disease, cholesterol, repair body tissues, blood pressure | Vitamin B, Vitamin K and Minerals | Cassava is eaten boiled, steamed, or fried | 2 |
| African Rice (Oryza glaberrima) | West Africa | Annual Plant | The root is eaten raw as a treatment for diarrhoea | Vitamin B, Iron | A staple food, highly appreciated for its taste and culinary qualities | 18 |
| Gum Arabic (Acacia senegal) Moringa oleifera | sub-Saharan Africa Southern to Northern Africa Tropical and subtropical regions | Deciduos tree | Use to treat several infections Antinflammatory Antioxidants Antimicrobial | Proteins, minerals Vitamins Minerals Aminocids | Candies and soft drinks dried seeds Soup, salad, with meat and fish | 19 20 |
According to WHO,23 herbal treatments represent the most popular form of traditional medicine used as 70% to 80% of primary health care. The high interest in traditional medicine in the African health system can be explained by two main reasons: the first is cultural and psychological (as mentioned above) and the second is inadequate access to allopathic medicines and Western forms of treatment, as most people in Africa cannot afford access to modern medical care because of the costs or lacks of providers of medical services.
Enormous benefits derivable from the simple, mostly vegetable-based diet often associated with the African cuisine are starting to be increasingly appreciated by the Western culture in the last decades. Some affluent people living in developed countries have elected by choice to subscribe to the simple vegetarian-based lifestyle of rural African with incredible results on their health.21 In fact, nonetheless the continent’s economic aspect has its influence, remains clear that the simplicity of the African diet is not dictated by poverty. The humanity’s oldest food plants have been nurturing and curing people since the beginning and the pharmaceutical potentials of African’s natural resources are immense: the ingredients used in traditional African diets should be harnessed aiming at reducing the disease burden in both rural and urban settings across the globe.
Among diverse medicinal plants from Africa which have short- as well as longterm potential to be developed as future phytopharmaceuticals to treat a myriad of pathophysiological conditions, in this review the attention is focused on a one of the most popular dietary supplements in Africa, a plant that has stood out in alternative medical therapies and is increasingly recognized in scientific publications (Figure 1) and commercialized by the rest of the world as a nutrient-rich superfood – Moringa oleifera (Figure 2).
Figure 1.
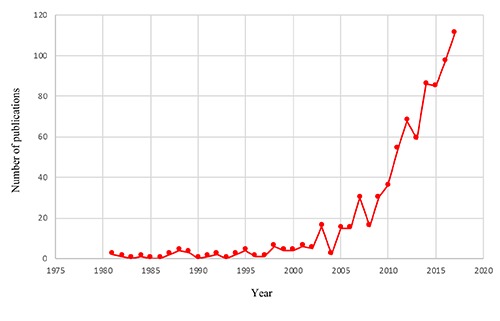
Number of publications on Moringa oleifera.
Figure 2.

Moringa oleifera tree.
Moringa oleifera - the miracle tree
M. oleifera is native to the sub- Himalayan tracts of India, Pakistan, Bangladesh and Afghanistan where it was first described around 2000 BC as a medicinal herb. The Moringa tree spread eastward (lower parts of China, Southeast Asia and the Philippines) and westward (Egypt, the Horn of Africa, around the Mediterranean, and finally to West Indies in America). It is called “Nebedaye”, which means “never die” in many African languages, also known as “the Miracle Tree” “drumstick tree” or “horseradish tree”. Moringa is grown mainly in semiarid, tropical, and subtropical areas in dry, sandy soil. It is very resistant, being able to withstand both severe drought and mild frost conditions. M. oleifera has long been used in herbal medicine by Indians and Africans and is often referred as panacea – used for treating more than 300 conditions – as an antioxidant, anticancer, anti-inflammatory, antidiabetic, antimicrobial etc. Nutritional potential of M. oleifera is notable: leaves are high in protein quality, seeds are abundant in lipids (mainly stearic acid, saturated palmitic acid and oleic acid), both seeds and pods contain high levels of calcium, potassium, sodium and iron.24 Moringa extracts have widespread use by doctors, healers, nutritionists and community leaders, to treat under-nutrition and anemia, especially in children and infants.25,26 Feeding animals with M. oleifera leaves results also in both weight gain and improved nutritional status.27
With its high nutritive values (rich in proteins, minerals and vitamins), every part of the tree is suitable for either nutritional or medicinal purposes (due to the presence of phytochemicals). Immature pods are consumed as highly nutritious vegetables but also bark, pods, leaves, nuts, seeds, tubers, roots and flowers – all are edible (Figure 3-5). Moreover, Moringa can be preserved by drying or freezing for a long time without loss of values.28
In addition to medicinal and nutritional uses, M. oleifera has many other applications (Table 2).29-36 Its seeds are used to extract oil rich in oleic acid, tocopherols and sterols that can be used in cooking as a substitute for olive oil, but also as for nonfood applications like biodiesel, cosmetics, and a lubricant for fine machinery.37,38 Moreover, after oil extraction, the seed cake can be used as an organic fertilizer to improve agricultural productivity.34
Table 2.
