Abstract
Purpose
To evaluate the outcomes of active surveillance (AS) on patients with low-risk prostate cancer (PCa) and to identify predictors of disease reclassification.
Methods
In 2005, we defined an institutional AS protocol (Sorveglianza Attiva Istituto Nazionale Tumori [SAINT]), and we joined the Prostate Cancer Research International: Active Surveillance (PRIAS) study in 2007. Eligibility criteria included clinical stage ≤T2a, initial prostate-specific antigen (PSA) <10 ng/mL, and Gleason Pattern Score (GPS) ≤3 + 3 (both protocols); ≤25% positive cores with a maximum core length containing cancer ≤50% (SAINT); and ≤2 positive cores and PSA density <0.2 ng/mL/cm3 (PRIAS). Switching to active treatment was advised for a worsening of GPS, increased positive cores, or PSA doubling time <3 years. Active treatment-free survival (ATFS) was assessed using the Kaplan-Meier method. Factors associated with ATFS were evaluated with a multivariate Cox proportional hazards model.
Results
A total of 818 patients were included: 200 in SAINT, 530 in PRIAS, and 88 in personalized AS monitoring. Active treatment-free survival was 50% after a median follow-up of 60 months. A total of 404/818 patients (49.4%) discontinued AS: 274 for biopsy-related reclassification, 121/404 (30%) for off-protocol reasons, 9/404 (2.2%) because of anxiety. Biopsy reclassification was associated with PSA density (hazard ratio [HR] 1.8), maximum percentage of core involvement (HR 1.5), positive cores at diagnostic biopsy (HR 1.6), older age (HR 1.5), and prostate volume (HR 0.6) (all p<0.01). Patients from SAINT were significantly more likely to discontinue AS than were the patients from PRIAS (HR 1.65, p<0.0001).
Conclusions
Five years after diagnosis, 50% of patients with early PCa were spared from active treatment. Wide inclusion criteria are associated with lower ATFS. However, at preliminary analysis, this does not seem to affect the probability of unfavorable pathology.
Keywords: Active surveillance, Active treatment-free survival, Low-risk prostate cancer, Outcomes
Introduction
The diagnosis of prostate cancer (PCa) has increased worldwide after prostate-specific antigen (PSA) screening became widespread in the early 1990s, although the mortality rate has remained stable or has slightly decreased. Many PSA-detected tumors are at low risk of progression according to the D'Amico classification and are not life-threatening (1). This is true also for the Italian population (2). Despite the fact that the PCa-specific mortality rate without any treatment is estimated to be <2% at 15 years for low-risk disease (3), standard clinical practice still considers radical treatments for these patients, such as prostatectomy or radiation therapy. Although early treatment and modern surgical and radiation techniques may reduce the side effects of therapy, the long survival time may increase the risk of long-term sequelae in this population. To reduce overtreatment of early, low-grade cancer, active surveillance (AS) programs are used in many countries with the aim to avoid or delay radical treatments and their side effects (4).
Our limited knowledge of the pathogenesis and natural history of PCa hinders our ability to accurately identify indolent PCa at diagnosis and during follow-up.
Active surveillance protocols are designed to include patients with clinically insignificant cancer, i.e., patients with an index lesion volume of ≤0.5 cm3, Gleason Pattern Score (GPS) ≤3 + 3, and low PSA.
These criteria are mostly based on the results of pathologic studies performed in the 1990s (5), and these clinical features are now being questioned. Nonetheless, expected PCa-specific mortality is very low for patients with PSA-detected, low-risk PCa that is conservatively managed, and the advantages of radical treatments remain unclear. In the Scandinavian Prostate Cancer Group-4 randomized study (6), which compared radical prostatectomy to watchful waiting in mainly clinically detected PCa cases, the advantages of radical treatment were limited to patients older than 65 years.
Moreover, the randomized Prostate Cancer Intervention versus Observation Trial reported that radical prostatectomy did not significantly reduce the all-cause and the PCa-specific absolute risks of mortality compared with observation (<3%) over at least 12 years of follow-up in patients with organ-confined disease diagnosed in the PSA era. A significant reduction was limited to the groups with high-risk PCa and to patients with PSA levels >10 ng/mL (7). Recently, in a population of patients with PSA-detected clinically localized PCas, the Prostate Testing for Cancer and Treatment (ProtecT) randomized trial showed that, after 10 years, PCa-specific mortality was very low (approximately 1%). There were also no significant differences among active monitoring (i.e., PSA kinetic monitoring), radical prostatectomy, and external beam radiotherapy (8). However, surgery and radiotherapy were associated with lower rates of disease progression and metastases. The inclusion of patients belonging to intermediate or high-risk classes, not included in the most recent AS protocols and monitoring without mandatory biopsies, may represent confounding factors in the ProtecT setting.
We describe an 11-year AS experience at the National Cancer Institute in Milan. This is the largest and the longest-running cohort in Italy. The main objectives of this work are to evaluate the outcomes of patients experiencing AS, to identify the factors associated with active treatment-free survival (ATFS), and to predict disease reclassification, i.e., the GPS or the number of positive cores at rebiopsy exceeding the eligibility criteria for AS.
Methods
In 2005, we began to manage all patients with PCa in a dedicated multidisciplinary team that included a urologist, a radiation oncologist, a psychologist, and an on-demand medical oncologist (9–10–11). Patients with low-risk PCa were offered curative options (radical prostatectomy, external beam radiation, brachytherapy), while AS was also proposed as an alternative to active therapies for patients satisfying specific criteria that are defined in the Sorveglianza Attiva Istituto Nazionale Tumori (SAINT) and Prostate Cancer Research International: Active Surveillance (PRIAS) protocols. The SAINT protocol began in March 2005 as a single-center cohort study. In November 2007, we joined the PRIAS study, which is coordinated by the Erasmus University Medical Center (Rotterdam, the Netherlands) (12). The aim of both studies is to validate AS, including the ability to defer radical therapy and its side effects, until there is evidence of nonindolent disease.
Details of the inclusion criteria and follow-up schedules for both protocols are reported below and in Table I. A limited proportion of selected patients at low and intermediate risk who did not meet all inclusion criteria for the SAINT and PRIAS protocols but wished to delay active treatment were followed in a personalized AS monitoring after approval of the multidisciplinary team, according to the SAINT follow-up schedule. Similarly, patients who did not meet the requirements of the inclusion protocol (e.g., PRIAS) during the AS follow-up but still matched the inclusion criteria of the other protocol (e.g., SAINT) were allowed to proceed with AS follow-up.
