Abstract
Background:
There is strong biologic plausibility to support change in albuminuria as a surrogate endpoint for progression of chronic kidney disease (CKD), but empirical evidence to supports its validity in epidemiologic studies is lacking.
Methods:
We analyzed 28 cohorts including 693,816 individuals (80% with diabetes) and 7,461 end-stage kidney disease (ESKD) events, defined as initiation of kidney replacement therapy. Percent change in albuminuria was quantified during a baseline period of 1, 2 and 3 years using linear regression. Associations with subsequent ESKD were quantified using Cox regression in each cohort, followed by random-effects meta-analysis. Further adjustment for regression dilution was used to take into account the high variability of albuminuria.
Findings:
Change in urine albumin-to-creatinine ratio (ACR) was consistently related to subsequent risk of ESKD. The adjusted hazard ratio of ESKD following a 30% decrease in ACR during a 2-year baseline period was 0.83 (95% CI 0.74–0.94); after further adjustment for regression dilution it was 0.78 (95% CI 0.66–0.92). Adjusted hazard ratios were relatively consistent across cohorts and subgroups but were somewhat stronger at higher ACR (p-interaction<0.05). In a group of persons with baseline ACR >30 mg/g, an average reduction in ACR of 30% over 2-years was estimated to confer >1% absolute reduction in 10-year ESKD risk, even at earlier stages of CKD. Results were generally similar when using change in urine protein-to-creatinine ratio (PCR), or when study populations from clinical trials were evaluated. Limitations include potential residual confounding, modeling assumptions, and heterogeneity.
Interpretation:
Change in albuminuria was consistently associated with subsequent risk of ESKD across a wide range of cohorts, lending support to using average change in albuminuria as a surrogate endpoint in clinical trials of CKD progression in the setting of elevated albuminuria.
Funding:
US National Kidney Foundation, National Institute of Diabetes and Digestive and Kidney Diseases.
Introduction:
A recent scientific workshop sponsored by the US National Kidney Foundation, in collaboration with the US Food and Drug Administration and European Medicines Agency (NKF-FDA-EMA) Workshop evaluated candidate surrogate endpoints for clinical trials of drugs to slow kidney disease progression, particularly among participants with early stages of chronic kidney disease (CKD). Change in albuminuria has been used for decades in some clinical trials for developing therapies to slow progression of CKD.1,2 However, substantial controversy exists about whether change in albuminuria meets the criteria for a surrogate outcome for end-stage kidney disease (ESKD) risk in phase 3 clinical trials. Criteria for surrogacy include biological plausibility, correlation with clinical trial estimates of clinical endpoints and observation of consistent risk associations with clinical endpoints across a wide range of settings.3 This study focuses on the latter aspect by examining the relationship of change in albuminuria to ESKD risk in a large individual-level meta-analysis. This complements parallel investigations in clinical trials of whether the effect of interventions on albuminuria agrees with their effects on ESKD risk.4,5
Previous meta-analyses of clinical trials conflict with respect to the strength of associations between change in albuminuria and risk of ESKD,4,6,7 leading to controversy8–11. Noted limitations leading to the aforementioned controversy were selected populations, small sample size, and relatively short duration of follow up. Observational studies can overcome these limitations. Published analyses of single cohorts have generally showed change in albuminuria was related to ESKD12–15 and cardiovascular16 risk, but had limited power to perform subgroup analyses and to investigate linearity and interactions. Only one study adjusted for imprecision in estimation of change in albuminuria. Imprecision in estimating albuminuria at the individual level is substantial17 and is therefore of particular importance because it leads to regression dilution which weakens empirical associations of changes in albuminuria with risk. In contrast, clinical trials compare groups of individuals and how average albuminuria reduction is related to risk.
The current investigation is published together with a companion meta-analysis of clinical trials to provide complementary approaches evaluating the association of albuminuria change to subsequent ESKD risk5. The clinical trial manuscript examines randomized effects at the trial level. The current investigation uses a global collaboration to precisely and rigorously model the individual level observational association. To assess consistency and generalizability, we examine the associations across a wide range of settings, albuminuria levels, diabetes, hypertension and antihypertensive medication use. We adjust for regression dilution to better estimate the association between changes in albuminuria and ESKD risk, focusing on changes that are of comparable magnitude to those observed in clinical trials. Finally, we estimate attributable risks of albuminuria reduction on ESKD for up to a decade of follow-up. Long follow-up is important for understanding the full implications of treatments evaluated in shorter term clinical trials.
Methods
Study selection criteria
Details of the CKD Prognosis Consortium (CKD-PC) are described elsewhere and in Appendices 1–2.18–22 Briefly, CKD-PC consists of more than 70 cohorts with at least 1,000 participants (not applied to cohorts predominantly enrolling persons with CKD) across over 40 countries, with data on serum creatinine and albuminuria, and with 50 or more events on outcomes of interest (either mortality or kidney outcome).18–22 CKD-PC is open to new cohorts and invitation to join was widely advertised in preparation for the NKF-FDA-EMA workshop. All cohorts with appropriate data, a repeated measure of albuminuria during an elapsed period of 8 months - 4 years and subsequent ESKD or mortality follow-up active during this phase opted into this study. We included 28 cohorts with 20 having follow-up on ESKD and 27 on mortality. Each meta-analysis for the present study was restricted to cohorts with participants aged ≥18 years. Data transfer and analysis took place between July 2015 and June 2018. To ensure that the results are consistent with participants enrolled in clinical trials, we performed the same analysis in 14 clinical trials included in the clinical trials database of CKD-EPI, including 9 clinical trials not included in CKD-PC.4 This study was approved by the institutional review board for use of de-identified data at the Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland, USA).
Procedures
Studies that measured albuminuria (measured as urine albumin-to-creatinine ratio [ACR] or urine albumin excretion rate [AER] converted to ACR) and proteinuria (measured as urine protein-to-creatinine ratio [PCR] or urine protein excretion rate [PER] converted to PCR) were analyzed separately (see supplement for details).23 We examined changes in albuminuria on the log scale to focus on relative changes, normalize the distribution and enable analysis of change across a wide range of baseline albuminuria levels (e.g. 30% decline is possible for all levels while a 300 mg/g decrease is only possible above this level of albuminuria). We expressed albuminuria changes as percent change; a change in albuminuria of +/−0.515 on the log (base2) scale corresponds to a 30% decrease and 43% increase in albuminuria (these percent changes are symmetric relative changes with 1/0.70=1.43). As the implications for the magnitude of change in albuminuria may vary depending on the time in which the change is observed, we defined three baseline periods (1, 2, and 3 years) to determine the change in albuminuria and repeated the analysis for each baseline period. For each baseline period, one-third of the total length of time was allowed as a window for determining the last available albuminuria to calculate the change, and the albuminuria closest to the end of the period of interest was selected for each participant. In the analysis of clinical trials in the CKD Epidemiology Collaboration (CKD-EPI), we defined a 0.5 year baseline period in addition to the 1-, 2-, and 3-year analyses, and we used +/− 3 months as the window for measurement. All covariates were assessed at the time of first albuminuria and all albuminuria measures within the baseline were used (Appendix shows details for methods).
eGFR was calculated using the CKD-EPI 2009 creatinine equation.22,24 In cohorts where the creatinine measurement was not standardized to isotope dilution mass spectrometry (IDMS), creatinine concentrations were reduced by 5%, the established calibration factor, and drift over time was corrected when possible.25 We defined diabetes as fasting glucose ≥7.0 mmol/L (126 mg/dl), non-fasting glucose ≥11.1 mmol/L (200 mg/dl), hemoglobin A1c ≥6.5%, use of glucose lowering drugs, or self-reported diabetes. Participants with a history of myocardial infarction, coronary revascularization, heart failure, or stroke were considered to have a history of cardiovascular disease (CVD). Smoking status was classified as never vs. former, or current smoker.
