Abstract
Background
In recent decades, tooth derivatives such as dentin (D) and enamel (E) have been considered as potential graft biomaterials to treat bone defects. This study aimed to investigate the effects of demineralization on the physical-chemical and biological behavior of D and E.
Methods
Human D and E were minced into particles (Ø<1 mm), demineralized and sterilized. Thorough physical-chemical and biochemical characterizations of native and demineralized materials were performed by SEM and EDS analysis and ELISA kits to determine mineral, collagen type I and BMP-2 contents. In addition, MG63 and SAOS-2 cells were seeded on tooth-derived materials and Bio-Oss®, and a comparison of cell responses in terms of adhesion and proliferation was carried out.
Results
The sterilization process, as a combination of chemical and thermal treatments, was found to be effective for all materials. On the other hand, D demineralization allowed preserving the collagen content, while increasing BMP-2 bioavailability. D and demineralized D (dD) displayed excellent biocompatibility, even greater than Bio-Oss®. Conversely, the high mineral content displayed by E, as confirmed by EDS analysis, inhibited cell proliferation. Of note, even though the demineralization process was somehow less effective in E than in D, demineralized E (dE) displayed increased BMP-2 bioavailability and improved performance in vitro compared with native E.
Conclusions
Our results substantiate the idea that the demineralization process lead to an increase of BMP-2 bioavailability, thus paving the way toward development of more effective, osteoinductive tooth-derived materials for bone regeneration and replacement.
Keywords: BMP-2, Demineralization, Dentin, Enamel, Osteoblast cell lines
Introduction
The need for off-the-shelf, readily available materials suitable for bone regeneration has inspired much research in the field of regenerative medicine. Nowadays, the commercial anorganic deproteinized bovine substitute Bio-Oss® represents the gold standard for regenerative dentistry worldwide (1, 2), because of its osteoconductive properties and biofunctionality leading to effective and reliable bone regeneration. Despite the widespread use of such a gold standard graft material in alveolar bone augmentations, great interest has recently been focused on autologous bone-like materials as suitable substrates for bone regeneration of alveolar defects. However, although autogenous bone grafts are currently used in dentistry, the major drawbacks of bone-harvesting procedures, such as the limited availability of bone tissue and the need for second surgery, have hindered their efficacy in replacement therapy (3). These issues, together with the shortcomings ensuing from allogeneic transplantation, such as the transmission of possible infections and the lack of osteointegration with host tissues, have spurred on researchers and clinicians to look for novel graft solutions by means of challenging biomaterial techniques. More specifically, the use of tooth-derived materials has recently attracted much interest due to the inherent wide availability of teeth that are extracted every day and discarded as waste.
Dentin (D), the bulk material of the tooth which closely resembles the chemical composition of bone (≈70% w/w of mineral phase, ≈20% of organic matrix and ≈10% of water) (4-5-6-7), is considered as a viable bone substitute. In fact, D, in the form of native D (8, 9), and D derivatives, such as demineralized (10-11-12-13) and deproteinized D (14), have been used as graft materials in bone repair processes. Some experimental evidence from a critical literature survey highlighted the role of demineralized D matrix in promoting osteodifferentiation in vitro (13) and, even more interestingly, in increasing osteoinduction in vivo (15, 16). Taken together, these works point to the use of demineralized D matrices for the treatment of bone defects.
Apart from D, teeth are composed of 2 other types of highly mineralized hard components, which are enamel (E) and cementum. It is worthy of note that E has also revealed some clinical potential to fill large bone defects, whether used alone or in combination with D.
This present study aimed to shed light on the effects of demineralization on the physical-chemical and biological behavior of D and E that display unique features per se. Besides, since D and E do play different osteoinductive roles, we compared the in vitro behavior of such materials whether or not subjected to demineralization process. Of note, their in vitro biocompatibility was assessed and compared with that of Bio-Oss®.
Materials and Methods
Materials and reagents
Demineralization and sterilization reagents were provided by TT Tooth Transformer S.r.l. (Milan, Italy). Bio-Oss® was purchased from Geistlich Pharma AG (Wolhausen, Switzerland).