Other traditional uses of M. oleifera.
| Traditional use | Mechanism of action | Part of the plant | Bioactive compounds | References |
|---|---|---|---|---|
| Skin care products | Antiseptic, anti-inflammatory, anti-senescent (antioxidants) | Seed oil | Tannins, saponins, flavonoids, terpenoids and glycosides, zeatin | 29 |
| Hair care products | Nutrient delivery to the hair follicles | Seed oil | Minerals and vitamins | 30 |
| Water purification | Cyanobacteria removal, coagulation/flocculation/ sedimentation | Seed | Coagulant protein | 31 |
| Snake bites and wounds | Anti-coagulation/ wound healing | Leaf and root | Thrombin and plasmin like proteases | 32 |
| Aphrodisiac | Stimulation of the sex drive | Leaf | Flavonoids, saponins and alkaloids | 33 |
| Fertilizer | Nutrient addition to the soil, behaving as a scavenger of certain nutrient | Seed cake left after oil extraction | Potassium, magnesium, calcium, phosphorus, nitrogen, copper, nickel | 34 |
| Breast milk production | Unknown | Leaf | Unknown | 35 |
| Machine lubricants | High kinematic viscosity | Seed oil | - | 36 |
Seeds are also a natural coagulant, containing a cationic protein that can clarify turbid water by precipitating organics and mineral particulates.39,40 Moringa seed extract has been shown to eliminate heavy metals (such as lead, copper, cadmium, chromium and arsenic) from water.41 Moreover, seed extracts have antimicrobial properties that inhibit bacterial growth, which finds implications in preventing waterborne diseases.
The properties of M. oleifera seeds have wide applicability in averting diseases and can enhance the quality of life in rural communities as it is highly abundant.
Other parts of Moringa are also used for non-medicinal purposes: the growth hormone from the Moringa leaves, called zeatin is an excellent foliar and has been shown to increase the crop yield by 25-30%.42 The gum from the tree for example, can be used in calico printing.43
Effects of Moringa oleifera on the prevention of chronic diseases
Chronic diseases, which until two decades ago were common only in highincome countries, are now becoming the dominant sources of morbidity and mortality worldwide (WHO 2002). Disease rates from these conditions are accelerating globally, advancing across every region and pervading all socioeconomic classes (79% of the deaths attributed to these diseases occur in the developing countries). Four of the most prominent chronic diseases – cardiovascular diseases (CVD), cancer, chronic obstructive pulmonary disease and type 2 diabetes – are linked by common and preventable biological risk factors, notably high blood pressure, high blood cholesterol and overweight, and by related major behavioral risk factors: unhealthy diet, physical inactivity and tobacco use. The fact that rates of cancers and cardiovascular disease (CVD) among migrants from lowrisk to high-risk countries almost always increase dramatically (in traditional African societies, for example, coronary artery disease (CAD) is virtually nonexistent, but rates among African Americans are similar to those among Caucasian Americans), confirm that the primary determinants of these diseases are both genetic and environmental factors, including diet and lifestyle.44 Increasing scientific evidence provides a sufficiently strong and plausible description of mechanisms linking diet to chronic diseases. Thus, healthy dietary/nutrition practice can modify the attributable risk of the undesirable development of chronic conditions and supplementation with medicinal plant compounds known for their beneficial effects can additionally contribute to this prevention.
Moringa is used traditionally for improving nutritional health particularly in the presence of underlying chronic conditions such as inflammation, infections or diabetes. This vast practice which is claimed by many cultures and communities based on real life experiences is now slowly being confirmed by scientific and clinical evidence (Figure 6), with no adverse effects reported in association with human studies. 45
Figure 6.
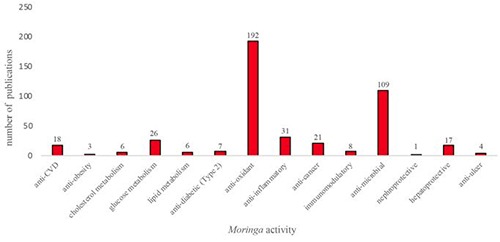
Number of publications on Moringa oleifera activity.
M. oleifera has potent hypocholesterolemic, hypolipidemic and antiatherosclerotic activity. Several studies showed the hypocholesterolemic and hypolipidemic effect of oral consumption of M. oleifera extracts in the context of high-fat diet46,47, prevention of liver inflammation48,49 and improvement in liver alterations due to diabetic- induced damage50-52. Moreover, Moringa leaf extract has also been reported to reduce the formation of atherosclerotic plaques.53
Although there are only a few studies in humans, the potential benefits of using M. oleifera for the treatment of hyperglycemia and dyslipidemia have been demonstrated: type-2 diabetes patients treated with leaf powder for 40 days, showed glycemia, total cholesterol, triglycerides and low-density lipoprotein and very-low-density lipoprotein cholesterol reduction.54
Scientific evidences document chemoprotective activity of M. oleifera (mainly leaf extracts) against heavy metal hepatoand neuro- toxicity in animal models.55-57 Furthermore, histological tests in animals showed that aqueous and alcoholic root, flower and leaf extracts induced reduction of drug-induced hepatic and renal damage. 58-60
Moringa has also remarkable antioxidant, anti-inflammatory and immunomodulatory activities. The antioxidant activity is particularly strong in leaves61, but also pods62 and seeds63 showed similar effects. Studies with normal and diabetic rats documented significant increase in the activity of the enzymes superoxide dismutase, catalase and glutathione S-transferase and decreased lipid peroxidation in response to treatment with aqueous leaf extracts.64 Clinical studies in humans showed that supplementation with leaf powder for 3 months significantly decreased the serum levels of malondialdehyde, generated by lipid peroxidation, and increased the levels of ascorbic acid, superoxide dismutase and glutathione peroxidase, which are indicators of the antioxidant property of the plant.65 The anti-inflammatory activity of M. oleifera has been observed after treatment with extracts of roots, stems, leaves, flowers, pods and seeds in studies on paw edema,66 airway inflammation,67 ulcerative colitis,68 atopic dermatitis69 even Parkinson’s disease. 70
