Abstract
Ninety percent of deaths from solid tumors have been ascribed to the invasion and metastatic dissemination of cancer cells. One of the most fundamental prerequisites of brain tumor stem cell invasion is their ability to penetrate and traverse through the basement membrane and stromal compartments displacing from their original point of origin and repopulating at a distant site and adjacent tissue. In order to propose a successful clinical strategy, the investigation of the molecular determinants of invasion must factor in measurements and predictors of the complexity of brain tumor invasion potential accounting for the physiological scenarios encountered in the tumor niche. This chapter will highlight some laboratory approaches such as: spot assay, 3D chemogradient chambers, Fluoroblok™ tumor invasion, organotypics, and 3D tumor spheroid assays that could help mitigate the investigation of a tumor stem cell’s invasion capacity through restrictive 3D environments otherwise seen in vivo.
Keywords: Motility, BTICs, Invasive capacity
1. Introduction
There is growing evidence that a small subset of cells, termed brain tumor initiating cells (BTICs), are responsible for new tumor formation due to their enhanced invasive and migratory capacity, enabling them to undergo a multistep process by augmenting natural cellular mechanisms that allow them to traverse across tissues in a perpetual and sometimes unstoppable manner. Invasion is defined as the destruction of surrounding matrices in a 3D system (in vivo) or 3D matrix (in vitro) requiring breakdown or lysis of extracellular components, manipulation of adhesion molecules, and remodeling of obstructive fiber networks that contain X, Y, and Z planes a BTIC must navigate through [1, 2]. This multi-step process requires distinct genetic players permitting (1) detachment of BTICs from the primary cancer, (2) destructing surrounding fibers and matrices, (3) intravasation through vessels entering the circulatory system or lymphatic system, (4) extravasation from circulation, (5) settlement, proliferation, and eventual outgrowth of new tumor at secondary site [3]. This step-wise process requires cytoskeleton mediated deviations starting with enhanced capacity to (1) polarize the body in a directional manner with the leading edge at the front and the lagging at the back, (2) this dynamic change potentiates a cell protrusion morphology resulting in a pseudopod, lamellipod, filopod, invadopods, and a few other phenotypes enabling contractile forwarding or retraction of the cell body, (3) vigorous interaction with actin and components of the extracellular matrix, (4) adherence and lysing of structural component, and finally (5) detachment of the trailing edge leading to propulsive movement forward [1, 4, 5]. This chapter will identify experimental assays for researchers in the field who desire to investigate migration and invasion in cancer; understanding such masked responses to environmental cues will help illuminate the causes giving rise to cancer invasiveness owing to the dismal prognosis seen in cancer patients today.
2. Materials
2.1. Spot Assay
Cells to be tested (must be labeled).
Eppendorf tubes.
Horse serum.
DNase I.
6-well plate.
Polycarbonate filters (with pore size of choice, we recommend size smaller than your cell of interest 1–3 μm).
PBS.
4% PFA.
HBSS.
Pipettes.
Cell dissociation media (trypsin/accutase).
Culture media.
Neurobasal media (NB).
l-glutamine.
Penicillin/streptomycin.
B27 supplement.
2.2. Fluoroblok™ Tumor Invasion for Coculture
Cells to be tested (cell type A).
Trypsin/Accutase/cell dissociation reagent.
Media of migratory cells (without serum).
Cells releasing Chemoattractant/soluble factors (cell type B).
DPBS.
Corning Fluoroblok™ cell culture inserts (24-well 8.0 μm pore size).
24-well Fluoroblok™ support.
Fetal bovine serum.
Calcien AM fluorescent.
DMSO.
HBSS ++.
Fluorescence plate reader with bottom reading capabilities.
Matrigel matrix.
Coating buffer for matrigel: 0.01 M Tris (pH 8.0), 0.7% NaCl.
0.2 μm sterile filter unit.
15 mL conical tube.
Ice.
Sterile forceps.
Sterile pipette tips.
2.3. Organotypics
Laminar flow culture hood.
Dissecting microscope.
HBSS plus Ca and Mg.
Microsurgical instruments (forceps, scissors, and scalpel).
