Abstract
Background
Spinal fusion is a common procedure used for surgical treatment of spinal deformity. In recent years, many bone graft substitutes (BGS) have been developed to provide good arthrodesis when the available autologous bone harvested from the patient is not enough. The aim of this study was to analyze the use of a new-generation composite material (RegenOss) made of Mg-hydroxyapatite nanoparticles nucleated on type I collagen to obtain long posterolateral fusion in adult scoliosis surgery.
Methods
A total of 41 patients who underwent spinal fusion for the treatment of adult scoliosis were retrospectively analyzed. According to Lenke classification, visual analog scale (VAS) score and Oswestry Disability Index (ODI) score, radiographic rates of bone union were evaluated before surgery and at 6, 12 and 36 months of follow-up. Fusion was considered to be successful when criteria for Lenke grade A or B were satisfied. Patient-related risk factors were considered for the evaluation of the final outcome.
Results
At 36-month follow-up, radiographic evidence of spinal fusion was present in the majority of patients (95.1%). A time-dependent statistically significant improvement was evidenced after surgery for all clinical outcomes evaluated. Based on the demographic data collected, there were no statistically significant factors determining fusion. The correction of deformity was maintained at different time points. No intraoperative or postoperative complications were recorded.
Conclusions
The present study demonstrated that RegenOss can safely be used to achieve good arthrodesis when associated with autologous bone graft to obtain long spinal fusion in the treatment of adult scoliosis.
Keywords: Biomaterial, Biomimetism, Bone graft substitute, Hybrid composite, Osteointegration, Regenerative bone, Spinal fusion
Introduction
Adult scoliosis is a spinal deformity in a skeletally mature patient with a Cobb angle of more than 10° in the coronal plain. Deformity in the adult spine can be a consequence of an adolescent deformity, a new presentation of deformity due to a degenerative process of the motion segment of the spine or the result of other causes such as tumor or osteoporotic fractures. Aebi scores describe 3 types of adult scoliosis: (i) primary degenerative scoliosis (de novo), (ii) progressive idiopathic scoliosis; (iii) secondary degenerative scoliosis (1).
In adult scoliosis, pain and disability correlate with segment degeneration, and sagittal imbalance are the most relevant findings to make a decision to undertake surgical treatment (2). Progression of neurological deficits or symptoms not responsive to conservative treatment for more than 6 months are additional indications to surgery. Instrumented spinal fusion is an effective treatment; however, the relatively limited amount of autologous local bone available for the graft makes it necessary to use bone from other sources or synthetic biomaterials.
Among the available graft options, an autologous iliac crest bone graft (ICBG) is considered the gold standard option for achieving arthrodesis, thanks to its excellent osteoinductive, osteoconductive and osteogenic properties. However, drawbacks such as morbidity at the harvesting site and limited availability may limit its use (3-4-5-6).
Local autograft bone (LAB), harvested from the lamina and the spinous processes during surgery, shows excellent fusion rates, reduced morbidity rates and shorter times in surgery (3, 7, 8); however, despite these excellent characteristics, the quantity and quality of LAB can be influenced by patient-related factors, particularly in children and older patients, resulting in a lack of material in sufficient amounts. Other bone graft substitutes developed as alternatives to autologous bone (i.e., allograft, bone morphogenetic proteins, demineralized bone matrix, platelet gel, mesenchymal stem cells) have shown variable results and disagreement in their clinical indications, being still the focus of investigations concerning their safety and efficacy in spinal applications (7, 9-10-11-12).
In this scenario, synthetic bone graft substitutes (BGSs) have been proposed and have available on the market since the 1980s as alternative osteoconductive materials to fill bony defects and smooth contour irregularities (3, 10, 13, 14). Usually derived from naturally occurring ceramics, these materials have been developed to provide porous scaffolds with a structural framework and mechanical stability, able to act as a cell anchorage site and thus enable new bone ingrowth.
The most common examples of ceramic-based materials are hydroxyapatite (HA), tricalcium phosphate (TCP) and calcium sulphate (CS), which have structural compositions that closely mimic the inorganic phase of bone. In spinal applications, the use of BGSs as viable replacements for autologous bone has been well-documented, showing how these materials, used as graft extenders, can provide comparable fusion rates to autologous bone, while avoiding donor site complications (15-16-17-18-19).
In particular, among the ceramic-based alternatives available, HA accounts for nearly 70 wt% of the mineral (inorganic) component of bone and teeth (20, 21), and is thus the most suitable material for bone replacement. In recent decades, new-generation BGSs have been developed to obtain enhanced mimicry of the biochemical and biophysical properties of the human bone. These “biomimetic” materials are manufactured by an assembling and mineralization process mimicking the cascade of phenomena yielding new bone formation in vivo (22), using equine-derived type I collagen, subjected to self-assembly and simultaneous mineralization with an apatite nanophase. In this complex process of biomineralization, therefore, an extracellular matrix (ECM)–mimicking matrix acts as an active template for the deposition of the mineral phase, and is also able to direct mineral deposition and limit crystal growth. The activation of these control mechanisms occurs through the linking of collagen functional groups (e.g., carbonyl groups) with calcium ions, which are then the nucleation sites for the mineral part. The highly regulated chemical-physical interaction between the inorganic (HA) and organic (type I collagen) phase is fundamental to allowing the formation of a composite material (bone) with unique properties of both stiffness (minerals) and elasticity (collagen) (23, 24).
