Abstract
STUDY OBJECTIVE:
Mucinous cystadenomas (MCAs) are benign epithelial ovarian tumors that occur rarely in children and adolescents. As children and adolescents typically have their childbearing years ahead of them, conservative therapy is indicated. However, there is concern that ovarian cystectomy may be associated with significant recurrence risk in MCA. Furthermore, GNAS gene mutations are associated with McCune-Albright Syndrome, which is associated with cystic ovaries. We sought to evaluate the outcomes of children and adolescents with MCA treated conservatively. A subset of patients underwent GNAS gene testing.
DESIGN AND PARTICIPANTS:
After Institutional Board Review approval, the pathology database of a large urban children’s hospital was queried to identify adolescents with MCA between the years 2008 and 2014. 14 patients, aged 8–18 years (median 14), were identified. A buccal swab for genetic testing was obtained from a subset of consenting patients.
MAIN OUTCOME MEASURES:
MCA recurrence; ovarian return to normal size; GNAS gene variants.
RESULTS:
Two patients underwent oophorectomies, while the remaining 12 underwent cystectomies. Follow-up ultrasound revealed slow return of ovary to normal size. Of the 10 patients with available follow-up data, there were no recurrences at a median of 225 days from surgery. Four patients consented to a buccal swab for genetic testing, and the GNAS gene was noted to have rare variants in 2 patients.
CONCLUSION:
This series supports the use of ovary-sparing surgery in the treatment of MCA. Further research exploring possible genetic variants such as the GNAS gene in children and adolescents diagnosed with MCA is warranted.
Keywords: Cystadenoma, Mucinous, Ovary, Adolescents, Children, Laparoscopy, Ovary-Sparing Surgery, cystectomy
Introduction
Despite being the most common childhood and adolescent tumor of the genital tract, ovarian tumors are still quite rare in females under the age of 21 1,2. Of these tumors, even fewer are categorized as benign mucinous cystadenomas (MCAs). Due to its limited prevalence, the data on the epidemiology and management of benign MCAs in adolescents are mostly extrapolated from other histological tumor types and case reports. There are approximately 10 reports of isolated cases or small series of adolescent patients treated for benign MCAs 1–10.
Although the current treatment for benign MCAs is typically unilateral salpingo-oophorectomy (USO) via laparotomy, many studies have shown not only efficacy, but also advantages, in the utilization of ovary-conserving procedures in the surgical management of benign adnexal pathology, especially in the adolescent population11–15. Presumably, benign MCAs could be managed similarly.
While generally considered the precursor lesion of mucinous cystadenocarcinoma, the tumorigenesis of benign MCA is still not fully understood. Genetic studies have identified KRAS mutations in up to 60% of benign MCAs 16–19. However, there may be other mutations that play a role in its tumorigenesis. P53, CDKN2A, SMAD4, BRAF, BRCA1, PTEN, PIK3CA, AATK, and RNF43 mutations in mucinous tumors have all been reported in the literature16,17,20,21. Recently, GNAS mutations have also been reported in mucinous tumors of the ovary and the gastrointestinal tract22,23. Moreover, GNAS activating mutations, encoding the stimulatory G-protein alpha subunit, are the cause of McCune-Albright Syndrome (MAS), a rare congenital condition also associated with large cystic ovaries in children and adolescents, characterized by café-au-lait skin pigementation, osseous fibrous dysplasia, and a variety of endocrine hyperfunctions 24,25.
The objective of this study was to describe our experience with benign MCAs in children and adolescents managed conservatively with ovary-sparing cystectomies. We also screened for germline GNAS sequence changes in a subset of patients with MCA.