Enzyme-linked immunosorbent assay (ELISA) kits (type I collagen: SEA571Hu; BMP-2: SEA013Hu) were purchased from Cloud-Clone Corp. (Katy, TX, USA). The bicinchoninic acid (BCA) protein assay kit was from ThermoFisher (Monza, Italy). MG63 (human osteosarcoma cell line) and SAOS-2 (human osteogenic sarcoma cell line) cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). AlamarBlue Cell Viability Assay was purchased from Life Technologies Italia (Monza, Italy), while UltraPure™ Agarose was from ThermoFisher. All other chemicals were from Sigma-Aldrich (Milan, Italy) if not otherwise specified.
Samples preparation
D and E particles were provided by TT Transformer S.r.l. Seven human teeth were extracted due to periodontal diseases from healthy patients (aged between 47 and 78 years) with informed consent. E was isolated from every tooth by means of a drill and collected, pooled and stored at room temperature (RT) until use. Dentin samples were pooled together, minced with a Tooth Transformer TT machine (TT Tooth Transformer S.r.l.), according to the manufacturer's instructions, to give D particles (hereafter referred to as D; Ø<1 mm).
Afterwards, E and D particles were subjected to demineralization and sterilization processes according to a single protocol and using 6 different solutions, both provided by TT Transformer S.r.l., to demineralize and sterilize teeth particles. Briefly, particles were (i) treated with demineralization reagent (reagent A) (1 mL/≈50 mg of particles) at 70°C under shaking by means of a thermomixer (1,000 rpm); (ii) washed sequentially with 2 solutions (reagents B and C) (1 mL/≈50 mg of particles) at RT for 2 minutes; (iii) treated with sterilization reagent (reagent D) (1 mL/≈50 mg of particles) at 70°C under shaking by means of a thermomixer (1,000 rpm). Particles were finally washed with reagent E and F (1 mL/≈50 mg of particles, twice) at RT for 2 minutes. Demineralized dentin and enamel particles (hereafter referred as to dDs and dEs, respectively) were next collected in sterile 1.5 mL polypropylene tubes and stored at RT until use.
In control samples, E and D were processed in parallel, except for the fact that demineralization and sterilization reagents (reagent A and reagent D, respectively) were replaced with sterile deionized water (dH2O). Bio-Oss® was used as the positive control in the experiments.
Evaluation of demineralization and sterilization process efficacy and reliability
Efficacy of the sterilization process
Before and at the end of the entire treatment, the microbial contamination of samples was investigated. Briefly, ≈30 mg of every kind of material was transferred into 50-mL polypropylene tubes containing 5 mL of Luria-Bertani (LB) nutrient broth, then incubated for 5 days at 37°C under orbital shaking (110 rpm). Afterwards, the number of viable bacteria was determined by means of the pour plate method. Briefly, the number of colony forming units (CFUs) counted on LB-agar Petri dishes after serial tenfold dilutions of each material suspension in LB, plating and 24-hour incubation at 37°C.
Morphological and chemical characterization
Surface morphology of treated (i.e., demineralized) and control samples was examined with an environmental scanning electron microscope (ESEM Zeiss EVO50; Carl Zeiss, Milan, Italy) connected to a secondary electron detector for energy dispersive X-ray (EDS) analysis. Briefly, samples were fixed in 1.5% (v/v) glutaraldehyde solution for 20 minutes, then dehydrated in graded ethanol series, and allowed to dry at RT. Following gold-sputtering, samples were mounted onto scanning electron microscope (SEM) stubs and examined using an accelerating voltage of 15 kV. SEM images were acquired at ×5,000 magnification. EDS analysis was also performed to investigate the surface composition (elemental analysis, atomic % of carbon: C, nitrogen: N, phosphorus: P and calcium: Ca) of 3 particles from each group. EDS spectra refer to the whole SEM image.
Evaluation of type I collagen and BMP-2 protein contents
ELISA was carried out to quantify type I collagen (COL-I) and bone morphogenetic protein-2 (BMP-2) content in demineralized and control samples. Briefly, following the treatment, ≈50 mg of particles from each experimental group was transferred into 1.5-mL polypropylene tubes, and 500 μL of extraction buffer (50 mM HEPES, pH 7.4; 1 mM phenylmethylsulfonyl fluoride [PMSF]; 2 μg/mL leupeptin; 2 μg/mL aprotinin; 1 μg/mL pepstatin and 1% [v/v] Triton X-100) was added to each sample. After incubation at 4°C overnight and 3 freezing/thawing cycles, samples were sonicated by means of a tip ultrasonicator, and protein was extracted. Total protein content was determined by BCA protein assay kit according to the manufacturer's instructions. For ELISA assays, 15 μg of total protein extracts was loaded, and tests were carried out according to the manufacturer's instructions. Results from ELISAs were normalized to the weight of each sample (expressed as grams of sample particles).