The primary outcome of interest was ESKD developing after the baseline period. We defined ESKD as initiation of kidney replacement therapy. ESKD cases during the baseline period were excluded from the relevant analyses. Since patients with CKD may die without reaching ESKD, we repeated the analysis for cardiovascular and all-cause mortality as well as non-cardiovascular mortality. Cardiovascular mortality was defined as death due to myocardial infarction, heart failure, stroke, or sudden cardiac death.
Statistical analysis
We applied a two-stage analytic approach, whereby each cohort was first analyzed separately, followed by a random-effects meta-analysis. The analysis overview and analytic notes for individual studies are described in Appendix 1. We imputed missing values of diabetes, history of CVD, smoking, systolic blood pressure, and total cholesterol using cohort-specific mean values if missing values were less than 50% (see Appendix 1 for details). Variables with more than 50% missing were not included in cohort-specific adjusted models. We quantified heterogeneity with the I2 statistic and χ2 test18 and explored sources of heterogeneity with Forest plots and random-effects meta-regression analysis. We performed analyses using Stata/MP 14 and 15 software (www.stata.com). We considered 2-sided P-values <0.05 as statistically significant.
We modeled the adjusted hazard ratios (HRs) of ESKD and mortality after the end of the baseline period as a spline function of change in albuminuria adjusted for the covariates. In each study, we fitted piece-wise linear splines for change in log albuminuria (five knots were placed: 1-fold change (e.g., no change) 2-fold change (e.g., 2-times and ½-times), and 4-fold change (4- and ¼-times) with 1-fold (no change) as the reference point. We adjusted Cox models for baseline age, sex, race/ethnicity (blacks vs. non-blacks), systolic blood pressure, total cholesterol, diabetes, history of CVD, current smoking, former smoking, and first eGFR and albuminuria. We assessed potential effect modifiers, including baseline albuminuria and diabetes, by incorporating interaction terms with continuous change in log albuminuria modeled as a linear spline with one knot at zero (no change). We illustrated the opposite effects of decreasing and increasing albuminuria as symmetric points on the log scale (30% decrease and 43% increase compared to 0% change). Robustness to further adjustment for hemoglobin A1c or expanding the outcome definition to include participants reaching eGFR < 15 ml/min/1.73m2 was tested in a large cohort which provided these data.
We adjusted the hazards ratios for regression dilution by dividing the log hazard ratio by the reliability coefficient of change in albuminuria.26 We derived the reliability coefficient for each study and baseline period as the ratio of the total variance less an estimate of the error variance to the total variance for change in albuminuria. We estimated the error variance of the 1-, 2-, and 3-year change in albuminuria as twice the albuminuria error variance estimated from repeat albuminuria measures in three studies with repeated albuminuria measures (details in Table S1). The primary analysis corrected the meta-analyzed hazard ratio for the median reliability coefficient, and sensitivity analyses corrected the meta-analyzed hazard ratio for the 25th and 75th percentile estimate of the reliability coefficients from the participating cohorts.
We translated meta-analyzed adjusted HRs for change in albuminuria to absolute risk of ESKD up to 10 years after the baseline period for the following combination of covariates: 60 year old, 50% female, non-black, no change albuminuria, first eGFR of 60 ml/min/1.73m2, a systolic blood pressure of 140 mm Hg, a total cholesterol of 5.2 mmol/L, 25% diabetes, 25% CVD, and 25% current and 25% former smoking. We estimated absolute risk by first fitting competing risk models which accounted for death as a competing event within each cohort. 27 We then fit a Weibull distribution to the adjusted baseline subhazard within each cohort and averaged these across all studies. This average adjusted subhazard was combined with regression dilution-adjusted meta-analyzed hazard ratios to estimate absolute risk. We calculated risk for a 30% decline from a baseline ACR of 30, 300 and 600 mg/g and baseline PCR of 50, 500 and 1000 mg/g as well as eGFR levels of 45 and 75 ml/min/1.73 m2 (Appendix 1).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. JC, YS, KM, and MEG had full access to all the data in the study and all authors had final responsibility for the decision to submit for publication, informed by discussions with collaborators.
Results:
The study population included 28 cohorts with a total sample size of 693,816 participants in the analysis which assessed change in albuminuria over a 2-year baseline period (Table 1). Among these cohorts, 20 had follow-up for ESKD, 27 had follow-up for mortality, of which 11 had follow-up for cardiovascular mortality (Table S2). Albuminuria was quantified with ACR in 16 cohorts, PCR in 3 cohorts and both in 9 cohorts. PCR was more likely to be available in cohorts with lower mean baseline eGFR. The total number of ESKD events after the two year baseline period was 7,461 (Table S2 with Tables S3-S6 showing results for 1 and 3 year baseline periods). Mean age was 63 (cohort IQR 58–66) years. A large number of both men and women were included with a wide range of diabetes prevalence, but only a minority of the participants were black. Mean baseline eGFR was 78 ml/min/1.73m2. Baseline albuminuria varied according to baseline eGFR, reflecting cohort entry criteria. Sixteen of the cohorts had a mean eGFR higher than 60 ml/min/1.73m2 and had a low median baseline ACR (2 to 18 mg/g), while twelve of the cohorts had a mean eGFR less than 60 ml/min/1.73m2 and had a wide range of median albuminuria (18 to 1118 mg/g). The median fold change in albuminuria was close to 1.0 with IQR of 0.61 to 2.17 (Table S3 and S5 show results for shorter and longer baseline periods).
Table 1.