Table I.
Inclusion criteria for the 2 active surveillance protocols at the National Cancer Institute in Milan
| SAINT | PRIAS | |
|---|---|---|
| PSA, ng/mL | ≤10 | <10 |
| Clinical stage | T1c and T2a; T1b if cancer ≤0.5 cm3 and negative biopsies of the peripheral zone | ≤T2c |
| GPS | 3 + 3 | 3 + 3 or 3 + 4 in age >70 years, tumor involving <10% core length |
| Number of positive cores | ≤20% of all cores until December 2011; ≤25% of all cores since December 2011 | ≤2 or ≤15% of all cores in case of saturation biopsy (>20 cores) with a maximum of 4 |
| PSA density | / | <0.2 ng/mL/cm3 |
| Core involvement | ≤50% of core involvement | / |
GPS = Gleason Pattern Score; PRIAS = Prostate Cancer Research International: Active Surveillance; PSA = prostate-specific antigen; SAINT = Sorveglianza Attiva Istituto Nazionale Tumori.
All biopsy specimens of patients suitable for AS and diagnosed outside the Fondazione IRCCS Istituto Nazionale dei Tumori were reviewed by expert uropathologists to confirm the PCa histologic features.
The SAINT and PRIAS protocols received approval from the local ethical committee, and all patients provided written informed consent before starting AS.
The SAINT protocol
Eligibility criteria for SAINT include histologically confirmed adenocarcinoma of the prostate, suitability for radical treatment, PSA at diagnosis ≤10 ng/mL, GPS ≤3 + 3, clinical stage T1c or T2a (2002-2009 TNM), positive cores in ≤20% of all biopsy cores and tumor involvement per biopsy core ≤50%.
In December 2012, the protocol was amended with the inclusion of the biopsy sampling criteria based on prostate volume, as was used in PRIAS. These criteria advise the following: at least 8 cores for volumes <40 cm3, 10 cores for 40-60 cm3, and 12 cores for >60 cm3, although a 12-core biopsy is now almost always obtained. Accordingly, the maximum number of allowed positive cores was changed to ≤25% of the total cores. Eligibility was also extended to patients with T1b disease and GPS 3 + 3 when cancer was found in less than 0.5 cm3 of the removed tissue or if they had negative peripheral zone biopsies.
Follow-up assessments included PSA measurements every 3 months, digital rectal examination (DRE) every 6 months, and rebiopsy scheduled at 1 and 2 years after diagnosis, as well as every subsequent 2 years.
Switching to active treatment was offered to patients with a PSA doubling time (DT) of <3 years, who showed disease upgrading (GPS >6) or upsizing (number of positive cores or tumor involvement per biopsy core exceeding the criteria for AS) at rebiopsy, or clinical progression >T2c as determined by DRE.
The PRIAS protocol
The original criteria for inclusion were suitability for curative treatment, PSA ≤10 ng/mL, GPS ≤3 + 3, stage ≤cT2c, 2 or fewer positive cores or less than 15% of cores positive in a saturation biopsy (≥20 total cores), and PSA density ≤0.2 ng/mL/cm3 (13). In PRIAS, the number of biopsy cores depends on the prostate volume as described previously (see SAINT protocol).
Since March 2015, there are no limits on the number of positive cores for patients with negative multiparametric magnetic resonance imaging (MRI) (Prostate Imaging-Reporting and Data System [PI-RADS] ≤2) or with MRI-targeted biopsies confirming GPS 3 + 3 in all the cores.
The PRIAS follow-up schedule differs from that of the SAINT protocol in the timing of the rebiopsy, which is at 1, 4, and 7 years after the diagnostic biopsy.
Active treatment is recommended for patients with PSA DT <3 years, clinical stage >T2c at DRE, >2 positive cores (upsizing), or GPS >6 (upgrading) at rebiopsy. The protocol also recommends an additional biopsy if PSA DT is 3-10 years, if the biopsy was not performed within 1 year. As mentioned, patients dropped off from the PRIAS protocol but still matching the SAINT inclusion criteria are allowed to continue AS.
Statistical analysis
Clinical variables related to tumor diagnosis were collected for each patient and are reported with summary statistics.
The causes of exit from AS, related or not to PCa reclassification, are also reported. They are established in a multidisciplinary consultation where, in case of exit for patient choice, the psychologist determines if the patient has dropped out owing to anxiety related to (untreated) PCa. Note that patients might change the reference protocol (SAINT, PRIAS, or personalized AS) during the follow-up. In the analysis, we considered the initial protocol as the reference protocol for those patients, while dropouts are considered as discontinuation from the last protocol followed in AS.
The ATFS was assessed using the Kaplan-Meier method. Patients who discontinued AS still remaining treatment-free (e.g., patients undergoing a watchful waiting approach) were considered as exits without events. Overall ATFS and ATFS related to biopsy reclassification, PSA DT, and anxiety were then calculated. The analysis was also repeated by comparing the SAINT, PRIAS, and personalized AS monitoring.
Age, DRE findings at diagnosis, PSA at diagnosis, PSA density, number of positive cores, percentage of positive cores, percentage of tumor involvement per biopsy core, number of total cores at diagnosis, and prostate volume were evaluated as potential risk factors for ATFS. Associations between these possible risk factors and biopsy-related ATFS were first determined with log-rank tests and univariate Cox proportional hazards models and log-rank tests. All significant variables at the univariate analysis were then introduced in a multivariate Cox model. Statistical analyses were performed using MedCalc software version 12.1.4 (MedCalc Software bvba, Mariakerke, Belgium) and KNIME software (KNIME GmbH, Germany) coupled to R (14).
Results
Patient characteristics
Between March 2005 and October 2016, 818 patients were enrolled: 200 in SAINT, 530 in PRIAS, and 88 monitored in a personalized strategy. Their characteristics at diagnosis are shown in Table II.
Table II.