Biological assessment in vitro
Cell culture
MG63 and SAOS-2 osteoblastic cell lines were used in in vitro cell culture experiments. MG63 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 1 mM sodium pyruvate, 10 mM HEPES buffer, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM glutamine and supplemented with 10% (v/v) fetal bovine serum (FBS) (hereafter referred to as MG63 medium), in a humidified atmosphere under constant supply of 5% CO2 and at 37°C. On the other hand, SAOS-2 cells were cultured at 37°C in a 5% CO2 humidified atmosphere in McCoy culture medium containing 1 mM sodium pyruvate, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM glutamine and supplemented with 10% (v/v) FBS (hereafter referred to as SAOS-2 medium).
For each experimental group, particles were transferred into 48-multiwell plates (≈75 mg/well; n≥4 per condition), coated with 100 μL/well of 1.5% (w/v) agarose gel. In each well, 400 μL of specific culture medium (MG63 or SAOS-2 medium) was added, then the plates were incubated at 37°C in a 5% CO2 humidified atmosphere to let particles settle down for 2 hrs. Cells were next seeded onto the particles (3 × 104 MG63 cells/cm2 in 400 μL of MG63 medium; 5 × 104 SAOS-2 cells/cm2 in 400 μL of SAOS-2 medium) and incubated at 37°C in a 5% CO2 humidified atmosphere for 3 and 7 days. Cells seeded onto Bio-Oss® were used as positive controls, while cells seeded onto polystyrene plates (hereafter referred to as CTRL) were used as internal controls for cell growth. Culture medium was changed at day 3 of culture.
Evaluation of cell adhesion and proliferation
At day 3 and day 7 of culture, cell viability was assessed using AlamarBlue cell viability assay. Briefly, at different time intervals, medium was removed, and each well was loaded with 400 μL of specific medium containing 40 μL of resazurin dye. Cells were incubated in standard culture conditions for 2 hours, and the fluorescence of the medium was read with a GENios Plus reader (Tecan, Monza, Italy) (λex = 540 nm; λem = 595 nm). Viability of CTRL cells was assigned as 100%.
At the end of culture period, SEM images were taken to show SAOS-2 morphology after adhesion to the particles. Briefly, samples were fixed in 1.5% (v/v) glutaraldehyde solution for 20 minutes, then dehydrated in graded ethanol series, and allowed to dry at RT. Afterwards, samples were gold-sputtered, and SEM images were then obtained as previously described (see the section “Morphological and chemical characterization”).
Statistical Analysis
Statistical analysis was carried out with GraphPad version 6 (GraphPad, La Jolla, CA, USA). All data were initially analyzed using the D’Agostino-Pearson omnibus normality test. Comparisons among groups were performed by 1-way analysis of variance (ANOVA) with post hoc Tukey test. Significance was achieved when the p value was <0.05. Data are expressed as means ± standard deviation (SD, n≥4).
Results and discussion
In the last few years, tooth derivatives have been attracting more and more interest in the fields of dentistry and tissue engineering because they closely resemble bone tissue. In addition, they are widely available, as teeth are extracted every day but are customarily discarded after extraction. In this scenario, we thus envisioned the use of tooth derivatives, namely D and E, as suitable substrates for tooth regeneration. In this study, D and E particles (Ø<1 mm) were obtained with a newly designed Tooth Transformer TT machine.
As demineralization is reported to improve the in vivo resorption rate of highly mineralized materials in the way that osteoconductivity and new bone formation are prompted (1, 2, 17, 18), we herein evaluated the physical-chemical features and the in vitro biological behavior of D and E particles, prepared following the predefined demineralization protocol provided by TT Transformer S.r.l. and envisioned in their prototype.
Evaluation of the sterilization process
Microbial contamination of bone tissue used in regenerative procedures has been found to impair osteogenesis and induce bone resorption with great bone volume losses (19). Graft materials must therefore be sterile, which means without microbial contaminants.