Baseline characteristics for cohorts with a 2-year baseline period.
| Cohort | Exposure | N | Age, years | % Female | % Black | Baseline eGFR, ml/min/1.72m2 | % DM | Baseline median ACR/PCR (IQR), mg/g | Median ACR/PCR fold change (IQR) |
|---|---|---|---|---|---|---|---|---|---|
| AASK | PCR* | 898 | 55 (11) | 39% | 100% | 47 (14) | 0% | 72 (28–285) | 1.19 (0.63–2.15) |
| ADVANCE | ACR | 9383 | 66 (6) | 43% | 0.33% | 78 (17) | 100% | 14 (7–38) | 1.01 (0.54–1.92) |
| BC CKD | ACR/PCR | 7855 | 70 (13) | 46% | 0% | 34 (15) | 51% | 103 (25–545) | 1.17 (0.63–2.33) |
| CanPREDDICT | ACR | 682 | 68 (12) | 37% | 1.2% | 28 (9) | 49% | 142 (28–616) | 1.04 (0.52–2.05) |
| CCF | ACR | 1739 | 71 (10) | 54% | 15% | 49 (11) | 85% | 19 (7–66) | 1.18 (0.62–2.15) |
| CPRD | ACR | 90172 | 63 (12) | 43% | 0% | 73 (21) | 95% | 10 (5–26) | 1.02 (0.60–1.95) |
| CRIC | PCR* | 2774 | 58 (11) | 45% | 40% | 45 (15) | 46% | 126 (54–602) | 1.08 (0.59–1.88) |
| Framingham | ACR | 893 | 60 (10) | 55% | 0% | 84 (19) | 9.2% | 7 (3–16) | 0.93 (0.52–2.04) |
| Geisinger | ACR/PCR | 26594 | 62 (14) | 50% | 2.3% | 83 (23) | 79% | 14 (6–41) | 1.07 (0.53–2.17) |
| GLOMMS 2 | ACR/PCR | 5953 | 66 (13) | 50% | 0% | 68 (20) | 8.0% | 9 (8–35) | 1.00 (0.87–1.83) |
| Maccabi | ACR | 117414 | 60 (13) | 48% | 0% | 81 (20) | 83% | 5 (5–20) | 1.00 (0.86–1.31) |
| MASTERPLAN | PCR* | 408 | 60 (13) | 31% | 0% | 37 (15) | 23% | 260 (98–840) | 1.00 (0.62–1.78) |
| MDRD | PCR* | 682 | 52 (12) | 39% | 6.7% | 36 (13) | 8.4% | 230 (60–1100) | 1.12 (0.63–1.93) |
| Mt Sinai BioMe | ACR/PCR | 2895 | 58 (13) | 63% | 33% | 76 (26) | 71% | 12 (5–55) | 1.10 (0.51–2.43) |
| NephroTest | PCR*/ACR* | 783 | 58 (15) | 33% | 13% | 44 (20) | 30% | 236 (110–720) | 1.06 (0.64–1.71) |
| NZDCS | ACR | 8698 | 61 (13) | 51% | 0.092% | 77 (22) | 100% | 2 (1–6) | 1.00 (0.42–2.37) |
| Optum/AMGA | ACR | 81653 | 63 (13) | 46% | 4.3% | 78 (24) | 78% | 14 (7–42) | 1.18 (0.67–2.13) |
| Pima | ACR/PCR | 2720 | 34 (14) | 62% | 0% | 120 (17) | 30% | 12 (7–24) | 1.09 (0.68–1.83) |
| PREVEND | ACR | 4941 | 54 (12) | 49% | 0.87% | 93 (15) | 6.6% | 7 (5–12) | 1.09 (0.87–1.35) |
| PSP-CKD | ACR/PCR | 3598 | 75 (10) | 53% | 0.94% | 49 (12) | 42% | 18 (11–39) | 1.04 (0.75–2.29) |
| Rancho Bernardo | ACR | 369 | 73 (9) | 60% | 0.27% | 70 (17) | 16% | 11 (7–18) | 1.27 (0.83–2.12) |
| RCAV | ACR | 301816 | 64 (10) | 3% | 17% | 79 (17) | 83% | 11 (5–33) | 1.12 (0.61–2.17) |
| RENAAL | ACR* | 1243 | 60 (7) | 36% | 15% | 40 (13) | 100% | 1118 (503–2203) | 0.85 (0.42–1.57) |
| SCREAM | ACR | 17811 | 53 (13) | 40% | 0% | 84 (27) | 46% | 18 (7–73) | 1.04 (0.55–1.97) |
| SRR-CKD | ACR | 420 | 65 (14) | 32% | 0% | 23 (7) | 35% | 117 (24–472) | 1.05 (0.48–2.95) |
| Sunnybrook | PCR/ACR | 1003 | 59 (17) | 42% | 0% | 59 (31) | 37% | 490 (175–1240) | 0.83 (0.42–1.55) |
| Takahata | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ZODIAC | ACR | 419 | 68 (10) | 58% | 0% | 68 (16) | 100% | 2 (1–5) | 1.22 (0.68–2.47) |
| Total | 693816 | 63 (12) | 25% | 9.1% | 78 (21) | 80% | 11 (5–33) | 1.12 (0.61–2.17) |
ACR: urine albumin-to-creatinine ratio; PCR: urine protein-to-creatinine ratio; DM: diabetes mellitus; IQR: interquartile range.
If both ACR and PCR are included, the first listed in the column is the larger sample size for this baseline period. All characteristics listed are for the larger sample.
Albuminuria is based on 24-hour urine in these studies as albumin excretion rate (AER) rather than ACR and protein excretion rate (PER) rather than PCR.
% Black is tabulated for use in categorization of race in the CKD-EPI eGFR equation. Publications from the individual cohorts describe race/ethnicity and geography in more detail.
Total number of ESKD, cardiovascular mortality, and all-cause mortality events are 7,461, 3,443, and 75,761 among 20, 11, and 25 cohorts. Table S2 provides events counts and additional covariates (1-year and 3-year baseline window data are detailed in Tables S4 and S6).
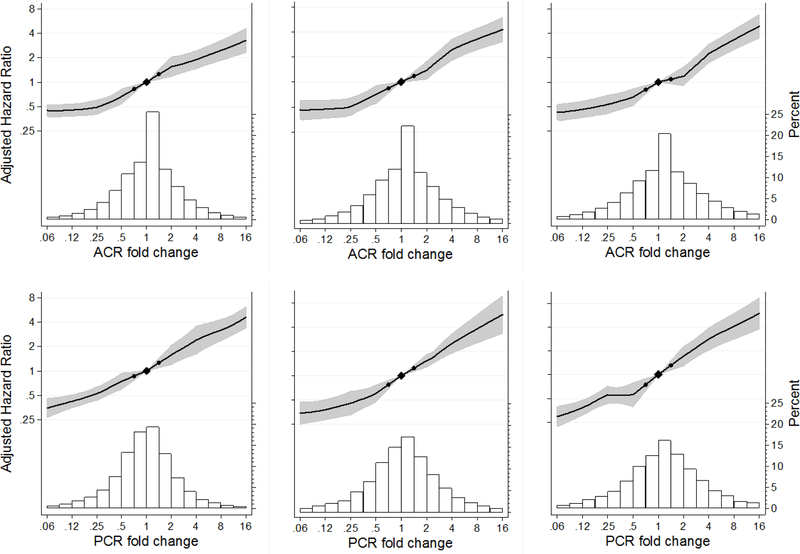
The adjusted hazard ratio of ESKD increased approximately linearly with the change in ACR and PCR on the log scale for 1, 2 and 3 year baseline periods (Figure 1). The 95% confidence intervals in all panels were narrow, such that even relatively small changes in albuminuria were significantly associated with the risk of ESKD. The relative hazard of ESKD following a 2-year 30% reduction in ACR was 0.83 (95% CI 0.74–0.94) after adjustment for age, sex, race/ethnicity (blacks vs. non-blacks), systolic blood pressure, total cholesterol, diabetes, history of CVD, current smoking, former smoking, and first eGFR and albuminuria (Table 2). The reliability of albuminuria change in the median cohort was 0.721 for the 2 year baseline period (detailed by cohort in Table S7). Using this reliability coefficient to adjust for regression dilution strengthened the relative hazard to 0.78 (95% CI 0.66–0.92). In analyses using reliability coefficients from the cohort in the 25th and 75th percentile, the corresponding hazard ratios were 0.76 (95% CI 0.63–0.91) and 0.80 (95% CI0.70–0.93). The reliability coefficients were higher at longer follow-up, indicating more precision in estimate of change in albuminuria, and were similar for ACR and PCR changes. Adjusted for regression dilution and baseline variables, the association of 30% reduction in albuminuria with ESKD was generally similar across different duration of the baseline period and measurement with ACR or PCR, although risk reduction was somewhat stronger for PCR studies with more than one year of follow-up (Table 2).
