Abstract
Atrial fibrillation (AF) is the most common dysrhythmia encountered in the United States. Symptoms may be similar to those of other cardiac conditions, which can delay the timely detection, diagnosis, and management of AF. This article provides an overview of AF and modalities used in remote monitoring.
Keywords: advanced practice registered nurse, atrial fibrillation, remote monitoring
Atrial fibrillation (AF) is the most common cardiac dysrhythmia.1 An estimated 2.7 to 6.1 million individuals are living with AF in the United States. By the year 2030, the number of Americans with AF will exceed 12 million.1–3 This is a result of the aging population and the number of individuals living with conditions associated with the development of AF, such as obesity, heart failure, and sleep apnea.1,4,5 Symptoms of AF can be vague or nonexistent and similar to those of other chronic conditions, making the diagnosis challenging.6–8 AF that remains undetected and untreated can lead to stroke and other thromboembolic events and can have a significant impact on quality of life (QoL).8,9
Patients who are asymptomatic or present with atypical symptoms of AF are often not diagnosed and therefore go untreated.10,11 AF can be missed through conventional monitoring approaches, such as 12-lead ECGs and ambulatory electrocardiography device monitoring, which captures ECG information during a finite time period. In addition, short-term ambulatory electrocardiography device and extended cardiac monitoring (for 30 days) is cumbersome. These monitoring approaches often fail to capture AF when episodes are not frequent, and thus, are not captured within the monitoring period.12,13
Newer remote monitoring (RM) devices can be worn or implanted for longer periods of time, allowing for ECG data to be collected continuously for extended time periods with less burden to the patient; these devices are more likely to identify subclinical AF and its associated triggers and symptoms.4,12–15 Additionally, RM used in conjunction with smartphone technology is easy to use in the real world setting and actively engages patients in selfmanagement.16,17 Many patients are already utilizing such mobile health technologies (smartwatches, sensors) on a daily basis to monitor their health.18,19 The advancements in RM have impacted clinical decision making and practice to enhance the diagnosis of AF.20
Pathophysiology
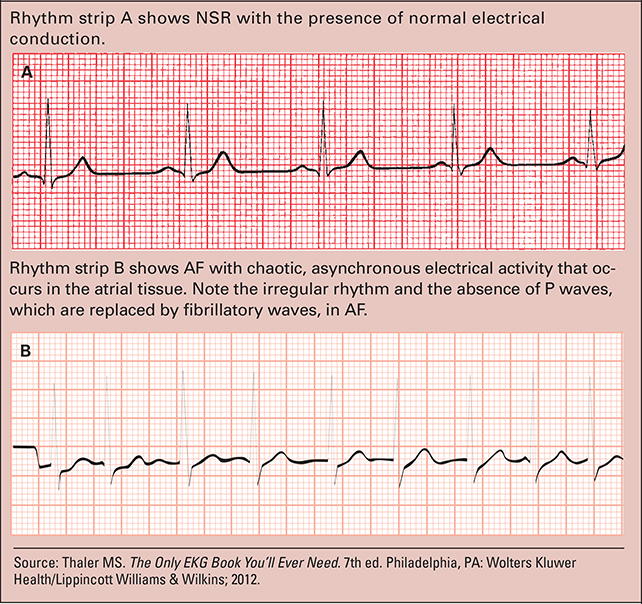
The pathophysiology of AF is complex and involves autonomic, neural, and genetic factors.21–23 The main mechanism that often onsets AF involves small reentrant or rapidly firing electrical impulses along the pulmonary veins and left atrium junction that result in atrial ectopic electrical activity, which ultimately replaces normal conduction within the heart.22 In normal sinus rhythm (NSR), the sinoatrial (SA) node regulates the heart rate at 60 to 100 beats/minute, and the a trioventricular (AV) node at 40 to 60 beats/minute (see Examples of NSR and AF).
