Abstract
Context:
Polycystic ovary syndrome (PCOS) is the commonest endocrine disorder affecting young women. Kisspeptins are a family of closely related peptides encoded by Kiss1 gene that controls the hypothalamic–pituitary–gonadal axis by binding to its receptor (GPR54) expressed in gonadotropin-releasing hormone (GnRH) neurons and releases GnRH. Since GnRH secretion is deregulated in PCOS, we hypothesized that dysregulated gonadotropin secretion in PCOS is reflected by kisspeptin levels.
Aim:
We aimed to measure serum kisspeptin levels of subjects with well-characterized PCOS versus controls and explore any correlation between kisspeptin and PCOS-related reproductive and metabolic disturbances.
Materials and Methods:
Consecutive women with PCOS manifesting from adolescence (n = 55) and adult controls (n = 110) were recruited. Pre-treatment baseline clinical, anthropometry, and biochemical parameters were measured in all. Serum kisspeptin and testosterone levels were determined by enzyme-linked immunosorbent assay method.
Results:
Serum kisspeptin and testosterone concentrations were significantly higher in women with PCOS (kisspeptin 4.873 nmol/L; testosterone 4.713 nmol/L) than controls (kisspeptin 4.127 nmol/L; testosterone 3.415 nmol/L; P < 0.05). Serum kisspeptin levels were positively associated with PCOS (odds ratio: 1.853; 95% confidence interval: 1.246–2.755; P = 0.002) in our studied population.
Conclusion:
Serum kisspeptin levels are higher in Sri Lankan women with PCOS manifesting from adolescence compared with controls regardless of body mass index. We propose serum kisspeptin concentration as a useful marker to recognize PCOS that manifests from adolescence.
KEY WORDS: Adolescence, GPR54, kisspeptin, KISS1, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is the commonest endocrine disorder in women of reproductive age.[1] The diagnosis of PCOS during adolescence is confirmed when all three classic features are evident, that is, oligo/amenorrhea, androgen excess (hirsutism), and polycystic ovaries.[2,3] This well-characterized phenotype is commonly associated with insulin resistance (IR), obesity, and future metabolic disease.[4,5,6,7] South Asians with PCOS have a notable ethnic variation in the manifestation of PCOS, occurring from a younger age and with a greater degree of IR when compared with white Europeans.[7,8] Its etiology, though yet unclear, is presumed to have an oligogenic basis interacting with environmental factors.[9,10,11]
Female reproductive function depends on proper development and regulation of hypothalamic–pituitary–gonadal (HPG) axis. The KISS1 gene has been recently recognized as an essential regulator of HPG axis, which is responsible for its timely activation at puberty and in normal cyclical function during adult life.[12,13] Kisspeptins are a family of closely related peptides encoded by KISS1 gene that act through G-protein-coupled receptor known as GPR54.[12,13,14] Although KISS1 gene was originally identified in 1996 as a suppressor of metastases of human malignant melanoma,[15] its role in reproduction was identified in 2003 with a better understanding of neuroendocrine regulation of human reproduction from puberty.[16,17] Kisspeptin binds to GPR54 receptor expressed in gonadotropin-releasing hormone (GnRH) neurons and participates in the control of HPG axis.[12,13,18,19] By binding to its receptor, kisspeptin stimulates the release of GnRH into portal circulation, which in turn stimulates the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from gonadotrophe cells of the anterior pituitary.[20] Since kisspeptin is a key central regulator of GnRH secretion, it can be hypothesized that the dysregulated gonadotropin secretion in PCOS is a reflection of altered kisspeptin inputs to GnRH neurons.
Any association of kisspeptin with PCOS is an unexplored area of study. Due to complex relationship between kisspeptin and HPG axis, this study was planned to measure the kisspeptin levels in PCOS and to analyze correlations between kisspeptin and PCOS.
We aimed to identify any relationship between kisspeptin levels in young Sri Lankan women with well-characterized PCOS, which manifested from adolescence, and compare with ethnically matched adult women without PCOS.