Figure 1.
Adjusted hazard ratio of ESKD and population distribution of change in albuminuria. Top row ACR, bottom row PCR, left to right 1, 2, and 3-year baseline period. Two-year albuminuria change is used for the primary analysis with 7,461 ESKD cases among 675,904 individuals in 20 cohorts. Black circles denote −30% and +43% change in albuminuria.
Table 2.
Hazard Ratio of ESKD with 30% ACR & PCR Reduction – Meta-Analysis Before and after Adjustment for Measurement Error
| Change Period | Hazard Ratio** HR (95% CI) | Regression Dilution adjusted HR estimates (95% CI)* | ||
|---|---|---|---|---|
| Assuming median reliability | Assuming low reliability | Assuming high reliability | ||
| ACR | ||||
| 1-year | 0.82 (0.74–0.91) | 0.75 (0.64–0.87) | 0.70 (0.58–0.85) | 0.78 (0.68–0.89) |
| 2-year | 0.83 (0.74–0.94) | 0.78 (0.66–0.92) | 0.76 (0.63–0.91) | 0.80 (0.69–0.93) |
| 3-year | 0.80 (0.71–0.90) | 0.76 (0.65–0.87) | 0.73 (0.62–0.86) | 0.77 (0.67–0.88) |
| PCR | ||||
| 1-year | 0.86 (0.76–0.97) | 0.80 (0.67–0.95) | 0.76 (0.61–0.94) | 0.82 (0.70–0.96) |
| 2-year | 0.77 (0.68–0.87) | 0.69 (0.58–0.83) | 0.67 (0.55–0.81) | 0.72 (0.62–0.84) |
| 3-year | 0.74 (0.61–0.89) | 0.68 (0.54–0.86) | 0.65 (0.50–0.84) | 0.70 (0.56–0.87) |
Based on estimates for ACR and PCR in 19 studies. Reliability estimates (λ) for 1, 2 and 3 year change had a median (IQR 25th to 75th percentile) of 0.677 (0.533–0.770), 0.721 (0.650–0.808) and 0.789 (0.713–0.852). The same reliability estimates were used for ACR and PCR.
Adjusted for age, sex, race/ethnicity (blacks vs. non-blacks), systolic blood pressure, total cholesterol, diabetes, history of cardiovascular disease, current smoking, former smoking, and first eGFR and albuminuria.
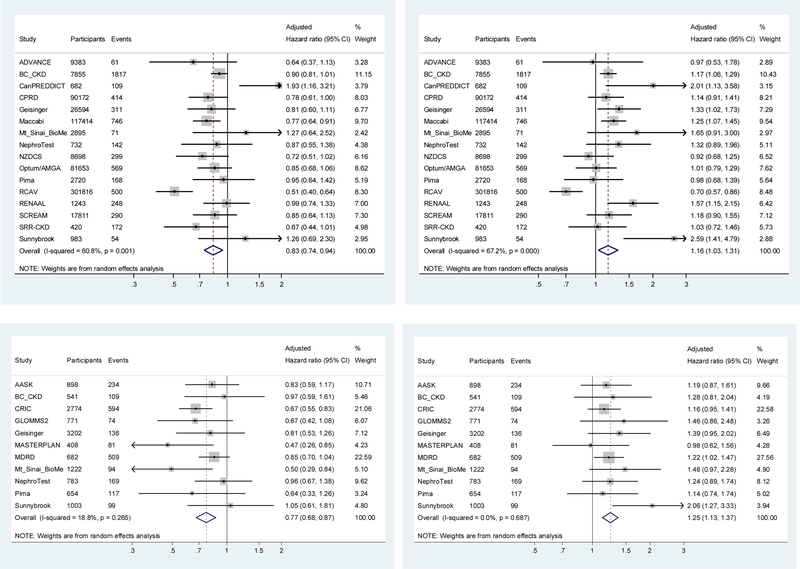
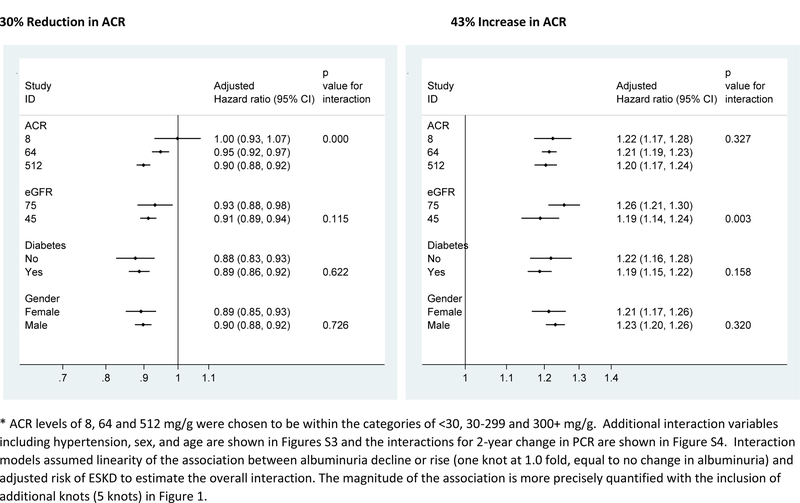
The associations between albuminuria change and ESKD in individual cohorts were consistent with the overall meta-analysis result in the majority of cohorts (Figure 2 and Figures S1-S2). The hazard ratio of ESKD associated with a 43% increase in albuminuria, measured using ACR or PCR, was opposite in direction but similar in magnitude to that of a 30% decrease in albuminuria. None of the covariates explained the between study variation in meta-regression. Interaction analysis showed that most associations between change in albuminuria and ESKD were similar across levels of other baseline covariates, including sex and, in the studies with available data, change in key covariates (e.g. use of renin-angiotensin-aldosterone system inhibitor use; see Figure 3 and Figures S3-S4). A notable exception was baseline albuminuria, where a 30% decrease in ACR was associated with a stronger adjusted relative hazard reduction in ESKD in higher compared to lower levels (p-interaction < 0.001 for ACR and 0.006 for PCR; detailed subgroup analyses are shown in Figures S5-S10). There was no interaction of baseline albuminuria and increase in albuminuria with ESKD (Figure 3). Other possible interactions with change in albuminuria for ESKD included a stronger association of decrease in ACR with stable blood pressure, increase in ACR with younger age, and increase in ACR with decrease in systolic blood pressure, and a weaker association of decrease in ACR in blacks. However, none of the p-values was significant after adjustment for multiple comparisons (Figure 3 and Figure S3; similarly trends were seen for decrease in PCR possibly interacting modestly with sex, race and decrease in blood pressure, Figure S4).