Due to the irregular impulses from the atria in AF, the heart can beat rapidly up to 200 beats/minute, which can result in symptoms and adverse reactions, such as heart failure, stroke, and even death.21–23 There are three classifications of AF that the advanced practice registered nurse (APRN) should be aware of, which include paroxysmal AF, persistent AF, and longstanding persistent AF (see Types of AF). It is important to identify paroxysmal AF, as it is more likely to progress to persistent AF in the future, and attempts to restore a NSR may become more difficult to achieve the longer a patient remains in AF due to atrial remodeling.1
Examples of NSR and AF
Risk factors associated with AF
AF is associated with many risk factors and is more common in men than women. The lifetime risk of developing AF in individuals age 40 or older is greater than 25% (see Risk factors associated with AF).23
Clinical presentation
AF can also be found incidentally, for instance on a routine ECG or an irregular pulse captured during a physical exam or in a preoperative setting. Other patients may experience symptoms (for example, AF with a rapid ventricular rate) prompting them to seek immediate medical evaluation or to visit the ED.24,25 Patients with AF may present with symptoms including fatigue, palpitations, syncope, hypotension, and dyspnea.6,21
Other patients may not recognize or experience symptoms when in AF and may attribute symptoms such as fatigue to age-related changes or coexisting chronic conditions (for example, heart failure, diabetes mellitus, hypertension) and therefore, may not seek further evaluation. Thus, it is important for the APRN to be aware of this and ask specific targeted questions during visits to help guide the recognition of possible symptoms associated with AF.
Heart failure is a common coexisting condition of AF, and many symptoms of heart failure overlap with those of AF, including shortness of breath, requiring differentiation, and tailored treatment of each specific condition.26 The APRN must recognize the challenges of identifying and classifying AF especially when patients have multiple coexisting conditions and/or when symptoms may or may not be reported.25,27 Using RM is ideally suited for distinguishing and documenting AF in such scenarios.
AF-focused history and clinical exam
A comprehensive medical history should be taken to determine if a family history of AF exists, and the individual’s risk for AF should be assessed. The first known documented episode of AF should also be included in the history along with the frequency, duration, precipitating factors, and modes of initiation or termination of AF (if available). If the patient has been treated for AF pharmacologically (beta-blockers for rate control or other antiarrhythmic medication for rhythm control) or procedurally (cardioversion or radiofrequency ablation), the response to treatment should be noted. Finally, any underlying heart conditions, comorbidities, and lifestyle factors that could affect AF (for example, alcohol consumption, obesity, and sleep apnea) should also be noted, and counseling/treatment to reduce associated risk factors should be taken.22
The APRN should perform a focused cardiovascular exam to identify any contributing factors (murmur, evidence of HF) to AF.7 Vital signs should be performed with particular attention to BP (undiagnosed hypertension) and heart rate (regularity, rate).7 Cardiac auscultation and palpation should be performed, noting any murmurs indicating the presence of valvular heart disease, displaced point of maximal impulse (suggesting left ventricular enlargement), abnormal heart sounds such as the presence of an S3 and prominent P2 (suggesting heart failure and pulmonary hypertension), which could all contribute to AF.23 The lungs should be auscultated for crackles and lower extremities examined for edema, indicating possible heart failure. Finally, a neurologic history and exam should be performed for signs of prior stroke.