The information stored in the organic phase (type I collagen) drives the mineralization process toward the development of nanostructured apatite platelets orientated along the long axis of the collagen, which is a feature considered to promote osteoblasts’ adhesion (25) (Fig. 1). Studies have also demonstrated that a multitude of doping ions (i.e., carbonate, magnesium), substituting either calcium (Ca) or phosphate (P) ions in the crystal lattice, characterize the formation of new bone (26-27-28). This phenomenon represents the major source of structural disorder in the mineral bone component, increasing its chemical reactivity and dissolution ability while maintaining a good affinity with osteoblast cells. Among the doping ions taking part to this process, Mg2+ ions, partially substituting calcium ions, are associated with the first stages and the very fast turnover of newly bone formation. The presence of Mg2+ ions increases the nucleation kinetic of the new mineral bone component while delaying the crystallization process, thus making it extremely active during remodeling (23). For the purpose of manufacturing a 3D fibrous mineralized construct which exhibits a very high degree of mimicry of the natural bone tissue, the assembling and simultaneous mineralization of type I collagen fibrils with biomimetic Mg-HA in aqueous media is provided, thus obtaining a newly developed 3D scaffold for bone regeneration made of magnesium-doped hydroxyapatite/type I collagen (MHA/Coll) (Fig. 2). The capability of this new-generation biomimetic BGS to promote bone regeneration and to assist new bone formation has been demonstrated in preliminary studies in animal models (29, 30), showing interesting results and therefore representing a valid alternative to ICBG for spinal applications – in particular for those procedures requiring enormous amounts of bone graft to be available for the treatment of long spinal segments (i.e., degenerative scoliosis).
Fig. 1.
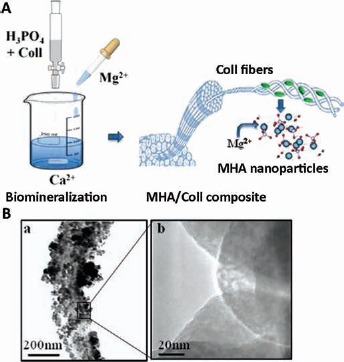
(A) Schematic representation of the biomineralization process used in the synthesis of hydroxyapatite/type I collagen (MGHA/Coll) scaffold. (B) Transmission electron microscopy (TEM) analysis: Collagen fiber completely covered with magnesium-doped hydroxyapatite (MHA) nanoparticles (A) and detail of the disordered crystalline structure of MHA nanoparticles (B).
Fig. 2.

The newly developed magnesium-doped hydroxyapatite/type I collagen (MHA/Coll) 3D scaffold developed for bone regeneration.
In light of these considerations, we retrospectively reviewed a case series of patients who underwent long spinal fusion with the use of a synthetic MHA/Coll composite scaffold (provided by Fin-Ceramica Faenza S.p.A., Faenza, Italy) mixed with LAB graft for the treatment of adult degenerative scoliosis. The final outcome was evaluated by the analysis of posterolateral fusion rates and clinical parameters. A number of patient-related factors were also considered in order to evidence their possible influence on the final outcome.
Materials and Methods
Patient population
This study was a retrospective study to investigate the efficacy of a new-generation composite material made of Mg-doped HA and type I collagen from an equine source, associated with an LAB graft in a spinal fusion for the treatment of adult degenerative scoliosis. Data were gathered through the review of patients’ case notes and relevant data records over 4 years (between 2011 and 2015) at the Niguarda Hospital (Milan, Italy), with a minimum of 3 years of follow-up. This retrospective study was conducted in conformity with the 1975 Declaration of Helsinki.
Specific inclusion criteria were (i) indication for multilevel instrumented posterolateral spinal fusion with or without interbody device; (ii) low-back pain, sciatica and/or neurogenic claudication for at least 6 months nonresponsive to previously administered conservative therapy (i.e., bed rest, bracing, antiinflammatory medications, physical therapy); (iii) preoperative radiographic analysis showing adult scoliosis type 1, 2 or 3 according to the Aebi classification system (1); (iv) aged 45 to 75 years; and (v) participant had personally signed and dated an informed consent document prior to any study-related procedure.
Exclusion criteria were (i) alcohol or drug abuse; (ii) drug therapy resulting in impaired bone regeneration (corticosteroids, chemotherapeutic drugs etc.); or (iii) active or systemic local infections, active malignancy, metabolic or hematic disorders. At pre-op, baseline characteristics (age, sex, body mass index, smoking habits, previous surgical procedures undertaken), clinical parameters (Oswestry Disability Index [ODI] and the visual analog scale [VAS]) were recorded, and radiographic analysis was carried out.
