Abstract
Purpose:
Stereotactic body radiation therapy (SBRT) is commonly used to treat primary or oligometastatic malignancies in the lung, but most of the available data that describe the safety and efficacy of SBRT are for smaller tumors. The purpose of this study was to evaluate the impact of tumor size, among other factors, on local control (LC) and radiation pneumonitis (RP) in patients who received lung SBRT.
Methods and materials:
This retrospective study included 144 patients with 100 primary (57.1%) and 75 metastatic (42.9%) lung tumors treated with SBRT between 2012 and 2018. Measurements of tumor size, treatment volume, histology, and radiation dose were evaluated for association with LC. Additional factors evaluated for association with the development of symptomatic RP included volume of the lung, heart, and central airway exposed to relevant doses of radiation.
Results:
The median follow-up time was 15.0 months (interquartile range, 8.0–26.0 months). LC rates at 12 and 24 months posttreatment were 95.1% and 92.7%, respectively. LC at 1 year was higher for tumors <5 cm in diameter than for tumors >5 cm in diameter (98.2% vs 79.8%, respectively; P < .01). On univariate analysis, LC was associated with a smaller gross tumor volume (GTV) diameter (P < .01), GTV volume (P < .01), planning target volume (PTV) diameter (P < .01), PTV volume (P < .01), and larger PTV-to-GTV ratio (P = .04). Tumor histology and treatment intent were not correlated with LC. RP was associated with a higher ipsilateral lung mean lung dose (P = .02), V2.5 (P = .03), V5 (P = .02), V13 (P = .03), V20 (P = .05), V30 (P = .02), V40 (P = .02), and V50 (P = .03), and several similar total lung dose parameters and heart maximum point dose (P = .02). The optimal mean ipsilateral lung dose cutoff predictive of RP was 8.6 Gy.
Conclusions:
A larger tumor size and smaller PTV-to-GTV ratio was associated with local recurrence of lung tumors treated with SBRT, but ipsilateral lung doses were most associated with symptomatic RP.
Introduction
Stereotactic body radiation therapy (SBRT) is commonly used to treat primary or metastatic malignancies in the lung in medically inoperable patients, and outcomes rival those of surgical resection.1–5 However, a large majority of studies have only assessed the efficacy of SBRT in the treatment of smaller lung tumors <5 cm, and retrospective series that assessed for an association between tumor size and local control (LC) offer conflicting findings.6–9
Larger tumors often also present a logistical challenge in treatment planning owing to their proximity to ≥1 normal organs at risk of toxicity, including healthy lung tissue, and thus puts limitations on the prescription dose or tumor coverage that can be safely achieved with the SBRT plan. Although lung SBRT generally results in limited rates of toxicity, symptomatic radiation pneumonitis (RP) is the most common and occurs in 9% to 28% of patients. However, there is no clear consensus on optimal planning parameters for a normal lung that should be used to prevent RP.10–21
In this retrospective study, we evaluated the impact of tumor size, among other factors, on the rates of LC and RP in patients who underwent lung SBRT at a single institution to determine predictive factors that may help guide management.
Methods and Materials
After obtaining approval from the local institutional review board, the medical records and radiation therapy treatments plans of all patients who underwent SBRT for either primary or metastatic lung tumors at our institution between October 2012 and January 2018 were reviewed. The indication for treatment generally fit into one of the following 3 categories: (1) early stage primary lung cancer treated with curative intent; (2) oligometastatic lung cancer with SBRT to the primary lung tumor if all other sites of disease were well controlled; or (3) oligometastasis to the lung from another primary tumor site if all other sites of disease were well controlled. In all cases, patients were either considered medically inoperable or electively chose SBRT rather than surgical resection of their tumor.
Post-treatment follow-up typically consisted of history and physical examinations, and either chest computed tomography (CT; +/− abdomen/pelvis) or positron emission tomography (PET)/CT of the whole body every 3 to 6 months for 2 years, and then at increasing intervals thereafter. Five different radiation oncologists cared for the included patients, with treatment and follow-up decisions at their discretion. Patients were excluded if they had undergone previous irradiation of the targeted site, were undergoing SBRT as a boost after conventionally fractionated radiation, received >5 fractions in their SBRT course, or had inadequate follow-up to evaluate tumor response or development of radiation pneumonitis.
CT simulation was carried out with the patient in a supine position with their arms above their head using a vacuum bag immobilization device. A 4-dimensional CT scan was used to capture tumor motion. The gross tumor volume (GTV) consisted of all known gross disease based on the planning CT and pretreatment PET/CT scans. An internal GTV included the union of the GTVs on respiratory correlated images from the 4-dimensional CT scan. The clinical target volume (CTV) included the entire GTV and internal GTV without additional margin added. The planning target volume (PTV) typically included the CTV plus a 5-mm isotropic expansion. However, margin size was at the discretion of the treating physician, and in some cases smaller or larger PTV margins were used depending on the risk of toxicity to neighboring structures. The volumetric ratio of PTV-to-GTV was collected to quantify the extent of tumor motion and margins used in each case. Organs at risk were contoured according to current Radiation Therapy Oncology Group consensus atlas, with the delineated normal lung volume excluding the GTV.22
All patients were prescribed 3 to 5 fractions of radiation therapy using highly conformal intensity modulated radiation or volumetric modulated arc therapy techniques. Radiation therapy treatment planning was carried out for the Varian TrueBeam linear accelerator (Varian Medical Systems, Palo Alto, CA) using the Eclipse treatment planning system (version 11). As indicated, accelerator beam gating was used for motion management during treatment.