Types of AF21
| Types of AF | |
|---|---|
| Paroxysmal | Episodes that start and stop spontaneously lasting <7 days |
| Persistent | Episodes lasting >7 days |
| Long-standing persistent | Sustained, lasting >12 months |
Diagnostic tests
A 12-lead ECG should be performed on all patients being evaluated for possible AF and may provide additional useful information: the presence or absence of left ventricular hypertrophy; bundle-branch and AV block; prior myocardial infarction; presence of other atrial dysrhythmias (atrial tachycardia, atrial flutter); and the basic P-wave duration and morphology, R-R intervals, QRS duration, and QT intervals.7,22
A transthoracic echocardiogram (TTE) should also be performed to evaluate the size and function of the left and right atria and ventricles.7 TTE can also determine the presence of left ventricular hypertrophy, pericardial disease, pulmonary hypertension, and valvular heart disease.1,21 Transesophageal echocardiogram (TEE) should be performed to evaluate the left atrium and particularly the left atrial appendage to rule out the presence of thrombus, especially in patients undergoing direct current cardioversion (DCCV) aimed at restoring a NSR.21
RM technologies to detect AF
A 12-lead ECG performed in the clinical setting offers only a brief snapshot of the cardiac rhythm at that point in time. RM is ideal for patients with rare, paroxysmal events to improve AF detection.27 There are many RM technologies available, each with unique features for AF and other dysrhythmia detection. The chosen RM device depends on the individual and their clinical characteristics (for example, frequency of palpitations or other symptoms).20
The Reveal LINQ is FDA approved and is the most common implantable cardiac monitor (ICM).28,29 The LINQ monitors heart rhythm, records events, and is the most accurate ICM available to date.10,11,30 It is implanted parallel to the sternum over the fourth intercostal space (2 cm from the left lateral edge of the sternum) and may remain implanted for up to 3 years. The LINQ also comes with a small, handheld device for recording any symptoms experienced by the patient so that they may later be correlated with ECG findings.29 The LINQ is approximately the size of a AAA battery and can be routinely inserted in outpatient settings (www.medtronicacademy.com ).30,31
Clinical trials have demonstrated that the LINQ adequately detects AF and may even be more accurate in classifying AF as compared with short-term monitoring devices (Holter monitor, extended cardiac monitoring).10,13,31 The LINQ is also highly sensitive in detecting AF duration among patients with a history of paroxysmal AF.11
The CRYSTAL-AF trial demonstrated that ICMs improve detection of silent or asymptomatic paroxysmal AF as the underlying cause of cryptogenic stroke.32 Researchers have demonstrated ICMs have accurately detected more than seven times more AF than noninvasive monitoring.32
ICMs are ideal for diagnosing potential AF in patients with infrequent episodes or stroke of unknown origin because it may remain implanted for a few years, thus capturing rare events that may otherwise be missed with short-term monitoring.27,30 It is also well-suited for long-term monitoring of patients after AF treatments, such as ablation, to monitor for AF recurrence.30 Though minimally invasive, the LINQ may not be appropriate for patients with more frequent events, as these events can likely be captured using a noninvasive RM.
The ZioPatch is a single-use, FDA-approved adhesive patch monitor that is applied to the patient’s left pectoral region (www.irhythmtech.com ).33,34 This single-lead ECG monitor allows continuous ECG monitoring for up to 14 days.35 The patch is lightweight and water-resistant, allowing patients to shower with the device and perform their usual activities of daily living and exercise.33 The correlation between symptoms and rhythm abnormalities recorded are possible by pressing the symptom button located on the ZioPatch and recording the details in a diary.14,33 After the monitoring period is complete, the patch is mailed to an ECG core lab, which analyzes the data and sends a report of ECG findings, ECG rhythm strips, and any recorded symptom details to the APRN.33
Multiple clinical trials have demonstrated that the ZioPatch correctly detects and identifies more AF episodes than the traditional 48-hour ambulatory electrocardiography device.14,33,36 In addition, due to low burden to the patient and reportedly high patient adherence with the device for the prescribed 14-day period, more infrequent events that occur in the setting of activity or exercise can better be identified as compared with the 48-hour ambulatory electrocardiography device.14,35,36
Studies have shown that the diagnostic yield increases incrementally for each day the ZioPatch is worn beyond 48 hours, and there is no significant diagnostic yield beyond 2 weeks for patients with palpitations or syncope/presyncope.35,36 Therefore, for patients referred for palpitations or syncope/near syncope that occurs frequently (over a 2-week period), this monitoring may be more appropriate than longer-term monitoring options such as the LINQ.36
| Risk factors | |
|---|---|