Figure 2.
Forest plot showing the individual study and meta-analyzed estimate of adjusted hazard ratio of ESKD associated with 2-year ACR change (top row) and PCR change (bottom row) for a 30% reduction (left side) 43% increase (right side)
Figure 3.
Interaction analysis of the adjusted hazard ratio of ESKD associated with 2-year change in ACR by baseline level of ACR, eGFR, and diabetes.*
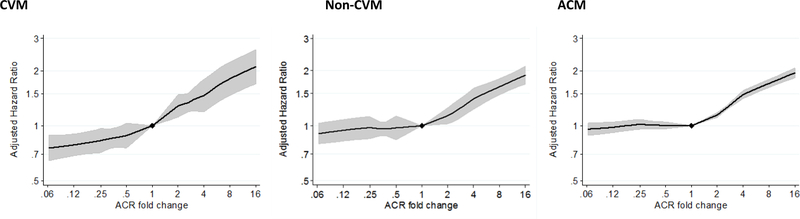
All-cause mortality and cardiovascular mortality were each related to change in ACR and PCR, but the relationships were weaker than for ESKD and in some cases non-linear (Figure 4 and Figure S11-S15). Specifically, ACR decrease and some PCR decrease segments were more weakly related than most corresponding increases in ACR or PCR during 1, 2 and 3 years of follow-up. A 43% increase in ACR was associated with an adjusted hazard ratio of cardiovascular mortality of 1.14 (1.06–1.22) while a 30% decrease has a weaker hazard ratio of 0.94 (0.87–1.02). Associations for non-cardiovascular mortality were weaker. While a 30% decrease in ACR was not significantly associated with all-cause mortality, a 43% increase in ACR over 2-years had an adjusted hazard ratio of mortality of 1.07 (1.05–1.09) with the corresponding adjusted hazard ratio for PCR weaker at 2-years (0.99 (0.88–1.11) but stronger at 1 and 3 years (1.09 (1.01–1.20) and 1.15 (0.99–1.33), correspondingly).
Figure 4.
Adjusted hazard ratio of cardiovascular (CVM), non-cardiovascular (Non-CVM) and all-cause mortality by 2-year change in albuminuria (ACR). 3,443 CVM, 13,175 non-CVM and 75,761 ACM cases among 125,000, 125,000 and 690,513 individuals in 11, 11 and 25 cohorts.
Nine of the 13 clinical trials in the CKD-EPI, comprising 11,272 participants, were not included in the CKD-PC analysis reported above (Table S8). Analysis of CKD-EPI trials showed that change in ACR had a strong linear association (on the log scale) with ESKD, overall and when limited to the nine trials not included in the main analysis (Figures S16-S17). The adjusted relative hazard of ESKD after a 2-year baseline period was 0.83 (95% CI 0.69–1.00), and 0.79 (0.63–1.00) after adjustment for regression dilution (Table S9-S10). Associations were even strong over shorter periods of time and for PCR. Changes in albuminuria across different baseline periods were moderately correlated (Table S11).
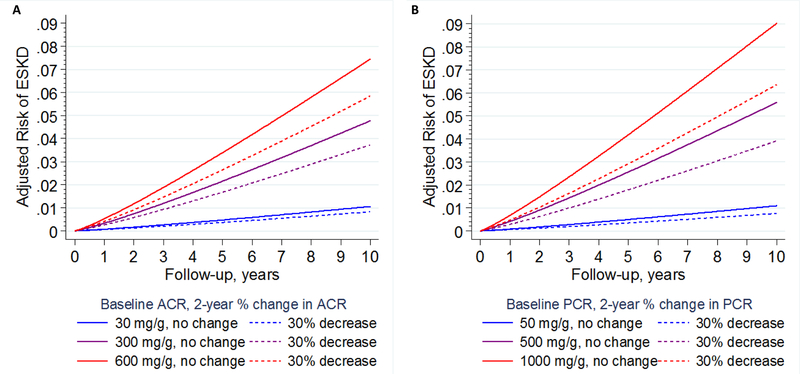
Sensitivity analysis in a cohort which had the relevant data showed that further adjustment for hemoglobin A1c or expanding the outcome definition to include participants reaching eGFR < 15 ml/min/1.73m2 or ESKD did not materially change the result (Figures S18-S19)._ Absolute risk of ESKD was calculated for up to 10 years of follow-up at different levels of baseline albuminuria and proteinuria (Figure 5). The apparent impact of a 30% reduction in albuminuria was accentuated with longer follow-up time and exceeded 1% at 10 years if baseline albuminuria was high (e.g., ACR 300 mg/g or PCR 500 mg/g).
Figure 5.
Risk of ESKD by years of follow-up for three levels of baseline albuminuria (ACR, panel A; PCR, panel B) and a 30% decline in albuminuria (dashed lines). Differences between lines with the same level of albuminuria indicate attributable risk for change in albuminuria adjusted for regression dilution. Analysis includes the competing risk of mortality with baseline subhazards meta-analyzed in 18 cohorts including 6,799 ESKD events and 584,489 individuals.
Discussion:
Change in albuminuria was strongly and robustly associated with subsequent risk of ESKD across a wide range of settings showing the value of repeat testing of albuminuria despite some guidelines recommendations of initial but not follow-up testing for albuminuria. The adjusted hazard ratio of ESKD following a 30% decrease in ACR during a 2-year baseline period was 0.83 (95% CI 0.74–0.94); after further adjustment for regression dilution it was 0.78 (95% CI 0.66–0.92). This 22% ESKD risk reduction was consistent whether albuminuria was quantified using albumin or protein and whether change was measured over one, two, or three years (20–32% across the different analyses), thus extending and expanding upon results from previous studies of single cohorts.12–15,28,29 Our results suggest a greater relative and absolute risk reduction at higher level of albuminuria, but no difference with use of renin-angiotensin aldosterone inhibitors. Even at a constant relative hazard, the absolute reduction in ESKD was higher at higher risk and greater than 1% at ACR >300 mg/g after ten years of follow-up. These analyses substantially advance our understanding of quantitative associations of early average change in albuminuria with the risk of the clinical endpoint of ESKD and their application as a surrogate endpoint to groups of individuals in clinical trials of limited duration.
The generalizability of our results is supported by consistency of the association across 28 cohorts, multiple subgroups, and observational analysis of 9 additional clinical trials. Previous observational analyses of albuminuria change and risk found increased risk of ESKD12–15,30 and cardiovascular mortality15,16 as well. These published associations are generally consistent with our findings but often focused on the risk implications of larger changes in albuminuria, since single cohorts have more limited power and poorer precision. Despite consistent association between change in albuminuria and ESKD incidence, the two are not equivalent, as one-third to one-half of ESKD cases developed without any increase in albuminuria during the baseline period, often when the baseline albuminuria was already high.