Materials and Methods
Sample size calculation
The following formula was used in sample size calculation of our ongoing study on association of single-nucleotide polymorphisms (SNPs) with PCOS and the same sample size subjects was used in this study on kisspeptin levels.[21]
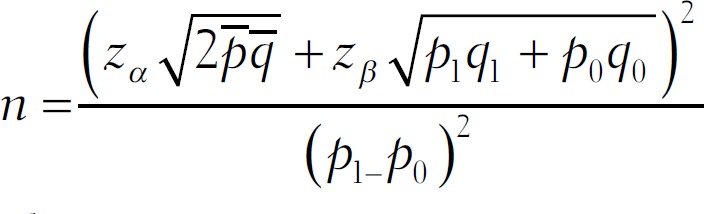
where
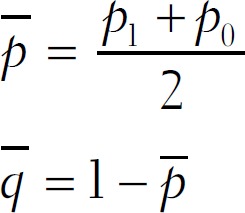
q1 = 1–p1
q0 = 1–p0
n = number of subjects in each group.
zα = corresponding to α (level of significance (95%)) = 1.96.
zβ = corresponding to β (probability of type II error; power of study is 80%, 1- β = 0.2).
p0 = proportion of exposure among control groups (prevalence of polymorphism in general population without PCOS).
The exposure rate of allele frequency of SNPs among controls (30%–44%) was based on literature from other countries as no studies have been done in Sri Lanka.[22,23,24,25]
The estimated proportion of 38% was used in sample size calculation.
R = odds ratio associated with exposure
The literature indicated odds ratio (OR) of occurence of any SNP among PCOS to be in the range of 1.1–2.1.[22,23,24,25]
This study used an OR of 2.7 in its sample size calculation because Sri Lankans with anovulatory PCOS manifest severe symptoms at a younger age, with greater IR and a higher prevalence of metabolic syndrome than white Europeans.[7] Therefore, association of kisspeptin with PCOS is likely to be much higher among Sri Lankan PCOS women.
P1 = Proportion of exposure among cases was calculated using the following formula
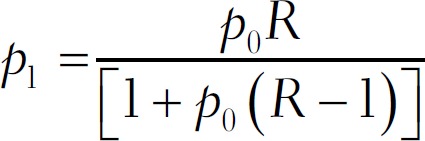
The number of consecutive subjects with confirmed PCOS commencing from adolescence was 50, to which 10% was added to make allowance for noncompliance/drop outs and so on. Therefore, the number of cases was 55. By selecting double the number of controls per case, the final total number of controls was 110.
Recruitment of subjects
This study was approved by the Ethical Review Committee, Faculty of Medicine, University of Colombo, Sri Lanka. Written informed consent was obtained from all participants. Cases were recruited from the Endocrine Clinic of the University Obstetrics and Gynaecology Department. Diagnosis of PCOS was based on Rotterdam criteria,[3] with diagnostic certification made by a single clinical lead.
Inclusion criteria
Inclusion criteria were women whose symptoms manifested from adolescent years (11–19 years WHO), with all three diagnostic criteria present from 16 to 19 years of age.[26]
Anovular PCOS or amenorrhea/oligomenorrhea: Anovular cycles are defined when the cycle length is more than 35 days and the lack of demonstrable ovulation by mid cycle and luteal phase ultrasound scans, and mid-luteal serum progesterone.[26] Amenorrhea is the absence of menstrual periods for 6 months or more in a woman who has previously been menstruating. Oligomenorrhea is the menstrual periods occurring at intervals of greater than 35 days, with only four to nine periods in a year.
Polycystic ovaries on ultrasound: It is defined by transvaginal or transabdominal ultrasound scan of ovaries, performed within the first 5 days from the onset of menstruation, and finding 12 or more follicles, measuring between 2 and 9 mm and/or an ovarian volume >10 cm3.[3]
Hyperandrogenism: Clinical evidence of hirsutism modified Ferriman–Gallwey score (mFG) ≥8, serum testosterone (T) >3.5 nmol/L, and/or free androgen index (FAI) >5.[26]
Exclusion criteria
Exclusion criteria included inherited disorders of IR such as Rabson–Mendenhall syndrome, Cushing syndrome, hyperprolactinemia, untreated primary hypothyroidism, congenital adrenal hyperplasia, or an androgen secreting ovarian/adrenal tumor; those taking corticosteroid, antiepileptic, or antipsychotic drugs; history of hormonal contraception within the previous 6 months; pregnancy; and the first postpartum year.