| Cardiac | Hypertension, heart failure, myocarditis, pericarditis, ischemia, valvular disease, myocardial infarction, hypertensive crisis, prior cardiac surgery |
| Pulmonary | Acute pulmonary disease, hypoxia related to chronic obstructive pulmonary disease, pneumonia, pulmonary edema, obstructive sleep apnea |
| Metabolic | Diabetes mellitus, hyperthyroidism, high catecholamine states, such as stress, infection, postsurgery |
| Neurologic | Subarachnoid hemorrhage, ischemic stroke |
| Drugs and other substances | Theophylline, alcohol, cocaine, amphetamines, caffeine |
| Other factors | Family history, obesity, advanced age, gender, race |
The AliveCor device is an FDA-approved mobile ECG system that leverages the Kardia smartphone app to capture dysrhythmias, including AF episodes.17,19 The device includes lightweight hardware that attaches or can be placed next to a smartphone or tablet and is able to record a 30-second ECG through the patients’ fingertips (https://www.alivecor.com ).19 The device records and sends single-channel (lead I) ECGs via Wi-Fi or cellular network transmission to the corresponding Kardia mobile application, where it can be displayed to the patient and shared with APRNs via the Health Insurance Portability and Accountability Act-compliant AliveCor cloud.19,37
The AliveCor device is highly sensitive (94%) and specific (98%) in capturing AF episodes.16,19 Kardia’s ECG algorithm identifies dysrhythmias, including AF.19 Patients may also document symptoms experienced at the time of the ECG recording and potential triggers of dysrhythmias (stress and/or exercise). Studies have found that AliveCor is easy to use, as most patients are able to independently record ECGs using the device after brief training.19,37 AliveCor can be especially beneficial for settings where AF is likely to go undetected.18,19 This is supported by a recent case report of a 58-year-old patient with AF who had multiple cardiac risk factors and procedures; the patient was given the AliveCor for symptom and rhythm monitoring in which self-monitoring was successfully implemented and detected recurrent AF.38
The ongoing iHEART study is a single-center, prospective, randomized controlled trial that will examine the utility in a larger cohort.37 A total of 300 participants with a recent history of AF will be enrolled and randomized 1:1 to receive an iPhone equipped with an AliveCor Mobile ECG and accompanying Kardia smartphone app (the iHEART intervention) versus AF standard of care for 6 months. The iHEART study is the first to investigate the utility of AliveCor in a “real world” setting to evaluate the ability to improve detection and treatment of recurrent AF.37
The AliveCor is most beneficial for patients whose AF episodes are frequent and long enough to be captured via a smartphone. Many patients want to know if their symptoms are related to their heart rhythm and whether lifestyle modifications may reduce AF symptoms based on self-monitoring data; these patients will likely appreciate the AliveCor device’s immediate display and interpretation of ECG rhythms.12,27,39
Patients with short symptomatic episodes may be less suited for AliveCor due to the time needed to capture on one’s smartphone; in these cases, other RM monitoring approaches may be more suitable. Importantly, APRNs should regularly review ECG data and communicate findings to their patients. Patients should document any symptomatic episodes that can be reviewed with their provider. RM does not replace treatment/management with a provider but is rather a tool to help guide it.
Treatment and management
The management of a patient with AF is dependent on multiple factors, including the clinical presentation, the frequency and duration of AF, and presence of coexisting risk factors. If urgent management is required due to the severity of symptoms and patient acuity (hypo-tension or rapid ventricular rate), the patient may need to undergo DCCV and/or medical therapy in an attempt to restore a NSR.21 A TEE should be performed to rule out the presence of a clot in the atrium of the heart prior to DCCV.21–23 For patients with longstanding, persistent AF, a joint decision between the patient and provider may be made to cease further procedural interventions to restore NSR and opt for pharmacologic rate control and anticoagulation.21 It is recommended that the risk of stroke and bleeding be discussed with patients considering anticoagulation therapy.40
Rate control is necessary to alleviate symptoms and prevent deterioration of ventricular function.21,22 Beta-blockers and/or calcium channel blockers such as metoprolol, propranolol, verapamil, and diltiazem are examples of some medications used to control heart rate.21 Digoxin may be added if the patient has heart failure due to its positive inotropic activity.23 In addition, antiarrhythmic medications such as amiodarone or dofetilide (which should only be used in a hospital setting with cardiac monitoring due to the risk of excessive QT prolongation) can also be used for rate control and/or in conjunction with rhythm control approaches.21 If the patient has no coronary artery disease, sodium channel blockers such as flecainide or a potassium channel blocker such as sotalol may be considered.21–23 Generally, a heart rate of less than 100 beats/minute is a target for adequate rate control.