Materials and Methods
Cases
After Institutional Review Board approval, we collected the demographic, clinical, pathologic, and outcome data for all patients who underwent surgery for benign MCA at Children’s National Medical Center, a large urban children’s hospital in Washington, DC from January 2008 to December 2014. Cases were identified through the institutional pathology database. Patients were included if they were under 21 years of age at the time of surgery and had an ovarian MCA noted on final pathology. Patients were excluded if they had borderline or malignant pathology. Data was extracted from chart review and compiled in an electronic database by one investigator and reviewed by the senior investigator for accuracy.
GNAS Sequencing Analysis
Authorization for DNA sequencing analysis was obtained from 4 patients. Informed assent and parental consent were obtained. Biological samples were obtained by saliva collection using OGR-500 (DNA Genotek Ink. Inc., Ottawa, Ontario, Canada). Results were de-identified, and patients were told they would not obtain the results. Total DNA was extracted from a 500 μL saliva aliquot using prepIT-L2P and ethanol precipitation as outlined by the manufacturer. All GNAS isoforms (OMIM: 139320; NM_001077490 – used as reference for mutation description) were analyzed. The complete GNAS-coding and surrounding intronic sequence was amplified 26. Primer sequence and PCR conditions are available upon request. Each PCR product was sequenced using BigDye® Terminator V3.1 (Life Technologies, Grand Island, NY), purified using ZR DNA Sequencing Clean-up Kit™ (Zymo Research, Irvine, CA), and analyzed by classical bidirectional Sanger sequencing. In silico analysis was performed by MutationTaster and Alamut version 2.7.2 (Interactive Biosoftware, Rouen, France) software. These programs use specific algorithms to calculate the probability of a DNA variant to cause a defect in the protein structure that can be deleterious for the protein’s function. These tools were used to predict the pathogenic potential of the identified variants in GNAS 27.
Results
Cases
Fourteen menarcheal and pre-menarcheal patients, ranging in age from 8–18 years, were identified. Clinical and surgical data are reported in Table 1. Of note, 2 patients had a surgical history significant for cystectomies for dermoid cysts. Two years prior to her surgery for benign MCA, Patient 3 had a dermoid cyst removed from her ipsilateral ovary. Patient 5 had a right ovarian torsion detorsed and bilateral dermoid cysts removed 3 years prior to her cystectomy for a left benign MCA. Other notable patient characteristics noted on medical histories are listed in Table 2.
Table 1:
Clinical and Surgical Characteristics
| Case No | Age (y) | Side | Preop Cyst Size (largest dimension cm) | Preop Cyst Volume (cm3) | Surgical Approach | Largest Incision Size (cm) | Length of Stay (day) | Surgery | Surgeon Type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | Left | Unavailable | Unavailable | Laparoscopy converted to laparotomy | 8 | 2 | Oophorectomy | General Pediatric |
| 2 | 8 | Right | 7.0 | 140 | Laparoscopy | 1 | 2 | Oophorectomy | General Pediatric |
| 3 | 12 | Left | 3.8 | 27 | Laparoscopy | 0.5 | 0 | Cystectomy | General Pediactric |
| 4 | 14 | Left | 19.4 | 4229 | Laparoscopy +Mini Lap | 3 | 0 | Cystectomy | Pediatric Gynecologist |