Biomaterial
The device employed in this study (RegenOss; provided by Fin-Ceramica Faenza S.p.A., Faenza, Italy) is a commercially available, porous, 3-dimensional composite bone substitute made of type I collagen fibers (from equine source) in which nano-sized (10-20 nm) crystals of biomimetic Mg-doped HA (Mg-HA) are nucleated at a 40%-60% ratio. The composite device is manufactured to be capable of reproducing the anatomical structure of the bone compartment as it occurs in the biological process of neo-ossification. The device has biocompatibility characteristics and a good safety profile (31), highlighted by toxicological studies carried out in accordance with the laws and regulations in force concerning Class III medical devices. Once the tissue regeneration process has been completed, the device is able to undergo resorption.
Surgical Procedure
All patients underwent decompression and spinal stabilization using instrumented fixation supports (pedicle screws/rods/cage) at at least 2 spinal levels between T4 and the ileum. All of the surgical procedures were performed by the same senior surgeon using the standard open posterior approach to the thoracolumbar spine. Patients underwent intravenous antibiotic treatment 30 minutes before surgery (cefazolin 2 g total amount). Pedicle titanium screws (Expedium system; DePuy Synthes) and PEEK interbody cages (T-PAL spacer system; DePuy Synthes) were used. A bleeding bone fusion bed was obtained through decortication of the posterolateral area from the transverse processes throughout the posterior aspect of the facet joints.
A synthetic magnesium-doped MHA/Coll BGS was shaped according to the defect to be treated (Fig. 3) and mixed with morselized autologous bone (ratio 1:1) (Fig. 4), previously harvested during laminectomy, spinosectomy. The mixture was used to provide posterolateral fusion. In 13 patients, a transforaminal approach to the intervertebral disc space was performed (TLIF). Local bone was placed in the anterior portion of the interbody space and used to fill the cage (with lordotic angle of 5°) prior to its placement. In all cases, the screw instrumentation was compressed to reproduce the normal lordotic curvature of the lumbar spine. In some cases, if required to restore good sagittal alignment, shortening of the posterior column with a Smith-Petersen osteotomy was performed. In a few cases, if required, a pedicle subtraction osteotomy was carried out to obtain more than 30 degrees of lordosis. The wound was sutured in 3 layers over 2 suction drainage tubes. Patients were intravenously treated with prophylactic antibiotic therapy immediately after surgery (cefazolin 2 g, total amount) and mobilized 2 to 3 days after surgery. A lumbar brace was used for about 1 month after surgery in all patients. Follow-up visits, including the recording of clinical parameters and radiological analysis, were conducted at 6, 12 and 36 months.
Fig. 3.
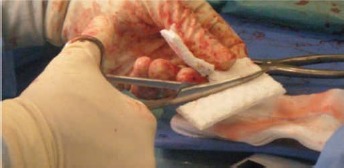
Intraoperative photograph showing the malleability and flexibility of the magnesium-doped hydroxyapatite/type I collagen 3D scaffold. The device can be cut and shaped according to the surgeon's needs.
Fig. 4.
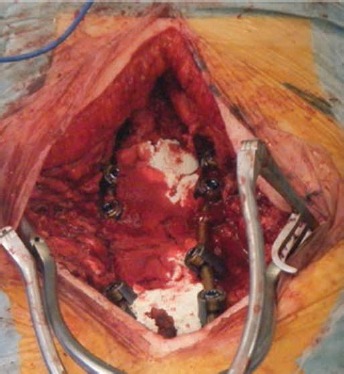
Intraoperative photograph showing the use of the magnesium-doped hydroxyapatite/type I collagen 3D scaffold in association with local autologous bone graft for posterior fusion in adult scoliosis surgery.
Clinical and radiological data
Adult scoliosis was classified preoperatively through radiographic analysis, according to the Aebi classification system (1) (Tab. I). All patients underwent complete standing radiography of the spine to evaluate the sagittal balance of the column. Changes in low-back pain and leg pain were evaluated before and after surgery. For pain scoring, the VAS score (scale range from 0 = no pain, to 10 = unbearable pain) was used to evaluate the patient's pain intensity. The ODI (0-20: minimal disability; 21-40: moderate disability; 41-60: severe disability; 61-80: crippling back pain; 81-100) was recorded for the quantification of the patient's disability. The degree of fusion was determined by 2 independent observers using plain radiography, including anteroposterior, lateral flexion/extension and Ferguson views. At follow-up visits (after 6, 12 and 36 months) radiological assessment of spinal fusion was graded by the Lenke classification system (Tab. II) (32). Fusion was considered to be successful when criteria for Lenke grade A or B were satisfied. At 36 months after surgery the sagittal vertical axis (SVA), which is the horizontal offset between a vertical plumb line from the center of the C7 vertebral body and the posterior superior corner of S1, was evaluated (33).
Table I.
AEBI classification of adult scoliosis
| Type 1: Primary degenerative scoliosis (de novo scoliosis) |
| Type 2: Progressive idiopathic scoliosis of the lumbar and/or thoracolumbar spine |
| Type 3a: Secondary adult scoliosis (for example: adjacent thoracic or thoracolumbar curve of idiopathic, neuromuscular or congenital origin) |
| Type 3b: Deformity progressing mostly due to bone weakness with, e.g., osteoporotic fracture with secondary deformity |
Aebi classification system used to classify adult scoliosis.