Rhythm control may be discussed between the patient and provider depending on the presentation, duration, and severity of the AF and associated symptoms.21 Electrical cardioversion may be performed under moderate sedation and analgesia, which delivers energy through the chest wall to convert the heart back into a NSR. This can be done alone or after maximizing beta-blocker or antiarrhythmic drug therapy in an attempt to restore and maintain a NSR.
Catheter ablation may be an appropriate therapy in some patients who cannot achieve rate control with medications.25 Catheter ablation had an estimated 80% success compared with pharmacologic interventions in persistent and paroxysmal AF.21 Catheter ablation can also improve QoL and alleviate symptoms when other treatment strategies have been unsuccessful.21,24 However, in cases with severe symptom progression, catheter ablation with AV nodal ablation and a pacemaker insertion may be an option.21,22 The APRN, as a critical member of the interdisciplinary team, must evaluate the risks and benefits of various medications and therapies in a personalized manner for each patient. Electrophysiology experts should be consulted when appropriate to optimize treatment and management.
Anticoagulation and CHA2DS2-VASc score
A CHA2DS2-VASc score should be documented for all patients with AF to determine thromboembolic risk and guide oral anticoagulation treatment (see CHA2DS2-VASc scoring).41 This risk stratification approach allows providers to stratify low-risk versus high-risk patients and guides anticoagulation by evaluating an individual’s risk factors.41 To calculate a CHA2DS2-VASc score, points are assigned for each risk factor with which the patient presents. If the score is 2 or greater, anticoagulant therapy is recommended per the current guidelines.7,41,42
Anticoagulation therapy options for outpatients include warfarin, the direct thrombin inhibitor dabigatran, or a factor Xa inhibitor, such as rivaroxaban, apixaban, and edoxaban. Warfarin requires blood test monitoring to ensure the international normalized ratio is maintained within a therapeutic range of 2.0 to 3.0 and slightly higher for those with mechanical heart valves.42 The direct thrombin inhibitors and factor Xa inhibitors require no blood test monitoring and thus, adherence to treatment may be difficult to assess. In addition, warfarin has a reversal agent (vitamin K), as well as dabigatran (idarucizumab); however, the factor Xa inhibitors currently do not (andexanet alfa is in clinical trials).7 Therefore, the APRN should consider the patient’s bleeding and fall risk before prescribing an oral anticoagulation agent.
Implications for the APRN
The APRN needs to be aware of the clinical presentation, risk factors, and currently available RM technologies that can aid in AF documentation. RM has evolved over time as the standard of care and has significantly improved the recognition of AF.20,21 However, challenges such as patient engagement with RM, which can wane over time, must be acknowledged.43 Additionally, RM may be difficult for some older patients who may be unfamiliar or have limited use with existing technologies such as smartphones and portal electronic devices, making RM use and training more challenging.
CHA2DS2-VASc scoring
| Risk factors | Points | |
|---|---|---|
| C | Congestive heart failure or left ventricular systolic dysfunction | 1 |
| H | Hypertension | 1 |
| A2 | Age ≥75 years | 2 |
| D | Diabetes mellitus | 1 |
| S2 | History of Stroke, transient ischemic attack, or thromboembolism | 2 |
| V | Vascular disease, including peripheral artery disease, myocardial infarction, or aortic plaque | 1 |
| A | Age 65–74 | 1 |
| Sc | Sex category (female) | 1 |
Source: Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refi ning clinical risk stratifi cation for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263-272. Used with permission.
Additionally, some patients may be unaware that their providers are using RM to enhance and not replace the care they provide. Researchers have found patients who have had more communication earlier with their provider regarding the utility and role of RM in their care were more likely to utilize RM technology.44 Researchers have also shown education of patients regarding the use of RM in the documenting dysrhythmias increases the likelihood of patient acceptance and adherence to RM.43 Patients and families who use RM report a sense of comfort and satisfaction in knowing that they are having their ECG vigilantly monitored and experience an improved QoL.45–47
As technology continues to evolve and RM becomes more integrated into clinical practice, APRNs will need to be knowledgeable about the various RM approaches. As essential members of the interdisciplinary team, APRNs will need to employ RM approaches to better recognize and treat AF and other dysrhythmias.
