Abstract
The current commercial production of natural astaxanthin is mainly carried out using Haematococcus pluvialis vegetative cells in the “two-stage” batch mode. The motile vegetative cells are more sensitive to stress than nonmotile vegetative cells, thereby affecting the overall astaxanthin productivity in H. pluvialis cultures. In this study, we compared the differences between motile cells and nonmotile cells in astaxanthin productivity, morphological changes, the mortality rate, and the diameter of the formed cysts. The experimental design was achieved by two different types H. pluvialis cell under continuous light of 80 μmol photons m−2 s−1 for a 9-day induction period. The highest astaxanthin concentration of 48.42 ± 3.13 mg L−1 was obtained in the nonmotile cell cultures with the highest the productivity of 5.04 ± 0.15 mg L−1 day−1, which was significantly higher than that in the motile cell cultures. The microscopic examination of cell morphological showed a large number of photooxidative damaged cells occurring in the motile cell cultures, resulting in higher cell mortality rate (22.2 ± 3.97%) than nonmotile cell cultures (9.6 ± 0.63%). In addition, the analysis results of cell diameter statistics indicated that nonmotile cells were more conducive to the formation of large astaxanthin-rich cysts than motile cells. In conclusion, the works presented here suggest that the accumulation of astaxanthin was significantly improved by nonmotile cells of H. pluvialis, which provided a possibility of optimizing the existing H. pluvialis cultivation strategy for the industrial production.
1. Introduction
Astaxanthin is a high-value red ketocarotenoid with powerful antioxidant capacity [1, 2] and widely used in nutraceuticals, aquaculture, cosmetics, food, and feed industries [3–6]. Because of the high market potential of natural astaxanthin, the efficient production of natural astaxanthin has become one of the main concerns in the industrial production of astaxanthin. The green microalga Haematococcus pluvialis is well known as the best source of natural astaxanthin, containing up to 4% of the total cellular dry weight, mainly corresponds to 3S, 3'S isomer, and it is cultivated in industrial scale [7, 8].
The common strategy for production of astaxanthin from H. pluvialis in industrial is “two-stage” batch method, consisting of a first step to sustain green vegetable cells rapid growth under favorable conditions (“green” stage) and then a second step carried out by exposing the cells into stress conditions inducing astaxanthin accumulation (“red” stage) [9–12]. At the red stage, the green vegetable cells transformed into red cysts with a thick cell wall by various stress conditions. Light intensity or nutrient depletion was considered as the major factors that stimulate the synthesis of astaxanthin in H. pluvialis [13, 14]. It has been reported that high temperature and high salt can also enhance the accumulation of astaxanthin [15–17]. But these stress factors may cause cell death, resulting in the fact that overall astaxanthin productivity in H. pluvialis cultures is low [18, 19].
The green vegetative cells of H. pluvialis typically include two cell types, motile- and nonmotile cells [20, 21]. The motile cells refer to the swimming cells driven by two flagella, including zoospores which came from asexual reproduction of H. pluvialis. The motile cells lost their flagella and developed into spherical nonmotile cells under adverse environment [8]. Most of the previous studies focused on the production of astaxanthin using H. pluvialis vegetable cells (the mixture of motile and nonmotile cells) [16, 17, 22, 23]. However, little information was reported on the accumulation of astaxanthin using the nonmotile cell of H. pluvialis.
In this study, with the goal of improving the production of astaxanthin by H. pluvialis, we mainly examined the astaxanthin accumulation of nonmotile cells of H. pluvialis during the induction period. Additionally, the morphology, mortality, and the diameter of red cysts formed were also investigated. The results obtained in this work suggest that nonmotile cells instead of motile cells to stress conditions can significantly improve the production of astaxanthin; this provided an optimized possibility for the existing strategy for the production of astaxanthin from H. pluvialis.