Average change in albuminuria as an endpoint in clinical trials overcomes the imprecision of its estimation at the individual level as a result of substantial biological variation and laboratory measurement error. Change in albuminuria has a (√2-times) greater coefficient of variation than albuminuria measured at a single visit; coefficients of variation for ACR in 24-hour urine AER collections, spot urine samples from first morning voids, and random spot urines have been estimated at 19%, 19% and 36%, respectively.17 Our updated estimates, which also include 8505 observations from the ALTITUDE trial where albuminuria was measured on three consecutive days at multiple follow-up study visits, show coefficients of variation of approximately 35% and 54% for first morning void and random urine albuminuria changes. This level of imprecision suggests that even without intervention, the inter-quartile range of change in albuminuria would be 20% decrease to 26% increase for a first-morning specimen, and 39% decrease to 65% increase for a random specimen, making it difficult to estimate the true treatment effects and risk associations at the individual level without correction for random variation. However, corrected for regression dilution, we found that even modest changes in the true albuminuria level are associated reliably with meaningful changes in subsequent risk of ESKD. The only other study to adjust for regression dilution, the ADVANCE-ON extended follow-up of a diabetes clinical trial, found similar strengthening of associations.30 The 20–32% reduction in ESKD risk we observed for a 30% reduction in albuminuria adjusted for regression dilution is similar in magnitude to the ~30% ESKD reduction associated with a 30% reduction in albuminuria found in a meta-regression of clinical trials in a companion manuscript.5 The companion meta-regression also quantifies the precision with which average changes in albuminuria can predict changes in ESKD risk in a trial and suggests that a minimum threshold average reduction of greater than 20–30% in albuminuria is needed to infer a clinical benefit. Both papers indicate change in albuminuria in more informative as a surrogate of ESKD at higher level of baseline albuminuria because of higher absolute risk reductions.
Absolute risks estimates may not generalize well across settings. However, the general trends and magnitude demonstrated here provide useful guidelines. As expected, reduction in ESKD risk from reduction in albuminuria increased markedly with longer follow-up time. Observational studies extend the relatively short period of follow-up in trials to suggest that after a decade of follow-up, the benefits associated with albuminuria reduction would be substantial, particularly for higher risk individuals. Risk itself is also strongly influenced by the baseline level of albuminuria and eGFR, as published previously.31 As a result, patients with high albuminuria can benefit substantially from albuminuria reduction, even if they have higher baseline eGFR and a lower short-term (but higher long-term) ESKD risk.
Our observational associations have a number of limitations. We cannot rule out residual confounding despite the temporal design where ESKD risk is observed after the change in albuminuria. Untreated cases of ESKD were not captured, representation of non-white groups is limited, and sex as a biological variable was not explicitly addressed. There is unexplained heterogeneity across studies in both methods and results despite a uniform analysis. Measurements of albuminuria were not standardized across cohorts, and reasons for changes in albuminuria are mostly unknown. Reliability of change in albuminuria used a median estimate across studies, and the residual error variance was based on three cohorts. The length of follow-up for events varied across cohorts. Results were not adjusted for diabetes control, the full range of concomitant diseases, or other risk factors after the baseline period, including subsequent changes in albuminuria. Interaction tests assumed linearity. This study also has significant strengths including the wide range of cohorts and large sample size, allowing investigation of various subgroups, adjustment for regression dilution and large number of sensitivity analyses. Relative changes in albuminuria can be applied to the full range of albuminuria and use each person as their own control, facilitating comparisons across groups which may differ in creatinine excretion such as men and women.
The observational association of change in albuminuria with subsequent risk of ESKD was consistent across a wide range of cohorts and subgroups. Even modest true changes in albuminuria such as a 30% decrease, associated with a significant reduction of ESKD risk when baseline albuminuria was elevated. This observational analysis provides a complementary line of evidence to intention to treat analyses in clinical trials.4,5 The latter are less susceptible to bias, but are also less precise, with our observational analyses representing larger and more heterogeneous groups. Taken together, the results from these two very different analyses and populations agree both qualitatively and quantitatively after adjustment for regression dilution. They support a role for average change in albuminuria as a surrogate endpoint for CKD progression, particularly in patients with higher baseline albuminuria.
Research in context
Evidence before this study
Change in albuminuria has biologic plausibility as a surrogate endpoint for kidney disease progression in clinical trials, and implementing therapies to slow progression of CKD. However, a review of the published data and discussions with experts showed substantial controversy about the extent to which change in albuminuria meets the criteria for a surrogate outcome for end-stage kidney disease (ESKD) risk. Two previous meta-analyses of clinical trials conflicted with respect to the strength of associations between change in albuminuria and risk of ESKD. Noted limitations were selected populations, small sample size, and relatively short duration of follow up. Observational studies can overcome these limitations. Published analyses of single cohorts have generally showed that change in albuminuria was related to ESKD and cardiovascular risk, but had limited power to perform subgroup analyses and to investigate linearity and interactions. The US National Kidney Foundation, in collaboration with the US Food and Drug Administration and European Medicines Agency sponsored a workshop to evaluated candidate surrogate endpoints for clinical trials of drugs to slow kidney disease progression, particularly among participants with early stages of chronic kidney disease (CKD). Planning began in 2015, cohorts were invited to contribute data in June 2015, detailed analyses with feedback from stakeholders were conducted subsequently and a workshop discussing the preliminary results was held in March 2018.
Added value of this study
This study provides a comprehensive individual-level meta-analysis of the association between change in albuminuria with subsequent risk of ESKD. A companion paper conducts a Bayesian summary of intention to treat analyses in 41 clinical trials of whether the effect of interventions on albuminuria agrees with their effects on ESKD risk. We analyzed 28 cohorts including 693,816 individuals and 7,461 end-stage kidney disease (ESKD) events. Change in urine albumin-to-creatinine ratio (ACR) was consistently related to subsequent risk of ESKD. The adjusted hazard ratio of ESKD following a 30% decrease in ACR during a 2-year baseline period was 0.83 (95% CI 0.74–0.94); after further adjustment for regression dilution it was 0.78 (95% CI 0.66–0.92). Adjusted hazard ratios were relatively consistent across cohorts and subgroups but were somewhat stronger at higher ACR. Among persons with ACR >30 mg/g, a reduction in ACR of 30% over 2-years was estimated to confer >1% absolute reduction in 10-year ESKD risk even at earlier stages of CKD with higher risks at later stages. Results were generally similar when using change in urine protein-to-creatinine ratio (PCR).
Implications of all the available evidence
Change in albuminuria is consistently associated with subsequent risk of ESKD across a wide range of cohorts and subgroups, lending support to use of average change in albuminuria as a surrogate endpoint for clinical trials including in early CKD. The magnitude of ESKD risk is related to the percent change in albuminuria, the baseline level of albuminuria and ESKD risk and the duration of follow up. Changes in albuminuria over a relatively short time period provide information about longer term risk of ESKD. Combined with data from clinical trials, simulations and biologic plausibility, these data provide a foundation for how to use average change in albuminuria as a surrogate endpoint for CKD progression.