Treatment response was evaluated using the Response Evaluation Criteria in Solid Tumors, version 1.1.23 LC was defined as a lack of progression in the irradiated tumor or adjacent tissue after SBRT. PET/CT scans and biopsy tissue samples were used in the follow-up period to help differentiate active tumor from RP or atelectasis. Persistent stable disease on a CT chest scan was classified as LC unless PET/CT scans suggested or biopsy test results confirmed otherwise. The Mann-Whitney U test was used to evaluate the following factors as possible predictors of LC: tumor size (based on measured single greatest dimension of GTV and equivalent sphere diameter and volume for GTV and PTV), volumetric ratio of PTV-to-GTV, biologically effective dose for an alpha-to-beta ratio of 10 Gy (BED10) of the radiation prescription, and maximum point dose (Dmax) within the PTV.
The χ2 test was used to evaluate the impact of tumor histology, use of concurrent systemic therapy, and treatment intent (curative SBRT for early stage lung cancer vs noncurative SBRT for metastatic cancer) on LC. The Kaplan-Meier method and log-rank test were used to compare the following: 1) LC of primary versus meta-static lung tumors, 2) LC of tumors <5 cm versus those >5 cm in the greatest dimension, and 3) LC of radio-sensitive versus radioresistant histologies (ie, renal cell carcinoma, sarcoma, and melanoma). Of note, the cutoff of 5 cm was used for this analysis because most prospective studies have only assessed the efficacy of SBRT to treat lung tumors <5 cm. Disease-free survival (DFS) for primary lung tumors, progression-free survival (PFS) for metastatic lung tumors, and overall survival (OS) for both primary and metastatic lung tumors were also assessed using the Kaplan-Meier method from the time of SBRT completion, with patients censored at the last follow-up. The follow-up duration was calculated up to the point of the last imaging study (for LC) or patient encounter (for survival and toxicity).
The cumulative incidence of RP was recorded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.24 Symptomatic RP was defined as grade ≥2. The Mann-Whitney U test was used to evaluate the following factors as possible predictors of RP: tumor size, volumetric ratio of PTV-to-GTV, PTV Dmax, BED3, maximum and mean dose to the heart and central airway (trachea through lobar bronchi), mean lung dose, and the volume of the lung that received at least 2.5 Gy (V2.5), 5 Gy (V5), 10 Gy (V10), 13 Gy (V13), 20 Gy (V20), 30 Gy (V30), 40 Gy (V40), 50 Gy (V50), and the prescription dose (VP). The lung parameters were evaluated for both the total and ipsilateral lung only. The χ2 test was used to evaluate the impact of concurrent systemic therapy use on RP. Recursive partitioning analysis was used for parameters associated with RP to determine an optimal dose constraint that is predictive of RP on the basis of the deviance statistic in the regression analysis.25
The statistical analysis was conducted using the Statistical Package for Social Sciences, version 24 (SPSS, Chicago, IL) and R statistical software (R, Vienna, Austria). A P-value of < .05 was considered statistically significant.
Results
Demographics
A total of 144 patients with 175 irradiated tumors were included in this study. Table 1 shows the patient and tumor characteristics. The median follow-up time for the survival and toxicity analyses was 15.0 months (inter-quartile range [IQR], 8.0–26.0 months). The median follow-up time for the LC analysis was 12.0 months (IQR, 6.0–22.0 months).
Table 1.
Patient and tumor characteristics
| Characteristics | n (%) |
|---|---|
| Sex | |
| Female | 69 (48) |
| Male | 75 (52) |
| Number of treated tumors | |
| 1 | 119 (83) |
| 2 | 20 (14) |
| 3–4 | 5 (4) |
| Primary tumor site | |
| Lung (early stage non-small cell) | 100 (57) |
| Lung (metastatic) | 41 (23) |
| Renal/urogenital | 10 (6) |
| Head and neck | 9 (5) |
| Sarcoma | 8 (5) |
| Other | 7 (4) |
| Histology | |
| Adenocarcinoma | 74 (42) |
| Squamous cell carcinoma | 50 (29) |
| Sarcoma | 7 (4) |
| Clear cell carcinoma | 5 (3) |
| Other/poorly differentiated carcinoma | 39 (22) |
| Tumor radiosensitivity | |
| Sensitive | 159 (91) |
| Resistant* | 16 (9) |
| Tumor location | |
| Left upper lobe | 54 (31) |
| Right upper lobe | 52 (30) |
| Right lower lobe | 37 (21) |
| Left lower lobe | 26 (15) |
| Right middle lobe | 6 (3) |
| Concurrent systemic therapy | |
| None | 153 (87) |
| Targeted therapy/immunotherapy | 14 (8) |
| Chemotherapy | 4 (2) |
| Combined chemotherapy/immunotherapy | 3 (2) |
| Combined hormonal/targeted therapy | 1 (1) |
Radioresistant histologies include renal cell carcinoma, sarcoma, and melanoma.