2. Materials and Methods
2.1. Algal Strain and Growth Conditions
The microalgae H. pluvialis CCMA-451 was obtained from CCMA (Center for Collections of Marine Algae, Xiamen University, Xiamen, China) and the accession number in the Genbank is MG847145.1. Stock cultures of H. pluvialis were maintained at 25 μmol photons m−2 s−1 in liquid Bold Basal Medium (BBM). Motile cells were grown photoautotrophic in BBM with 0.75 g L−1 NaNO3 under continuous low light (25 μmol photons m−2 s−1) for 5 days. For the preparation of nonmotile cells, the vegetative cells from the stock cultures were collected and concentrated by centrifugation (2000 rpm, 2 min) and the supernatant was removed. The collected cells were transferred, in 1-L glass columns (inner diameter 5 cm) containing 600 mL of phosphate-starvation medium, under low light conditions for 5 days. To increase the quantity of nonmotile cells, cells that settled at the bottom of the glass columns were collected and washed with fresh aseptically medium several times to remove remaining motile cells. In the experiments, the cultures of two types of cell (motile and nonmotile cells) were adjusted with induction medium (Table S1) to achieve 0.5 × 106 cell mL−1 of cell density and then exposed to continuous light of 80 μmol photons m−2 s−1 at 25 ± 1°C for 9 days. All the cultures were aerated with 1.5% (v/v) CO2 continuously at 0.5 vvm. Illumination was provided from the side by LED plant which grows white lights (Xiamen Top-Succeed Photobiology Technology Co., Ltd., Xiamen, China). Each type of cells was repeated in triplicate.
2.2. Morphological Observation
The algal cells were examined using Leica DM750 light microscope and taken photos with Leica ICC50 W camera. Leica application software was used for picture editing.
2.3. Determination of Cell Number and Cell Diameter
The samples were fixed with Lugol's iodine solution first, then cell numbers were counted using a Neubauer improved cell counting chamber under Leica DM750 light microscope and measured as cells mL−1. The living and dead cells were identified according to the cell morphology shown in Figure S1.
The cell diameter of red cysts was determined using a Leica application software with an internal reticle scale.
2.4. Pigment Extraction and Analysis
The astaxanthin concentration was determined photometrically [24, 25]. The samples were collected by centrifugation at 7000 rpm for 5 min. Then the pellet was treated with 5 mL solution of 5% (w/v) KOH in 30% (v/v) methanol in a 75°C water bath for 10 min to remove the chlorophyll. The remaining pellet was then extracted with DMSO after adding 25 μL acetic acid at 75°C for 10 min. This last step was repeated several times to colorless and recover the astaxanthin. The absorbance of the combined extracts was measured at 492 nm (E1 cm1%=2220), and the astaxanthin concentration was calculated with
| (1) |
in which C is the astaxanthin concentration (mg L−1), Va is the volume of extracted pigment sample (mL), Vb is the volume of sample (mL), and f is the dilution ratio of measuring the absorbance.
The astaxanthin productivity (mg L−1 day−1) was calculated with
| (2) |
in which the AXt and AX0 were the astaxanthin concentration of day t and day 0, respectively.
3. Results and Discussion
3.1. Astaxanthin Accumulation
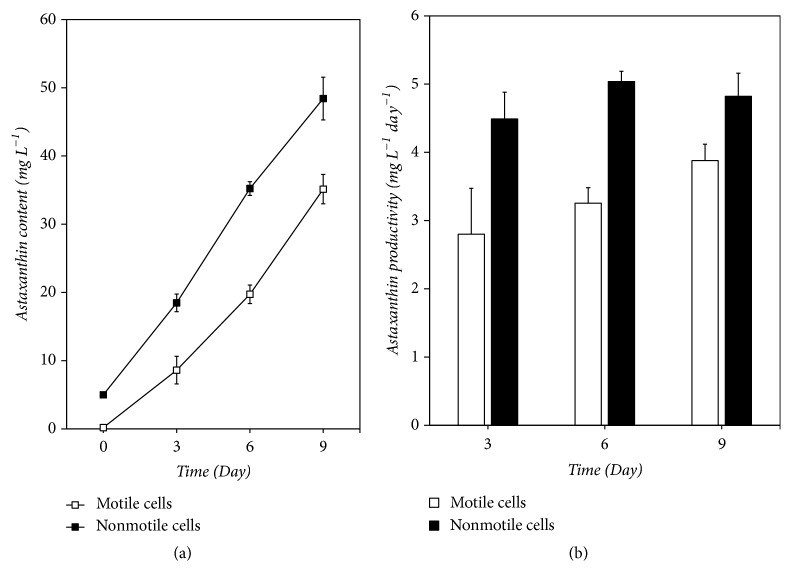