In the present study, we first checked the effectiveness of the sterilization downstream to treatments the teeth particles had undergone, in line with the conditions set out in the protocol envisaged by the TT device during tooth sterilization and demineralization. It is worthy of note that a small microbial load was found in D samples only (Fig. 1), whereas no apparent contamination was detected in any other untreated material. Such a low microbial content was probably due the mechanical action of the dental drill used to remove E from D. Most important, no contamination was found in dD samples, thus demonstrating that the chemicals used and the sequence of thermal cycles applied to E and D samples subjected to sterilization and demineralization processes allowed tooth sterilization.
Fig. 1.
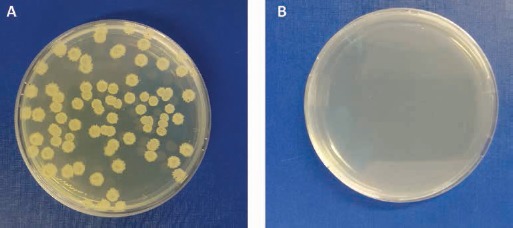
Qualitative observation of microbial contamination in dentin (D) particles (A), after plating onto agar plates. No microbial contamination was found in demineralized dentin (dD) particles after sterilization (B).
Effect of demineralization on surface morphology and chemical composition of D and E
The hard tissues of human teeth, including E and D, are mainly composed of an inorganic mineral phase – i.e., hydroxyapatite (HAp) – and an organic protein matrix. The mineral phase accounts for ≈70% of the native D weight (40%-45% in volume) (5, 6), while E consists of 96% HAp and a complex crystalline lattice organization (7, 20).
It is now acknowledged that extreme demineralization can damage D structure and affect in some way the composition and function of odontogenic factors; conversely, inadequate demineralization may lead to D with poor odontogenic properties. Further, some experimental evidence has highlighted the role played by surface properties of grafts in their in vitro and in vivo biological properties and functions (21).
Since thorough analysis of such properties is essential to predict their final performance, we decided to have a look at the morphology and topography of the different materials by means of SEM, and we carried out EDS analyses to shed light on the chemical compositions of each, whether or not subjected to the demineralization process. SEM micrographs revealed the presence of mineral crystals surrounding the rim of dentinal tubules (Fig. 2A), as confirmed by the Ca and P peaks in EDS spectrum in the D samples (Fig. 2B). dD displayed a substantial removal of mineral components (Fig. 2D), leading to a very smooth D surface. Further, it is worthy of note that the lumen of dentinal tubules was wider in dD samples than in untreated D particles (Fig. 2A and D respectively). EDS analysis confirmed these visual and qualitative findings, with significant changes in Ca and P content after the demineralization treatment (Fig. 2E). In fact, the decrease in % Ca and % P was ≈45% in dD samples compared with their D counterparts. On the other hand, the rise in C content (%) in dD samples support the idea that the protein component (and content) of D was preserved.
Fig. 2.
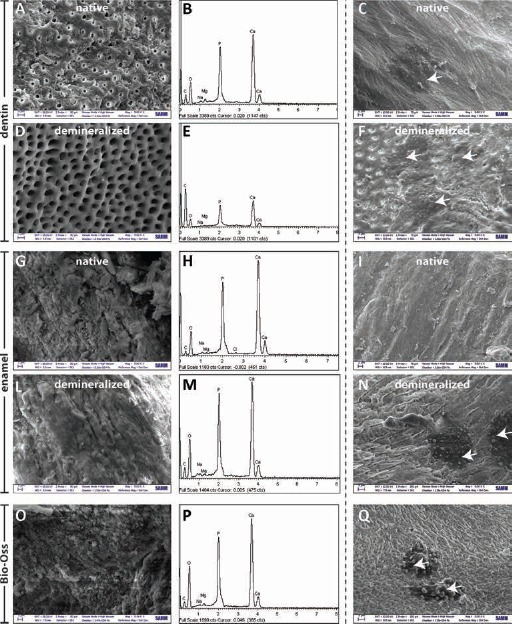
SEM images (magnification: ×5,000) and the corresponding energy dispersive X-ray (EDS) spectra of: dentin (D) particles (A, B); demineralized dentin (dD) particles (D, E); enamel (E) particles (G, H); demineralized enamel (dE) particles (L, M); and Bio-Oss® (O, P). SEM micrographs (magnification: ×5,000) after 7 days of SAOS-2 cell culture onto: D particles (C), dD particles (F), E particles (I), dE particles (N) and Bio-Oss® (Q). Scale bar: 3 µm in A, D, F, N, Q; scale bar: 2 µm in C, G, I, L, O.
