Abstract
Background:
Curcuma longa has strong anti-inflammatory effect. This study aims to evaluated the level of anti-Toxoplasma immunoglobulin G and immunoglobulin M (IgG-IgM) antibody after intervention with C. longa extract in early pregnant mice with acute toxoplasmosis.
Materials and Methods:
We evaluated 20 early pregnant mice that were divided into five groups, four mice in each. Group 1-4 received injections of Toxoplasma gondii tachyzoites. Three days later, G1 and G2 were given orally 125 mg/kg/day and 500 mg/kg/day of C. longa extract, respectively. G3 was given 60 mg/kg/day of spiramycin (positive control), and G4 was given 0.2 ml of distilled water (negative control). G5 underwent no intervention at all. Blood samples were obtained serially (before and 3 days after injection of tachyzoites, 3 days and 7 days after intervention) to assess anti-Toxoplasma IgG-IgM antibody levels by enzyme-linked immunosorbent assay methods.
Results:
Anti-Toxoplasma IgG-IgM antibody levels increased significantly 3 days after injection of tachyzoites (P < 0.05), but decreased significantly (P < 0.05) 3 days, and 7 days after administration of C. longa extract dose 125 mg, 500 mg, and spiramycin 60 mg, and there was no significant difference between these three groups. Anti-Toxoplasma IgG-IgM antibody levels increased significantly (P < 0.05) 3 days, 6 days, and 10 days after injections of tachyzoites on G4. The IgG-IgM antibody levels fluctuated on G5 and considered as insignificant (P > 0.05).
Conclusion:
The administration of C. longa extract at a dose of 125 mg/kg/day for 7 days effectively decreased anti-Toxoplasma IgG-IgM antibody level in early pregnant mice with acute toxoplasmosis.
Keywords: Curcuma longa, early pregnancy, immunoglobulin G, immunoglobulin M, toxoplasmosis
INTRODUCTION
Toxoplasmosis is a zoonotic disease caused by the Toxoplasma gondii.[1] Humans can be infected through several ways,[2] if infected during early pregnancy can lead to congenital abnormalities, fetal death, and abortion.[3] T. gondii infection will trigger the production of antibody level. The antibodies level is associated with virulence, and the number of parasites.[4,5] Examination of immuno-globulin G and -immuno-globulin M (IgG-IgM) antibody to taking diagnosis and also to determine the efficacy of a therapy.[6,7] The diagnosis of acute infection depends on detection of Toxoplasma-specific IgG and IgM antibodies.[8] IgG avidity test and IgM analysis are considered to be suitable methods for determining acute infections. Recently, recombinant fusion proteins have been used in an attempt to improve serologic tests for diagnosis of T. gondii.[9,10]
Appropriate therapy in early pregnancy in the acute phase of toxoplasmosis will help to decrease the probability of transmission to the fetus, and prevent potential damage in case of transplacental infection. However, these therapies may be teratogenic and/or may sometimes generate intolerance to women. Therefore, it is very important to treat only patient whose acute infection has been proven. Consequently, an accurate diagnosis of this infection phase is necessary.[11]
As revealed by Serranti et al., until now there has been no ideal drug as toxoplasmosis therapy in pregnant women.[12] Spiramycin is still the drug of choice, but it proves unable to eradicate infection in the fetus. Spiramycin works as bacteriostatic and bactericidal proven to decrease the rate of growth of bacteria/parasite load.[13,14] Damage to embryonic tissue cells/placental tissue cells may occur directly from the parasite, but embryonic cell tissue damage or placental tissue may also occur indirectly from excessive pro-inflammatory levels[15,16,17] that cannot be controlled/influenced by spiramycin.
Yellow turmeric (Curcuma longa) extract, which has been shown to be an anti-inflammatory agent[18] and also has antimicrobial, although it is more likely to be simply anti-inflammatory.[19,20] This study aims to demonstrate the effectiveness of the C. longa as a treatment in early pregnant mice with acute toxoplasmosis by analyzing anti-Toxoplasma IgG-IgM antibody level, hoping that our result will provide additional information for further research.