The ability of astaxanthin accumulation is the key parameter for evaluating the application potential of algae strains in H. pluvialis astaxanthin production. It was reported that astaxanthin synthesis can occur in both motile and nonmotile cells of H. pluvialis [20, 21, 26]; however the differences of the astaxanthin accumulation between them still unknown. To compare the differences in astaxanthin accumulation between motile and nonmotile cells, we examined the contents and productivity of astaxanthin in the two cultures. As shown in Figure 1, the nonmotile cell cultures exhibited maximum astaxanthin content (Figure 1(a)). Considering that the initial astaxanthin content in both cultures was different, we furtherly calculated the astaxanthin productivity and the results is shown in Figure 1(b). The value of astaxanthin productivity in the nonmotile cells cultures was ranged from 4.49 ± 0.39 to 5.04 ± 0.15 mg L−1 day−1 and the maximum value occurred on day 6. It was significantly higher than that of the motile cell cultures. For the motile cell cultures, the value of astaxanthin productivity ranged from 2.80 ± 0.67 to 3.88 ± 0.24 mg L−1 day−1. The astaxanthin productivity was affected by many factors, such as strains, bioreactors, stress conditions, and initial biomass density in the red stage [8, 11, 16, 27]. A highest astaxanthin productivity of 17.1 mg L−1 day−1 was obtained at 0.8 g L−1 initial biomass density in an outdoor photobioreactor by Wang et al. [27]. In our recent work, the highest astaxanthin productivity in nonmotile cells cultures reached 11.8 mg L−1 day−1 at 0.5 g L−1 initial biomass density under high light conditions (unpublished). Therefore, there was much room for improvement in the production of astaxanthin by nonmotile cells of H. pluvialis.
Figure 1.
The astaxanthin concentration (a) and productivity (b) of H. pluvialis cultures.
Astaxanthin was regarded as a long-term defense mechanism in H. pluvialis, serving as a physicochemical barrier, protecting the cell survival under stress conditions [28, 29]. The astaxanthin accumulation generally was accompanied with the formation of encystment [28, 30–32]. Encystment was also considered as a manifestation of the natural algal defense system [33, 34]. Both astaxanthin accumulation and encystment were the responses of H. pluvialis cells to unfavorable conditions; they all needed extra energy consuming. For nonmotile cells, the secondary carotenoids and carbohydrate, which accumulated during the process of transformation, as the precursor and energy provider accelerated the astaxanthin synthesis [35]. On the other hand, due to the high susceptibility to stress conditions [27, 36], cell death occurred largely in motile cells cultures, accordingly the astaxanthin production rate was lower than in nonmotile cells cultures.
3.2. Microscopic Examination
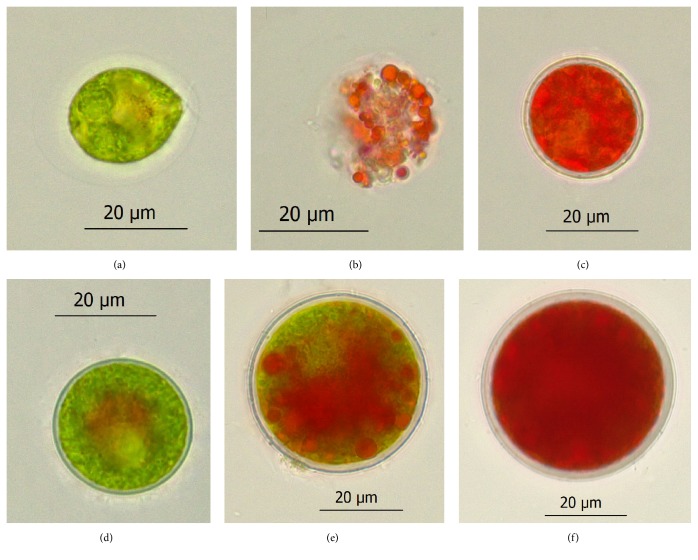
Changes in cell morphology of motile and nonmotile cells were observed under the light microscope, and the results are shown in Figure 2. The motile cells were green, ellipsoidal, or pear-shaped, with two isometric flagella at the anterior end (Figure 2(a)). Nonmotile cells were spherical, without flagella, little orange-red pigmentation observed in the mid-region (Figure 2(d)). After being induced for 3 days, some motile cells became vacuolated (Figure 2(b)), whereas the nonmotile cells were intact (Figure 2(e)). After being induced for 9 days, almost all of the cells in both of cultures transformed into red cysts with thickened cell walls and accumulated astaxanthin (Figures 2(c) and 2(f)). Moreover, some dead or damaged cells were observed, especially in motile cells cultures.