Conversely, demineralization treatment was found to have a weaker effect on E samples compared with D samples. Indeed, dE samples displayed a modest reduction of ≈9% in Ca and P content (Fig. 2H-M) compared with untreated E, and no gross morphological differences were observed between these samples (Fig. 2G-L).
It is worthy of note that Bio-Oss® displayed similar Ca and P content to dE (Fig. 2P). SEM pictures showed mineral crystals studding the surface of Bio-Oss® particles (Fig. 2O).
Table I summarizes the results for the chemical composition of the different D and E samples.
Table I.
Energy dispersive X-ray (EDS) analysis performed on native and demineralized samples
| Sample | C (%) | O (%) | P (%) | Ca (%) |
|---|---|---|---|---|
| D | 24.02 | 49.98 | 8.59 | 16.56 |
| dD | 60.02 | 26.06 | 5.04 | 8.59 |
| E | 12.90 | 55.21 | 11.04 | 20.25 |
| dE | 16.13 | 58.26 | 9.99 | 15.02 |
| Bio-Oss® | 15.95 | 62.32 | 8.62 | 12.45 |
Bio-Oss® was used for comparison.
D = dentin; dD = demineralized dentin; E = enamel; dE = demineralized enamel.
Overall, the demineralization process was very effective on D, while weaker on the E particles. Moreover, dD samples displayed no damage to the extracellular matrix (ECM) in the way that protein components were preserved after treatment, as demonstrated by the C peak in the EDS spectra and quantification of %C on the dD surface.
Effect of demineralization on COL-I and BMP-2 contents in D and E
Apart from the mineral phase, D displays an organic ECM mostly comprising collagenous and noncollagenous proteins. COL-I represents ≈90% of the ECM components of D (5, 6), whereas other noncollagenous proteins, such as osteocalcin, osteonectin and osteopontin which are present in small amounts, play a key roles in matrix mineralization (22).
Furthermore, bioactive growth factors (GFs), such as transforming growth factor-β (TGF-β) and BMPs, which are known to be present in and released from D, are involved in dental tissue repair (23, 24). In fact, as BMPs have a role during embryonic tooth development (25) in stimulating osteodifferentiation and in inducing bone formation (11), these GFs have been extensively studied for tissue regeneration purposes. Unfortunately, only a limited amount of BMPs can be extracted from teeth. In this regard, some reports have highlighted the limited osteogenic potential displayed by teeth because of the significant amount of mineral components that, in some way, do entangle BMPs and restrict their bioavailability (26). Through the reduction of the mineral phase, demineralization is thus believed to favor the release of such GFs from the tooth matrix (27).
In this study, we quantified COL-I and BMP-2 content in E and D particles, whether demineralized or not. ELISA performed on D and E particles singled out greater amounts of COL-I and BMP-2 in demineralized samples, as compared with untreated particles (Fig. 3A and B). More specifically, D samples displayed a significantly higher COL-I content compared with E particles (Fig. 3A). Notably, a slightly higher COL-I content was found in both dD and dE samples than in the untreated counterparts, although no significant difference was found. Such a finding provides compelling evidence that the demineralization process did not damage the ECM of D. Besides, COL-I was invariably present also in E samples (E and dE), as reported in Figure 3A but at ≈10-fold lower content compared with D (Fig. 3: D vs. E, p<0.05; dD vs. dE, p<0.05). Even though generally higher BMP-2 levels were found in E samples, a similar trend between demineralized and untreated pairs in terms of BMP-2 content was observed as well, as reported in Figure 3B. Indeed, a significant ≈2.5-fold increase in BMP-2 levels was observed in dD samples compared with D (Fig. 3B: D vs. dD, p<0.05) and a very mild rise in dE compared with untreated E particles (Fig. 3B: E vs. D, p>0.05).
Fig. 3.