Table II.
Lenke classification of posterolateral fusion success
| Grade A: Definitely solid, with bilateral trabeculated stout fusion masses present. |
| Grade B: Possibly solid, with a unilateral large fusion mass and a contra-lateral small fusion mass. |
| Grade C: Probably not solid, with a small fusion mass bilaterally. |
| Grade D: Definitely not solid, with bone graft resorption or obvious pseudarthrosis bilaterally. |
Lenke classification system used to radiographically evaluate the degree of fusion.
The number of vertebral levels fused, the postoperative sagittal balance and postoperative complications were also recorded. A number of parameters which might influence the degree of fusion were considered, among which were age, sex, number of levels fused during surgery (<4 or ≥4 levels), previous spinal surgeries undertaken (yes/no), interbody fusions and smoking habits (yes/no). A single senior author examined all of the images and recorded data.
Statistical Analysis
Values are presented as number (no.), mean or percentage, as appropriate. Differences of clinical values (ODI and VAS) among the follow-up periods were analyzed using the Friedman ANOVA test. Stratification groups were correlated to ODI and VAS values at different follow-up periods by the Wilcoxon matched-pairs test. The level of statistical significance was set at a p value <0.05.
Results
According to the inclusion criteria, 41 patients were included in this retrospective study. Table III shows the patients’ demographic characteristics. There were 31 women and 10 men. The mean patient age was 62.9 years (standard deviation [SD] = 8.21 years). There were 22 participants younger than 65 years. Mean BMI (kg/m2) was 24.5 (SD = 3.6). Smokers comprised 29.3% of the sample population. Twelve patients (29.3%) had had previous surgical treatments for degenerative scoliosis. Thirteen patients (31.7%) had undergone an interbody fusion. The type of adult scoliosis was evaluated according to the Aebi classification system (Tab. III). Mean preoperative VAS score was 8.6. The mean preoperative ODI score was 72.
Table III.
Baseline characteristics
| Baseline characteristics | |
|---|---|
| Patients, no. | 41 |
| Female, no. (%) | 31 (75.6) |
| Male, no. (%) | 10 (24.4) |
| Mean age | 62.9 |
| Age <65, no. (%) | 22 (53.6) |
| Age >65, no. (%) | 19 (46.4) |
| Mean BMI | 24.5 |
| Smokers, no. (%) | 12 (29.3) |
| Previous surgeries | |
| Interbody fusion, no. (%) | 13 (31.7) |
| Aebi classification | |
| De novo scoliosis, no. (%) | 31 (75.6) |
| Idiopathic scoliosis, no. (%) | 4 (9.8) |
| Iatrogenic scoliosis, no. (%) | 6 (14.6) |
| Mean pre-op VAS | 8.6 |
| Mean pre-op ODI | 72 |
Patient demographic and clinical data at pre-op. Data are reported as numbers (no.) and percentage (%), or means where indicated.
ODI = Oswestry Disability Index; VAS = visual analog scale.
All of the patients underwent at least the fusion of 2 vertebral levels (Tab. IV). The postoperative radiographs (Figs. 5 and 6) showed the progressive incorporation of synthetic bone graft in the fusion area with time. Table V reports bony fusion as evaluated by the Lenke classification system. After 6 months, a good level of arthrodesis was achieved by the majority of patients (grade A or B). Only 1 patient showed no fusion (Lenke grade D). At 12-month follow-up, more than half of the patients (56%) already showed complete fusion mass (grade A). Only 2 patients still manifested partial and incomplete fusion (grade C). There were no patients showing a bone union failure. After 36 months, the majority of patients showed complete (grade A, 61%) (Fig. 7) or almost complete (grade B, 34%) bone union. Only 2 patients still manifested partial and incomplete fusion (grade C). The lack of bone union failure was confirmed at 36 months of follow-up. No baseline or clinical differences affected the outcome of patients with a delayed fusion as compared with patients already showing Lenke grade A or B at 6 months (Fisher's exact test: 2-sided p>0.05).
Table IV.
Number of levels fused
| Number of levels fused | No. of patients (%) |
|---|---|
| 2 levels | 4 (9.8) |
| 3 levels | 8 (19.5) |
| 4 levels | 8 (19.5) |
| 5 levels | 5 (12.2) |
| 6 levels | 2 (4.9) |
| 7 levels | 6 (14.6) |
| 8 levels | 3 (7.3) |
| 9 levels | 1 (2.4) |
| 10 levels | 4 (9.8) |
Number of levels fused during surgery. Data are reported as numbers (no.) and percentage (%), or means where indicated.
Fig. 5.
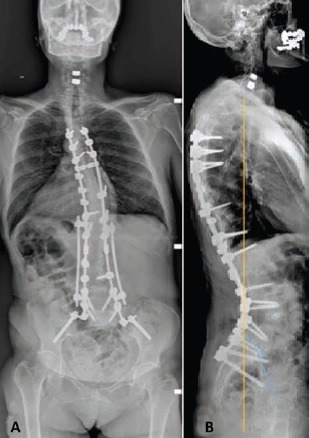
Patient's X-ray images. (A) The patient underwent T4-ileum fusion, L4 pedicle subtraction osteotomy (PSO) and L3-L4 extreme lateral interbody fusion (XLIF). (B) The patient shows good sagittal balance at 36-month follow-up (sagittal vertical axis [SVA] 0.3 cm).