MATERIALS AND METHODS
This study was conducted using 20 early pregnant mice and was divided into five groups (G1-G5) randomly, and 4 mice each group. At the end of the study, the mice were sacrificed then buried in specific places. The procedures for animal preservation and intervention in this study were according to the procedures of the U. K. Animal (Scientific Procedures) Act, 1986 and associated guidelines. This research had been approved by the animal ethics research committee of the Faculty of Medicine, University of Hasanuddin, Makassar, Indonesia.
Modeling and intervention sampling
The adult female mice Balb/c fulfilling the inclusion criteria (age 11–13 weeks, weight 18–20 g, active movement) were placed in a stable with a healthy and proven mature male mice. Mice were pregnant if a plug is found in the vagina and is said to be a 0 day pregnancy. gestational age in this study 0–2 days. The 20 early pregnant mice and were divided into five groups (G1-G5) randomly, and 4 mice each group. The G1-G4 was injected with 10 tachyzoites of T. gondii RH strain intraperitoneal, and G5 without infection. Three days after injection of tachyzoites, G1 and G2 were given C. longa extract 125 and 500 mg/kg/day respectively, G3 (positive control) was given spiramycin of 60 mg/day, and G4 (negative control) was given 0.2 ml of distilled water. Each intervention was administered orally using cannulas for 7 days. G5 underwent no intervention at all.
Examination of blood samples
Blood samples were taken from the tail vein serially (1 day before tachyzoites injection, 3 days after tachyzoites injection, and 3 and 7 days after the intervention). Anti-Toxoplasma IgG/IgM antibody levels were determined by enzyme-linked immunosorbent assay (ELISA) method, using a qualitative mouse antibody IgG (toxoplasma[TP]-IgG) ELISA kit and a qualitative mouse antibody IgM (TP-IgM) ELISA kit.
Statistical analysis
Research data were obtained, tabulated, and processed using SPSS version 20 software. The data were analyzed statistically using paired t-test and one-way ANOVA test. The P < 0.05 is used.
RESULTS
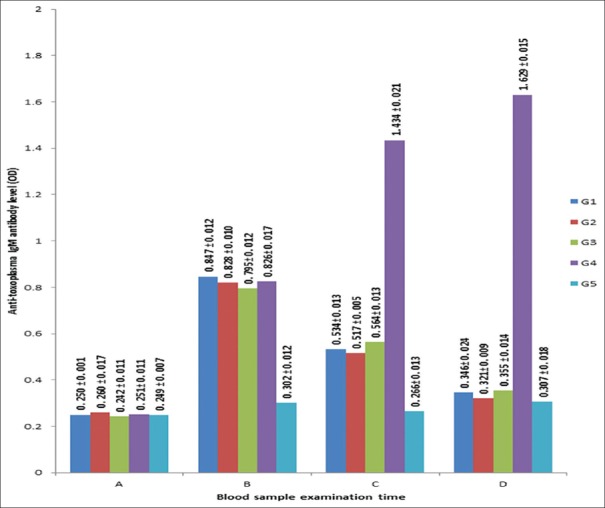
Anti-Toxoplasma IgM antibody results were shown in Figure 1. Anti-Toxoplasma IgM antibody level increased significantly (P < 0.05) 3 days after tachyzoites injection. Anti-Toxoplasma IgM antibody level in G1-G3 decrease significantly (P < 0.05) 3 days, and 7 days after treatment with C. longa extract doses of 125 mg/kg/day, 500 mg/kg/day, and spiramycin 60 mg/kg/day, but no significant differences among these groups (P > 0.05). Anti-Toxoplasma IgM antibody level in G4 increased significantly (P < 0.05) at 3, 6, 10 days after injection of tachyzoites. Anti-Toxoplasma IgM antibody level in G5 fluctuated at the same period, but no significant changes (P > 0.05).
Figure 1.