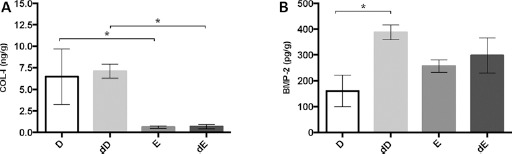
Protein quantification via ELISA performed on dentin and enamel particles subjected or not to demineralization process. (A) COL-I; (B) BMP-2. Protein content was normalized with respect to the weight of each sample (expressed as grams of sample particles). Data are expressed as means ± SD. D = dentin; dD = demineralized dentin; dE = demineralized enamel; E = enamel.
∗p<0.05.
Overall, we would like to point out that the demineralization process we have adopted allowed the preservation of some of the structural and functional ECM proteins in D and E, as opposed to reports in previous studies that extensive demineralization and process thereof resulted in severe BMP depletion in bone and/or bone-like tissues (28). Our results substantiate the idea that the demineralization process we used may allow us to increase the bioavailability of BMP-2, possibly resulting in more osteoinductive materials (29).
Effect of D and E substrates on cell behavior
An essential aspect of bone tissue engineering is the identification of suitable scaffold materials to properly support cell growth and eventually tissue regeneration in vitro and in vivo. Evaluation of the overall biocompatibility, and specifically of cell–biomaterial interactions, is thus crucial in the very first assessment of a biomaterial in vitro. In this regard, a number of studies have been carried out thus far on different cell lines, such as gingival fibroblasts (14), dental follicle cells (13), MG63 and SAOS-2 cells (8) to study the cell response to D substrates. Further, as D was shown to stimulate new bone formation in vivo and was thoroughly incorporated into bony sites (1, 14, 15, 30), D is nowadays considered one of the most suitable substitutes in bone repair and regeneration.
In the present work, we studied and compared the effect of different substrates – namely, dentin (in the form of D and dD), enamel (E and dE) and Bio-Oss® – on cell behavior in vitro. As shown in Figure 4, D and E, substrates accounted for a very different cell response, regardless of the treatment the materials underwent. In more detail, MG63 cells displayed a greater proliferation rate when seeded on D compared with when seeded on E particles (Fig. 4A, B). This behavior was already apparent after 3 days of culture, and it was maintained up to day 7 (Fig. 4A, B). Nevertheless, no difference was observed when MG63 cells were cultured on D and dD and Bio-Oss®. Conversely, SAOS-2 cells were more prone to proliferation when cultured on D (both D and dD) compared with Bio-Oss®, at any time point considered in this study (Fig. 4C, D).
Fig. 4.
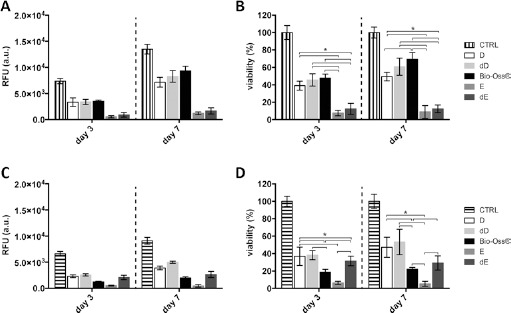
Evaluation of MG63 (A, B) and SAOS-2 (C, D) cell proliferation on dentin (D) and enamel (E) particles subjected or not to demineralization process. MG63 and SAOS-2 cells cultured onto polystyrene plates were used as the internal reference (i.e., positive controls [CTRL] for cell growth; 100% cell viability). Data are expressed as arbitrary units (RFU) as well as percentage of cell viability with respect to the CTRL. Bio-Oss® was used as a positive control. Data are expressed as mean ± SD; D = dentin; dD = demineralized dentin; E = enamel; dE = demineralized enamel.
∗p<0.05.
Although the demineralization of D did not influence cell proliferation rate, as no difference was found between D and dD samples (Fig. 4C, D), the chemical and thermal treatment performed on E gave rise to an increase in cell growth in dE samples at any time of culture. SEM images shown in Figure 2 allow us to appreciate qualitatively the adhesion of SAOS-2 on different materials. Roughly elongated cells were found on D (Fig. 2C), dD (Fig. 2F), dE (Fig. 2N) and Bio-Oss® (Fig. 2Q), while no cells were seen on the surface of native E particles 7 days postseeding (Fig. 2I). Such observations strengthen the results from viability assays performed on SAOS-2 cells.