Footnotes
The authors have disclosed that they have no financial relationships related to this article.
Contributor Information
Kathleen T. Hickey, CUMC School of Nursing, New York, N.Y..
Teresa C. Riga, Columbia University Medical Center, New York, N.Y..
Shazia A. Mitha, CUMC School of Nursing, New York, N.Y..
Meghan J. Reading, Columbia University School of Nursing, New York, N.Y..
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statis-tics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morin DP, Bernard ML, Madias C, Rogers PA, Thihalolipavan S, Estes NA 3rd. The state of the art: atrial fibrillation epidemiology, prevention, and treatment. Mayo Clin Proc. 2016;91(12):1778–1810. [DOI] [PubMed] [Google Scholar]
- 4.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123(14):1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. Atrial Fibrillation Fact Sheet. 2015; www.cdc.gov/dhdsp/data_statis-tics/fact_sheets/fs_atrial_fibrillation.htm.
- 7.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Rev Esp Cardiol (Engl Ed). 2017;70(1):50. [DOI] [PubMed] [Google Scholar]
- 8.Verdino RJ. Untreated atrial fibrillation in the United States of America: understanding the barriers and treatment options. J Saudi Heart Assoc. 2015;27(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherian TS, Shrader P, Fonarow GC, et al. Effect of atrial fibrillation on mortality, stroke risk, and quality-of-life scores in patients with heart failure (from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation [ORBIT-AF]). Am J Cardiol. 2017;119(11):1763–1769. [DOI] [PubMed] [Google Scholar]
- 10.Mittal S, Rogers J, Sarkar S, et al. Real-world performance of an enhanced atrial fibrillation detection algorithm in an insertable cardiac monitor. Heart Rhythm. 2016;13(8):1624–1630. [DOI] [PubMed] [Google Scholar]
- 11.Sanders P, Pürerfellner H, Pokushalov E, et al. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: results from the Reveal LINQ Usability Study. Heart Rhythm. 2016;13(7):1425–1430. [DOI] [PubMed] [Google Scholar]
- 12.Turakhia MP, Kaiser DW. Transforming the care of atrial fibrillation with mobile health. J Interv Card Electrophysiol. 2016;47(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker LR, Pokushalov E, Sanders P, Lindborg KA, Maus B, Pürerfellner H. Continuous cardiac monitoring around atrial fibrillation ablation: insights on clinical classifications and end points. Pacing Clin Electrophysiol. 2016;39(8):805–813. [DOI] [PubMed] [Google Scholar]
- 14.Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol. 2015;5:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin BS, Wong AM, Tseng KC. Community-based ECG monitoring system for patients with cardiovascular diseases. J Med Syst. 2016;40(4):80. [DOI] [PubMed] [Google Scholar]
- 16.Chan PH, Wong CK, Pun L, et al. Head-to-head comparison of the alivecor heart monitor and microlife watchBP office AFIB for atrial fibrillation screening in a primary care setting. Circulation. 2017;135(1):110–112. [DOI] [PubMed] [Google Scholar]
- 17.FDA. AliveCor Heart Monitor. 2017.
- 18.Evans GF, Shirk A, Muturi P, Soliman EZ. Feasibility of using mobile ECG recording technology to detect atrial fibrillation in low-resource settings. Glob Heart. 2017;12(4):285–289. [DOI] [PubMed] [Google Scholar]
- 19.Haberman ZC, Jahn RT, Bose R, et al. Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol. 2015;26(5):520–526. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/ telemetry. Heart Rhythm. 2017;14(7):e55–e96. [DOI] [PubMed] [Google Scholar]
- 21.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–2104. [DOI] [PubMed] [Google Scholar]
- 22.Michaud GF, Stevenson WG. Supraventricular tachyarrhythmias In: Harrison’s Principles of Internal Medicine, 19e New York, NY: McGrawHill; 2014. [Google Scholar]
- 23.Sabatine MS. Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 24.AHA. What are the symptoms of atrial fibrillation (AFib or AF)? 2016. www.heart.org/HEARTORG/Conditions/Arrhythmia/AboutArrhythmia/What-are-the-Symptoms-of-Atrial-Fibrillation-AFib-or-AF_UCM_423777_Article.jsp-.WK7o0xLysdV.