Supplementary Material
Acknowledgements
CKD-PC investigators/collaborators (study acronyms/abbreviations are listed in appendix 2 in the Supplement):
AASK: Brad Astor, Larry Appel, Tom Greene, Teresa Chen; ADVANCE: John Chalmers, Mark Woodward, Hisatomi Arima, Vlado Perkovic; BC CKD: Adeera Levin, Ognjenka Djurdjev; CanPREDDICT: Adeera Levin, Ognjenka Djurdjev, Mila Tang; CCF: Joseph Nally, Sankar Navaneethan and Jesse Schold; CPRD: Misghina Weldegiorgis, William Herrington, Margaret Smith, Mark Woodward; CRIC: Harold Feldman, Yenchih Hsu; Framingham: Caroline Fox, Shih-Jen Hwang; Geisinger: Alex R. Chang, H. Lester Kirchner, Jamie Green, Kevin Ho; GLOMMS 2: Corri Black, Angharad Marks, Gordon Prescott, Laura Clark, Nick Fluck; Maccabi: Varda Shalev, Gabriel Chodick; MASTERPLAN: Jack Wetzels, Peter Blankestijn, Arjan van Zuilen, Jan van den Brand; MDRD: Mark Sarnak, Lesley Inker; Mt Sinai BioMe: Erwin Bottinger, Girish N Nadkarni, Stephen B Ellis, Rajiv Nadukuru; NephroTest: Benedicte Stengel, Marie Metzger, Martin Flamant, Pascal Houillier, Jean-Philippe Haymann, Marc Froissart; NZDCS: Timothy Kenealy, C Raina Elley, John F Collins, Paul L Drury; Optum/AMGA: Nikita Stempniewicz, John Cuddeback, Elizabeth Ciemins, Rich Stempniewicz; Pima: Robert G. Nelson, William C. Knowler; PREVEND: Ron T Gansevoort, Hiddo JL Heerspink, Stephen JL Bakker; PSP-CKD: Nigel Brunskill, Rupert Major, James F. Medcalf, David Shepherd; Rancho Bernardo: Simerjot K. Jassal, Elizabeth Barrett-Connor, Jaclyn Bergstrom, Joe Ix; RCAV: Csaba Kovesdy, Miklos Z Molnar, Keiichi Sumida; RENAAL: Dick de Zeeuw, Hiddo JL Heerspink, Barry Brenner; SCREAM: Juan Jesus Carrero, Abdul Rashid Qureshi, Carl-Gustaf Elinder, Björn Runesson; SRR-CKD: Marie Evans, Mårten Segelmark, Maria Stendahl, Staffan Schön; Sunnybrook: David Naimark, Navdeep Tangri, Maneesh Sud; Takahata: Tsuneo Konta, Atsushi Hirayama, Kazunobu Ichikawa; ZODIAC: Henk JG Bilo, Gijs WD Landman, Kornelis JJ van Hateren, Nanne Kleefstra
CKD-PC Steering Committee: Josef Coresh (Chair), Ron T Gansevoort, Morgan E. Grams, Stein Hallan, Csaba P Kovesdy, Andrew S Levey, Kunihiro Matsushita, Varda Shalev, Mark Woodward
CKD-PC Data Coordinating Center: Shoshana H Ballew (Assistant Project Director), Jingsha Chen (Programmer), Josef Coresh (Principal Investigator), Morgan E Grams (Director of Nephrology Initiatives), Lucia Kwak (Programmer), Kunihiro Matsushita (Director), Yingying Sang (Lead Programmer), Mark Woodward (Senior Statistician)
CKD-EPI investigators/collaborators (not included in CKD-PC):
ALTITUDE Hans-Henrik Parving; CSG: Roger A. Rodby, Richard D. Rohde; HALTPKD_B: Ronald Perrone; Guangzhou: Fan Fan Hou; IDNT: Ed Lewis, Lawrence G. Hunsicker; ORIENT: Enyu Imai; REIN: Giuseppe Remuzzi, Piero Ruggenenti; REIN-2: Giuseppe Remuzzi, Piero Ruggenenti; ROAD: Fan Fan Hou;
Collaborators for AASK, ADVANCE, MASTERPLAN, MDRD, and RENAAL are previously listed for CKD-PC.
The planning and operations committee of the NKF-FDA-EMA workshop on Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of Chronic Kidney Disease contributed to the design and critical review of these analyses.
Planning and Operations Committees: Andrew Levey (Chair), Ron Gansevoort, Josef Coresh, Dick de Zeeuw, Kai-Uwe Eckardt, Hrefna Gudmundsdottir, Adeera Levin, Romaldas Maciulaitis, Tom Manley, Vlado Perkovic, Kimberly Smith, Norman Stockbridge, Aliza Thompson, Thorsten Vetter, Kerry Willis, Luxia Zhang
Some of the data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Funding: The CKD-PC Data Coordinating Center is funded in part by a program grant from the US National Kidney Foundation (which in turn receives support from industry) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100446-01). A variety of sources have supported enrollment and data collection including laboratory measurements, and follow-up in the collaborating cohorts of the CKD-PC. These funding sources include government agencies such as national institutes of health and medical research councils as well as foundations and industry sponsors listed in appendix 3 in the supplement. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. A wide group of individuals provided input about the research as part of NKF-FDA-EMA Workshop and its preparation meetings.
Footnotes
Conflict of Interest Disclosures: All authors will complete and submit the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Josef Coresh, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Hiddo JL Heerspink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, the Netherlands.
Yingying Sang, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Kunihiro Matsushita, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Johan Arnlov, School of Health and Social Studies, Dalarna University, Falun, Sweden and Department of Neurobiology, Care Sciences and Society, Division of Family Medicine and Primary Care, Karolinska Institutet, Huddinge, Sweden.
Brad C Astor, Departments of Medicine and Population Health Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI.
Corri Black, Institute of Applied Health Sciences, University of Aberdeen, Aberdeen, UK and Public Health, NHS Grampian, Summerfield House, Aberdeen, UK.
Nigel J Brunskill, Department of Infection Immunity and Inflammation, University of Leicester, UK and John Walls Renal Unit, University Hospitals of Leicester, UK.
Juan-Jesus Carrero, Department of Medical Epidemiology and Biosatistics (MEB), Karolinska Institutet, Stockholm, Sweden.
Harold I Feldman, Department of Biostatistics, Epidemiology and Informatics, and the Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
Caroline S Fox, National Heart, Lung, and Blood Institute’s Framingham Heart Study, Center for Population Studies, Framingham, Massachusetts and Division of Endocrinology, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts.
Lesley A Inker, Division of Nephrology at Tufts Medical Center, Boston, MA.
Areef Ishani, Veterans Administration Health Care System, Minneapolis, MN, and Department of Medicine, University of Minnesota, Minneapolis, MN.
Sadayoshi Ito, Division of Nephrology, Endocrinology, and Vascular Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan.