Control sample
Concurrently asymptomatic, normo-androgenic, normal cycling since adolescence, unmedicated, consenting women of reproductive age in whom PCOS was objectively excluded by clinical, biochemical, and ultrasound assessment were recruited as controls. The control subjects were recruited from a single work setting where health promotion programs were conducted from 2012 (3 years before the study). Working women of similar ethnic and social background as the affected subjects were invited to participate in the study.
Clinical, biochemical, and endocrine evaluation
Clinical evaluation
Clinical evaluation was by a questionnaire-based interview regarding sociodemographic factors, detailed menstrual and obstetric histories, infertility if relevant, the onset and degree of clinical symptoms of PCOS, drug history, family history of diabetes, and other cardiovascular risk factors. Detailed physical examination included measurement of standing height to the closest centimeter[27] and weight in kilograms to calculate the body mass index (BMI), waist and hip circumference and waist-to-hip ratio (WHR), resting blood pressure,[28] hirsutism (FG score), frontal balding, distribution of acne, and acanthosis nigricans.[29]
Evaluation of the modified FG score was done by a single medically qualified clinical lead of the Department of Obstetrics and Gynecology, Faculty of Medicine, University of Colombo.
Ultrasound examinations were performed by a single trained medically qualified ultrasonographer under the supervision of the radiology lead of De Soysa Hospital for Women, Colombo.
Biochemical and endocrine evaluation
Biochemical and endocrine evaluation involved determining the kisspeptin and testosterone levels in serum by enzyme-linked immunosorbent assay kit (ELISA kit). Venous blood was collected from each subject and serum was extracted. Serum kisspeptin and testosterone levels were measured with ELISA kits (Phoenix Pharmaceuticals Inc., Belmont, CA, USA and Teco Diagnostics, Anaheim, CA, USA) as per manufactures’ recommendation. Analytical sensitivity of kisspeptin assay is 0.08 ng/mL and testosterone assay is 0.06 ng/mL. Samples were tested along with the standards and positive controls. Standards, controls, and samples were assayed in duplicates. Concentration of the samples was determined from standard curve of known concentrations. Routine laboratory tests performed to diagnose/monitor PCOS (follicular phase FSH, LH, thyroid-stimulating hormone, and fasting blood glucose/75 g oral glucose tolerance test) which were carried out at quality-controlled laboratory of the National Hospital Colombo were recorded.
Statistical analysis
Kolmogorov–Smirnov test was used to test the normality of distribution. Values with a biological distribution are presented as mean ± standard deviation. Comparison of means between those with PCOS and controls was performed with independent sample t-test for normal values. Comparisons between the four groups were performed with multivariate general linear model-based one-way analysis of variance. Post hoc analysis was performed with Dunnette's T3 test. Calculation of Spearman's coefficient was used to assess correlation of plasma kisspeptin with each parameter. Determining the best predictors of PCOS was evaluated by multiple logistic regression analysis with backward LR procedure. All analyses were performed by SPSS software (v. 18.0 SPSS, Inc., Chicago, IL, USA). The level of significance was set as 5%.
Results
Demographic, clinical, and hormonal characteristics of women with PCOS and controls are summarized in Table 1. No significant difference in age, fasting blood glucose level, and WHR was observed. Women with PCOS had significantly higher BMI and mFG score.
Table 1.