| 5 | 14 | Left | 7.7 | 228 | Laparoscopy | 0.5 | 0 | Cystectomy | Pediatric Gynecologist |
| 6 | 17 | Right | 11.4 | 153 | Laparoscopy | 1.2 | 0 | Cystectomy | Pediatric Gynecologist |
| 7 | 18 | Right | 6.9 | 153 | Laparoscopy | 1.2 | 0 | Cystectomy | Pediatric Gynecologist |
| 8 | 11 | Left | 26.0 | 7280 | Laparotomy | 6 | 2 | Cystectomy | Pediatric Gynecologist |
| 9 | 13 | Right | 20.0 | 3000 | Mini Lap | 5 | 0 | Cystectomy | Pediatric Gynecologist |
| 10 | 14 | Left | 4.2 | 62 | Laparoscopy converted to mini-lap | Unavailable | 0 | Cystectomy | Pediatric Gynecologist |
| 11 | 17 | Left | 16.0 | 1949 | Mini Lap | 3 | 0 | Cystectomy | Pediatric Gynecologist |
| 12 | 13 | Right | 9 | 347 | Laparoscopy | 1 | 0 | Cystectomy | Pediatric Gynecologist |
| 13 | 14 | Right | 17 | 1550 | Mini Lap | 3 | 0 | Cystectomy | Pediatric Gynecologist |
| 14 | 15 | Right | Unavailable | Unavailable | Laparoscopy | 1 | 0 | Cystectomy | Pediatric Gynecologist |
Table 2:
Significant Past Medical History (PMH)
| Patient No | Significant PMH |
|---|---|
| 2 | Vesicoureteral reflex with recurrent urinary tract infections and ureterostomy |
| 3 | Dermoid cyst in ipsilateral ovary prior to MCA |
| 5 | Bilateral dermoid cysts prior to MCA |
| 7 | Biliary atresia with Kasai procedure in infancy, acute cholangitis, cleft mitral valve with repair and recurrent pericarditis |
| 11 | Depression and papillary thyroid cancer (after her mucinous cystadenoma) |
| 12 | Asthma |
| 13 | Postural tachycardia syndrome |
| 14 | Polycystic ovarian syndrome, acanthosis nigricans, menorrhagia, depression |
There was some preoperative spillage of mucinous cystic fluid in Patient 10, whose cyst ruptured during a soccer game. She presented after the traumatic incident with ascites and a visibly ruptured cyst. There were no intraoperative complications. Patient 13 was readmitted on postoperative day 5 with an infected hematoma of the right ovary. Patient 8 was postoperatively transfused one unit of blood by the medical team. Ten of the 11 patients treated by a pediatric gynecologist were discharged on the day of surgery.
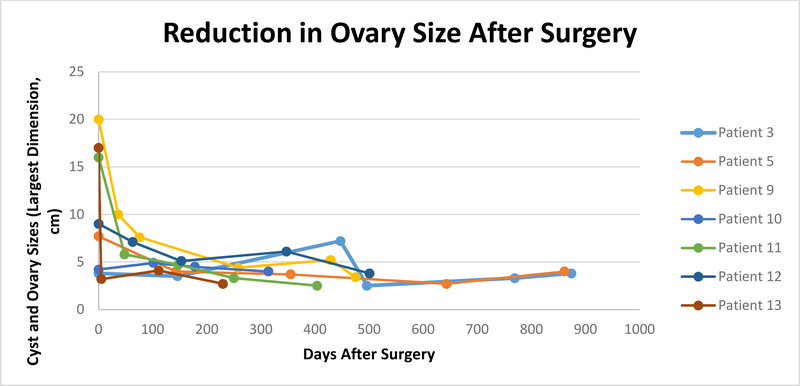
The MCAs ranged in size from 3.8–26 cm (median, 10.2 cm). Follow-up ultrasounds were available for 10 of the 12 cystectomy patients. Seven of the patients had ≥3 follow-up sonograms. Mean time from first to third ultrasound was 225 days (range, 202–500 days). All of the ovaries had returned to size within normal limits. Figure 1 shows trends in size reduction. Some radiology reports documented the ovaries as “normal in size and appearance”; these were excluded from the figures.
Figure 1:
Cyst and ovary size on ultrasound or CT for seven subjects with ≥ 3 follow-up ultrasounds. First data point shows cyst size preoperatively; subsequent data points show ovaries postoperatively.
There were no recurrences in either ovary noted on follow-up ultrasound for the 10 patients with available follow-up data. Patient 8 had hyperechoic or soft tissue structures visualized on multiple follow-up ultrasounds and recurrence could not be ruled out. She was referred for an MRI per her provider, which then showed both ovaries as normal. Patients 3, 9, 11, 12, and 13 also had unclear findings on early follow-up ultrasounds that all eventually resolved.