The immunomodulatory effects of Moringa have been extensively studied in models of Lipopolysaccharide (LPS) stimulated macrophages.71-74 Furthermore, antimicrobial activity of root, stem, leaf, flower, pod and seed extracts has been demonstrated in numerous studies on Gram-positive (Enterococcus faecalis, methicillinresistant Staphylococcus aureus and Staphylococcus epidermidis) and Gram-negative bacteria (Salmonella enterica, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli) isolated from clinical samples.75-82 The antibacterial potential of the Moringa crude extracts was comparable to that of the commonly used antibiotics. Several studies have demonstrated the antifungal activity of seed, pod and root extracts.83-85
Moringa has been shown effective in inhibiting the growth of several human cancer cells: acute myeloid leukemia lymphoblastic leukemia and hepatocellular carcinoma cells,86 pancreatic87 and breast cancer cells.88 Some animal studies have also confirmed the efficacy of leaf extracts in preventing cancer in rats with hepatic carcinomas induced by diethyl nitrosamine89 and in suppressing azoxymethane-induced colon carcinogenesis in mice.90
Other investigators studied the effects of oral administration of hydromethanolic and methanolic leaf extracts on a mouse melanoma model – after 15 days, tumor growth was delayed and mouse lifespan significantly increased.91
Bioactive compounds in Moringa oleifera
A bioactive plant compound (BPC) is defined as any non-nutritive constituent of food plants that has an effect on the organism consuming it. BPC include molecules that are present in small quantities in plants and can promote good health in human body. Typical BPCs are plant secondary metabolites that are not essential (i.e. they have no function in plant growth), but still play an important role to the plant’s survival. 92
M. oleifera has been recognized to contain a great number of bioactive compounds. Moringa leaves are reported to be rich sources of vitamins, carotenoids, polyphenols, phenolic acids, flavonoids, alkaloids, glucosinolates, isothiocyanates, tannins and saponins.93 Many studies confirmed the statement that Moringa leaves are the main source of the numerous pharmacological properties attributed to this plant; i.e., the antioxidant activity of leaf extracts due to the high contents of polyphenols underlies Moringa’s antiinflammation, hepatoprotective, antibacterial and antitumor effects.94,20
However, all other tissues of this plant: roots, bark, gum, fruit (pods), flowers, seed, and seed oil can be used in the treatment of various diseases, including inflammation or infectious diseases along with cardiovascular, gastrointestinal, hematological and neoplastic diseases.20 The therapeutic potential of the leaves is due to the great amount of bioactive compounds including steroids, glycosides, quercetin, terpenoids, gallic acid, caffeic acid, phytosterols and many others.93-95,20
It was recently discovered that the Moringa calluses contain a large amount of microRNA, a proposed bioactive compound. This compound was reported to exhibit certain chemopreventive activity, by blocking the increase of breast cancer volume (personal communication).
Moringa leaves have been reported to be a rich source of carotenoids, proteins, calcium, potassium and vitamins.55 Vitamin A deficiency is associated with chronic conditions including night blindness, increased risk of resistance to severe infection and impaired embryonic development and spermatogenesis in males.96 Moringa leaves have large amounts of vitamin A and β-carotene, which can be converted to vitamin A when the body’s vitamin A stores are depleted. Moringa leaves have even 10 times higher vitamin A concentration than carrots; moreover, some studies reported a higher content of different micro-nutrients in Moringa compared to those found in distinct types of food (i.e., 12 times higher vitamin C concentration then orange).45,97
Furthermore, it has been proposed that M. oleifera leaves extracts contain a large number of phenolic compounds such as kaempferol, quercetin, catechin, gallic acid, caffeic acid, p-coumaric acid, vanillin, ferulic acid, protocatechuic acid, cinnamic acid, flavonoids and epicatechin. These secondary metabolites identified from Moringa extract have been shown to protect against chronic diseases through the action of various biological profiles including antioxidant, anti-tuberculosis, analgesic, anticancer, anti-diabetic, antispasmodic, diuretic, antihypertensive, cholesterol lowering, antioxidant, antibacterial and antimicrobial and antimalarial activities exhibited by this plant.97,98 Phenolic acid has antioxidant and anti-inflammatory properties due to its particular chemical structure: this compound neutralizes free radicals and other reactive oxygen species (ROS) by donating hydrogen atoms.99 It has been recently proposed that the protective effect of different polyphenols, such as quercetin or resveratrol, can modulate the synthesis of microRNA.100 Quercetin was found in dried Moringa leaves in high concentration:20 it has been reported that quercetin rich food intake influences the expression as many as 198 miRNAs in lung cancer tissues.101 Moreover, plant miRNAs are involved in regulating of biosynthesis of secondary metabolites.102 MicroRNA156, very common in Moringa seed, targeting squamosa promoter binding protein-like 9 (SPL9) involved in the biosynthesis of glucisinolates and flavonoids.102,103
Tannins and saponins are other natural compounds very common in Moringa leaves. These compounds exhibit anti-cancer and anti-inflammatory properties.43
Moringa oleifera in African diet
In African and Asian cuisine there are a lot of recipes based on M. oleifera leaves, seeds, flowers and fruits. In fact, Moringa leaves are used to prepare dishes in Ghana, Nigeria, Ethiopia, East Africa and Malawi.104 In Cameroon, Moringa is consumed as a vegetable, is used to prepare soup but also to prepare dishes with meat and fish (personal communication). Leaves can be used fresh or as dry powder. Fresh leaves are often used in the same way as spinach or to prepare salads, sauces and soups. Dried leaves are often milled and could be used to confer a spicy taste to dishes, also combined with other ingredients. Flowers are either cooked or fried and may be combined with relishes. Fruits, especially when are green, are consumed as vegetables while once collected they can be boiled and added to dishes. Seeds are used in different ways: they can be boiled, after they are removed from the pods, fried or they can be used to produce edible oil. Moringa can be added to meat and fish to increase the taste.