Demographic, clinical, biochemical and hormonal characteristic of the study and control populations
| PCOS (n=55) | Controls (n=110) | P | |
|---|---|---|---|
| Demographic | |||
| Age (years) | 24.67±0.883 | 33.80±0.528 | 0.061 |
| Clinical | |||
| BMI (kg/m2) | 26.89±0.716 | 25.25±0.344 | 0.007 |
| mFG score | 8±0.445 | 3±0.222 | 0.006 |
| WC:HC | 0.839±0.008 | 0.824±0.004 | 0.114 |
| Biochemical/Hormonal | |||
| FBG (mg/dL) | 98.81±2.08 | 108.69±2.74 | 0.284 |
| Kisspeptin (nmol/L) | 4.873±0.238 | 4.127±0.132 | 0.033 |
| Testosterone (nmol/L) | 4.713±0.458 | 3.415±0.256 | 0.018 |
| TSH (µIU/mL) | 1.96±0.346 | Not done | - |
| FSH (mIU/mL) | 5.5±0.430 | Not done | - |
| LH (mIU/mL) | 7.34±1.198 | Not done | - |
PCOS: polycystic ovary syndrome, BMI: body mass index, mFG: modified Ferriman–Gallwey score, WC: HC: waist circumference: hip circumference, FBG: fasting blood glucose level, TSH: thyroid-stimulating hormone, FSH: follicle-stimulating hormone, LH: luteinizing hormone Data are given as mean±standard error of the mean
Serum kisspeptin and testosterone concentrations were significantly higher [Table 1, Figures 1 and 2] in women with PCOS (kisspeptin 4.873 nmol/L ± 1.76; testosterone 4.713 nmol/L ± 3.39) than controls (kisspeptin 4.127 nmol/L ± 1.38; testosterone 3.415 nmol/L ± 2.68; P < 0.05).
Figure 1.
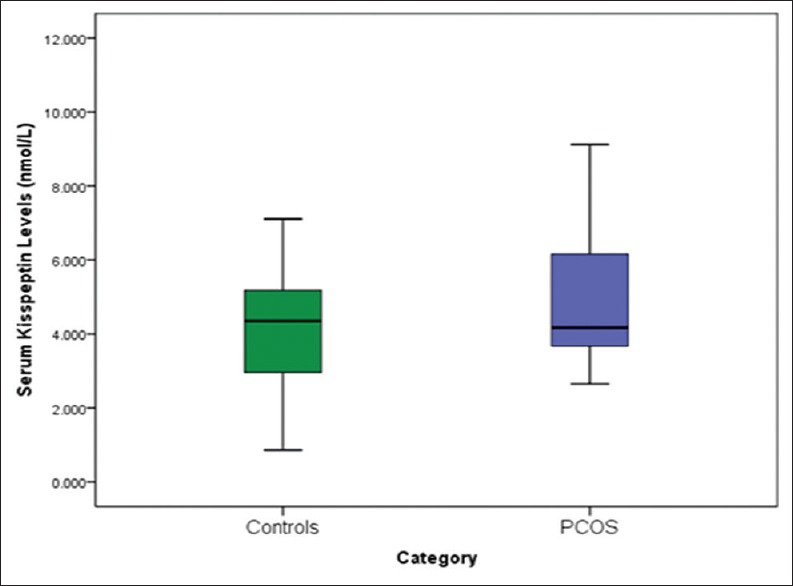
Serum kisspeptin levels of women with PCOS and controls
Figure 2.