Simerjot Jassal, Division of General Internal Medicine, Department of Medicine, VA San Diego Healthcare System, University of California San Diego, San Diego, CA.
Tsuneo Konta, Department of Public Health and Hygiene, Yamagata University Faculty of Medicine, Yamagata, Japan.
Kevan Polkinghorne, Department of Nephrology, Monash Medical Centre, Melbourne, Australia; Department of Medicine, Monash University, Melbourne, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Solfrid Romundstad, Department of Clinical and Molecular Medicine, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU), Trondheim, and Department of Internal Medicine, Levanger Hospital, Health Trust Nord-Trøndelag, Levanger, Norway.
Marit D Solbu, Section of Nephrology, Division of Internal Medicine, University Hospital of North Norway, Tromsø, Norway, and Metabolic and Renal Research Group, UiT The Arctic University of Norway, Tromsø, Norway.
Nikita Stempniewicz, AMGA, Alexandria, VA, USA.
Benedicte Stengel, Inserm UMR1018, CESP Center for Research in Epidemiology and Population Health, Team 5, Villejuif, France, UVSQ and UMRS 1018, Paris-Sud University, Villejuif, France.
Marcello Tonelli, Department of Medicine, Division of Nephrology, University of Calgary, Calgary, Alberta, Canada.
Mitsumasa Umesawa, Department of Public Health, Dokkyo Medical University, Tochigi, Japan and Ibaraki Health Plaza, Ibaraki Health Service Association, Mito.
Sushrut S Waikar, Renal Division, Brigham and Women’s Hospital, Harvard Medical School, Boston MA.
Chi-pang Wen, China Medical University Hospital, Taichung, Taiwan, and Institute of Population Health Sciences, National Health Research Institutes, Miaoli County, Taiwan.
Jack FM Wetzels, Department of Nephrology, Radboud Institute of Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Mark Woodward, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, The George Institute for Global Health, University of Oxford, UK and The George Institute for Global Health, University of New South Wales, Australia.
Morgan E Grams, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Csaba P Kovesdy, University of Tennessee Health Science Center, Memphis, TN and Memphis Veterans Affairs Medical Center, Memphis, TN.
Andrew S Levey, Division of Nephrology at Tufts Medical Center, Boston, MA.
Ron T Gansevoort, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
References:
- 1.Parving HH, Andersen AR, Smidt UM, Friisberg B, Svendsen PA. Reduced albuminuria during early and aggressive antihypertensive treatment of insulin-dependent diabetic patients with diabetic nephropathy. Diabetes Care Jul-Aug 1981;4(4):459–463. [DOI] [PubMed] [Google Scholar]
- 2.de Zeeuw D Should albuminuria be a therapeutic target in patients with hypertension and diabetes? Am J Hypertens November 2004;17(11 Pt 2):11S–15S; quiz A12–14. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis December 2014;64(6):821–835. [DOI] [PubMed] [Google Scholar]
- 4.Heerspink HJ, Kropelin TF, Hoekman J, de Zeeuw D, Reducing Albuminuria as Surrogate Endpoint Consortium. Drug-Induced Reduction in Albuminuria Is Associated with Subsequent Renoprotection: A Meta-Analysis. J. Am. Soc. Nephrol August 2015;26(8):2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heerspink H, Tom G, Tighiouart H, et al. Change in Albuminuria as a Surrogate End Point for Kidney Disease Progression in Clinical Trials: A Meta-analysis of Treatment Effects of 41 Randomized Trials. Lancet Diabetes Endocrinol 2018;(in press). [DOI] [PubMed]
- 6.Inker LA, Levey AS, Pandya K, Stoycheff N, Okparavero A, Greene T. Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient meta-analysis. Am J Kidney Dis July 2014;64(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inker LA, Mondal H, Greene T, et al. Early Change in Urine Protein as a Surrogate End Point in Studies of IgA Nephropathy: An Individual-Patient Meta-analysis. Am J Kidney Dis September 2016;68(3):392–401. [DOI] [PubMed] [Google Scholar]
- 8.Thompson A Proteinuria as a surrogate end point--more data are needed. Nat Rev Nephrol March 6 2012;8(5):306–309. [DOI] [PubMed] [Google Scholar]
- 9.Cravedi P, Ruggenenti P, Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol March 6 2012;8(5):301–306. [DOI] [PubMed] [Google Scholar]
- 10.Lambers Heerspink HJ, Gansevoort RT. Albuminuria Is an Appropriate Therapeutic Target in Patients with CKD: The Pro View. Clin J Am Soc Nephrol June 5 2015;10(6):1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LF, Lewis J. Albuminuria is Not an Appropriate Therapeutic Target in Patients with CKD: The Con View. Clin J Am Soc Nephrol June 5 2015;10(6):1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrero JJ, Grams ME, Sang Y, et al. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int January 2017;91(1):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in Albuminuria and Subsequent Risk of Incident Kidney Disease. Clin J Am Soc Nephrol December 7 2017;12(12):1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usui T, Kanda E, Iseki C, Iseki K, Kashihara N, Nangaku M. Observation period for changes in proteinuria and risk prediction of end-stage renal disease in general population. Nephrology (Carlton) September 2018;23(9):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmieder RE, Mann JF, Schumacher H, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J. Am. Soc. Nephrol July 2011;22(7):1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brantsma AH, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT, Group PS. Extended prognostic value of urinary albumin excretion for cardiovascular events. J. Am. Soc. Nephrol September 2008;19(9):1785–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J. Am. Soc. Nephrol February 2009;20(2):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet June 12 2010;375(9731):2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int June 2011;79(12):1341–1352. [DOI] [PubMed] [Google Scholar]
- 20.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes in both general and high-risk populations. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int July 2011;80(1):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int June 2011;79(12):1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA May 9 2012;307(18):1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem January 2009;55(1):24–38. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med May 5 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate with Standardized Serum Creatinine Values. Clin. Chem April 1 2007;53(4):766–772. [DOI] [PubMed] [Google Scholar]
- 26.Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement Error in Nonlinear Models: A Modern Perspective, Second Edition Second ed. Boca Raton FL: Chapman & Hall/CRC, Taylor & Francis Group; 2006. [Google Scholar]
- 27.Grams ME, Coresh J, Segev DL, Kucirka LM, Tighiouart H, Sarnak MJ. Vascular disease, ESRD, and death: interpreting competing risk analyses. Clin J Am Soc Nephrol October 2012;7(10):1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eijkelkamp WB, Zhang Z, Remuzzi G, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol May 2007;18(5):1540–1546. [DOI] [PubMed] [Google Scholar]
- 29.Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med April 25 2005;165(8):947–953. [DOI] [PubMed] [Google Scholar]
- 30.Jun M, Ohkuma T, Zoungas S, et al. Changes in Albuminuria and the Risk of Major Clinical Outcomes in Diabetes: Results From ADVANCE-ON. Diabetes Care January 2018;41(1):163–170. [DOI] [PubMed] [Google Scholar]
- 31.Tangri N, Grams ME, Levey AS, et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA January 12 2016;315(2):164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.