Patient characteristics at prostate cancer diagnosis
| Total cohort (n = 818) | SAINT (n = 200) | PRIAS (n = 530) | Personalized AS (n = 88) | |
|---|---|---|---|---|
| Age, y | 66 (60-71) | 66 (61-72) | 65 (61-71) | 69 (63-75) |
| PSA at diagnosis, ng/mL, % | ||||
| ≤3 | 7.9 | 3.5 | 8.3 | 15.3 |
| 3-5 | 28.2 | 20.5 | 34.2 | 9.4 |
| 5-8 | 47.2 | 55.0 | 48.5 | 21.2 |
| >8 | 16.7 | 21.0 | 9.1 | 54.1 |
| Gleason Pattern Score, % | ||||
| 3 + 2 | 0.4 | 0.0 | 0.0 | 3.6 |
| 3 + 3 | 98.3 | 99.5 | 100.0 | 84.5 |
| 3 + 4 | 1.3 | 0.5 | 0.0 | 11.9 |
| Number of positive cores, % | ||||
| 1 | 64.1 | 56.6 | 69.2 | 48.8 |
| 2 | 25.6 | 18.7 | 28.9 | 21.3 |
| 3 (or more) | 10.3 | 24.7 | 1.9 | 30.0 |
| Number of total cores (diagnosis) | 12 (11-16) | 12 (9-15) | 12 (12-16) | 12 (10-15) |
| Number of total cores (12-month rebiopsy) | 12 (10-12) | 10 (10-12) | 12 (10-12) | 12 (10-12) |
| Clinical T stage, % | ||||
| T1b | 1.8 | 1.5 | 0.4 | 11.8 |
| T1c | 91.3 | 92.0 | 91.9 | 85.9 |
| T2a | 6.9 | 6.5 | 7.7 | 2.4 |
| Prostate volume, cm3 | 46 (36-63) | 36 (29-50) | 50 (38-65) | 52 (41-78) |
| PSA density, ng/mL/cm3 | 0.12 (0.08-0.16) | 0.18 (0.11-0.25) | 0.11 (0.8-0.14) | 0.16 (0.10-0.25) |
AS = active surveillance; PRIAS = Prostate Cancer Research International: Active Surveillance; PSA = prostate-specific antigen; SAINT = Sorveglianza Attiva Istituto Nazionale Tumori.
Median (interquartile range) are reported for continuous variables and percentages for categorical parameters.
The median age at enrollment was 66 years (range 42-79 years), and median PSA at diagnosis was 5.7 ng/mL (range 0.29-22.68 ng/mL). Most of the patients (93.1%) had nonpalpable disease (clinical T1c).
Note that the SAINT cohort, where the inclusion of patients with >2 positive cores at biopsy (provided that positive cores were ≤20% of all cores, or ≤25% in the amended version) was allowed, included 24.7% of patients with >2 cores showing tumor involvement. In PRIAS, only 1.9% of patients had more than 2 positive cores at the enrollment following a diagnostic saturation biopsy or recent MRI-based staging.
Different entry criteria of AS protocols implied significant differences in clinical characteristics for the 2 largest cohorts: PSA density and number of positive cores were statistically lower in the PRIAS vs the SAINT group (p<0.001, both), while median prostate volume was higher for PRIAS patients (p = 0.0001).
Active treatment-free survival
The Kaplan-Meier curve for all causes of ATFS is shown in Figure 1. The median follow-up was 59 months (range 4-145 months).
Fig. 1.
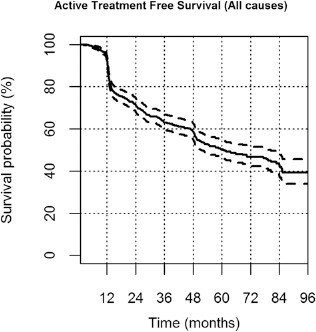
Active treatment-free survival (ATFS) in the whole active surveillance cohort. Kaplan-Meier curve for ATFS with discontinuation for any reason. Continuous and dotted lines represent actuarial survival and 95% confidence intervals, respectively.
The all-cause ATFS at 24 and 60 months was equal to 71% (95% confidence interval [CI] 69%-73%) and 50% (95% CI 48%-52%), respectively.
Overall, 404/818 patients (49.4%) had discontinued AS at the time of analysis. Median permanence in AS was 27.9 months (range 2.3-143.34 months).
Details about AS dropout are given in Table III. In particular, 274 patients discontinued AS because of protocol criteria for reclassification, primarily due to upgrading/upsizing at rebiopsy in 255/404 (63.1%) patients. More than half of these patients (139/255) discontinued AS after the first rebiopsy. Nonetheless, among patients who underwent a rebiopsy at 1 year, 39.8% had a negative histology for PCa.
Table III.
Reasons for discontinuing active surveillance, n (%)
| All | SAINT | PRIAS | Personalized AS | |
|---|---|---|---|---|
| Total number of dropped out patientsa | 404/818 (49.4) | 135/200 (67.5) | 222/530 (41.9) | 47/88 (53.4) |
| Non-PCa reclassification | 130/404 (32.2) | 40/135 (29.6) | 68/222 (30.6) | 22/47 (46.8) |
| Patient choice (anxious preoccupation) | 9 (2.2) | 2 (1.5) | 7 (3.2) | 0 (0) |
| Patient choice (other than anxiety) | 59 (14.6) | 19 (14.1) | 31 (14) | 9 (19.1) |
| Change of hospital | 7 (1.7) | 1 (0.7) | 6 (2.7) | 0 (0) |
| Lost at follow-up | 10 (2.5) | 3 (2.2) | 4 (1.8) | 3 (6.4) |
| Non-prostate cancer death | 5 (1.2) | 1 (0.7) | 2 (0.9) | 2 (4.3) |
| Worsening of performance status or age >80 y | 35 (8.7) | 14 (10.4) | 13 (5.9) | 8 (17.0) |
| Other reasons | 5 (1.2) | 0 (0) | 5 (2.3) | 0 (0) |
| Prostate cancer reclassification | 274/404 (67.8) | 95/135 (70.4) | 154/222 (69.4) | 25/47 (53.2) |
| PSA DT <3 y | 19 (4.7) | 5 (3.7) | 14 (6.3) | 0 (0) |
| Upgrading at 1-y rebiopsy | 53 (13.1) | 22 (16.3) | 25 (11.3) | 6 (12.8) |
| Upsizing at 1-y rebiopsy | 35 (8.7) | 12 (8.9) | 17 (7.7) | 6 (12.8) |
| Upgrading and upsizing at 1-y rebiopsy | 51 (12.6) | 14 (10.4) | 34 (15.3) | 3 (6.4) |
| Upgrading at >1-y rebiopsy | 45 (11.1) | 17 (12.6) | 23 (10.4) | 5 (10.6) |
| Upsizing at >1-y rebiopsy | 30 (7.4) | 16 (11.9) | 11 (5) | 3 (6.4) |
| Upgrading + upsizing at >1-y rebiopsy | 41 (10.1) | 9 (6.7) | 30 (13.5) | 2 (4.3) |
This number includes all patients who discontinued active surveillance, irrespective to whether they had undergone active treatment or not at the time of analysis.