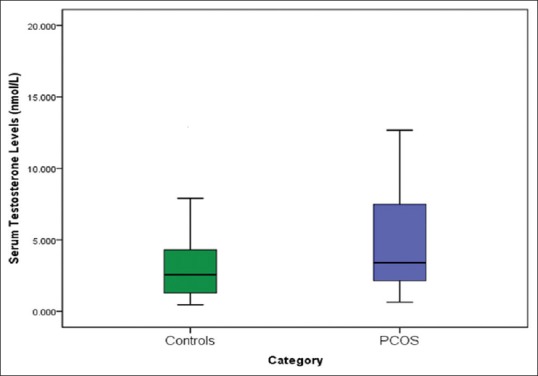
Serum testosterone levels of women with PCOS and controls The horizontal line in the middle of each box indicates the median (50th percentile) testosterone values, whereas the top and bottom borders of the box mark the 25th and 75th percentiles, respectively. The whiskers above and below the box indicate the maximum and minimum testosterone levels, respectively
When subjects were further subdivided based on BMI (BMI < 25 kg/m2 and BMI ≥ 25 kg/m2) in both groups, kisspeptin levels continued to be higher in those with PCOS subgroups regardless of BMI [Table 2, Figure 3]. When obese and overweight women (BMI ≥ 25 kg/m2) and lean (BMI < 25kg/m2) women were analyzed within their own group, PCOS group showed higher kisspeptin levels than controls (4.795 versus 4.263 nmol/L and 5.000 versus 3.995 nmol/L, respectively). Although kisspeptin levels were significantly higher in PCOS group than controls (P < 0.05), there was no significant difference between the subgroups – obese and overweight PCOS versus obese and overweight controls and lean PCOS versus lean controls (post hoc P > 0.05). Similar results were observed with serum testosterone concentration. Serum testosterone was higher in women with PCOS regardless of BMI. However, when obese and overweight women were analyzed within their own groups, testosterone levels were significantly higher in PCOS group than controls (P = 0.002) [Table 2].
Table 2.
Demographic, clinical, biochemical and hormonal characteristics of subgroups of women with PCOS and controls based on BMI
| BMI <25 | BMI ≥25 | |||||
|---|---|---|---|---|---|---|
| PCOS (n=21) | Controls (n=56) | P | PCOS (n=34) | Controls (n=54) | P | |
| Age (Years) | 24.52±5.36 | 33.45±5.46 | 0.894 | 24.76±7.29 | 34.17±5.66 | 0.044 |
| BMI (Kg/m2) | 22.29±2.24 | 22.58±2.02 | 0.917 | 29.73±4.61 | 28.01±2.65 | 0.001 |
| mFG score | 8±5 | 3±0 | 0.375 | 3±6 | 1±0 | 0.001 |
| Kisspeptin (nmol/L) | 5.000±1.79 | 3.995±1.42 | 0.131 | 4.795±1.81 | 4.263±1.34 | 0.136 |
| Testosterone (nmol/L) | 4.594±2.84 | 3.58±3.07 | 0.898 | 4.786±3.67 | 3.244±2.20 | 0.002 |
Data are given as mean±standard error of the mean
Figure 3.
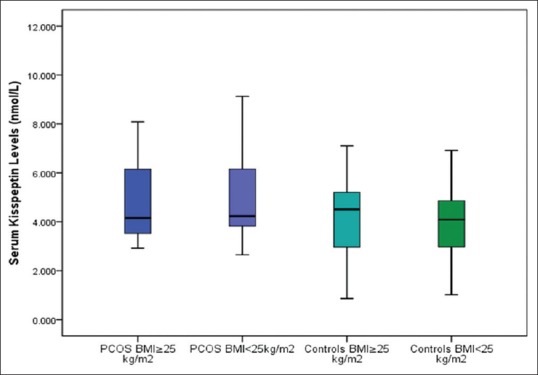
Serum kisspeptin levels of PCOS subjects and controls with regard to BMI The horizontal line in the middle of each box indicates the median (50th percentile) kisspeptin values, whereas the top and bottom borders of the box mark the 25th and 75th percentiles, respectively. The whiskers above and below the box indicate the maximum and minimum kisspeptin levels, respectively
The best predictors of PCOS were evaluated by multiple logistic regression model and were statistically significant with a Hosmer and Lemeshow test value of 0.79 (P > 0.1 was taken as significant). The model also explained 71% (Nagelkerke R2) of the variance among those with PCOS and correctly classified 92% of the cases, which confirms a good fit. In addition, we found that kisspeptin levels [OR: 1.853; 95% confidence interval (CI): 1.246–2.755; P = 0.002] and mFG scores (OR: 2.158; 95% CI: 1.719–2.708; P = 0.0001) alone were positively associated with PCOS in our studied population [Table 3]. Thus, our results suggest that kisspeptin might be an independent marker for PCOS.
Table 3.