Fig. 6.
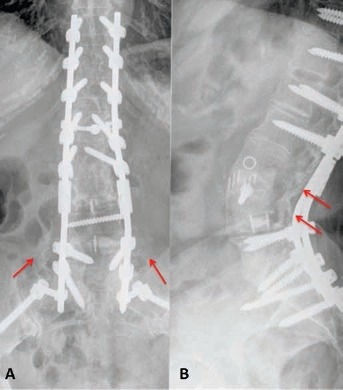
Example of posterior and lateral X-ray image at 36 months of follow-up showing successful posterolateral fusion as confirmed by the presence of mature bony trabeculae (arrows), equivalent to Lenke classification grade A, in patient who underwent T10-ileum fusion, L3-L4 anterior column realignment (ACR), L4 pedicle subtraction osteotomy (PSO) and L3-L4+L4-L5 extreme lateral interbody fusion (XLIF) (image kindly provided by the Department of Orthopaedic Surgery, Niguarda Hospital, Milan, Italy).
Table V.
Evaluation of spinal fusion (Lenke classification)
| Lenke classification | |||
|---|---|---|---|
| 6 months | 12 months | 36 months | |
| Grade A | 10 (24.4%) | 23 (56.1%) | 25 (61.0%) |
| Grade B | 21 (51.2%) | 16 (39.0%) | 14 (34.1%) |
| Grade C | 9 (22.0%) | 2 (4.9%) | 2 (4.9%) |
| Grade D | 1 (2.4%) | 0 (0.0%) | 0 (0.0%) |
Values are represented as numbers of patients (%).
Fig. 7.
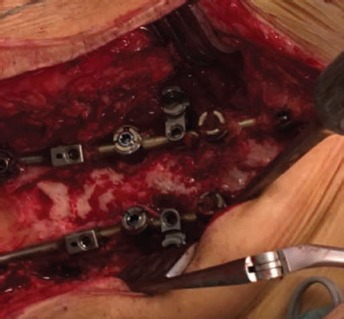
Intraoperative photograph of fusion area at 30 months (case of revision surgery in adult scoliosis). Histological analysis performed in a 63-year-old patient who underwent revision surgery, showing newly formed bone and the complete osteointegration of the device at 30 months (image kindly provided by the Department of Orthopaedic Surgery, Niguarda Hospital, Milan, Italy).
Maximum flexion-extension postoperative radiographs, performed at 36 months after surgery, did not show any mechanical instability. No hardware failure was recorded. Correction of the scoliosis deformity and sagittal balance were maintained similarly and satisfactorily at final follow-up in all patients. SVA was between 0 and 5 points in all patients, and was also evaluated at 36-month follow-up (Tab. VI) (34). According to the Schwab criteria for good sagittal alignment, all patients showed SVA of between 0 and 5 cm (35).
Table VI.
Sagittal Vertical Axis stratification
| Sagittal vertical axis points | No. of patients (%) |
|---|---|
| SVA 0 | 10 (24.4) |
| SVA 1 | 9 (22.0) |
| SVA 2 | 4 (9.8) |
| SVA 2.5 | 1 (2.4) |
| SVA 3 | 9 (22.0) |
| SVA 4 | 7 (17.1) |
| SVA 5 | 1 (2.4) |
Sagittal vertical axis (SVA) recorded at 36-month follow-up. For each level, data are reported as number (n) of patients and relative percentage (%).
The mean ODI decreased from 72 (baseline value) to 34.6, 25.5 and 23, at 6, 12 and 36 months, respectively (p<0.0001 for all time points vs. pre-op measure) (Tab. VII).
Table VII.
Oswestry Disability Index (ODI) evaluation
| Oswestry disability index | ||||
|---|---|---|---|---|
| Baseline | 6 months | 12 months | 36 months | |
| No. | 41 | 41 | 41 | 41 |
| Mean | 72 | 34.6 | 25.5 | 23.0 |
| SD | 5.79 | 8.79 | 14.91 | 12.65 |
| Change from baseline | ||||
| Mean | - | -37.4 | -46.5 | -49 |
| p value | - | <0.0001 | <0.0001 | <0.0001 |
Time course of oswestry disability index (ODI) scores. ODI values significantly decreased for all follow-up time points, vs. basal values.
Mean pain intensity, measured on the 10-point VAS, was 8.6 at baseline. The mean VAS score decreased from 8.6 to 4.5 after 6 months post-op, then from 4.5 to 2.8 at 12-month follow-up, and from 2.8 to 2.2 at 36-month follow-up. At the different time points, the decrease of VAS score was significant, compared with the pre-op measure (p<0.0001) (Tab. VIII). No demographic or surgical factors affected either fusion or clinical values, which were all statistically significantly different at the follow-up periods (6, 12 and 36 months) compared with their preoperative values (p≤0.0005, at least). No intraoperative or postoperative complications were recorded.