Figure 2.
Photomicrographs of H. pluvialis cells grown in the red stage. Green motile cell (a) and nonmotile cell (d). The motile cell (b) and nonmotile cell (e) after exposure to phosphate and nitrate starvation at 80 μmol photons m−2 s−1 for 3 days. The red cyst formed after 9-day induction in the motile cells cultures (c) and nonmotile cells cultures (f).
3.3. The Cell Mortality Rate
High light and nutrient depletion were the major environmental factors that promote astaxanthin accumulation [37, 38], but they were able to cause the production of toxic reactive oxygen species (ROS) to result in the cell photooxidative death [39, 40], thereby affecting the overall astaxanthin productivity in H. pluvialis cultures. In general, algal cells can dissipate excess light energy and relax ROS production to reduce the photooxidative damage; however it was suggested that these strategies were not sufficient to protect motile cells from environmental stress [23]. In contrast, the nonmotile cells can cope with and survive under stress conditions using several strategies, such as downregulating the linear electron transport through decreasing the level of cytochrome f and consuming excess electrons produced by PSII via a significantly enhanced plastid terminal oxidase pathway (POTX) [23]. Furthermore, secondary carotenoids as antioxidants and photoprotective agents, which accumulated during the transformation of nonmotile cells by motile cells, could protect nonmotile cells from environmental stress [33, 41]. Nonmotile cells were also able to effectively convert fixed photochemical energy into storage starch under phosphate-starvation condition and subsequently convert it into storage neutral lipids [23]. In this study, the initial cell mortality rate was 1.0 ± 0.30% and 1.9 ± 0.15% in motile and nonmotile cell cultures, respectively. After induction for 9 days, the cell mortality rate in nonmotile cells cultures reached 9.6 ± 0.63%; it was significantly lower than that in motile cells cultures (22.2 ± 3.97%) (Table 1). It is indicated that the nonmotile cells may have a stronger tolerance to the adverse environment than motile cells. The difference of tolerance to the adverse environment between the two cells may be one of the reasons for the difference in astaxanthin content between the two cultures. In addition, the relatively low mortality of the nonmotile cell cultures may be helpful for enhancing the content of astaxanthin and the stability of astaxanthin production.
Table 1.
The average cyst diameter, astaxanthin content, and cell mortality rate in motile and nonmotile cell cultures on day 9 of the induction period.
| Cultures | Average cyst diameter (μm) | Astaxanthin content (pg cell−1) | The cell mortality rate (%) |
|---|---|---|---|
| Motile cell cultures | 22.30 ± 4.74 | 22.79 ± 0.75 | 22.20 ± 3.97 |
| Nonmotile cell cultures | 34.82 ± 5.62 | 44.30 ± 4.47 | 9.60 ± 0.63 |
3.4. The Size of the Red Cysts Formed
The cell size of different types of H. pluvialis varies greatly, the range is from 8 to 50 μm [30]. However, the reasons for such a large variation in cell size remain unknown, the correlation between cell type and the size of the red cysts formed are also unclear. In this study, we observed that most of the red cysts in nonmotile cells cultures were bigger than that in motile cells cultures and measured the cell diameter of red cysts in both cultures. As shown in Figure 3 and Table 1, the red cyst diameter in nonmotile cells culture ranged from 20.19 to 49.35μm, the average was 34.82 ± 5.62 μm, whereas, in the motile cell cultures, the diameter of red cyst ranged from 13.45 to 46.69 μm and the average was 22.30 ± 4.74 μm. When environmental or culture conditions became less favorable, motile cells lost their flagella and developed into nonmotile form [22]. Once the culture conditions became unfavorable, cells entered a resting stage to accumulate astaxanthin with the formation of cysts cells (encystment) [25]. Encystment was believed to control by unknown intracellular signaling chains leading to gene expression initiated by environmental signals [42]. Previous studies have demonstrated the formation of H. pluvialis encystment accompanied by massive accumulation of carbohydrate, fatty acids, and secondary carotenoids [25, 43]. Thus, it was possible that the enlargement of cells was in order to provide more storage space for these increased compounds.