- 25.Simantirakis EN, Papakonstantinou PE, Chlouverakis GI, et al. Asymptomatic versus symptomatic episodes in patients with paroxysmal atrial fibrillation via long-term monitoring with implantable loop recorders. Int J Cardiol. 2017;231:125–130. [DOI] [PubMed] [Google Scholar]
- 26.Gigli L, Ameri P, Secco G, et al. Clinical characteristics and prognostic impact of atrial fibrillation in patients with chronic heart failure. World J Cardiol. 2016;8(11):647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olgun Kucuk H, Kucuk U, Yalcin M, Isilak Z. Time to use mobile health devices to diagnose paroxysmal atrial fibrillation. Int J Cardiol. 2016;222:1061. [DOI] [PubMed] [Google Scholar]
- 28.FDA. Reveal LINQ insertable cardiac monitor (Model LNQ11). 2017.
- 29.Medtronic. Patient Assistant Model 96000. 2017. www.medtronicdiagnostics.com/us/cardiac-monitors/reveal-linq/reveal-linq-cardiac-monitor/patient-assistant/index.htm.
- 30.Steffel J, Wright DJ, Schäfer H, Rashid-Fadel T, Lewalter T. Insertion of miniaturized cardiac monitors outside the catheter operating room: experience and practical advice. Europace. 2017;19(10):1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pürerfellner H, Sanders P, Pokushalov E, Di Bacco M, Bergemann T, Dekker LR. Miniaturized Reveal LINQ insertable cardiac monitoring system: first-in-human experience. Heart Rhythm. 2015;12(6):1113–1119. [DOI] [PubMed] [Google Scholar]
- 32.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486. [DOI] [PubMed] [Google Scholar]
- 33.Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(1):95.e11–95.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FDA. Zio Patch. 2017.
- 35.Turakhia MP, Hoang DD, Zimetbaum P, et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013;112(4):520–524. [DOI] [PubMed] [Google Scholar]
- 36.Cheung CC, Kerr CR, Krahn AD. Comparing 14-day adhesive patch with 24-h Holter monitoring. Future Cardiol. 2014;10(3):319–322. [DOI] [PubMed] [Google Scholar]
- 37.Hickey KT, Hauser NR, Valente LE, et al. A single-center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovasc Disord. 2016;16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickey KT, Dizon J, Frulla A. Detection of recurrent atrial fibrillation utilizing novel technology. J Atr Fibrillation. 2013;6(4):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc. 2016;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frost JL, Campos-Outcalt D, Hoelting D, et al. Atrial fibrillation guideline summary. Ann Fam Med. 2017;15(5):490–491. [Google Scholar]
- 41.Lip GY, Halperin JL. Improving stroke risk stratification in atrial fibrillation. Am J Med. 2010;123(6):484–488. [DOI] [PubMed] [Google Scholar]
- 42.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e152S–e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alkhaldi G, Hamilton FL, Lau R, Webster R, Michie S, Murray E. The Effectiveness of prompts to promote engagement with digital interventions: a systematic review. J Med Internet Res. 2016;18(1):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ottenberg AL, Swetz KM, Mueller LA, Gerhardson S, Mueller PS. “We as Human Beings Get Farther and Farther Apart”: the experiences of patients with remote monitoring systems. Heart Lung. 2013;42(5):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hickey KT, Biviano AB, Garan H, et al. Evaluating the utility of Mhealth ECG heart monitoring for the detection and management of atrial fibrillation in clinical practice. J Atr Fibrillation. 2017;9(5):1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leahy RA, Davenport EE. Home monitoring for cardiovascular implantable electronic devices: benefits to patients and to their follow-up clinic. AACN Adv Crit Care. 2015;26(4):343–355. [DOI] [PubMed] [Google Scholar]
- 47.Ricci RP, Morichelli L, Varma N. Remote monitoring for follow-up of patients with cardiac implantable electronic devices. Arrhythm Electrophysiol Rev. 2014;3(2):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]