Genetic Data
Two different rare variants were found in 2 (50%) of 4 samples. In the sample GSA1, the variant c.1127C>T (p.P376L, rs61749697) was found. The affected amino acid is moderately conserved throughout species; however, the expressed isoform differs between different species. As a result, in some of them, the region of the protein, where the mutation is located, is missing, not allowing a comparison. In silico, or computer simulation, analysis shows that the variant c.1127C>T is probably benign and may not affect the protein function. Its frequency in the general population varies from 1.8% (1000 genomes project) to 3.05% (ExAC project). The second variant, found in the sample GSA3, was c.1654C>T (p.R552W). It is a novel variant that has not been described before; however, in silico analysis predicts this variant as benign and shows that the protein region where the variant c.1654C>T is located is not fully conserved among different species.
Discussion
Benign MCAs, though rare in childhood, represent an area for further research in pediatric and adolescent gynecology. Reports suggest that they are usually unilateral but can grow large in size. The size of the tumor, while not indicative of malignancy, combined with the concern for recurrence, often leads the surgeon to perform an oophorectomy 1,2,19,28. Data from our case series review suggest that benign MCAs as large as 26 cm in children and adolescents can be managed conservatively with cystectomy and without an associated increased risk of recurrence. To our knowledge, this is the largest series reporting on benign MCAs in this patient population.
One argument for treating young girls and adolescents with oophorectomy for benign MCAs is size. These tumors can become notably large, with multiple articles reporting tumors as large as 30 cm 15. Our series demonstrates that cystectomies can be safely performed in cases involving such large tumors, with eventual return to normal ovarian size and volume. While there were a number of postoperative abnormal sonographic findings, when conservatively followed with non-invasive imaging, all of the abnormal findings were resolved.
Another argument for oophorectomy is the risk of recurrence, potentially requiring more surgery. Most of the literature on both children and adults suggests that the risk of recurrence in a contralateral or even ipsilateral adnexa is very low, and there have been no reported deaths related to benign MCA 15. In 2010, however, Ben Ami et al published a report of 42 patients, aged 17–78 years, diagnosed with benign MCAs 28. Over a 10-year study period, they noted 3 recurrences (7.1%), which is higher rate than that of any other published report. All 3 patients, with a median age of 21.7 ±4.5 years, had undergone laparoscopic cystectomies. Of note, all 3 also had intraoperative spillage of the cyst contents, recurrence in the ipsilateral ovary, and recurrence within the initial year of surgery. These are unique characteristics necessitating further study, especially since these patients were not far out from adolescence. Historically, there has been some concern for the development of pseudomyxoma peritonei secondary to intraoperative spillage of mucinous contents; however, more recent data do not support this theory, showing that pseudomyxoma peritonei most commonly originates from the appendix 19. Our report, however, describes 14 patients followed for over 1 year, with no recurrences or episodes of pseudomyxoma peritonei. We documented one preoperative cyst rupture in Patient 10, but she did not develop a recurrence during the follow-up time. The minilap technique allows the surgeon to carefully inspect the ovary and ensure there is no remnant cyst wall. The patient is also spared from a large incision and can be discharged on the day of surgery – two of the main benefits of minimally invasive surgery, which includes both laparoscopy and mini-laparotomy in this series.
Preoperative evaluation of pelvic masses in children and adolescents requires a thorough review of imaging and laboratory work-up in order to plan for surgical approach. Tumor markers may be helpful in diagnosis, though nonspecific. Theses include, but are not limited to, beta-human chorionic gonadotropin, alpha fetoprotein, lactate dehydrogenase, inhibin, carcineoembryonic antigen, cancer antigen 125, and cancer antigen 19–9 13. In this series, the patients’ radiologic findings demonstrated cystic masses with no solid components. Normal tumor markers were noted when evaluated. Cystectomy may not be indicated in cases with complex pelvic masses, which include solid areas not consistent with dermoid cyst, and/or when accompanied by abnormal tumor markers.