Figure 3.
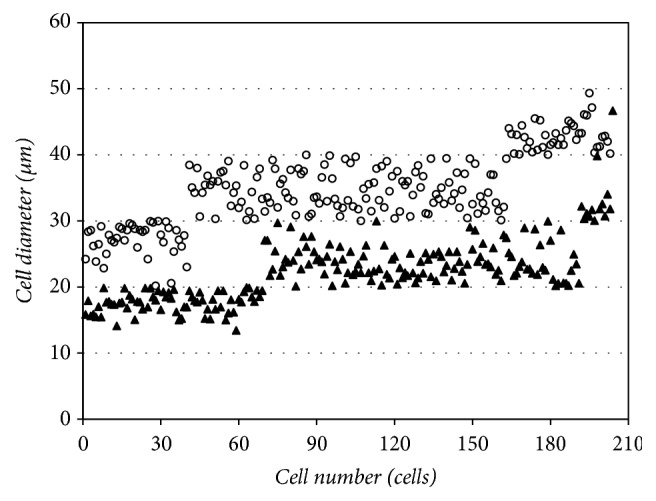
The distribution of H. pluvialis cysts in different diameter ranges in the motile cell culture (black triangle) and the nonmotile cell culture (white circle).
Cell diameter statistics showed that 33.7% of red cysts in the motile cell cultures were less than 20 μm in cell diameter, 59.5% were in the range of 20 to 30 μm, and 6.3% were in the range of 30 to 40 μm, while the values of the same range in nonmotile cells cultures were 0%, 19.7%, and 60.6%, respectively. In addition, about 19.7% of red cysts in the nonmotile cells cultures were bigger than 40 μm, while the cell percentage of this range in motile cells cultures was only 0.5% (Figure 3). We furtherly calculated the astaxanthin content of single red cyst in both cultures; the results showed that the average astaxanthin content of a single red cyst in the nonmotile cell cultures was 1.94 times as high as that in the motile cell cultures (Table 1).
These results supported the fact that the nonmotile-type cells can form larger red cysts and accumulate higher astaxanthin than motile-type cells, which suggest that increasing the number of nonmotile-type cells has a positive effect on improving the astaxanthin content of H. pluvialis.
4. Conclusions
In this study, we have determined the fact that the accumulation of astaxanthin is significantly improved by the nonmotile cells of H. pluvialis compared with motile cells. We show that nonmotile cells have higher astaxanthin productivity than motile cells. The results of the cell mortality rate indicate that the nonmotile cells may have a stronger tolerance to the adverse environment than motile cells. In addition, the analysis results of cell diameter statistics indicate that nonmotile cells are more conducive to the formation of large astaxanthin-rich cysts than motile cells. These results may account for the improvement of astaxanthin accumulation in nonmotile cell cultures. Our work presented here provides a possibility of optimizing the existing cultivation strategy by the nonmotile cells as the primary cell type to improve the stability and overall astaxanthin productivity in industrial cultivation of H. pluvialis.