Multiple regression analysis of possible factors affecting PCOS
| Variables | OR | 95% CI | Wald | P | |
|---|---|---|---|---|---|
| Minimum | Maximum | ||||
| BMI | 1.061 | 0.943 | 1.194 | 0.971 | 0.324 |
| mFG | 2.158 | 1.719 | 2.708 | 44.000 | 0.000 |
| Kisspeptin | 1.853 | 1.246 | 2.755 | 9.279 | 0.002 |
| Testosterone | 1.289 | 0.714 | 2.324 | 0.710 | 0.40 |
PCOS: polycystic ovary syndrome, OR: odds ratio, CI: confidence interval, BMI: body mass index, mFG: modified Ferriman–Gallwey score
Discussion
The pathogenesis of PCOS remains unclear with multiple factors that determine its expression. A single cause for PCOS is unlikely.[30] Several studies have been carried out to determine the role of kisspeptin in PCOS. Chen et al.[31] Jeon et al.,[32] and Yilmaz et al.[33] have reported significantly higher kisspeptin levels in women with PCOS when compared with control subjects that are similar to our current findings. Furthermore, Yilmaz et al. showed that women with PCOS exhibit higher kisspeptin levels than controls, even after controlling for BMI.[33] Our study also demonstrates that kisspeptin levels were significantly higher in PCOS group than ethnically matched controls, with no significant difference observed based on BMI. Although it can be argued that the relatively small sample sizes within each subgroup may explain the lack of any relationship of kisspeptin with BMI, it is noteworthy that the overall kisspeptin levels showed a positive correlation with BMI, serum testosterone, and mFG scores that did not reach significance. In addition, in this study, cases and control samples were not strictly age-matched where the mean age of controls was higher than PCOS subjects, although this did not reach statistical significance. This may have led to the lack of an overt relationship between kisspeptin and BMI. One reason for us selecting an older cohort of volunteer women to recruit controls was to ensure that they had regular cycles since adolescence, had achieved their fertility, and thereby deemed to clearly not have any form of even the milder phenotype of PCOS.
Nevertheless, Panidis et al.[34] indicated that obese and overweight women with PCOS had significantly lower kisspeptin levels when compared with normal weight women with the syndrome, and that kisspeptin was negatively associated with Free androgen index (FAI). In their study, normal weight women with PCOS and obese controls showed less Insulin resistance (IR) and higher kisspeptin levels when compared with obese women with PCOS. Their contradictory findings may be due to the number of control women being relatively small, and the kisspeptin levels were significantly and inversely correlated with IR. Women with PCOS and BMI >25 kg/m2 being more insulin-resistant with no lean healthy women being included in the control group might have led to an overall increase in IR with a resulting decrease in kisspeptin levels. Therefore, Panidis et al. concluded that the increase in free androgen levels, as a result of IR, is associated with a relatively lower circulating kisspeptin levels.[34] Furthermore, a prospective study by Ozay et al.[35] carried out in a larger group did not find any difference between kisspeptin levels of PCOS group and controls.
The distribution of phenotypes of PCOS and their relationship with metabolic syndrome among Sri Lankan women have been determined in previous studies. But the association of kisspeptin levels with PCOS has not been studied in Sri Lankan women. To the best of our knowledge, this study is the first to determine any association between kisspeptin levels and well-characterized PCOS manifesting from adolescence in South Asian women. Interestingly, the results of the studies by Yilmaz et al.[33] (Turkish population), Chen et al.[31] (Chinese population), and Jeon et al.[32] (Korean population) were similar to our results. However, Panidis et al.[34] who studied European Caucasian women (Greece) reported contradictory results. Since Asian studies reported similar results to those of our study when compared with European study, it might suggest that the ethnic origin of woman may also have an effect on the expression of kisspeptin. In addition, variation in study design, sample size, demographic, and genetic characteristics of differing study populations may also lead to discrepancies in study results.