Anti-Toxoplasma immuno-globulin M antibody levels before and after intervention. Early pregnant mice group (g). G1 were injected of tachyzoites and intervened with Curcuma longa extract 125 mg/kg/day, G2 were injected of tachyzoites and intervened with Curcuma longa extract 500 mg/kg/day, G3 were injected of tachyzoites and intervened with Spiramycin 60 mg/kg/day, G4 were injected of tachyzoites and intervened with distilled water 0.2 ml/day, G5 were not injected nor intervented. Time of sample collection: A (1 day before tachyzoites injection), B (3 days after tachyzoites injection), C (3 days after the intervention), D (7 days after the intervention). Optical density
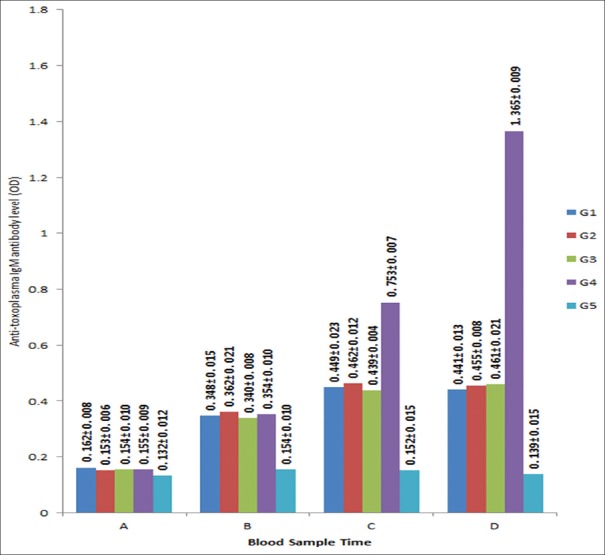
Anti-Toxoplasma IgG antibody results were shown Figure 2. Anti-Toxoplasma IgG antibody level increased significantly (P < 0.05) 3 days after tachyzoites injection. Anti-Toxoplasma IgG antibody level in G1-G3 decrease significantly (P < 0.05) 3 days, and 7 days after treatment with C. longa extract doses of 125 mg/kg/day, 500 mg/kg/day, and spiramycin 60 mg/kg/day, but no significant differences among these groups (P > 0.05). Anti-Toxoplasma IgG antibody level in G4 increased significantly (P < 0.05) at 3, 6, 10 days after injection of tachyzoites. Anti-Toxoplasma IgG antibody level in G5 fluctuated at the same period, but no significant changes were noted (P > 0.05).
Figure 2.
Anti-Toxoplasma immuno-globulin G antibody levels before and after intervention. Early pregnant mice group (g), G1 were injected with tachyzoites and intervened with Curcuma longa extract 125 mg/kg/day, G2 were injectected with tachyzoites and intervened with Curcuma longa extract 500 mg/kg/day, G3 were injected of tachyzoites and intervened with Spiramycin 60 mg/kg/day, G4 were injected of tachyzoites and intervened with distilled water 0.2 ml/day, G5 were neither injected nor intervented. Time of sample collection: A (1 day before tachyzoites injection), B (3 days after tachyzoites injection), C (3 days after the intervention), D (7 days after the intervention). Optical density
DISCUSSION
The infection of T. gondii will trigger the humoral and cellular immune response. Humoral immunity such as anti-Toxoplasma IgG-IgM antibody level increase in the event of infection of T. gondii.[21] In our study, after injection of T. gondii tachyzoites, the anti-toxoplasma IgG-IgM antibody level increased significantly (P < 0.05) after 3 days, 6 days, and 10 days Figure 1 and Figure 2.