Acknowledgments
This research was mainly supported by Xiamen Southern Ocean Technology Center of China (no. 14CZP035HJ09) and partly funded by Xiamen Scientific and Technologic Projects (nos. 3052Z20031086 and 3052Z20123004), Marine Science Base Scientific Research Training and Scientific Research Ability Enhancement Project of Xiamen University (no. J1210050), Xiamen University Training Program of Innovation and Entrepreneurship for Undergraduates (no. 2016X0619), and the Special Funds for Scientific Research of Marine Public Welfare Industry (no. 201305016).
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supplementary Materials
Table S1: chemical compositions of induction culture media for H. pluvialis used in this study. Figure S1: the cell morphology of living and dead cells of H. pluvialis.
References
- 1.Guerin M., Huntley M. E., Olaizola M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends in Biotechnology. 2003;21(5):210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 2.De Morais M. G., Vaz B. D. S., De Morais E. G., Costa J. A. V. Biologically active metabolites synthesized by microalgae. BioMed Research International. 2015;2015:15. doi: 10.1155/2015/835761.835761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai M. G., Li F. Recent advances in Haematococcus pluvialis scale culture technology. Journal of Xiamen University (Natural Science) 2016;55(5):733–741. [Google Scholar]
- 4.Zou T.-B., Zhu S.-S., Luo F., et al. Effects of astaxanthin on reverse cholesterol transport and atherosclerosis in mice. BioMed Research International. 2017;2017:6. doi: 10.1155/2017/4625932.4625932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higuera-Ciapara I., Félix-Valenzuela L., Goycoolea F. M. Astaxanthin: a review of its chemistry and applications. Critical Reviews in Food Science and Nutrition. 2006;46(2):185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 6.Cai M. G., Wang S. L., Li W. Q., et al. Advances in application studies of natural astaxanthin in aquaculture. Journal of Oceanography in Taiwan Strait. 2001;22(3):377–385. [Google Scholar]
- 7.Klochkova T. A., Kwak M. S., Han J. W., Motomura T., Nagasato C., Kim G. H. Cold-tolerant strain of Haematococcus pluvialis (Haematococcaceae, Chlorophyta) from Blomstrandhalvøya (Svalbard) Algae. 2013;28(2):185–192. doi: 10.4490/algae.2013.28.2.185. [DOI] [Google Scholar]
- 8.García-Malea M. C., Gabriel Acién F., Río E. D., et al. Production of astaxanthin by haematococcus Pluvialis: Taking the one-step system outdoors. Biotechnology and Bioengineering. 2009;102(2):651–657. doi: 10.1002/bit.22076. [DOI] [PubMed] [Google Scholar]
- 9.Kang C. D., Lee J. S., Park T. H., Sim S. J. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Applied Microbiology and Biotechnology. 2005;68(2):237–241. doi: 10.1007/s00253-005-1889-2. [DOI] [PubMed] [Google Scholar]
- 10.Fábregas J., Otero A., Maseda A., Domínguez A. Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. Journal of Biotechnology. 2001;89(1):65–71. doi: 10.1016/S0168-1656(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 11.Aflalo C., Meshulam Y., Zarka A., Boussiba S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnology and Bioengineering. 2007;98(1):300–305. doi: 10.1002/bit.21391. [DOI] [PubMed] [Google Scholar]
- 12.Xi T., Kim D. G., Roh S. W., Choi J.-S., Choi Y.-E. Enhancement of astaxanthin production using Haematococcus pluvialis with novel LED wavelength shift strategy. Applied Microbiology and Biotechnology. 2016;100(14):6231–6238. doi: 10.1007/s00253-016-7301-6. [DOI] [PubMed] [Google Scholar]
- 13.Huang S. Y., Qi A. X., Li Z., et al. Initial studies of the effects of stress conditions on astaxanthin accumulation of Haematococcus pluvialis. Studia Marina Sinica. 2009;(49):144–151. [Google Scholar]