In Africa, but also in other countries, Moringa is increasingly used as a food fortificant. There are a lot of study showing the potential use of M.oleifera to prepare bread,105 cake,106 yoghurt,107 soups108 and herbal biscuits.109
Cross-kingdom regulation: A potential mechanism of action?
Our bodies need nutrients for normal growth, maintenance, repair and reproduction. The composition of our diet requires a fine balance between two different types of nutrients: macro- (carbohydrates, proteins and fats) and micronutrients (vitamins, minerals and trace elements). Recently, it has become evident that nutrition not only does provide macro and micronutrients, but plants used as food, can deliver different molecules with pharmacological properties.110 Among these, bioactive compounds (especially secondary metabolites) and plant microRNAs provide organisms with bioactive principles required for gene regulation, disease prevention and overall well-being.
MicroRNAs are a class of evolutionarily conserved small non-coding RNAs of 19-24 nucleotides in length that regulate gene expression in eukaryotes. In humans, miRNA binds to the 3’untranslated region of target mRNA through different sequence complementarity: incomplete complementarity results in inhibition of translation, while perfect complementarity leads to mRNA degradation.111 In plants, a near perfect complementarity with the open reading frame of protein coding gene leads to mRNA degradation.112 It has been calculated that more than 60% of all animal mRNAs are miRNA targets.113
In 2012, Zhang and collaborators demonstrated for the first time that osamiR168a and other exogenous microRNA abundant in rice plants could pass through the mouse gastrointestinal (GI) tract and enter into the circulation and various organ of mice. Functional studies in vitro and in vivo demonstrated that osa-miR168a binds the human/mouse low-density lipoprotein receptor adapter protein 1 (LDLRAP1) mRNA, inhibits the expression of protein in liver, and decreases the LDL removal from mouse plasma.114 For the first time, Zhang and collaborators demonstrated that miRNAs contained in vegetal food regulate mRNA translation in a manner of mammalian functional miRNAs.
In a mouse feeding experiments, Liang et al. showed that dietary bol-miR172, very common in B.oleracea, can survive through the GI tract and enter the bloodstream and various organ of mice.115 Further works were able to detect miR2911, derived from honeysuckle (Lonicera japonica), from sera and urine of mice fed a plant-chow diets enriched with honeysuckle; the same miRNA showed an anti-viral effect against influenza A viruses.116 These results suggest that miR2911, an atypical miRNA found in a well-known Chinese herb, may represent a natural novel drug against different types of influenza viruses. Another group showed that oral administration of plant miRNA159 suppressed the growth of xenograft breast cancer in mice;117 another study from an Italian research group demonstrated an interesting action of plant miRNA168 in reduction of inflammation by binding to Toll-like receptor 3 of dendritic cells.118
It is now well accepted that a regular consumption of fruits and vegetables, associated with daily physical activity, may reduce the onset of many chronic diseases, like cardiovascular, obesity, diabetes and cancer. Food plants release into the human body several natural bioactive compound with powerful antioxidant properties. These natural antioxidants from plant are mainly polyphenols (phenolic acids, flavonoids, anthocyanins, lignans and stilbenes), carotenoids (xanthophylls and carotenes) and vitamins (vitamin E and C).119 Considering their important health effects, the mechanism of action of polyphenols has been widely studied. Recently, a polyphenol regulatory modulation on human microRNA expression has been demonstrated - this study highlighted another crosskingdom mechanism: modulation of endogenous microRNAs by polyphenols in mammalian cell homeostasis.120
Conclusions
African diet is prevalently vegetarian, and the plants used by traditional cuisine are not only highly nutritive, but many are potent medicinal remedies at the same time. Amongst the bioactive compounds responsible for the beneficial effects, polyphenols and microRNA prevail. Recently, a new mechanism of genetic regulation has been identified, where the exogenous plant derived microRNAs are capable of fine-tuning mammalian gene expression, and the polyphenols from the plants are capable of regulating endogenous mammalian microRNA levels. This cross-kingdom regulation represents a bursting field of research with immense potential for the formulations of nutraceutical compounds and functional foods based on medicinal plants. One of the most commonly used plants with remarkable nutritional value and medicinal properties in African continent - M. oleifera - has recently been sequenced for microRNA and, consecutively, analyzed to point out the cross-kingdom interaction on its microRNAs. Moringa’s medicinal and nutritional uses and bioactive compound composition in the context of the potential cross-kingdom regulation place this plant in the spotlight of the nutraceuticals and functional foods field.
Figure 3.

Moringa oleifera pods.
Figure 4.

Moringa oleifera flower.
Figure 5.

Moringa oleifera leaves.
Acknowledgments
We would like to thank Dr. Mark Olson for kindly providing the photographs of Moringa oleifera for this work.
Funding Statement
Funding: none.