DT = doubling time; PRIAS = Prostate Cancer Research International: Active Surveillance; PSA = prostate-specific antigen; SAINT = Sorveglianza Attiva Istituto Nazionale Tumori.
Only 19/404 patients (4.7%), all in the first period of our experience, were excluded from AS on the bases of short PSA DT alone as in more recent years they underwent further examinations (such as rebiopsy or multiparametric MRI).
Accordingly, PSA DT <10 years prompted additional biopsies in 171 patients. The findings in these patients resulted in upgrading and upsizing in 23/171 (13%), in upgrading in 13/171 (8%), and in upsizing in 7/171 (4%). Additionally, 56/171 (33%) of patients had a positive biopsy still satisfying AS criteria, while 72/171 (42%) had a negative biopsy.
Note that only 9/404 patients (2.2%) discontinued AS for anxious preoccupation of disease progression, while 81/404 patients (20.0%) were withdrawn for personal choice not anxiety-related or refusal to undergo rebiopsy or to continue follow-up.
In general, 335/404 (82.9%) patients who discontinued AS were offered radical treatment, while 46/404 (11.4%) began a watchful waiting approach following the guidelines (age >80 years) or patient wishes or worsening of performance status.
Figure 2 shows ATFS curves according to whether discontinuation of AS was related to biopsy findings, PSA DT, or anxious preoccupation.
Fig. 2.
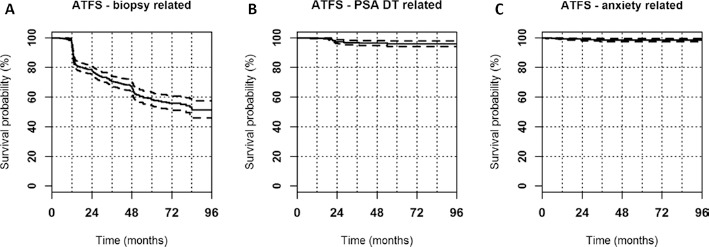
Active treatment-free survival (ATFS) stratified by causes of discontinuation from active surveillance. Kaplan-Meier curves for ATFS according to whether discontinuation of active surveillance was related to biopsy findings (A), prostate-specific antigen doubling time (DT) (B), or patient anxiety (C). Continuous and dotted lines represent actuarial survival and 95% confidence intervals, respectively.
Comparison among the AS protocols
The ATFS curves were also analyzed for the 3 cohorts individually (Fig. 3). Patients enrolled in the SAINT protocol were significantly more likely to discontinue AS than patients in the PRIAS and personalized AS groups for both all causes and biopsy-related causes of exit (all p<0.0001).
Fig. 3.
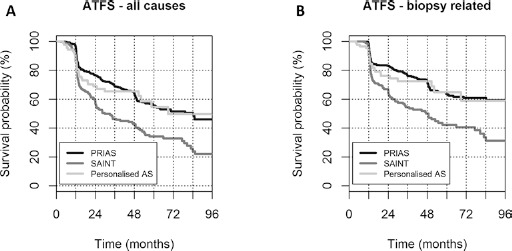
Comparison of active treatment-free survival among the different protocols. Kaplan-Meier curves for active treatment-free survival (ATFS) in the Sorveglianza Attiva Istituto Nazionale Tumori (SAINT), Prostate Cancer Research International: Active Surveillance (PRIAS), and personalized active surveillance (AS) cohorts in patients who discontinued for any reason (A) or because of biopsy findings (B).
Overall, 52% of patients who underwent radical treatment chose external beam radiotherapy (37.2% of them with concomitant hormonal therapy because of reclassification to intermediate/high risk), and 8% chose brachytherapy. Additionally, 40% of patients underwent radical prostatectomy, since our institute suggests it as a secondary option in older men (>70 years).
Among the 107 patients treated at our institute, 9 (8.4%) showed biochemical failure at the time of the analysis, 5/86 (5.8%) were treated with radiation therapy, and 4/22 (18%) were treated with surgery.
Table IV reports the 53 available pathologic features of patients who were enrolled after 2007 (when PRIAS started) and underwent radical prostatectomy. Differences in adverse findings between the 2 protocols were not significant (p>0.05, Barnard test for superiority). However, the lowest p values were found for clinical staging ≥pT3a (p = 0.07), with higher percentages in the SAINT group.
Table IV.
Comparison of adverse pathologic findings in specimens for patients enrolled after 2007 (when PRIAS started) who were treated with radical prostatectomy at our institute, n (%)
| Adverse finding | SAINT | PRIAS | p value |
|---|---|---|---|
| Stage ≥ pT3a | 4/13 (31) | 4/38 (11) | 0.07 |
| GPS ≥ 4 + 3 | 2/14 (14) | 7/39 (18) | 0.58 |
| Positive margin | 4/10 (40) | 7/37 (19) | 0.11 |
| Perineural invasion | 4/8 (50) | 13/23 (57) | 0.41 |
| Extracapsular invasion | 3/12 (25) | 5/34 (15) | 0.32 |
| Seminal vesicles invasion | 2/12 (17) | 1/35 (3) | 0.09 |
p Values for Barnard test for superiority are reported.
GPS = Gleason Pattern Score; PRIAS = Prostate Cancer Research International: Active Surveillance; SAINT = Sorveglianza Attiva Istituto Nazionale Tumori.
Predictors of disease reclassification
Among patients who discontinued AS for biopsy-related reasons (upgrading and/or upsizing), ATFS was significantly lower for older patients (hazard ratio [HR] 1.4, p = 0.006, Fig. 4A) and for patients with higher PSA density (HR 2.2, p<0.0001, Fig. 4B). In addition, ATFS was significantly lower for patients with a higher percentage of tumor involvement per biopsy core (HR 1.6, p = 0.001, Fig. 4C), with >1 positive core at diagnostic biopsy (HR 1.5, p = 0.001, Fig. 4D) and prostate volume <50 cm3 (HR 0.4, p<0.0001, Fig. 4E). A lower number of cores at diagnostic biopsy showed significant association with lower ATFS (HR 0.7, p = 0.002, Fig. 4F). Clinical stage and PSA values at diagnosis were not significantly associated with biopsy-related ATFS at univariate analysis.