Spiramycin is one of the macrolide antibiotics; it inhibits the synthesis of both in vivo and in vitro bacterial proteins with various potentials. Macrolides are generally bacteriostatic, although some of these drugs may be bactericidal at very high concentrations.[13] Spiramycin is the drug of choice of toxoplasmosis, but it has been proven failed to eradicate fetal toxoplasmosis. Pyrimethamine and sulfadiazine are given when the polymerase chain reaction test is positive, as it may penetrate the placenta, but pyrimethamine and sulfadiazine are not given in suspected infections especially in the first trimester of pregnancy because of the potential teratogenic and toxic effects on fetal bone marrow.[14]
Curcumin is a major component of C. longa,[22] it has been shown to be a potent anti-inflammatory agent,[18,23,24] with additional antimicrobial,[25,26] and antioxidant properties.[27] Al-Zanbagi et al., reported that administration of C. longa extract dose of 100 mg/kg/day and doses of 200 mg/kg/day for 7 days, intraperitoneal injected female mice 2 × 102 toxoplasma/ml, is highly effective in inhibiting the rate of growth of tachyzoites 98.6% and 99.2%, compared to control group, as well as spiramycin dose of 100 mg/kg/day and 200 mg/kg/day inhibited tachyzoites growth rate of 71% and 94%.[17]
In our study, we found that anti-Toxoplasma IgG-IgM antibody level decreased significantly 3 days and 7 days after intervention with C. longa extract doses of 125 mg/kg/days, 500 mg/kg/days, and spiramycin 60 mg/kg/day. The decrease in levels of anti-Toxoplasma IgG-IgM antibody in the group that were given the extract of C. longa was greater than the spiramycin group (positive control), but the difference was not significant. Decreased levels of anti-Toxoplasma IgG-IgM antibodies in this study may be related to effectiveness of C. longa as anti-inflammatory,[18,23,24] antimicrobial,[25,26] and antioxidant,[27] thus inhibiting the rate of growth of tachyzoites. Based on the results of this study and the results of previous researchers who have been reported, the C. longa extract may be considered as an alternative treatment in early pregnancy with acute toxoplasmosis, but further research is required with larger sample and a more comprehensive research.
CONCLUSION
The administration of C. longa extract dose of 125 mg/kg/day for 7 days effectively decreased anti-Toxoplasma IgG-IgM antibody level in early pregnant mice with acute toxoplasmosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Burhanuddin, Prof. Nurpujiastuti A. Taslim, Prof. Nusratuddin Abdullah, Dr. Carmen Siagian, Hasnawati Saleh Ph. D, and the laboratory staffs of Molecular Biology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University, Makassar and also to Phytochemistry Laboratory, Faculty of Pharmacy, Hasanuddin, Makassar, for their help in this study.
REFERENCES
- 1.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: From animals to humans. Int J Parasitol. 2000;30:1217–58. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham FG, Leveno KJ, Bloom SL. Williams Obstetrics. 24th ed. New York: McGraw-Hill Education; 2014. Infectious diseases: Toxoplasmosis; pp. 1254–6. [Google Scholar]
- 3.Khurana S, Bagga R, Aggarwal A, Lyngdoh V, Shivapriya, Diddi K, et al. Serological screening for antenatal toxoplasma infection in India. Indian J Med Microbiol. 2010;28:143–6. doi: 10.4103/0255-0857.62492. [DOI] [PubMed] [Google Scholar]
- 4.Angeloni MB, Guirelli PM, Franco PS, Barbosa BF, Gomes AO, Castro AS, et al. Differential apoptosis in BeWo cells after infection with highly (RH) or moderately (ME49) virulent strains of Toxoplasma gondii is related to the cytokine profile secreted, the death receptor Fas expression and phosphorylated ERK1/2 expression. Placenta. 2013;34:973–82. doi: 10.1016/j.placenta.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Jennes M, De Craeye S, Devriendt B, Dierick K, Dorny P, Cox E, et al. Strain- and dose-dependent reduction of Toxoplasma gondii burden in pigs is associated with interferon-gamma production by CD8+lymphocytes in a heterologous challenge model. Front Cell Infect Microbiol. 2017;7:232. doi: 10.3389/fcimb.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murat JB, Hidalgo HF, Brenier-Pinchart MP, Pelloux H. Human toxoplasmosis: Which biological diagnostic tests are best suited to which clinical situations? Expert Rev Anti Infect Ther. 2013;11:943–56. doi: 10.1586/14787210.2013.825441. [DOI] [PubMed] [Google Scholar]