Even though a genetic analysis was performed only in a small number of patients for whom we had DNA, it showed rare GNAS variants in 2 of the 4 patients. Although the variants c.1127C>T and c.1654C>T are predicted as benign in silico, their presence in vivo could be pathogenic. They could, for example, lead to a small but important change in protein function or other modest effect that leads to MCA formation under the influence of other factors, genetic or environmental. These genetic data warrant further investigation to confirm the presence of rare GNAS variants among patients with MCA, and to study the functional effects of these variants. It should be noted that a 2015 exome sequencing project of benign, borderline, and malignant mucinous ovarian tumors also identified activating GNAS mutations in the serum and tissue samples 22; 9.1% (2/22) of benign cystadenomas, 6.9% (2/29) of borderline, and 3.3% (1/30) of malignant tumors had GNAS mutations. Further research might consider evaluating the cyst wall of the MCA for activating mutations in the alpha subunit of the G3 protein such as those associated with MAS.
In conclusion, this study reports on a sizeable population of adolescents treated with a minimally invasive, ovary-sparing approach for benign MCAs. We have demonstrated feasibility and safety, with no evidence of increased risk of recurrence. This ovary-sparing, minimally invasive approach may be considered for other adolescent females with benign MCAs. Genetic testing showed GNAS variants to be potentially involved in the predisposition for the development of these lesions, although these data are limited and require validation in a larger number of patients and/or lesions.
Acknowledgements:
These data were presented at the 30th Annual Clinical and Research Meeting for The North American Society for Pediatric and Adolescent Gynecology (NASPAG) in April 2016.
This research was supported in part by the Intramural Research Program of Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paran TS, Mortell A, Devaney D, Pinter A, Puri P. Mucinous cystadenoma of the ovary in perimenarchal girls. Pediatric surgery international 2006;22:224–7. [DOI] [PubMed] [Google Scholar]
- 2.Cevik M, Guldur ME. An extra-large ovarian mucinous cystadenoma in a premenarchal girl and a review of the literature. Journal of pediatric and adolescent gynecology 2013;26:22–6. [DOI] [PubMed] [Google Scholar]
- 3.Flotho C, Rückauer K, Duffner U, Bergsträβer E, Böhm N, Niemeyer CM. Mucinous cystadenoma of the ovary in a 15-year-old girl. Journal of pediatric surgery 2001;36:1–3. [DOI] [PubMed] [Google Scholar]
- 4.Olesen H, Eisum A. [Recurrent mucineous cystadenoma in an 11-year-old premenarchal girl]. Ugeskrift for laeger 2001;163:6601–2. [PubMed] [Google Scholar]
- 5.Baksu B, Akyol A, Davas I, Yazgan A, Ozgul J, TanƖk C. Recurrent mucinous cystadenoma in a 20-year-old woman: Was hysterectomy inevitable? Journal of Obstetrics and Gynaecology Research 2006;32:615–8. [DOI] [PubMed] [Google Scholar]
- 6.Alobaid AS. Mucinous cystadenoma of the ovary in a 12-year-old girl. Saudi medical journal 2008;29:126–8. [PubMed] [Google Scholar]
- 7.Parmentier B, Vaz E, Chabaud-Williamson M, et al. Mucinous cystadenoma arising 3 years after ovarian-sparing surgery for mature teratoma in a child. Journal of Pediatric Surgery 2010;45:e9–e12. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama A, Fukikoshi M, Saka R, et al. Minilaparotomy Approach for Giant Mucinous Cystadenoma of the Ovary in Children: Report of Two Cases. The Showa University Journal of Medical Sciences 2010;22:135–41. [Google Scholar]