Table VIII.
Visual Analog Scale (VAS) evaluation
| Visual analog scale | ||||
|---|---|---|---|---|
| Baseline | 6 months | 12 months | 36 months | |
| No. | 41 | 41 | 41 | 41 |
| Mean | 8.6 | 4.5 | 2.8 | 2.2 |
| SD | 0.73 | 1.19 | 1.74 | 1.56 |
| Change from baseline | ||||
| Mean | - | -4.1 | -5.8 | -6.4 |
| p value | - | <0.0001 | <0.0001 | <0.0001 |
Time course of visual analog scale (VAS) score. Mean VAS values significantly decreased for all follow-up time points, vs.basal values.
Discussion
Currently, no BGS is better than an autologous bone graft. In long spinal fusion, the relatively limited quantity of local bone LAB graft means there is a need for other sources. Harvesting autologous bone graft from the iliac crest is one of the standard procedures, but possible complications associated with this technique are well known: donor site morbidity, postoperative pain, hematoma, infections and increased blood loss, which may occur in 25%-30% of patients, thus limiting its use (3). Another alternative is the use of allograft bone (harvested from a cadaveric donor), but this is often associated with potential risk of disease transmission, bacterial contamination or host-related reactions (36).
The results of the present study demonstrated that the use of the newly developed MHA/Coll 3D scaffold (RegenOss; provided by Fin-Ceramica Faenza S.p.A., Faenza, Italy) as BGS for bone regeneration is effective to achieve long spinal fusion in the surgical treatment of adult scoliosis. Moreover, this material can safely be mixed with autologous bone, as demonstrated by the absence of any adverse events recorded. We also considered a number of patient-related risk factors, which might influence the final outcome, but no correlations with either the degree of fusion or any clinical parameters were found.
The RegenOss scaffold evaluated in the present work was obtained by a bioinspired synthesis method (biomineralization) enabling the mineralization of type I collagen fibrils with an ion-doped apatitic nanophase, similar to what occurs during the formation of natural bone (22). Therefore, the scaffold exhibits physicochemical, morphological, structural and ultrastructural features very close to those of newly formed bone tissue (37).; as a result, the presence of a plethora of compositional and morphological cues offered by this scaffold can trigger several biochemical mechanisms instructing cells toward tissue regeneration.
In particular, the osteointegrative capabilities of this new scaffold have been recently reported by Grigolo and colleagues (38). The author reported the clinical case of a 65-year-old patient who underwent a long posterolateral fusion for treatment of adult scoliosis with severe sagittal imbalance and, 1 year later, revision surgery due to the rupture of a metallic bar. During the revision surgery, a histological examination was performed, showing the complete osteointegration of the graft, and evidencing the safety and efficacy of the implanted material (Fig. 8).
Fig. 8.
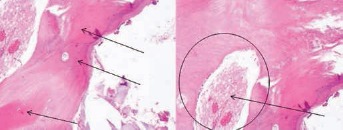
Histological analysis performed in a 63-year-old patient after revision surgery, showing newly formed bone tissue marked by purple-red staining (black arrows) and the complete osteointegration of the device (represented by the white area in the black circle) at 30 months post-op.
The use of ceramic-based BGSs in spinal applications has been widely investigated during recent years. Nickoli and colleagues (39) reviewed 30 clinical studies using ceramic-based materials as bone graft extenders in the lumbar spine. In 10 studies, involving more than 450 patients, the use of ceramics plus local autograft evidenced a fusion rate of around 90%. Lee and coworkers (40) reported no difference between the patient group treated with HA (87%) and the control group treated with ICBG (89%) in terms of fusion rates. Korovessis et al (41) concluded that HA together with the use of instrumentation and autologous bone provides good performance and a solid dorsal fusion within the expected time. Mashhadinezhad et al (42) evaluated the degree of fusion after applying HA inserted into cages for interbody fusion. The authors reported no difference between fusion rates achieved with HA compared with ICGB at 12-month follow-up, showing that application of HA granules, even inserted in cages, proved to be an effective treatment also for interbody fusion applications.
All of these data again confirm the safety and effectiveness of HA-based bone grafts in different spinal applications. From this perspective, data from the present retrospective study, in which we showed an improvement of bony fusion of about more than 90% at 36 months after surgery, are in line with results reported above. Moreover, the safety and efficacy of RegenOss in providing bone union was reiterated by the lack of baseline or clinical factors affecting the final outcome, as measured by Lenke grades A and B, at 6-month follow-up.
HA provides an osteoconductive matrix and shows osteogenic properties, but it generally lacks osteoinductive potential. For this reason, rates of successful arthrodesis can be increased by using bioceramics in conjunction with a source of cells such as a local autograft, which can be indicated to reduce the need for ICBG and concomitantly increase the quantity of bone graft available during surgery. The advantages provided by the use of synthetic bone grafts include their immediate availability and their unlimited quantity and good safety profile, which make them an essential resource, especially for those surgical procedures requiring a large amount of available bone graft.