- 7.Dard C, Fricker-Hidalgo H, Brenier-Pinchart MP, Pelloux H. Relevance of and new developments in serology for toxoplasmosis. Trends Parasitol. 2016;32:492–506. doi: 10.1016/j.pt.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Beaman MH, McCabe RE, Wong SY, Remington JS. Toxoplasma gondii. In: Mandel GL, Bennett JE, Dolin R, editors. Principles and Practices of Infectious Diseases. 4th ed. New York, USA: Churchill and Livingstone, Inc; 1995. pp. 2455–75. [Google Scholar]
- 9.Johnson AM, Illana S. Cloning of Toxoplasma gondii gene fragments encoding diagnostic antigens. Gene. 1991;99:127–32. doi: 10.1016/0378-1119(91)90044-c. [DOI] [PubMed] [Google Scholar]
- 10.Tenter AM, Johnson AM. Recognition of recombinant Toxoplasma gondii antigens by human sera in an ELISA. Parasitol Res. 1991;77:197–203. doi: 10.1007/BF00930858. [DOI] [PubMed] [Google Scholar]
- 11.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–96. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serranti D, Buonsenso D, Valentini P. Congenital toxoplasmosis. Departement of pediatrics, catholic university of sacred heart, Rome (Italy) Euro review for medical and pharmacological sciences. 2011;15:193–8. [PubMed] [Google Scholar]
- 13.Brisson-Noël A, Trieu-Cuot P, Courvalin P. Mechanism of action of spiramycin and other macrolides. J Antimicrob Chemother. 1988;22(Suppl B):13–23. doi: 10.1093/jac/22.supplement_b.13. [DOI] [PubMed] [Google Scholar]
- 14.Valentini P, Buonsenso D, Barone G, Serranti D, Calzedda R, Ceccarelli M, et al. Spiramycin/cotrimoxazole versus pyrimethamine/sulfonamide and spiramycin alone for the treatment of toxoplasmosis in pregnancy. J Perinatol. 2015;35:90–4. doi: 10.1038/jp.2014.161. [DOI] [PubMed] [Google Scholar]
- 15.Senegas A, Villard O, Neuville A, Marcellin L, Pfaff AW, Steinmetz T, et al. Toxoplasma gondii-induced foetal resorption in mice involves interferon-gamma-induced apoptosis and spiral artery dilation at the maternofoetal interface. Int J Parasitol. 2009;39:481–7. doi: 10.1016/j.ijpara.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Hampson J, McLaughlin PJ, Johnson PM. Low-affinity receptors for tumour necrosis factor-alpha, interferon-gamma and granulocyte-macrophage colony-stimulating factor are expressed on human placental syncytiotrophoblast. Immunology. 1993;79:485–90. [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Zanbagi NA. In vivo effect of some home spices extracts on the Toxoplasma gondii tachyzoites. J Family Community Med. 2009;16:59–65. [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Gupta SC, Sung B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–92. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K, et al. Areview on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One. 2015;10:e0121313. doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simanjuntak TP, Hatta M, Sirait RH, Karo M, Sirait LI, Aritonang TR, et al. Analysis concentration of Toxoplasma gondii on anti-toxoplasma IgG-IgM antibody Levels, and the outcomes of pregnancy in mice Balb/c. Open J Obstet Gynecol. 2017;7:281–9. [Google Scholar]
- 22.Araújo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–8. doi: 10.1590/s0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- 23.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern Med Rev. 2009;14:141–53. [PubMed] [Google Scholar]
- 24.Guo YZ, He P, Feng AM. Effect of curcumin on expressions of NF-κBp65, TNF-α and IL-8 in placental tissue of premature birth of infected mice. Asian Pac J Trop Med. 2017;10:175–8. doi: 10.1016/j.apjtm.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Li LM, Li J, Zhang XY. Antimicrobial and molecular interaction studies on derivatives of curcumin against Streptococcus pneumonia which caused pneumonia. E J Biotechnol. 2016;19:8–14. [Google Scholar]
- 26.Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K, et al. Areview on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mişe Yonar S, Yonar ME, Ural MŞ. Antioxidant effect of curcumin against exposure to malathion in Cyprinus carpio. Cell Mol Biol (Noisy-le-grand) 2017;63:68–72. doi: 10.14715/cmb/2017.63.3.13. [DOI] [PubMed] [Google Scholar]