Fig. 4.
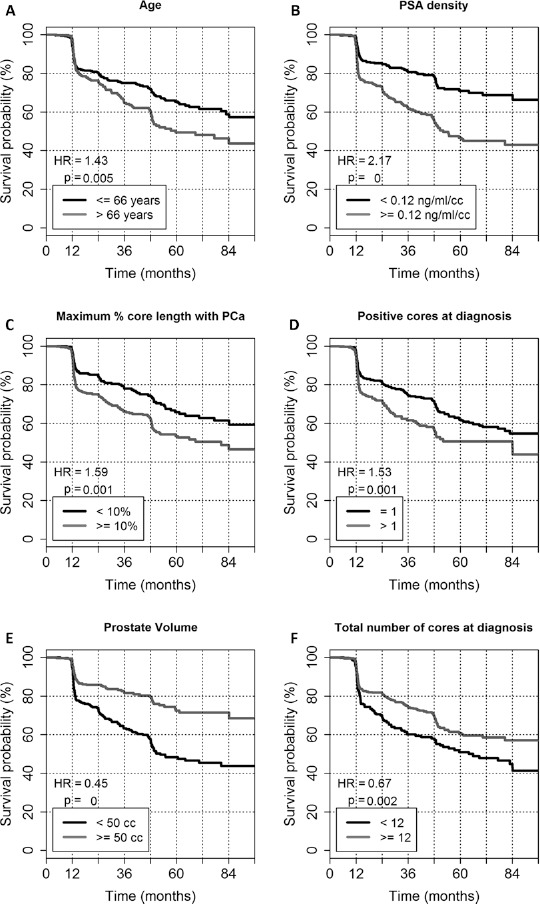
Factors associated with biopsy-related active treatment-free survival (ATFS). Kaplan-Meier curves of biopsy-related ATFS stratified by age (A), prostate-specific antigen (PSA) density (B), maximum percentage of core length containing prostate cancer (PCa) (C), number of positive cores at diagnosis (D), total number of cores at diagnosis (E), and prostate volume (F). Hazard ratios (HR) and p values for log-rank test are reported.
Table V summarizes the results of univariate and multivariate Cox regression analyses. Multivariate analysis confirms the results of univariate analysis, except for the number of cores at diagnostic biopsy.
Table V.
Results of univariate and multivariate cox proportional hazard model for biopsy-related active treatment-free survival
| Factorsa | Univariate cox model | Multivariate cox model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| PSA density ≥0.12 ng/mL/cm3 | 2.2 | 1.66-2.83 | <0.0001 | 1.8 | 1.28-2.41 | 0.0006 |
| Prostate volume ≥50 cm3 | 0.4 | 0.34-0.58 | 0.0001 | 0.6 | 0.40-0.78 | 0.0007 |
| Maximum tumor involvement per biopsy core ≥10% | 1.6 | 1.23-2.06 | 0.001 | 1.5 | 1.09-1.96 | 0.0124 |
| Positive cores at diagnosis >1 | 1.5 | 1.17-1.99 | 0.001 | 1.6 | 1.18-2.09 | 0.002 |
| Number of cores at diagnosis ≥12 | 0.7 | 0.51-0.88 | 0.002 | 0.8 | 0.57-1.05 | 0.0978 |
| Age >66 y | 1.4 | 1.11-1.83 | 0.006 | 1.5 | 1.13-1.97 | 0.0055 |
| Clinical stage ≥T2a | 1.4 | 0.87-2.69 | 0.147 | |||
| PSA ≥8 ng/mL | 1.1 | 0.85-1.22 | 0.772 | |||
All factors were reduced to dichotomous variables following the cutoff values reported in the Table.
CI = confidence interval; HR = hazard ratio; PSA = prostate-specific antigen.
Discussion
Most of the new PCa diagnoses involve patients with low-volume and low-grade cancer (1, 2). Many of these patients have a disease with a very long natural history, which may frequently behave as indolent cancers (15). The long-term benefits of therapy with radical intent are uncertain in these patients, and any intervention may represent an overtreatment (6, 7). Active surveillance is now embraced by the major international guidelines as an alternative to active treatment for selected early-stage tumors (16–17–18). Nonetheless, the most critical caveat of AS policy remains the reliability of initial tumor classification and its reevaluation during follow-up. In recent years, various AS series have been started worldwide, which have adopted protocols with different inclusion and dropout criteria and follow-up schedules (19–20–21–22–23–24–25–26). The rates of discontinuation among these studies vary, especially due to biopsy findings. Therefore, establishing definitive conclusions regarding the impact of diagnostic features on AS permanence is not straightforward. It is also difficult to have robust data on the final prognosis of patients undergoing AS when compared with those undergoing immediate treatment with radical intent.
The long natural history of low-grade PCa requires a long period before PCa-related events can occur, and a limited number of AS studies have reached intermediate or long-term follow-up. This is the largest and the longest cohort in Italy. It includes some patient heterogeneity due to the 2 different protocols (SAINT and PRIAS) and the inclusion of a small proportion of patients in personalized AS monitoring.
Our 50% 5-year ATFS is in agreement with the 48% reported in the worldwide PRIAS study (13), although it appears to be lower than those reported in other single studies (Tab. VI). The reported 5-year ATFS ranges from 48% to 85.7%, with most studies (7/10) in the 48%-70% range. Two of the 3 AS studies with ATFS >70% have particular enrollment characteristics. For example, Soloway et al (21) included in AS only patients who only had positive cores with very low cancer involvement (<20%), while Eggener et al (20) requested a confirmatory biopsy before enrollment in AS (and they have a very short follow-up, 29 months, entailing large CIs in the 5-year ATFS estimation). The steep decrease of ATFS at 1 year confirms that the first rebiopsy is a crucial step for verifying the diagnostic findings and for providing a prompt reclassification of higher-risk patients. Note, however, that only recently additional information from MRI was included among the enrollment criteria for PRIAS patients. Probably the introduction of multiparametric MRI examinations on a large scale, along with advances in genetic/genomic markers, might lead to a more reliable classification of indolent PCa and sensibly modify the ATFS curves in the following years.