Based on our results, D exhibited superior in vitro performance compared with E and Bio-Oss® (the last being the gold standard graft material used in dental clinics). Our preliminary in vitro findings support the use of D for bone replacement as a viable alternative to commercial Bio-Oss®. Further, D and dD samples affected the behavior of MG63 and SAOS-2 cells in the same way, thus demonstrating that the demineralization process we followed did not negatively impact D biocompatibility, as previously shown in other works (31).
There is a growing awareness that in vivo demineralized matrices are even more bioactive than native (i.e., undemineralized) substrates, as recently demonstrated by Li et al (13) and other scientists (32, 33). From this perspective, and in light of these very promising results obtained in vitro, we would expect similar behavior to occur also in vivo. Further in vivo investigation is thus urged to strengthen our findings, and to shed light on how demineralization influences the cell-driven resorption of such tooth graft materials.
Conclusion
Since there is a wide availability of teeth, which are extracted but discarded every day, many researchers have envisioned the potential to use tooth-derived materials as grafts for bone regeneration and replacement. In this view, understanding how these materials behave in vitro and in vivo is of great importance in the area of dentistry and bone tissue engineering.
The physical-chemical properties of tooth-derived materials used in this study displayed a great influence on cell behavior in vitro. Specifically, the high mineral content of E, as confirmed by EDS analysis, was found to inhibit cell proliferation in some way. Of note, a partial demineralization of E gave rise to an increased bioavailability of BMP-2 and to a significant improvement of its biological performance in vitro. Irrespective of the demineralization, the ECM of D invariably exhibited excellent biocompatibility, even greater than that of the widely used Bio-Oss®. Further, the demineralization process of D we used allowed preserving the organic content and increasing the BMP-2 bioavailability, yielding an overall increase in biocompatibility, compared with that of untreated D. Putting this in perspective, the possibility to finely tune the degree of mineralization (and thus of demineralization) may open new routes toward the development of a more and more effective osteoinductive material for bone regeneration and replacement.
Acknowledgments
The authors would like to thank Dr. E. Minetti for providing the dentin and enamel used in this study.
Disclosures
Financial support: This work was supported by TT Tooth Transformers S.r.l.
Conflict of interest: None of the authors has any financial interest related to this study to disclose.
References
- 1.Kim YK Lee J Yun JY Yun PY Um IW Comparison of autogenous tooth bone graft and synthetic bone graft materials used for bone resorption around implants after crestal approach sinus lifting: a retrospective study. J Periodontal Implant Sci 201444(5)216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pang KM Um IW Kim YK Woo JM Kim SM Lee JH Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: a prospective randomized clinical trial in comparison with anorganic bovine bone. Clin Oral Implants Res 2016. [DOI] [PubMed] [Google Scholar]
- 3.Rogers GF Greene AK Autogenous bone graft: basic science and clinical implications. J Craniofac Surg 201223(1)323–327. [DOI] [PubMed] [Google Scholar]
- 4.Tabatabaei FS Tatari S Samadi R Moharamzadeh K Different methods of dentin processing for application in bone tissue engineering: A systematic review. J Biomed Mater Res A 2016104(10)2616–2627. [DOI] [PubMed] [Google Scholar]
- 5.Marshall GW Jr Marshall SJ Kinney JH Balooch M The dentin substrate: structure and properties related to bonding. J Dent 199725(6)441–458. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M Kulkarni AB Young M Boskey A Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 20113(2)711–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YK Lee J Um IW et al. Tooth-derived bone graft material. J Korean Assoc Oral Maxillofac Surg 201339(3)103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheven BA Marshall D Aspden RM In vitro behaviour of human osteoblasts on dentin and bone. Cell Biol Int 200226(4)337–346. [DOI] [PubMed] [Google Scholar]