Autoclaved Pasteur pipettes.
10 mm petri dishes.
24-well culture plate.
Organotypic culture media: Minimal Essential Medium (MEM) (sigma, St. Louis, MO, USA) containing 25% heat-inactivated horse serum (Gibco/BRL, Bethesda, MD), 25% HBSS (Gibco/BRL, Bethesda, MD) with 25.8 mg/mL of glucose, and 12 mg/mL of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (sigma, St. Louis, MO, USA) and 1% 0.2 M, glutamine (Gibco/BRL, Bethesda, MD) pH 7.2.
Microtome or vibratome capable of 300 μm tissue thickness.
12 mm culture plate inserts (Millipore, Billerica, MA).
Angled dissecting microscope or surgical microscope.
2.4. 3D Tumor Spheroid Invasion
Cell line tested (must form spheres which BTICs usually do).
Ultra-low attachment 96-well plate.
5× basement membrane extract (BME) or matrigel coating solution, keep at 4 °C.
Culture medium of cell to be tested.
Cytometer or any form of cell counter.
Inverted microscope.
Sterile pipettes.
PBS.
Cell dissociation solution.
Wash buffer.
Laminar flow and incubator.
Multichannel pipette.
Ice bucket.
3. Methods
3.1. Spot Assay (Fig. 1)
Fig. 1.
Spot Assay: The assessment of migration is dependent on the distance traveled from the middle spot of which the cells were dropped. The principal relies on placing a polycarbonate filter in the middle of a well and dropping 1ul of cell concentration on the top of the filter. Over time, the cell of interest will migrate away from the point of origin whereby the filter is then fixed, imaged and analyzed for distance cells traveled in response to exogenous supplements. Adapted from: Dose-dependent effect of EGF on migration and differentiation of adult subventricular zone astrocytes. Perez-Gonzalez O, Quinones-HinojosaA. Glia.58 (8),2010. Pages:975–983
3.1.1. Making of Neurobasal Media (NB)
DMEM/F12 HEPES.
2 mM l-glutamine.
B27 serum-free supplement.
1% penicillin/streptomycin.
hEGF 20 ng/mL.
hFGF 10 ng/mL.
3.1.2. Preparation of Cell Transplantation
Dissociate labeled cells to be tested from flask.
Spin down dissociated cells according to pelleting protocols.
Wash cell pellet in NB medium supplemented with 10 μg/mL DNase I.
Centrifuge 5 min, 200 × g.
Remove media.
Resuspend pellet with NB for a final concentration of 1 × 105 cells per μl.
3.1.3. Spot Assay Drop
Place 1.5–2 mL of NB medium inside each well of a 6-well plate.
Place one polycarbonate filter inside each well allowing it to float.
Place 1 μL drop of cell suspension in the middle of the filter.
Decide your time point (note: allow for a number of days in vitro (DIV) to assess the migratory capacity of cells: recommended 24–72 h).
When cell migration time point is reached, replace media with 4% PFA fixative at 37 °C for 1 h.
Take the plate, incubate it at 4 °C for 6 h in the same fixative.
Image your cells in a fluorescence microscope.
Photograph each replicate.
Use imaging software (ex: NIH IMAGE) for cell migration analysis.
Assess distance of furthest cell traveled from center point.
3.2. Fluoroblok™ Tumor Invasion for Coculture (Fig. 2)
Fig. 2.
Fluoroblok invasion assay. This technique follows the same premise as the transwell migration assay except the porous membrane is overlaid with a layer of a matrix a cell must transfer through
Prepare coating buffer: 0.01 M Tris (pH 8.0), 0.7% NacL. Filter using a 0.2 μm sterile filter unit.
Thaw matrigel on ice.
Once matrigel is thawed, swirl it several times to ensure it is evenly dispersed.
Prepare coating solution to place inside transwell by mixing matrigel at a final concentration of 200–300 μg/mL with coating buffer until the final volume comes to 2.0 mL and place solution on ice until ready for use.
Prepare calcein AM labeling by diluting stock solution with DMSO for a final concentration of 1 mg/mL.