- 9.Leys CM, Gasior AC, Hornberger LL, St. Peter SD Laparoscopic resection of massive ovarian mucinous cystadenoma. Journal of Laparoendoscopic & Advanced Surgical Techniques 2012;22:307–10. [DOI] [PubMed] [Google Scholar]
- 10.Skondras I, Gavera S, Achilleos O, Kapouleas G, Aivazoglou T, Passalidis A. Giant Mucinous Ovarian Cystadenoma in 13-year-old Premenarchal Girl REVISTA SOCIETĂŢII ROMÂNE DE CHIRURGIE PEDIATRICĂ 2012:50–53. [Google Scholar]
- 11.Berger-Chen S, Herzog TJ, Lewin SN, et al. Access to conservative surgical therapy for adolescents with benign ovarian masses. Obstetrics & Gynecology 2012;119:270–5. [DOI] [PubMed] [Google Scholar]
- 12.Rossi BV, Ference EH, Zurakowski D, et al. The clinical presentation and surgical management of adnexal torsion in the pediatric and adolescent population. Journal of pediatric and adolescent gynecology 2012;25:109–13. [DOI] [PubMed] [Google Scholar]
- 13.Eskander R, Bristow RE. Adnexal masses in pediatric and adolescent females: a review of the literature. Current Obstetrics and Gynecology Reports 2012;1:25–32. [Google Scholar]
- 14.Bristow RE, Nugent AC, Zahurak ML, Khouzhami V, Fox HE. Impact of surgeon specialty on ovarian-conserving surgery in young females with an adnexal mass. Journal of adolescent health 2006;39:411–6. [DOI] [PubMed] [Google Scholar]
- 15.Chaitin BA, Evans HL, Gershenson DM. Mucinous tumors of the ovary. A clinicopathologic study of 70 cases. Cancer 1985;55:1958–62. [DOI] [PubMed] [Google Scholar]
- 16.Shih I-M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. The American journal of pathology 2004;164:1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter SM, Gorringe KL, Christie M, Rowley SM, Bowtell DD, Campbell IG. Pre-invasive ovarian mucinous tumors are characterized by CDKN2A and RAS pathway aberrations. Clinical Cancer Research 2012;18:5267–77. [DOI] [PubMed] [Google Scholar]
- 18.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-ras mutations in mucinous ovarian tumors. Cancer 1997;79:1581–6. [DOI] [PubMed] [Google Scholar]
- 19.Hart WR. Mucinous tumors of the ovary: a review. International Journal of Gynecologic Pathology 2005;24:4–25. [PubMed] [Google Scholar]
- 20.Coleman WB, Tsongalis GJ. Molecular Pathology: The Molecular Basis of Human Disease: Elsevier Science; 2009. [Google Scholar]
- 21.Ryland GL, Hunter SM, Doyle MA, et al. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. The Journal of pathology 2013;229:469–76. [DOI] [PubMed] [Google Scholar]
- 22.Ryland GL, Hunter SM, Doyle MA, et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome medicine 2015;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Science translational medicine 2011;3:92ra66–92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis 2008;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pienkowski C, Lumbroso S, Bieth E, Sultan C, Rochiccioli P, Tauber M. Recurrent ovarian cyst and mutation of the Gs alpha gene in ovarian cyst fluid cells: what is the link with McCune-Albright syndrome? Acta paediatrica (Oslo, Norway : 1992) 1997;86:1019–21. [DOI] [PubMed] [Google Scholar]
- 26.Faucz FR, Horvath AD, Azevedo MF, et al. Is IGSF1 involved in human pituitary tumor formation? Endocrine-related cancer 2015;22:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nature methods 2014;11:361–2. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Ami I, Smorgick N, Tovbin J, Fuchs N, Halperin R, Pansky M. Does intraoperative spillage of benign ovarian mucinous cystadenoma increase its recurrence rate? American journal of obstetrics and gynecology 2010;202:142 e1–e5. [DOI] [PubMed] [Google Scholar]