References
- 1.Pickford M. Palaeoenvironments and hominoid evolution. Z Morphol Anthropol 2002;83:337-48. [PubMed] [Google Scholar]
- 2.National Research Council Policy and Global Affairs Development Security Cooperation Lost Crops of Africa: Volume II Vegetables National Academies Press; 2006. [Google Scholar]
- 3.Harris JB. The Africa Cookbook: Tastes of a Continent. New York: Simon & Schuster; 1998. [Google Scholar]
- 4.Lentz C, ed. Changing Food Habits: Case Studies from Africa, South America, and Europe. Sydney, Australia: Harwood Academic Publishers; 1999. [Google Scholar]
- 5.Fiple KF, Ornelas KC, eds. The Cambridge World History of Food, Volumes. 1 and 2. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 6.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Ann Rev Genomics Hum Genet 2008;9:403-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lock JM Africa Ecosystem of. Levin S. A. ed. Encyclopedia of Biodiversity, vol.1, Academic Press, London, 2001. [Google Scholar]
- 8.Mahomoodally MF. Traditional medicines in Africa: an appraisal of ten potent african medicinal plants. Evid Based Complement Alternat Med. 2013;2013:617459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu K, Wang L, Zhang Y. The clinical research of Okra in treatment of diabetic nephropathy. Jilin Med J 2005. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-JLYX200510009.htm [Google Scholar]
- 10.Liu SY, Chang TW, Lin YK, et al. Studies on the varietal characters, production potential, phytochemical properties, and antioxidant effect of Dioscorea spp. J Agric Res China 1999;48:1e22. [Google Scholar]
- 11.Chiu CS, Deng JS, Chang HY, et al. Antioxidant and anti-inflammatory properties of Taiwanese yam (Dioscorea japonica Thunb. var. pseudojaponica (Hayata) Yamam.) and its reference compounds. Food Chem 2013;141:1087e96. [DOI] [PubMed] [Google Scholar]
- 12.Farombi EO, Britton G, Emerole GO. Evaluation of the antioxidant activity and partial characterization of extracts from browned yam flour diet. Food Res Int 2000;33:493e9. [Google Scholar]
- 13.Chen H, Wang C, Chang CT, Wang T. Effects of Taiwanese yam (Dioscorea japonica Thunb var. pseudojaponica Yamamoto) on upper gut function and lipid metabolism in Balb/c mice. Nutrition 2003;19:646e51. [DOI] [PubMed] [Google Scholar]
- 14.Wu WH, Liu LY, Chung CJ, et al. Estrogenic effect of yam ingestion in healthy postmenopausal women. J Am Coll Nutr 2005;24:235e43. [DOI] [PubMed] [Google Scholar]
- 15.Asare AT, Agbemafle R, Adukpo GE, et al. Assessment of functional properties and nutritional composition of some cowpea (Vigna unguiculata L.) genotypes in Ghana. J Agricul Biol Sci 2013;8:465-9. [Google Scholar]
- 16.Klopfenstein CF, Hoseney RC. Nutritional properties of sorghum and the millet: chemistry and technology, American association of cereal chemists. Inc St Paul 1995;125-168. [Google Scholar]
- 17.Fasasi OS. Proximate, antinutritional factors and functional properties of processed Pearl Millet (Pennisetum glaucum) J Food Technol 2009;3:92-7. [Google Scholar]
- 18.Linares OF. African rice (Oryza glaberrima): History and future potential. Proc Natl Acad Sci USA 2002;99:16360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council Policy and Global Affairs Development Security Cooperation Lost Crops of Africa: Volume I Vegetables National Academies Press; 1996. [Google Scholar]
- 20.Vergara-Jimenez M, Almatrafi MM, Fernandez ML. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants (Basel) 2017;6:E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwu MM Food as Medicine: Functional Food Plants of Africa, CRC Press 2016. [Google Scholar]
- 22.Ghalioungui P. The House of Life: Magic and Medical Science in Ancient Egypt. BM Israel, Amsterdam, 1973. [Google Scholar]
- 23.WHO regional office for Africa. Traditional Medicine. Available from: http://www.afro.who.int/healthtopics/traditional-medicine [Google Scholar]
- 24.Raimunda S, Nogueira B, Jamille AS, et al. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pac J Trop Med 2017;10:621-30. [DOI] [PubMed] [Google Scholar]
- 25.Yang R, Chang L, Hsu J, et al. Nutritional and functional properties of moringa leaves from germplasm, to plant, to food, to health. Am Chem Soc 2006;1-17. [Google Scholar]
- 26.Thurber MD, Fahey JW. Adoption of Moringa oleifera to combat undernutrition viewed through the lens of the "Diffusion of innovations" theory. Ecol Food Nutr 2009;48:212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendieta-Araica B, Spörndly E, Reyes-Sánchez N, Spörndly R. Feeding Moringa oleifera fresh or ensiled to dairy cows: effects on milk yield and milk flavor. Trop Anim Health Prod 2011;43:1039-47. [DOI] [PubMed] [Google Scholar]
- 28.Katayon S, Noor MJ, Asma M, et al. Effects of storage conditions of Moringa oleifera seeds on its performance in coagulation. Bioresour Technol 2006;97:1455-60. [DOI] [PubMed] [Google Scholar]
- 29.Leone A, Spada A, Battezzati A, et al. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int J Mol Sci 2016;17:E2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhammad N, Muhammad I. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis 2016; 15: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kweku Amagloh F, Benang A. Effectiveness of Moringa oleifera seed as coagulant for water purification. Available from: https://www.sswm.info/sites/default/files/reference_attachments/AMAGLOH%20BENANG%202009%20Effectiveness%20of%20Moringa%20Seed%20as%20Coagulant%20for%20Water%20Purification.pdf [Google Scholar]