Prepare working solutions of calcein for a final volume of 4 μg/mL and use 500 mL/well in HBSS++ for a 2 μg/well concentration for cell quantification.
3.2.1. Permeable Support Coating of Matrigel
Under sterile conditions, assemble transwells inside 24-well support plates to prepare for matrigel coating.
With ice cold pipette tips, carefully add 0.1 mL of the diluted matrigel coating to each permeable membrane. Avoid air bubbles and minimize contact with walls.
Incubate plates with coated matrigel inside transwells at 37 °C for 2 h. Do not let matrigel matrix layer dry out.
Prepare an equal number of controls.
3.2.2. Preparation of Cell A (Cell to Be Tested) and Cell Type B (Cell to Be Invaded)
Prepare cell suspension in culture medium for cell A and cell B.
Spin and pellet cells at 1 × 105 cells/mL for cell A.
Determine optimal dilution/concentration for cell type B *(A: B --1:2 or 1:3. Meaning if you plate cell type A at 1 × 105 cells/mL, for a 1:2 concentration, your cell B seeded at the surface of well would be 2 × 105 cells/mL).
Add the 1:2 or 1:3 dilution of cell B at the bottom for a final cell volume of 500 μL/well.
Add 200 μL/well of cell A (cells invading the matrix) inside well and on the top of a matrigel invasion chamber.
Make sure there are no air bubbles.
Incubate invasion chambers overnight (22–24 h) in a humidified tissue culture at 37 °C, 5% CO2 atmosphere (see Note 1).
3.2.3. Measurement of Cell Invasion
When ready to measure invasion, carefully remove medium from apical membrane chambers which can be accomplished by careful aspiration.
It is advisable to use forceps to flip and flick the inside of the contents inside a biohazard bin. Do not touch the bottom surface of the insert system.
After removing the inside contents of the invasion chambers, transfer the inserts in another 24-well support fluoroblok plate containing 500 μL of 4 μg/mL Calcein AM in HBSS++.
Incubate for 1 h at 37 °C, 5% CO2 (see Note 2).
For a bottom plate reader, it is recommended that the dimensions of your plate and the gains setting must be adjusted to quantitate fluorescent average appropriately.
Start with a midpoint setting and allow the bottom plate reader to read fluorescence calcien AM moiety.
Fluorescence of invaded cells is read at wavelength 494 excitation and 517 emission on a bottom-reading fluorescent plate reader.
Data is expressed as % invasion = mean RFU of cells invaded through matrigel matrix/mean RFU of cells migrated through uncoated membrane (see Note 3).
The results could be verified by using an inverted fluorescent microscope and is especially helpful to do this the first time you do this experiment.
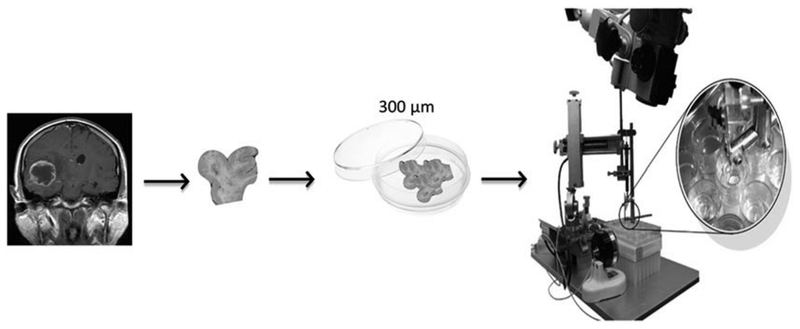
3.3. Organotypics (Fig. 3)
Fig. 3.