Table VI.
Comparison of patient outcomes, eligibility criteria, and biopsy frequency in cohorts of patients in active surveillance
| Institution | No. of patients | Differences in entry criteria | Median age, y | Median follow-up, mo | Triggers for active treatment | Active treatment-free survival | Cancer- specific survival, % |
|---|---|---|---|---|---|---|---|
| UCSF (19) | 810 | <33% positive cores,≤50% of core involvement | 62 (mean) | 60 | Biopsy every 12-24 mo, based on clinical risk | 60% at 5 y | 100 |
| Four North American centers (20) | 262 | GPS ≤3 + 3, ≤3 positive cores | 64 | 29 | Biopsy after 18 mo and then every 1-3 y, prompted by a change in clinical status | 91% at 2 y; 75% at 5 y | 100 |
| Miami (21) | 230 | <3 positive cores; ≤20% of core involvement | 63 (mean) | 44 (mean) | Biopsy every 12 mo | 85.7% at 5 y | 100 |
| Sunnybrook (22) | 993 | Low-risk GPS 3 + 4 or PSA <15 in older 70s | 68 | 82 | PSA; biopsy after 12 mo then at 3-4 y | 75.7% at 5 y; 3.5% at 10 y; 55% at 15 y | 98.1 at 10 y; 94.3 at 15 y |
| JHU (23) | 1,298 | PSAD<0.15; <3 positive cores; ≤50% of core involvement; low risk (18% patients) | 66 | 60 | Biopsy every 12 mo | 59% at 5 y; 41% at 10 y | 99.9 at 15 y |
| PRIAS (13) | 5,302 | PSAD<0.2; <3 positive cores; GPS 3 + 4 (1% patients) | 65.9 | 19 | PSA; biopsy after 1-4-7 y and yearly if PSA DT in the range 3-10 y | 48% at 5 y; 27% at 10 y | 99 at 10 y |
| Goteborg (24) | 439 | ≤2 adjacent cores; <2 mm core involvement | 65 | 72 | PSA; DRE; biopsy (confirmatory then not regulated) | 61.8% at 5 y; 45.4% at 10 y | 99.7 |
| Royal Marsden (25) | 471 | PSA <15 ng/mL; GPS ≤3 + 4; ≤50% positive cores | 66 | 68 | PSA; biopsy (GPS ≤4 + 3) | 70% at 5 y | 99.6 |
| Copenhagen (26) | 167 | ≤3 positive cores; ≤50% of core involvement | 65 | 41 | PSA; biopsy (confirmatory then not regulated) | 60% at 5 y | 100 |
| Present study | 818 | See text for details | 66 | 59 | See text for details | 71% at 2 y; 50% at 5 y | 100 |
DRE = digital rectal examination; DT = doubling time; GPS = Gleason Pattern Score; JHU = Johns Hopkins University; PRIAS = Prostate Cancer Research International: Active Surveillance; PSA = prostate-specific antigen; UCFS = University of California San Francisco.
Biopsy-related reclassification was also the primary reason for exit from AS. Multivariate analysis showed that patients with higher PSA density, higher maximum percentage of tumor length per biopsy core, higher number of positive cores at diagnosis, lower prostate volume, and older age have an increased reclassification risk.
Prostate-specific antigen density was already reported to be an independent predictor of adverse histologic findings at repeat biopsy in other cohorts of patients in AS (27–28–29–30), with proposed cutoff values ranging from 0.08 to 0.15 ng/mL/cm3. Margel et al (28) also reported that older age was associated with cancer grade/volume progression. The role of prostate volume in predicting cancer reclassification highlights the difficulty of achieving adequate prostate sampling in the presence of large volumes. As in several other studies (22, 20, 31), PSA at diagnosis did not appear to be associated with overall ATFS.
The ATFS curve was significantly lower in the SAINT cohort compared to the PRIAS and personalized AS cohorts. All factors that were related to either selection criteria (i.e., PSA density and number of positive cores) or random sampling (i.e., prostate volume and age) had more detrimental values in SAINT than in PRIAS, mainly because of differences in eligibility criteria. The PRIAS protocol, indeed, defines upper bounds on PSA density and the number of positive cores, which led to statistically significant differences in the 2 groups. The median PSA density was 0.11 ng/mL/cm3 in PRIAS vs 0.18 ng/mL/cm3 in SAINT (p<0.001), while 31% of PRIAS patients had more than 1 positive core at diagnosis compared to 43% of patients enrolled in SAINT (mean 1.4 vs 1.8; p<0.0001). The median prostate volume was 50 cm3 in PRIAS vs 36 cm3 in SAINT (p = 0.0001). Nonetheless, these statistically significant differences may not represent relevant differences in terms of clinical outcomes. A difference of 12% in the proportion of patients with more than 1 positive biopsy core would not be sufficient for excluding patients from an AS protocol. The larger inclusion criteria of the SAINT protocol, combined with its tighter biopsy schedule, may have affected the significant differences in ATFS and biopsy-related ATFS between the protocols. However, the impact on prognosis appears to be less important.
Our preliminary analysis on a limited sample of surgical specimens showed that adverse pathology at radical prostatectomy was not significantly different among patients who dropped out from the 2 AS protocols. Notably, the percentage of patients with pathologic GPS ≥4 + 3 were comparable in the 2 groups (18% in PRIAS vs 14% in SAINT, p = 0.58), as seen in Table IV. Similar results were obtained in the experience of Johns Hopkins University (JHU) (32). Compared to other series, JHU adopted stringent selection criteria (corresponding to the original Epstein criteria for insignificant PCa and to the very low risk category of National Comprehensive Cancer Network classification) for most patients. Overall, PCa deaths or metastasis onset were extremely rare (<0.4%) at 15 years of follow-up. A higher reclassification rate was observed in the low-risk group compared with the very low risk (42.2% vs 27.4%, p <0.001), but no differences in Gleason score upgrading (18.1% vs 12.6%, p = 0.70) were found in the radical prostatectomy specimens (23).
It should be mentioned that the population with personalized AS monitoring showed ATFS that was as high as PRIAS, despite the larger selection criteria. This cohort was composed of significantly older patients (median age 69 years) and showed a high percentage of patients who switched to watchful waiting because of age or comorbidities (17%) or due to personal choice, not anxiety (19.1%), such as the choice to avoid rebiopsy. In this population, the large fraction of dropout without a reclassification event could be responsible for the higher ATFS.