- 32.Satish A, Sairam S, Ahmed F, Urooj A. Moringa oleifera Lam: protease activity against blood coagulation cascade. Pharmacognosy Res 2012;4:44-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabsattroo T, Wattanathorn J, Iamsaard S, et al. Moringa oleifera extract enhances sexual performance in stressed rats. J Zhejiang Univ Sci B 2015;16:179-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emmanuel SA, Emmanuel BS, Zaku SG, Thomas SA. Biodiversity and agricultural productivity enhancement in Nigeria: Application of processed Moringa oleifera seeds for improved organic farming. Biol J N Am 2011;2:867-71. [Google Scholar]
- 35.Estrella CP, Mantaring JBV, David GZ, et al. A double-blind, randomized controlled trial on the use of malunggay (Moringa oleifera) for augmentation of the volume of breast milk among non-nursing mothers of preterm infants. Available from: https://miracletrees.org/moringadoc/moringa_breastfeeding_study.pdf [Google Scholar]
- 36.Mohd Mizuan O. An investigation of using moringa oleifera seeds oil as lubricant. 2013. Available from: http://iportal.ump.edu.my/lib/item?id=chamo:83486&theme=UMP2 [Google Scholar]
- 37.Tsaknis J, Lalas S, Gergis V, et al. Characterization of Moringa oleifera variety Mbololo seed oil of Kenya. J Agric Food Chem 1999;47:4495-9. [DOI] [PubMed] [Google Scholar]
- 38.Fahey J. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Trees Life J 2005;1:1-33. [Google Scholar]
- 39.Ndabigengesere A, Subba Narasiah K. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res 1998;32:781-91. [Google Scholar]
- 40.Lurling M, Beekman W. Anticyanobacterial activity of Moringa oleifera seeds. J Appl Phycol 2010;23:503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravikumar K, Sheeja AK. Heavy metal removal from water using Moringa oleifera seed coagulant and double filtration. Int J Sci Eng Res 2013;4:10-3. [Google Scholar]
- 42.Hala H, El-Nour A, Ewais NA. Effect of Moringa oleifera Leaf Extract (MLE) on Pepper Seed Germination, Seedlings Improvement, Growth, Fruit Yield and its Quality. Middle East J Agric Res 2017;6:448-63. [Google Scholar]
- 43.Gopalakrishnan L, Doriya K, Kumara DS. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci Human Wellness 2016;5:49-56. [Google Scholar]
- 44.WHO. Diet Nutrition and the Prevention of Chronic Diseases. 2003. Available from: http://apps.who.int/iris/bitstream/10665/42665/1/WHO_TRS_916.pdf [PubMed] [Google Scholar]
- 45.Stohs SJ, Hartman MJ. Review of the Safety and Efficacy of Moringa oleifera. Phytother Res 2015;29:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghasi S, Nwobodo E, Ofili JO. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed Wistar rats. J Ethnopharmacol 2000;69:21-5. [DOI] [PubMed] [Google Scholar]
- 47.Mehta K, Balaraman R, Amin AH, et al. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J Ethnopharmacol 2003;86:191-5. [DOI] [PubMed] [Google Scholar]
- 48.Almatrafi MM, Vergara-Jimenez M, Murillo AG, et al. Moringa Leaves Prevent Hepatic Lipid Accumulation and Inflammation in Guinea Pigs by Reducing the Expression of Genes Involved in Lipid Metabolism. Int J Mol Sci 2017;18:E1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helmy SA, Morsy NFS, Elaby SM, Ghaly MAA. Hypolipidemic Effect of Moringa oleifera Lam Leaf Powder and its Extract in Diet-Induced Hypercholesterolemic Rats. J Med Food 2017;20:755-62. [DOI] [PubMed] [Google Scholar]
- 50.Olayaki LA, Irekpita JE, Yakubu MT, Ojo OO. Methanolic extract of Moringa oleifera leaves improves glucose tolerance, glycogen synthesis and lipid metabolism in alloxan-induced diabetic rats. J Basic Clin Physiol Pharmacol 2015;26:585-93. [DOI] [PubMed] [Google Scholar]
- 51.Omodanisi EI, Aboua YG, Chegou NN, Oguntibeju OO. Hepatoprotective, Antihyperlipidemic, and Anti-inflammatory Activity of Moringa oleifera in Diabetic-induced Damage in Male Wistar Rats. Pharmacognosy Res 2017;9:182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan W, Parveen R, Chester K, et al. Hypoglycemic Potential of Aqueous Extract of Moringa oleifera Leaf and In Vivo GC-MS Metabolomics. Front Pharmacol 2017;12:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chumark P, Khunawat P, Sanvarinda Y, et al. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol 2008;116:439-46. [DOI] [PubMed] [Google Scholar]
- 54.Kumari DJ. Hypoglycaemic effect of Moringa oleifera and Azadirachta indica in type 2 diabees mellitus. Bioscan 2010;5:211-4. [Google Scholar]
- 55.Toppo R, Roy BK, Gora RH, et al. Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Vet World 2015;8:537-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adeyemi SO, Aroge SC, Akanji A. Moringa oleifera-based diet protects against nickel-induced hepatotoxicity in rats. J Biomed Res 2017;31:350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Igado OO, Glaser J, Ramos-Tirado M, et al. Isolation of a novel compound (MIMO2) from the methanolic extract of Moringa oleifera leaves: protective effects against vanadium-induced cytotoxity. Drug Chem Toxicol 2017;19:1-10. [DOI] [PubMed] [Google Scholar]
- 58.Sharifudin SA, Fakurazi S, Hidayat MT, et al. Therapeutic potential of Moringa oleifera extracts against acetaminophen- induced hepatotoxicity in rats. Pharm Biol 2013;51:279-88. [DOI] [PubMed] [Google Scholar]
- 59.Ouédraogo M, Lamien-Sanou A, Ramdé N, et al. Protective effect of Moringa oleifera leaves against gentamicin- induced nephrotoxicity in rabbits. Exp Toxicol Pathol 2013;65:335-9. [DOI] [PubMed] [Google Scholar]
- 60.Gupta A, Gautam MK, Singh RK, et al. Immunomodulatory effect of Moringa oleifera Lam. extract on cyclophosphamide induced toxicity in mice. Indian J Exp Biol 2010;48:1157-60. [PubMed] [Google Scholar]