Human Organotypics. The organotypic assay is a powerful in vitro system that allows for the specific analysis of brain cell migration and invasion ex vivo. Organotypic tissue slices are 300um in thickness and stay viable for several days. One could study the mechanical capabilities of cells in a platform that maintains brain cytoarchitecture in a 3D fashion. Brain tumor cells are labeled and incorporated on top of the tissue slice where their penetration and displacement over several days are assessed and measured in a 3 dimensional manner using a two-photon microscope. Adapted from: Neurosphere culture and human organotypic model to evaluate brain tumor stem cells. Guerrero-Cázares H, Chaichana KL, Quiñones-Hinojosa A. Methods Mol Biol. 2009;568:73–83. doi: 10.1007/978-1-59745-280-9_6. Preservation of glial cytoarchitecture from ex vivo human tumor and non-tumor cerebral cortical explants: A human model to study neurological diseases. Chaichana KL, Capilla-Gonzalez V, Gonzalez-Perez O, Pradilla G, Han J, Olivi A, Brem H, Garcia-Verdugo JM, Quiñones-Hinojosa A. J Neurosci Methods. 2007 Aug
Tissue sample (human or rodent) must be transported in saline solution, PBS, or cell culture media, and kept on ice until processing.
Inside of a culture hood, place the dissecting microscope, the sterile surgical instruments, and the 10 mm petri dish (all the instruments need to be previously cleaned with ethanol).
Fill a petri dish half full with the HBSS high glucose solution under the hood.
Place the tissue sample into the filled petri dish and with the surgical instruments remove the necrotic areas and blood vessels from the tissue under the dissecting microscope.
Remove organotypic culture media from the refrigerator and place under the hood for 20–30 min to pre-warm the solution.
Using sterile technique in the hood, add 1 mL pre-warm organotypic culture media into each 24-well culture plate, and using forceps place a 12 mm Millipore insert into each incubation media-filled well. Cover the plate and set aside in the hood (see Note 4).
With the tissue chopper, cut the tissue to have slices of thickness of 350 microns.
Place the slides into a new petri dish containing high-glucose HBSS.
Carefully separate each transverse section of tissue with curved tweezers or by squirting solution onto the tissue and transfer each individual section of tissue onto the Millipore inserts.
Place approximately a tissue section in each well.
The plate with the organotypic cultures is then placed into an incubator at 37 °C and 5% CO2.
The organotypic culture media is carefully changed every 2 days (see Note 5).
3.4. 3D Tumor Spheroid Invasion (Fig. 4)
Fig. 4.
3D Tumor Spheroid Invasion: The principal of this assay is simple and heavily dependent on brain tumor stem cell propensity to aggregate in spheres. The tumor spheroid is embedded in a basement like setting usually composed of extracellular matrix components and entrapped within a well. The invasive capacity of these cells are measured by assessing the ability of a single cell to break from the tumor sphere bulk and invade adjacent space by evaluating the distance traveled from the point of origin. The analysis is based on sequential time point images of the tumor spheroid taken over a period of 3–4 days and analyzed through imaging software to determine distance traveled. Figures donated from Hugo Guerrero-Cazares
Pre-warm media along with chemoattractant media (conditioned media/cytokine media/media with your soluble factor of interest).
Harvest migratory cell of interest by washing cells to be studied (BTICs) from flask with PBS and shake flask back and forth to remove cell debris.
Aspirate PBS from flask.
Add trypsin or cell dissociation reagent and incubate for 5–10 min or until cells detach and floating in flask.
Collect floated cells.
Place collected cells in a 15 mL conical tube.
Spin cells at 200 × g from 5 min.
Aspirate the supernatant and resuspend pellet in 1 mL.
Count cell pellet to acquire final cell concentration in 1 mL.
For tumor spheroid per well, obtain 0.5–2 104 cells/mL and optimize seeding so that the diameter of spheroid formation after 4 days is seen to be around 300–500 μm.
Transfer the cell suspension to a sterile reservoir and, using a multichannel pipette, dispense 200 μL/well into ultra-low attachment (ULA) 96-well round-bottom plates.
Transfer the plates to an incubator (37 °C, 5% CO2, 95% humidity).
Visually confirm tumor spheroid formation after 3–4 days and proceed with the 3D invasion assay.
3.4.1. Preparation of BMM
Thaw BMM on ice.
Allow sterile filter tips to be on ice overnight.
Place the 4-day old spheres from the 96-well plates on ice.
Gently remove 100 μL old media using a multichannel pipette.
sure to angle the tips in such a way so as to not disrupt the spheroid and/or create bubbles.
Using the cold pipette tips, transfer BMM to ice cold tubes (see Note 6).