In this retrospective study, we evidenced the safety and efficacy of this new-generation BGS for long fusion in spinal surgery. This BGS, by virtue of its malleability which makes it adaptable to cover complex shape defects, is particularly recommended for overlapping long segments of the spinal column. In addition, its regenerative properties, which allow bone ingrowth and tissue remodeling, are of relevant interest in scoliosis surgery.
One limitation of this study was the lack of a control group treated with autologous bone alone. Furthermore, a longer-term observation is needed to evaluate the fate of the synthetic bone graft–assisted spinal fusion, because a rigid spinal system can mask possible pseudoarthrosis during the first few years after surgery.
Conclusion
This study suggests that the use of this newly developed MHA/Coll 3D scaffold as a BGS in long spinal fusion for the treatment of adult scoliosis was safe and effective in providing spinal arthrodesis without any adverse effects or inflammatory response in all of the 41 patients treated for adult degenerative scoliosis. Surgical results showed equivalent or superior results compared with previous published data associated with the use of the gold standard ICBG. No patient-related factors affected the final outcome, underlying the notion that the use of this bone graft is effective independently of the patient's clinical condition, and it can be safely associated with autologous bone in this surgery. Use of the 3D scaffold provided new bone formation, allowing spinal arthrodesis and therefore representing a valid support to instrumentation devices for the achievement of spinal fusion. Further investigations are needed to support long-term efficacy and additional indications for its use.
Abbreviations
- BGS
Bone graft substitutes
- CS
Calcium sulphate
- HA
Hydroxyapatite
- ICBG
Iliac crest bone graft
- LAB
Local autograft bone
- MHA/Coll
Magnesium-doped hydroxyapatite/type I collagen
- ODI
Oswestry Disability Index
- SVA
Sagittal vertical axis
- TCP
Tricalcium phosphate
- TLIF
Transforaminal lumbar interbody fusion
- VAS
Visual analog scale
Disclosures
Financial support: None.
Conflict of interest: None.
References
- 1.Aebi M The adult scoliosis. Eur Spine J 200514(10)925–948. [DOI] [PubMed] [Google Scholar]
- 2.Berjano P Lamartina C Classification of degenerative segment disease in adults with deformity of the lumbar or thoracolumbar spine. Eur Spine J 201423(9)1815–1824. [DOI] [PubMed] [Google Scholar]
- 3.Park JJ Hershman SH Kim YH Updates in the use of bone grafts in the lumbar spine. Bull Hosp Jt Dis 201371(1)39–48. [PubMed] [Google Scholar]
- 4.Dimar JR II Glassman SD Burkus JK Pryor PW Hardacker JW Carreon LY Two-year fusion and clinical outcomes in 224 patients treated with a single-level instrumented posterolateral fusion with iliac crest bone graft. Spine J 20099(11)880–885. [DOI] [PubMed] [Google Scholar]
- 5.Gruskay JA Basques BA Bohl DD Webb ML Grauer JN Short-term adverse events, length of stay, and readmission after iliac crest bone graft for spinal fusion. Spine 201439(20)1718–1724. [DOI] [PubMed] [Google Scholar]
- 6.Silber JS Anderson DG Daffner SD et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 200328(2)134–139. [DOI] [PubMed] [Google Scholar]
- 7.Hsu WK Nickoli MS Wang JC et al. Improving the clinical evidence of bone graft substitute technology in lumbar spine surgery. Global Spine J 20122(4)239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eder C Chavanne A Meissner J et al. Autografts for spinal fusion: osteogenic potential of laminectomy bone chips and bone shavings collected via high speed drill. Eur Spine J 201120(11)1791–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer CR Cassilly R Cantor W et al. A systematic review of comparative studies on bone graft alternatives for common spine fusion procedures. Eur Spine J 201322(6)1423–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A Kukkar N Sharif K Main BJ Albers CE El-Amin SF III Bone graft substitutes for spine fusion: a brief review. World J Orthop 20156(6)449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder J Kueper J Leon K Liebergall M Stem cells for spine surgery. World J Stem Cells 20157(1)186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannan A Dodwad S-NM Hsu WK Biologics in spine arthrodesis. J Spinal Disord Tech 201528(5)163–170. [DOI] [PubMed] [Google Scholar]
- 13.Campana V Milano G Pagano E Barba M Cicione C Salonna G Lattanzi W Logroscino G Bone substitutes in orthopaedic surgery: from basic science to clinical practice J Mater Sci Mater Med. 201425(10)2445–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masashi M Hiroshi T Jeffrey C.W Ahmet A An update on bone substitutes for spinal fusion. Eur Spine J 2009(18)783–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsaleh KA Tougas CA Roffey DM Wai EK Osteoconductive bone graft extenders in posterolateral thoracolumbar spinal fusion: a systematic review. Spine 201237(16)E993–E1000. [DOI] [PubMed] [Google Scholar]