- 61.Paula PC, Sousa DO, Oliveira JT, et al. Protein Isolate from Moringa oleifera Leaves Has Hypoglycemic and Antioxidant Effects in Alloxan- Induced Diabetic Mice. Molecules 2017;22:E271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gull I, Javed A, Aslam MS, et al. Use of Moringa oleifera Flower Pod Extract as Natural Preservative and Development of SCAR Marker for Its DNA Based Identification. Biomed Res Int 2016;2016:7584318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Randriamboavonjy JI, Rio M, Pacaud P, et al. Moringa oleifera Seeds Attenuate Vascular Oxidative and Nitrosative Stresses in Spontaneously Hypertensive Rats. Oxid Med Cell Longev 2017;2017:4129459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaiswal D, Rai PK, Mehta S, et al. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med 2013; 6: 426-32. [DOI] [PubMed] [Google Scholar]
- 65.Kushwaha S, Chawla P, Khurana DS. Effect of supplementation of drumstick (Moringa oleifera) and amaranth (Amaranthus tricolor) leaves powder on lipid profile in postmenopausal women. Int J Sci Res Publ 2012;2:162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Càceres A, Saravia A, Rizzo S, et al. Pharmacologie properties of Moringa oleifera. 2: screening for antispasmodic, antiinflammatory and diuretic activity. J Ethnopharmacol 1992;36:233-7. [DOI] [PubMed] [Google Scholar]
- 67.Mahajan SG, Banerjee A, Chauhan BF, et al. Inhibitory effect of n-butanol fraction of Moringa oleifera Lam. seeds on ovalbumin-induced airway inflammation in a Guinea pig model of asthma. Int J Toxicol 2009;28:519-27. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y, Wu AG, Jaja-Chimedza A, et al. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS One 2017;12:e0184709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi EJ, Debnath T, Tang Y, et al. Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance. Biomed Pharmacother 2016;84:870-7. [DOI] [PubMed] [Google Scholar]
- 70.Giacoppo S, Rajan TS, De Nicola GR, et al. The Isothiocyanate Isolated from Moringa oleifera Shows Potent Anti- Inflammatory Activity in the Treatment of Murine Subacute Parkinson's Disease. Rejuvenation Res 2017;20:50-63. [DOI] [PubMed] [Google Scholar]
- 71.Arulselvan P, Tan WS, Gothai S, et al. Anti-Inflammatory Potential of Ethyl Acetate Fraction of Moringa oleifera in Downregulating the NF-κB Signaling Pathway in Lipopolysaccharide- Stimulated Macrophages. Molecules 2016;21:E1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fard MT, Arulselvan P, Karthivashan G, et al. Bioactive Extract from Moringa oleifera inhibits the Proinflammatory mediators in Lipopolysaccharide stimulated macrophages. Pharmacogn Mag 2015;11:S556-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan WS, Arulselvan P, Karthivashan G, Fakurazi S. Moringa oleifera Flower Extract Suppresses the Activation of Inflammatory Mediators in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages via NF-κB Pathway Mediators Inflamm 2015;2015:720171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajan TS, Giacoppo S, Iori R, et al. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016;112:104-15. [DOI] [PubMed] [Google Scholar]
- 75.Nikkon F, Saud ZA, Rahman MH, Haque ME. In vitro antimicrobial activity of the compound isolated from chloroform extract of Moringa oleifera Lam. Pak J Biol Sci 2003;6:1888-90. [Google Scholar]
- 76.Doughari JH, Pukuma MS, De N. Antibacterial effects of Balanites aegyptiaca L. Drel. and Moringa oleifera Lam. on Salmonella typhi. Afr J Biotechnol 2007;6:2212-5. [Google Scholar]
- 77.Sayeed MA, Hossain MS, Chowdhury MEH, Haque M. In vitro antimicrobial activity of methanolic extract of Moringa oleifera Lam. fruits. J Pharmacogn Phytochem 2012;1:94-8. [Google Scholar]
- 78.Onsare JG, Kaur H, Arora DS. Antimicrobial activity of Moringa oleifera from different locations against some human pathogens. Acad J Med Plants 2013;1:80-91. [Google Scholar]
- 79.Arora DS, Onsare JG. In vitro antimicrobial evaluation and phytoconstituents of Moringa oleifera pod husks. Ind Crop Prod 2014;52:125-35. [Google Scholar]
- 80.Oluduro AO. Evaluation of antimicrobial properties and nutritional potentials of Moringa oleifera Lam. leaf in South-Western Nigeria. Malays J Microbiol 2012;8:59-67. [Google Scholar]
- 81.Rahman MA, Rahman MM, et al. Antibacterial activity of leaf juice and extracts of Moringa oleifera Lam. against some human pathogenic bacteria. CMU J Nat Sci 2009;8:219. [Google Scholar]
- 82.Idris A, Abubakar U. Phytochemical and antibacterial investigations of moringa (Moringa oleifera) leaf extract on selected bacterial pathogens. J Microbiol Antimicrob 2016;8:28-33. [Google Scholar]
- 83.Rahman MS, Zerin L, Anwar MN. Antibacterial and antifungal activity of Moringa oleifera stem bark. Chittagong Univ J Biol Sci 2008;3: 109-17. [Google Scholar]
- 84.Ishnava KB, Chauhan KH, Bhatt CA. Screening of antifungal activity of various plant leaves extracts from Indian plants. Arch Phytopathol Plant Prot 2012;45:152-60. [Google Scholar]
- 85.Padla EP, Solis LT, Levida RM, et al. Antimicrobial isothiocyanates from the seeds of Moringa oleifera Lam. Z Naturforsch C 2012;67:557-64. [DOI] [PubMed] [Google Scholar]
- 86.Khalafalla MM, Abdellatef E, Dafalla HM, et al. Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma. Afr J Biotechnol 2010;9:8467-71. [Google Scholar]
- 87.Berkovich L, Earon G, Ron I, et al. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med 2013;13:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adebayo IA, Arsad H, Samian MR. Antiproliferative effect on breast cancer (MCF7) of Moringa oleifera seed extracts. Afr J Tradit Complement Altern Med 2017;14:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sadek KM, Abouzed TK, Abouelkhair R, Nasr S. The chemo-prophylactic efficacy of an ethanol Moringa oleifera leaf extract against hepatocellular carcinoma in rats. Pharm Biol 2017;55:1458-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Budda S, Butryee C, Tuntipopipat S, et al. Suppressive effects of Moringa oleifera Lam pod against mouse colon carcinogenesis induced by azoxymethane and dextran sodium sulfate. Asian Pac. J Cancer Prev 2011;12:3221-8. [PubMed] [Google Scholar]