Although the number of events and the follow-up period still limit definitive conclusions in regards to cancer outcome, the current biochemical failure rate of 8.4% is comparable to other experiences. In the JHU cohort, biochemical failure was 9.4% in the group of 192 patients (75.3%) who underwent delayed radical treatment and had a posttreatment follow-up >1 year (32).
These preliminary results suggest that overtreatment of patients with GPS 3 + 3 at diagnosis remains relevant, irrespective of the differences between the AS programs. The Canadian group, which was one of the pioneers in AS and has published data with the longest follow-up, already found that the cumulative non-PCa-specific risk of death was 9.2-fold higher than the overall risk of death in a cohort of mainly low-risk patients (22). Similarly, the Goteborg randomized screening trial, at 15 years, found a 96% PCa-specific survival in a cohort of very low-, low-, and intermediate-risk patients against an overall survival of 51% (24).
Overall, only 9/818 (1.1%) patients in our series discontinued AS because of anxiety, similar to the 1.6% rate recorded by van den Bergh et al (33) and others (34). These results are in agreement with a previous published investigation that showed a low level of regret in patients who had previously followed an active AS protocol (35). Our multidisciplinary approach (11), which included psychological support, could have played a major role in the low rate of discontinuations due to anxious preoccupation in our institution. A quality of life analysis of 487 patients who completed the self-reported questionnaires at the beginning and during AS revealed that these patients had high levels of physical, social, and emotional well-being (data not shown). We also observed a positive style of coping with cancer. In particular, we noted that anxious preoccupation regarding the presence of an untreated cancer and the idea of disease progression was very low at the start of AS and decreased over time (36). A recent systematic review on the published literature confirmed that anxiety, depression, and distress did not represent a major burden for most AS patients in their first few years (37).
Notably, the multidisciplinary approach also appears to favor selection of AS (38, 39). In our experience, the percentage of patients with low-risk PCa who chose AS is very high, and it nearly doubled from 2006 to 2010 (44% vs 73%), with a concomitant reduction in the percentage of patients who underwent radiotherapy (30% vs 13%) or prostatectomy (17% vs 6%) (10).
The choice of discontinuing AS without a protocol indication is often related to other causes not related to anxiety for having a PCa. Primarily, a long follow-up with favorable outcomes (stable biopsies and PSA values) reduces the level of alertness in a small, but significant, fraction of patients. This is somewhat overcome by our strict management of AS. All patients are contacted every 3 months in order to recall their PSA examinations and every 6 months before the multidisciplinary consultation. However, some of them still prefer to reduce cancer monitoring. The refusal to undergo rebiopsy is another cause of AS discontinuation, especially for patients who had some discomfort after the biopsies. In addition, some patients who needed other pelvic surgical interventions during the follow-up (e.g., transurethral resection of the prostate) have opted for radical prostatectomy. Finally, in a few cases, the diagnosis of a second primary cancer forced the treatment of the PCa.
The main limitation of this study is the short duration of median follow-up relative to the long life expectancy of patients with low-risk PCa. Accordingly, it is not yet possible to reliably assess overall survival, disease-specific survival, or biochemical relapse in these untreated patients.
Conclusion
At 5 years from diagnosis, approximately 50% of patients with early PCa enrolled in AS are spared from active treatments and their side effects, as they do not show disease reclassification or progression. Repeated biopsies help define a progressive, better selection of patients with low-risk PCa during AS but might represent a significant burden in terms of tolerability, suggesting the need for new strategies to improve patient selection.
Prostate-specific antigen density, age, prostate volume, percentage of core length containing cancer, and the number of positive cores at diagnostic biopsy are associated with biopsy-related active treatment-free survival. Moreover, anxious preoccupation does not appear to be a major reason to switch from AS to radical therapies.
Heterogeneity among 2 AS programs does not seem to be clinically relevant for reclassification and does not affect the most important endpoints, including the probability of unfavorable pathology and biochemical recurrence. A longer follow-up duration, however, is needed to explore the long-term outcomes of these patients.
Acknowledgments
The authors thank the Italo Monzino Foundation, Foundation proADAMO Onlus, and Associazione Italiana Ricerca sul Cancro (AIRC), Special Program “Innovative Tools for Cancer Risk Assessment and Early Diagnosis”, 5×1000 (project 12162).
Disclosures
Financial support: No financial support was received for this submission.
Conflict of interest: None of the authors has conflict of interest with this submission.
References
- 1.Trama A Foschi R Larrañaga N et al. EUROCARE-5 Working Group. Survival of male genital cancers (prostate, testis and penis) in Europe 19992007: Results from the EUROCARE-5 study. Eur J Cancer. 2015;51 (15):2206–2216. [DOI] [PubMed] [Google Scholar]
- 2.Trama A Botta L Nicolai N et al. Prostate Cancer High Resolution Study Working Group. Prostate cancer changes in clinical presentation and treatments in two decades: an Italian population-based study. Eur J Cancer. 2016;67:91–98. [DOI] [PubMed] [Google Scholar]
- 3.Parker C Muston D Melia J Moss S Dearnaley D A model of the natural history of screen-detected prostate cancer, and the effect of radical treatment on overall survival. Br J Cancer. 2006;94 (10):1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangma CH Bul M van der Kwast TH et al. Active surveillance for low-risk prostate cancer. Crit Rev Oncol Hematol. 2013;85 (3):295–302. [DOI] [PubMed] [Google Scholar]
- 5.Stamey TA Freiha FS McNeal JE Redwine EA Whittemore AS Schmid HP Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71 (3) (Suppl):933–938. [DOI] [PubMed] [Google Scholar]
- 6.Bill-Axelson A Garmo H Holmberg L et al. Long-term distress after radical prostatectomy versus watchful waiting in prostate cancer: a longitudinal study from the Scandinavian Prostate Cancer Group-4 randomized clinical trial. Eur Urol. 2013;64 (6): 920–928. [DOI] [PubMed] [Google Scholar]
- 7.Wilt TJ Brawer MK Jones KM et al. Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367 (3):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamdy FC Donovan JL Lane JA et al. ProtecT Study Group. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375 (15): 1415–1424. [DOI] [PubMed] [Google Scholar]
- 9.Valdagni R Salvioni R Nicolai N et al. In regard to Kagan: The multidisciplinary clinic (Int J Radiat Oncol Biol Phys 2005;61: 967968). Int J Radiat Oncol Biol Phys. 2005;63 (1):309–310. [DOI] [PubMed] [Google Scholar]
- 10.Valdagni R Prostate cancer units: has the time come to discuss this thorny issue and promote their establishment in Europe? Eur Urol. 2011;60 (6):1193–1196. [DOI] [PubMed] [Google Scholar]
- 11.Magnani T Valdagni R Salvioni R et al. The 6-year attendance of a multidisciplinary prostate cancer clinic in Italy: incidence of management changes. BJU Int. 2012;110 (7):998–1003. [DOI] [PubMed] [Google Scholar]
- 12.van den Bergh RC Roemeling S Roobol MJ Roobol W Schröder FH Bangma CH Prospective validation of active surveillance in prostate cancer: the PRIAS study. Eur Urol. 2007;52 (6):1560–1563. [DOI] [PubMed] [Google Scholar]
- 13.Bokhorst LP Valdagni R Rannikko A et al. PRIAS study group. A Decade of Active Surveillance in the PRIAS Study: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. Eur Urol. 2016;70 (6):954–960. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria URL https://www.R-project.org/.