- 16.Lerner T Bullmann V Schulte TL Schneider M Liljenqvist U A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J 200918(2)170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linovitz RJ Peppers TA Use of an advanced formulation of beta-tricalcium phosphate as a bone extender in interbody lumbar fusion. Orthopedics 200225(5 Suppl)s585–s589. [DOI] [PubMed] [Google Scholar]
- 18.Dai L-Y Jiang L-S Single-level instrumented posterolateral fusion of lumbar spine with beta-tricalcium phosphate versus autograft: a prospective, randomized study with 3-year follow-up. Spine 200833(12)1299–1304. [DOI] [PubMed] [Google Scholar]
- 19.Muschik M Ludwig R Halbhübner S Bursche K Stoll T Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J 200110(Suppl 2)S178–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H Lee J Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater 20117(7)2769–2781. [DOI] [PubMed] [Google Scholar]
- 21.Gao C Deng Y Feng P et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci 201415(3)4714–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tampieri A Celotti G Landi E Sandri M Roveri N Falini G Biologically inspired synthesis of bone-like composite: self-assembled collagen fibers/hydroxyapatite nanocrystals. J Biomed Mater Res A 200367(2)618–625. [DOI] [PubMed] [Google Scholar]
- 23.Minardi S Corradetti B Taraballi F et al. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 2015(62)128–137. [DOI] [PubMed] [Google Scholar]
- 24.Tampieri A Sprio S Sandri M Valentini F Mimicking natural bio-mineralization processes: a new tool for osteochondral scaffold development. Trends Biotechnol 201129(10)526–535. [DOI] [PubMed] [Google Scholar]
- 25.Weiner S Biomineralization: a structural perspective. J Struct Biol 2008163(3)229–234. [DOI] [PubMed] [Google Scholar]
- 26.Bigi A Foresti E Gregorini R Ripamonti A Roveri N Shah JS The role of magnesium on the structure of biological apatites. Calcif Tissue Int 199250(5)439–444. [DOI] [PubMed] [Google Scholar]
- 27.Celotti G Tampieri A Sprio S et al. Crystallinity in apatites: how can a truly disordered fraction be distinguished from nanosize crystalline domains? J Mater Sci Mater Med 200617(11)1079–1087. [DOI] [PubMed] [Google Scholar]
- 28.Landi E Tampieri A Celotti G Langenati R Sandri M Sprio S Nucleation of biomimetic apatite in synthetic body fluids: dense and porous scaffold development. Biomaterials 2005. 26(16)2835–2845. [DOI] [PubMed] [Google Scholar]
- 29.Kon E Delcogliano M Filardo G et al. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J Orthop Res 201028(1)116–124. [DOI] [PubMed] [Google Scholar]
- 30.Kon E Filardo G Delcogliano M et al. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord 201011(1)220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbanti Brodano G Griffoni C Zanotti B et al. A post-market surveillance analysis of the safety of hydroxyapatite-derived products as bone graft extenders or substitutes for spine fusion. Eur Rev Med Pharmacol Sci 201519(19)3548–3555. [PubMed] [Google Scholar]
- 32.Lenke LG Bridwell KH Bullis D Betz RR Baldus C Schoenecker PL Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord 19925(4)433–442. [DOI] [PubMed] [Google Scholar]
- 33.Ailon T Sure DR Smith JS Shaffrey CI Surgical considerations for major deformity correction spine surgery. Best Pract Res Clin Anaesthesiol 201630(1)3–11. [DOI] [PubMed] [Google Scholar]
- 34.Jackson RP McManus AC Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size: a prospective controlled clinical study. Spine 199419(14 Suppl)1611–1618. [DOI] [PubMed] [Google Scholar]
- 35.Schwab F Patel A Ungar B Farcy J-P Lafage V Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine 201035(25)2224–2231. [DOI] [PubMed] [Google Scholar]
- 36.Delloye C Cornu O Druez V Barbier O Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br 200789(5)574–579. [DOI] [PubMed] [Google Scholar]
- 37.Sprio S Sandri M Panseri S et al. Hybrid scaffolds for tissue regeneration: chemotaxis and physical confinement as sources of biomimesis. J Nanomater 2012(2012)e418281.. [Google Scholar]
- 38.Grigolo G Dolzani P Giannetti C et al. Use of a fully-resorbable, biomimetic composite hydroxyapatite as bone graft substitute for posterolateral spine fusion: a case report. Int J Clin Exp Med 20169(11)22458–22462. [Google Scholar]
- 39.Nickoli MS Hsu WK Ceramic-based bone grafts as a bone grafts extender for lumbar spine arthrodesis: a systematic review. Global Spine J 20144(3)211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH Hwang C-J Song B-W Koo KH Chang BS Lee CK A prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. J Biomed Mater Res A 200990(3)804–810. [DOI] [PubMed] [Google Scholar]
- 41.Korovessis P Koureas G Zacharatos S Papazisis Z Lambiris E Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease: autograft versus coralline hydroxyapatite. Eur Spine J 200514(7)630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mashhadinezhad H Samini F Zare R Comparison of outcomes and safety of using hydroxyapatite granules as a substitute for autograft in cervical cages for anterior cervical discectomy and interbody fusion. Arch Bone Jt Surg 20142(1)37–42. [PMC free article] [PubMed] [Google Scholar]