Gently dispense 100 μL of BMM into the U-bottom 96-well plate aiming the tip toward the inside of the well to avoid spheroid disturbance.
Repeat all steps for all groups and allow for at least n = 5 replicates.
To remove any bubble formations, use a sterile needle to remove or pop them.
Using a microscope, visually check the position of the spheroid as it must be positioned in the middle of the well (see Note 7).
After visualizing spheroid and confirming central position, transfer the plate to incubator and allow BMM extract to solidify.
After 1 h, gently add 100 μL of growth medium. At this point, you can add cytokines/drugs/growth medium/etc. for screening and testing reagents.
3.4.2. Image Acquisition and Analysis
Use an inverted microscope at 10 × objective (or 4 × if invaded area is too large).
Record spheroid image per well at intervals starting from t = 0 up to t = 96 h.
Save each image acquired, individually.
Open stage graticule image on your software analysis of choice.
Input measurements: units/objective.
For calibration, take images of a stage graticule (1 mm) using both 10 × and 4 × objectives (and save images).
Measure the area of spheroid covered by outlining the distance from t = 0 origin to your endpoint.
Export measurements of different parameters (area, diameter, perimeter, etc.) to a spreadsheet. Record on the spreadsheet the relevant image information (e.g., well number, relative time point).
Plot the mean area of replicate spheroids (invasion) versus time using scientific graphing and statistical software. Alternatively, calculate the change in spheroid area at each time point, relative to the area at t = 0. Then plot invasion (% t0) versus time as a linear graph.
4. Notes
Depending on your A: B dilution, you may want to optimize parameters to determine if migratory and invasive capacity is enough to augment an overnight migration. It is also acceptable to include a serum gradient to potentiate migration by adding 2–5% FBS/FCS.
The Calcein AM solution is not removed from the lower chamber before reading fluorescence because it is a non-fluorescent vital dye that is converted into green fluorescent calcein by cytosolic esterases—although it is recommended.
The Fluoroblok chambers effectively block the passage of light from 490–700 nm at >99% efficiently, labeled cells that have not invaded do not have to be removed such as the earlier Boyden chamber described in this chapter. The cells that have invaded through the matrix and are found at the bottom of the membrane are no longer shielded from the light source and are thus detected.
Slowly place the inserts into each well to avoid bubble formation.
The media needs to be changed using autoclave Pasteur pipettes attached to a vacuum system. It is recommended refilling each insert with 1 mL of organotypic media.
It is recommended for your tested cell line to use growth factor to stimulate invasion as you would use a serum gradient to stimulate migration.
If spheroid is not found in middle, then centrifuge the plate at 300 × g for 3–5 min at 4°°C to allow for central positioning of spheroids.
Acknowledgments
AQH was supported by the Mayo Clinic Professorship and a Clinician Investigator award as well as the NIH (R43CA221490, R01CA200399, R01CA183827, R01CA195503, R01CA216855). MLV was supported by CONACYT and PECEM from the National Autonomous University of Mexico. We would like to thank Hugo Guerrero-Cazares for his contribution to figures in this chapter.
References
- 1.Nina Kramera AW, Ungera C, Rosnera M, Krupitzab G, Hengstschlägera M, Dolznig H (2012) In vitro cell migration and invasion assays. Mutat Res 752:10–24 [DOI] [PubMed] [Google Scholar]
- 2.Smith CL, Kilic O, Schiapparelli P, Guerrero-Cazares H, Kim DH, Sedora-Roman NI et al. (2016) Migration phenotype of brain-Cancer cells predicts patient outcomes. Cell Rep 15 (12):2616–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 728(1–2):23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith CL, Chaichana KL, Lee YM, Lin B, Stanko KM, O’Donnell T et al. (2015) Pre-exposure of human adipose mesenchymal stem cells to soluble factors enhances their homing to brain cancer. Stem Cells Transl Med 4 (3):239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbadi S, Rodarte JJ, Abutaleb A, Lavell E, Smith CL, Ruff W et al. (2014) Glucose-6-phosphatase is a key metabolic regulator of glioblastoma invasion. Mol Cancer Res 12 (11):1547–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]