- 15.Albertsen PC Hanley JA Fine J 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293 (17):2095–2101. [DOI] [PubMed] [Google Scholar]
- 16.Van der Kwast TH Roobol MJ Defining the threshold for significant versus insignificant prostate cancer. Nat Rev Urol. 2013: 473–82. [DOI] [PubMed] [Google Scholar]
- 17.Mohler JL Armstrong AJ Bahnson RR et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14 (1):19–30. [DOI] [PubMed] [Google Scholar]
- 18.Heidenreich A Bastian PJ Bellmunt J et al. European Association of Urology. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65 (1):124–137. [DOI] [PubMed] [Google Scholar]
- 19.Welty CJ Cowan JE Nguyen H et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193 (3):807–811. [DOI] [PubMed] [Google Scholar]
- 20.Eggener SE Mueller A Berglund RK et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2013;189:19–25, discussion S25. [DOI] [PubMed] [Google Scholar]
- 21.Soloway MS Soloway CT Eldefrawy A Acosta K Kava B Manoharan M Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur Urol. 2010;58 (6):831–835. [DOI] [PubMed] [Google Scholar]
- 22.Klotz L Vesprini D Sethukavalan P et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33 (3):272–277. [DOI] [PubMed] [Google Scholar]
- 23.Tosoian JJ Mamawala M Epstein JI et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol. 2015;33 (30):3379–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godtman RA Holmberg E Khatami A Stranne J Hugosson J Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Göteborg randomised population-based prostate cancer screening trial. Eur Urol. 2013; 63 (1):101–107. [DOI] [PubMed] [Google Scholar]
- 25.Selvadurai ED Singhera M Thomas K et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol. 2013;64 (6):981–987. [DOI] [PubMed] [Google Scholar]
- 26.Thomsen FB Røder MA Hvarness H Iversen P Brasso K Active surveillance can reduce overtreatment in patients with low-risk prostate cancer. Dan Med J. 2013;60 (2):A4575. [PubMed] [Google Scholar]
- 27.Bul M Zhu X Valdagni R et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63 (4):597–603. [DOI] [PubMed] [Google Scholar]
- 28.Margel D Nandy I Wilson TH Castro R Fleshner N Predictors of pathological progression among men with localized prostate cancer undergoing active surveillance - a sub-analysis of the REDEEM (REduction by Dutasteride of clinical progression Events in Expectant Management of prostate cancer) study. J Urol. 2013;190 (6):2039–2045. [DOI] [PubMed] [Google Scholar]
- 29.San Francisco IF Werner L Regan MM Garnick MB Bubley G DeWolf WC Risk stratification and validation of prostate specific antigen density as independent predictor of progression in men with low risk prostate cancer during active surveillance. J Urol. 2011;185 (2):471–476. [DOI] [PubMed] [Google Scholar]
- 30.Iremashvili V Soloway MS Rosenberg DL Manoharan M Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol. 2012;187 (5):1594–1599. [DOI] [PubMed] [Google Scholar]
- 31.Bul M van den Bergh RC Rannikko A et al. Predictors of unfavourable repeat biopsy results in men participating in a prospective active surveillance program. Eur Urol. 2012;61 (2):370–377. [DOI] [PubMed] [Google Scholar]
- 32.Tosoian JJ JohnBull E Trock BJ et al. Pathological outcomes in men with low risk and very low risk prostate cancer: implications on the practice of active surveillance. J Urol. 2013;190 (4):1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Bergh RC Essink-Bot ML Roobol MJ Schröder FH Bangma CH Steyerberg EW Do anxiety and distress increase during active surveillance for low risk prostate cancer? J Urol. 2010;183 (5):1786–1791. [DOI] [PubMed] [Google Scholar]
- 34.Kazer MW Psutka SP Latini DM Bailey DE Jr. Psychosocial aspects of active surveillance. Curr Opin Urol. 2013;23 (3):273–277. [DOI] [PubMed] [Google Scholar]
- 35.Repetto C Rancati T Magnani T et al. What if…: decisional regret in patients who discontinued active surveillance. Tumori. 2016;102 (6):562–568. [DOI] [PubMed] [Google Scholar]
- 36.Bellardita L Rancati T Alvisi MF et al. Predictors of health-related quality of life and adjustment to prostate cancer during active surveillance. Eur Urol. 2013;64 (1):30–36. [DOI] [PubMed] [Google Scholar]
- 37.Bellardita L Valdagni R van den Bergh R et al. How does active surveillance for prostate cancer affect quality of life? A systematic review. Eur Urol. 2015;67 (4):637–645. [DOI] [PubMed] [Google Scholar]
- 38.Aizer AA Paly JJ Zietman AL et al. Multidisciplinary care and pursuit of active surveillance in low-risk prostate cancer. J Clin Oncol. 2012;30 (25):3071–3076. [DOI] [PubMed] [Google Scholar]
- 39.Aizer AA Paly JJ Efstathiou JA Multidisciplinary care and management selection in prostate cancer. Semin Radiat Oncol. 2013;23 (3):157–164. [DOI] [PubMed] [Google Scholar]