Moreover, the heterogeneous nature of genetic etiology of PCOS might also play a role in any variation in serum kisspeptin levels. PCOS is a complex endocrine disorder that results from the interaction of susceptible and protective genomic variants in several genes under the influence of environmental factors.[9] Any variation in interlinking genetic factors might affect the expression of KISS1 gene, which in turn may result in alteration of serum kisspeptin levels. Such a phenomenon may thereby play a putative role in the pathogenesis of PCOS along with polymorphisms in other genes involved in its metabolic and reproductive pathways.
The mechanism of kisspeptin for regulating gonadotropin remains unknown. Although the clinical and the biochemical features of PCOS have been extensively studied, the molecular basis and polymorphism of KISS1 and GPR54 genes in relation to PCOS are not well elucidated and need further study.
Conclusion
In conclusion, this study indicates that Sri Lankan women with well-characterized PCOS manifesting from adolescence exhibit significantly higher kisspeptin levels than ethnically matched controls. Furthermore, our findings confirm that kisspeptin levels are positively associated with PCOS in our studied population. The clinical relevance of this study is foreseen along the lines of biochemical profiling of the well-characterized PCOS phenotype through kisspeptin pathway linked to puberty and adolescence. We propose that increased kisspeptin levels can be used as an early marker of PCOS to help recognize the condition from post-puberty. We recommend confirmation from further large-scaled studies.
Financial support and sponsorship
This study was funded by National Research Council of Sri Lanka (NRC grant no. 15-149).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank all the staff of the Obstetrics and Gynaecology Department and Unit of De Soysa Hospital for Women, Colombo, for their assistance and support with ultrasound scanning; all staff of the Reproductive Biology and Endocrinology Laboratory, at the Department of Obstetrics and Gynecology, Faculty of Medicine, and the patients and their families for their cooperation. They also thank the staff of Bank of Ceylon, Head Office, Colombo, for their voluntary participation in the study. They specially thank Dr. Anoma Senanayake for her support during ultrasound examinations; and Dr. Sumudu Jayasinghe and Dr. Dakshila Galappathi for their support in coordinating patient evaluation and sample collections.
References
- 1.Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, et al. Polycystic ovary syndrome: The spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107–11. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 2.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific Publications; 1992. pp. 377–84. [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Alexander CJ, Tangchitnob E, Lepor NE. Polycystic ovary syndrome: A major unrecognized cardiovascular risk factor in women. Rev Cardiovasc Med. 2009;10:83–90. [PubMed] [Google Scholar]
- 5.Apridonidze T, Essah PA, Iurno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–35. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 6.Barber TM, Bennett AJ, Groves CJ, Sovio U, Ruokonen A, Martikainen H, et al. Association of variants in the fat mass and obesity associated (FTO) gene with polycystic ovary syndrome. Diabetologia. 2008;51:1153–8. doi: 10.1007/s00125-008-1028-6. [DOI] [PubMed] [Google Scholar]
- 7.Wijeyaratne CN, Udayangani SAD, Balen AH. Ethnic-specific polycystic ovary syndrome: Epidemiology, significance and implications. Expert Rev Endocrinol Metab. 2013;8:71–9. doi: 10.1586/eem.12.73. [DOI] [PubMed] [Google Scholar]
- 8.Kumarapeli V, Seneviratne Rde A, Wijeyaratne CN, Yapa RM, Dodampahala SH. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka. Am J Epidemiol. 2008;168:321–8. doi: 10.1093/aje/kwn137. [DOI] [PubMed] [Google Scholar]
- 9.Kandarakis ED, Piperi C, Argyrakopoulou G, Spina J, Papanastasiou L, Bergiele A, et al. Polycystic ovary syndrome: The influence of environmental and genetic factors. Hormones Rev. 2006;5:17–34. doi: 10.14310/horm.2002.11165. [DOI] [PubMed] [Google Scholar]