- 14.Imamoglu E., Sukan F. V., Dalay M. C. Effect of different culture media and light intensities on growth of Haematococcus pluvialis. International Journal of Natural & Engineering Sciences. 2007;1(3):05–09. [Google Scholar]
- 15.Hong M.-E., Hwang S. K., Chang W. S., Kim B. W., Lee J., Sim S. J. Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber–Weiss reaction. Applied Microbiology and Biotechnology. 2015;99(12):5203–5215. doi: 10.1007/s00253-015-6440-5. [DOI] [PubMed] [Google Scholar]
- 16.Harker M., Tsavalos A. J., Young A. J. Factors responsible for astaxanthin formation in the chlorophyte Haematococcus pluvialis. Bioresource Technology. 1996;55(3):207–214. doi: 10.1016/0960-8524(95)00002-X. [DOI] [Google Scholar]
- 17.Cordero B., Otero A., Patiño M., Arredondo B. O., Fabregas J. Astaxanthin production from the green alga Haematococcus pluvialis with different stress conditions. Biotechnology Letters. 1996;18(2):213–218. doi: 10.1007/BF00128682. [DOI] [Google Scholar]
- 18.Kobayashi M., Kurimura Y., Kakizono T., Nishio N., Tsuji Y. Morphological changes in the life cycle of the green alga Haematococcus pluvialis. Journal of Fermentation and Bioengineering. 1997;84(1):94–97. doi: 10.1016/S0922-338X(97)82794-8. [DOI] [Google Scholar]
- 19.Grunewald K., Hagen C. Extrusion of secondary carotenoid containing vesicles from flagellates of Haematococcus pluvialis (Volvocales; Chlorophyceae) Journal of Applied Botany and Food Quality. 2000;74(3-4):141–144. [Google Scholar]
- 20.Han D., Li Y., Hu Q. Biology and commercial aspects of Haematococcus pluvialis. In: Richmond A., Emeritus, Qiang H., editors. Handbook of Microalgal Culture: Applied Phycology and Biotechnology. New Jersey, NJ, USA: John Wiley & Sons, Ltd.; 2013. pp. 388–405. [Google Scholar]
- 21.Han D., Wang J., Sommerfeld M., Hu Q. Susceptibility and protective mechanisms of motile and non motile cells of haematococcus pluvialis (chlorophyceae) to photooxidative stress. Journal of Phycology. 2012;48(3):693–705. doi: 10.1111/j.1529-8817.2012.01147.x. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y., Cai M. G., Huang S. Y., et al. Haematococcus pluvialis culture in photobioreactor. Marine Sciences. 2010;34(10):83–89. [Google Scholar]
- 23.Li X. M., Qi A. X., Cai M. G., et al. Large culture for Haematococcus pluvialis outside and accumulation of astaxanthin. Journal of Xiamen University (Natural Science) 2006;45:245–249. [Google Scholar]
- 24.Sun H., Liu B., Lu X., Cheng K.-W., Chen F. Staged cultivation enhances biomass accumulation in the green growth phase of Haematococcus pluvialis. Bioresource Technology. 2017;233:326–331. doi: 10.1016/j.biortech.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Boussiba S., Vonshak A. Astaxanthin accumulation in the green alga haematococcus pluvialis. Plant & Cell Physiology. 1991;32(7):1077–1082. doi: 10.1093/oxfordjournals.pcp.a078171. [DOI] [Google Scholar]
- 26.Hagen C., Grünewald K., Schmidt S., Müller J. Accumulation of secondary carotenoids in flagellates of haematococcus pluvialis (chlorophyta) is accompanied by an increase in per unit chlorophyll productivity of photosynthesis. European Journal of Phycology. 2000;35(1):75–82. doi: 10.1017/S0967026299002528. [DOI] [Google Scholar]
- 27.Wang J., Han D., Sommerfeld M. R., Lu C., Hu Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. Journal of Applied Phycology. 2013;25(1):253–260. doi: 10.1007/s10811-012-9859-4. [DOI] [Google Scholar]
- 28.Wang B., Zarka A., Trebst A., Boussiba S. Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. Journal of Phycology. 2003;39(6):1116–1124. doi: 10.1111/j.0022-3646.2003.03-043.x. [DOI] [Google Scholar]
- 29.Li Y., Sommerfeld M., Chen F., Hu Q. Consumption of oxygen by astaxanthin biosynthesis: A protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae) Journal of Plant Physiology. 2008;165(17):1783–1797. doi: 10.1016/j.jplph.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Boussiba S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiologia Plantarum. 2000;108(2):111–117. doi: 10.1034/j.1399-3054.2000.108002111x./. [DOI] [Google Scholar]