- 91.Purwal L, Pathak AK, Jain UK. In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacol Online 2010;1:655-65. [Google Scholar]
- 92.Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr 2011. 2:32-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leone A, Spada A, Battezzati A, et al. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int J Mol Sci 2015;16:12791-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mbikay M. Therapeutic Potential of Moringa oleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: A Review. Front Pharmacol 2012;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brilhante RSN, Sales JA, Pereira VS, et al. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pac J Trop Med 2017;10:621-30. [DOI] [PubMed] [Google Scholar]
- 96.Lopez-Teros V, Ford JL, Green MH, et al. Use of a "Super-child" Approach to Assess the Vitamin A Equivalence of Moringa oleifera Leaves, Develop a Compartmental Model for Vitamin A Kinetics, and Estimate Vitamin A Total Body Stores in Young Mexican Children. J Nutr 2017;147:2356-63. [DOI] [PubMed] [Google Scholar]
- 97.Abdull Razis AF, Ibrahim MD, Kntayya SB. Health benefits of Moringa oleifera. Asian Pac J Cancer Prev 2014;15:8571-6. [DOI] [PubMed] [Google Scholar]
- 98.Saini RK, Sivanesan I, Keum YS. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. Biotech 2016;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010;2:1231-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lançon A, Michaille JJ, Latruffe N. Effects of dietary phytophenols on the expression of microRNAs involved in mammalian cell homeostasis. J Sci Food Agric 2013;93:3155-64. [DOI] [PubMed] [Google Scholar]
- 101.Lam TK, Shao S, Zhao Y, et al. Influence of quercetin-rich food intake on microRNA expression in lung cancer tissues. Cancer Epidemiol Biomarkers Prev 2012;21:2176-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gupta OP, Karkute SG, Banerjee S, et al. Contemporary Understanding of miRNA-Based Regulation of Secondary Metabolites Biosynthesis in Plants. Front Plant Sci 2017;8:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pirrò S, Zanella L, Kenzo M, et al. MicroRNA from Moringa oleifera: Identification by High Throughput Sequencing and Their Potential Contribution to Plant Medicinal Value. PLoS One 2016;11:e0149495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Agbogidi O, Ilondu E. Moringa oleifera Lam: its potentials as a food security and rural medicinal item. J Biol Innov 2012;1:156-67. [Google Scholar]
- 105.Chinma C, Abu J, Akoma S. Effect of germinated tigernut and moringa flour blends on the quality of wheat-based bread. Food Process Preserv 2014;38:721-7. [Google Scholar]
- 106.Kolawole F, Balogun M, Opaleke D, Amali H. An evaluation of nutritional and sensory qualities of wheat-moringa cake. Agrosearch 2013;13:87-94. [Google Scholar]
- 107.Hekmat S, Morgan K, Soltani M, Gough R. Sensory evaluation of locally- grown fruit purees and inulin fibre on probiotic yogurt in mwanza, Tanzania and the microbial analysis of probiotic yogurt fortified with Moringa oleifera. J Health Popul Nutr 2015;33:60–7. [PMC free article] [PubMed] [Google Scholar]
- 108.Babayeju A, Gbadebo C, Obalowu M, et al. Comparison of Organoleptic properties of egusi and efo riro soup blends produced with moringa and spinach leaves. Food Sci Qual Manag 2014;28:15-8. [Google Scholar]
- 109.Alam M, Alam M, Hakim M, et al. Development of fiber enriched herbal biscuits: a preliminary study on sensory evaluation and chemical composition. Int J Nutr Food Sci 2014;3:246-50. [Google Scholar]
- 110.Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv 2015;33:1582-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pirrò S, Minutolo A, Galgani A, et al. Bioinformatics Prediction and Experimental Validation of MicroRNAs Involved in Cross- Kingdom. Interaction. J Comput Biol 2016;23:976-89. [DOI] [PubMed] [Google Scholar]
- 112.Sunkar R, Zhu JK. Novel and stressregulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 2004;16:2001-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Witkos TM, Koscianska E, Krzyzosiak WJ. Practical Aspects of microRNA Target Prediction. Curr Mol Med 2011;11:93-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang L, Hou D, Chen X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 2012;22:107-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang G, Zhu Y, Sun B, et al. Assessing the survival of exogenous plant microRNA in mice. Food Sci Nutr 2014;2:380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou Z, Li X, Liu J, et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res 2015;25: 39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chin AR, Fong MY, Somlo G, et al. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res 2016;22:107-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cavalieri D, Rizzetto L, Tocci N, et al. Plant microRNAs as novel immunomodulatory agents. Sci Rep 2016;6: 25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu DP, Li Y, Meng X, et al. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int J Mol Sci 2017;18:E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blade C, Baselga-Escudero L, Arola-Arnal A. microRNAs as new targets of dietary polyphenols. Curr Pharm Biotechnol 2014;15:343-51. [DOI] [PubMed] [Google Scholar]