- 10.Kandarakis ED, Christakou C, Kandarakis H, Alexandraki KI. Early onset adiposity: A pathway to polycystic ovary syndrome in adolescents? Hormones. 2007;6:210–7. [PubMed] [Google Scholar]
- 11.Kandarakis ED, Christakou C, Palioura E, Kandaraki E, Livadas S. Does polycystic ovary syndrome start in childhood? Pediatr Endocrinol Rev. 2008;5:904–11. [PubMed] [Google Scholar]
- 12.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–43. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: Physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;23:1731–7. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 16.Roux deN, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KISS1-derived peptide receptor GPR54. Proc Natl Acad Sci. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 18.Tassignyded AX, Colledge WH. The role of kisspeptin signaling in reproduction. Physiology. 2010;25:207–17. doi: 10.1152/physiol.00009.2010. [DOI] [PubMed] [Google Scholar]
- 19.Popa SM, Clifton DK, Steiner RA. The role of Kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–38. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- 20.Clarke IJ, Cummins JT. GnRH pulse frequency determines LH pulse amplitude by altering the amount of releasable LH in the pituitary glands of ewes. J Reprod Fertil. 1985;2:425–31. doi: 10.1530/jrf.0.0730425. [DOI] [PubMed] [Google Scholar]
- 21.Schlesselman J. Vol. 354. New York: Oxford University Press; 1982. Case-control studies: Design, conduct, analysis. [Google Scholar]
- 22.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:884–9. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valkenburg O, Uitterlinden AG, Piersma D, Hofman A, Themmen AP, de Jong FH, et al. Genetic polymorphisms of GnRH and gonadotrophic hormone receptors affect the phenotype of polycystic ovary syndrome. Hum Reprod. 2009;24:2014–22. doi: 10.1093/humrep/dep113. [DOI] [PubMed] [Google Scholar]
- 24.Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nature genetics. 2011;43:55–9. doi: 10.1038/ng.732. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nature genetics. 2012;44:1020–5. doi: 10.1038/ng.2384. [DOI] [PubMed] [Google Scholar]
- 26.Boteju WMM, Karunarathna GDKN, Udayangani SAD, Silva KGH, Wijeyaratne CN. Markers of hyperandrogenism in South Asians with polycystic ovary syndrome. Sri Lanka J Diabetes, Endocrinol Metabol. 2014;4:3–8. [Google Scholar]
- 27.Stewart A, Marfell-Jones M. New Zealand: Lower Hutt; 2011. International Society for the Advancement of Kinanthropometry. [Google Scholar]
- 28.AAMI Standards Philosophy and Strategy – Key Elements – Standards – Association for the Advancement of Medical Instrumentation. [Last accessed on 2016 Dec 01]. Available from: http://www.aami.org .
- 29.Wijeyaratne CN, Seneviratne RdeA, Dahanayake S, Kumarapeli V, Palipane E, Kuruppu N, et al. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): Results of a large database from a specialist Endocrine Clinic. Hum Reprod. 2011;26:202–13. doi: 10.1093/humrep/deq310. [DOI] [PubMed] [Google Scholar]
- 30.Jakubowski L. Genetic aspects of polycystic ovary syndrome. Endokrynologia Polska. 2005;56:285–93. [PubMed] [Google Scholar]
- 31.Chen X, Mo Y, Li L, Chen Y, Li Y, Yang D. Increased plasma metastin levels in adolescent women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2010;149:72–6. doi: 10.1016/j.ejogrb.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Jeon YE, Lee KE, Jung JA, Yim SY, Kim HY, Seo SK, et al. Kisspeptin, leptin and retinol binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Invest. 2013;75:268–74. doi: 10.1159/000350217. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz SA, Kerimoglu OS, Pekin AT, Incesu F, Dogan NU, Celik C, et al. Metastin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2014;180:56–60. doi: 10.1016/j.ejogrb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Panidis D, Rousso D, Koliakos G, Kourtis A, Katsikis I, Farmakiotis D, et al. Plasma metastin levels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil Steril. 2006;85:1778–83. doi: 10.1016/j.fertnstert.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 35.Ozay OE, Ozay AC, Acar B, Cagliyan E, Seçil M, Küme T. Role of kisspeptin in polycystic ovary syndrome (PCOS) Gynecol Endocrinol. 2016;32:718–22. doi: 10.3109/09513590.2016.1161019. [DOI] [PubMed] [Google Scholar]