- 31.Montsant A., Zarka A., Boussiba S. Presence of a nonhydrolyzable biopolymer in the cell wall of vegetative cells and astaxanthin-rich cysts of haematococcus pluvialis (chlorophyceae) Marine Biotechnology. 2001;3(6):515–521. doi: 10.1007/s1012601-0051-0. [DOI] [PubMed] [Google Scholar]
- 32.Hagen C., Siegmund S., Braune W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. European Journal of Phycology. 2002;37(2):217–226. doi: 10.1017/S0967026202003669. [DOI] [Google Scholar]
- 33.Hagen C., Braune W., Björn L. O. Functional aspects of secondary carotenoids in haematococcus lacustris (Volvocales). III. action as a “sunshade”. Journal of Phycology. 1994;30(2):241–248. doi: 10.1111/j.0022-3646.1994.00241.x. [DOI] [Google Scholar]
- 34.Hagen C., Braune W., Greulich F. Functional aspects of secondary carotenoids in Haematococcus lacustris [Girod] Rostafinski (Volvocales) IV. Protection from photodynamic damage. Journal of Photochemistry and Photobiology B: Biology. 1993;20(2-3):153–160. doi: 10.1016/1011-1344(93)80145-Y. [DOI] [Google Scholar]
- 35.Schoefs B., Rmiki N.-E., Rachadi J., Lemoine Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Letters. 2001;500(3):125–128. doi: 10.1016/S0014-5793(01)02596-0. [DOI] [PubMed] [Google Scholar]
- 36.Olaizola M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. Journal of Applied Phycology. 2000;12(3–5):499–506. doi: 10.1023/A:1008159127672. [DOI] [Google Scholar]
- 37.Hu Z., Li Y., Sommerfeld M., Chen F., Hu Q. Enhanced protection against oxidative stress in an astaxanthin- overproduction Haematococcus mutant (Chlorophyceae) European Journal of Phycology. 2008;43(4):365–376. doi: 10.1080/09670260802227736. [DOI] [Google Scholar]
- 38.Li Y., Sommerfeld M., Chen F., Hu Q. Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae) Journal of Applied Phycology. 2010;22(3):253–263. doi: 10.1007/s10811-009-9453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giuseppe T., Avigad V. Environmental stress physiology with reference to mass cultures. In: Richmond A., Emeritus, Qiang H., editors. Handbook of Microalgal Culture: Applied Phycology and Biotechnology. New Jersey, NJ, USA: John Wiley & Sons, Ltd.; 2013. pp. 90–91. [Google Scholar]
- 40.Choi Y. E., Yun Y.-S., Park J. M. Evaluation of factors promoting astaxanthin production by a unicellular green alga, Haematococcus pluvialis, with fractional factorial design. Biotechnology Progress. 2002;18(6):1170–1175. doi: 10.1021/bp025549b. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder W. A., Johnson E. A. Singlet oxygen and peroxyl radicals regulate carotenoid biosynthesis in Phaffia rhodozyma. The Journal of Biological Chemistry. 1995;270(31):18374–18379. doi: 10.1074/jbc.270.31.18374. [DOI] [PubMed] [Google Scholar]
- 42.Villalobo E., Moch C., Perasso R., Baroin-Tourancheau A. Searching for excystment-regulated genes in Sterkiella histriomuscorum (ciliophora, oxytrichidae): A mRNA differential display analysis of gene expression in excysting cells. Journal of Eukaryotic Microbiology. 2001;48(3):382–390. doi: 10.1111/j.1550-7408.2001.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi M., Kakizono T., Nagai S. Astaxanthin production by a green alga, Haematococcus pluvialis accompanied with morphological changes in acetate media. Journal of Fermentation and Bioengineering. 1991;71(5):335–339. doi: 10.1016/0922-338X(91)90346-I. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: chemical compositions of induction culture media for H. pluvialis used in this study. Figure S1: the cell morphology of living and dead cells of H. pluvialis.
Data Availability Statement
No data were used to support this study.