- 9.Binderman I Hallel G Nardy C Yaffe A Sapoznikov L A novel procedure to process extracted teeth for immediate grafting of autogenous dentin. J Interdiscipl Med Dent Sci 20142(154)6–11. [Google Scholar]
- 10.Koga T Minamizato T Kawai Y et al. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLoS ONE 201611(1)e0147235.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yagihashi K Miyazawa K Togari K Goto S Demineralized dentin matrix acts as a scaffold for repair of articular cartilage defects. Calcif Tissue Int 200984(3)210–220. [DOI] [PubMed] [Google Scholar]
- 12.Guo W He Y Zhang X et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials 200930(35)6708–6723. [DOI] [PubMed] [Google Scholar]
- 13.Li R Guo W Yang B et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 201132(20)4525–4538. [DOI] [PubMed] [Google Scholar]
- 14.Moharamzadeh K Freeman C Blackwood K Processed bovine dentine as a bone substitute. Br J Oral Maxillofac Surg 200846(2)110–113. [DOI] [PubMed] [Google Scholar]
- 15.Kim KW Bone induction by demineralized dentin matrix in nude mouse muscles. Maxillofac Plast Reconstr Surg 201436(2)50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murata M Akazawa T Takahata M et al. Bone induction of human tooth and bone crushed by newly developed automatic mill. J Ceram Soc Jpn 2010118(1378)434–437. [Google Scholar]
- 17.Linden GJ Bone induction in implants of decalcified bone and dentine. J Anat 1975119(Pt 2)359–367. [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YK Lee J Kim KW Um IW Murata M Ito K Analysis of organic components and osteoinductivity in autogenous tooth bone graft material. J Korean Assoc Maxillofac Plast Reconstr Surg 201335(6)353–359. [Google Scholar]
- 19.Verdugo F Castillo A Moragues MD Pontón J Bone microbial contamination influences autogenous grafting in sinus augmentation. J Periodontol 200980(8)1355–1364. [DOI] [PubMed] [Google Scholar]
- 20.Jernvall J Thesleff I Tooth shape formation and tooth renewal: evolving with the same signals. Development 2012139(19) 3487–3497. [DOI] [PubMed] [Google Scholar]
- 21.Suchanek W Yashima M Kakihana M Yoshimura M Processing and mechanical properties of hydroxyapatite reinforced with hydroxyapatite whiskers. Biomaterials 199617(17)1715–1723. [DOI] [PubMed] [Google Scholar]
- 22.Boskey AL Roy R Cell culture systems for studies of bone and tooth mineralization. Chem Rev 2008108(11)4716–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakashima M Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev 200516(3)369–376. [DOI] [PubMed] [Google Scholar]
- 24.Iohara K Nakashima M Ito M Ishikawa M Nakasima A Akamine A Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 200483(8)590–595. [DOI] [PubMed] [Google Scholar]
- 25.Yang W Harris MA Cui Y Mishina Y Harris SE Gluhak-Heinrich J Bmp2 is required for odontoblast differentiation and pulp vasculogenesis. J Dent Res 201291(1)58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urist MR Strates BS Bone morphogenetic protein. J Dent Res 197150(6)1392–1406. [DOI] [PubMed] [Google Scholar]
- 27.Blum B Moseley J Miller L Richelsoph K Haggard W Measurement of bone morphogenetic proteins and other growth factors in demineralized bone matrix. Orthopedics 200427(1 Suppl)s161–s165. [DOI] [PubMed] [Google Scholar]
- 28.Pietrzak WS Ali SN Chitturi D Jacob M Woodell-May JE BMP depletion occurs during prolonged acid demineralization of bone: characterization and implications for graft preparation. Cell Tissue Bank 201112(2)81–88. [DOI] [PubMed] [Google Scholar]
- 29.Sloan AJ Lynch CD Dental tissue repair: novel models for tissue regeneration strategies. Open Dent J 20126(1)214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YK Kim SG Yun PY et al. Autogenous teeth used for bone grafting: a comparison with traditional grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol 2014117(1)e39–e45. [DOI] [PubMed] [Google Scholar]
- 31.Tabatabaei FS Tatari S Samadi R Torshabi M Surface characterization and biological properties of regular dentin, demineralized dentin, and deproteinized dentin. J Mater Sci Mater Med 201627(11)164. [DOI] [PubMed] [Google Scholar]
- 32.Kim YK Kim SG Oh JS et al. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol 201111(8)7442–7445. [DOI] [PubMed] [Google Scholar]
- 33.Akazawa T Murata M Hino J et al. Surface structure and biocompatibility of demineralized dentin matrix granules soaked in a simulated body fluid. Appl Surf Sci 2012(262)51–55. [Google Scholar]