The median single greatest dimension of the irradiated tumors was 2.8 cm (IQR, 2.0–4.3 cm), the median GTV equivalent sphere diameter was 2.2 cm (IQR, 1.6–3.0), and the GTV volume was 5.9 cm3 (IQR, 2.1–14.4 cm3). The median PTV equivalent sphere diameter was 4.0 cm (IQR, 3.2–5.0 cm) and PTV volume was 34.4 cm3 (IQR, 17.8–63.6 cm3). The median PTV-to-GTV ratio was 5.1 (IQR 3.5–9.0). There was no significant difference between the median GTV diameter of patients with early stage lung cancer and those with oligometastatic disease (2.4 vs 2.2 cm, respectively).
The most common SBRT prescriptions consisted of 50 Gy delivered in 5 fractions (28.6%), 50 Gy in 4 fractions (24.6%), and 54 Gy in 3 fractions (13.1%). The median BED10 for all regimens was 105.60 Gy (IQR, 100.0–112.5 Gy), and the median BED3 was 240.0 Gy (IQR, 216.7–258.3 Gy). Nineteen percent of tumors received a prescription with BED10 <100 Gy, and 12.6% of tumors received a prescription with BED10 <80 Gy. The median PTV Dmax was 62.5 Gy (IQR, 60.0–62.5 Gy). Intensity modulated radiation and volumetric modulated arc therapy accounted for 39 (22.3%) and 136 (77.7%) of treatments, respectively.
Tumor response analysis
Overall, 167 patients (95.4%) demonstrated LC. The cumulative proportion of LC at 6, 12, 18, 24, and 36 months was 98.1%, 95.1%, 92.7%, 92.7%, and 92.7%, respectively (Fig 1). A univariate comparison between LC and local recurrence groups with regard to tumor size and dose parameters is shown in Table 2. Larger tumor size and smaller PTV-to-GTV ratio were associated with reduced LC, but BED10 and PTV Dmax were similar in both groups and not significantly associated with improved LC. There were also no observed differences in LC for radiosensitive histologies compared with radioresistant histologies (95.0% vs 100%, respectively; P = .34), early stage versus metastatic tumors (94.8% vs 96.0%, respectively; P = .72), and use of concurrent systemic therapy or radiation alone (95.5% vs 95.4%, respectively; P = .95).
Figure 1.
Kaplan-Meier plots showing (A) overall survival for primary (solid) and metastatic (dashed) tumors, (B) local control for both primary and metastatic tumors, (C) PFS for patients with metastatic tumors, and (D) DFS for primary tumors. Abbreviations: PFS = progression-free survival; DFS = disease-free survival.
Table 2.
Univariate comparison of LC versus local recurrence groups
| Parameter | LC (+) N = 167 Median (IQR) |
LC (−) N = 8 Median (IQR) |
Absolute difference | Percentage difference | P-value |
|---|---|---|---|---|---|
| Tumor diameter (cm) | 2.8 (2.0–4.2) | 5.1 (3.6–5.7) | +2.3 | 58.6 | <.01 |
| GTV diameter (cm) | 2.2 (1.6–2.9) | 4.0 (2.9–4.8) | +1.8 | 44.3 | <.01 |
| GTV volume (cm3) | 5.7 (2.0–12.6) | 33.8 (13.4–57.9) | +28.1 | 83.2 | <.01 |
| PTV diameter (cm) | 3.9 (3.2–4.8) | 6.0 (4.8–6.2) | +2.1 | 35.0 | <.01 |
| PTV volume (cm3) | 31.1 (17.3–57.7) | 115.0 (57.9–121.4) | +83.9 | 72.9 | <.01 |
| Prescription BED10 | 105.6 (100.0–112.5) | 100.0 (79.0–112.5) | −5.6 | 5.6 | .21 |
| PTV Dmax (Gy) | 62.5 (60.0–62.5) | 62.5 (42.5–62.5) | 0 | 0 | .43 |
| PTV-to-GTV ratio | 5.7 (3.6–9.2) | 3.3 (2.3–4.6) | −2.4 | 72.7 | .04 |
Abbreviations: BED10 = biologically effective dose at an alpha:beta ratio of 10 Gy; Dmax = maximum point dose within the PTV; GTV = gross tumor volume; IQR = interquartile range; LC = local control; PTV = planning target volume.
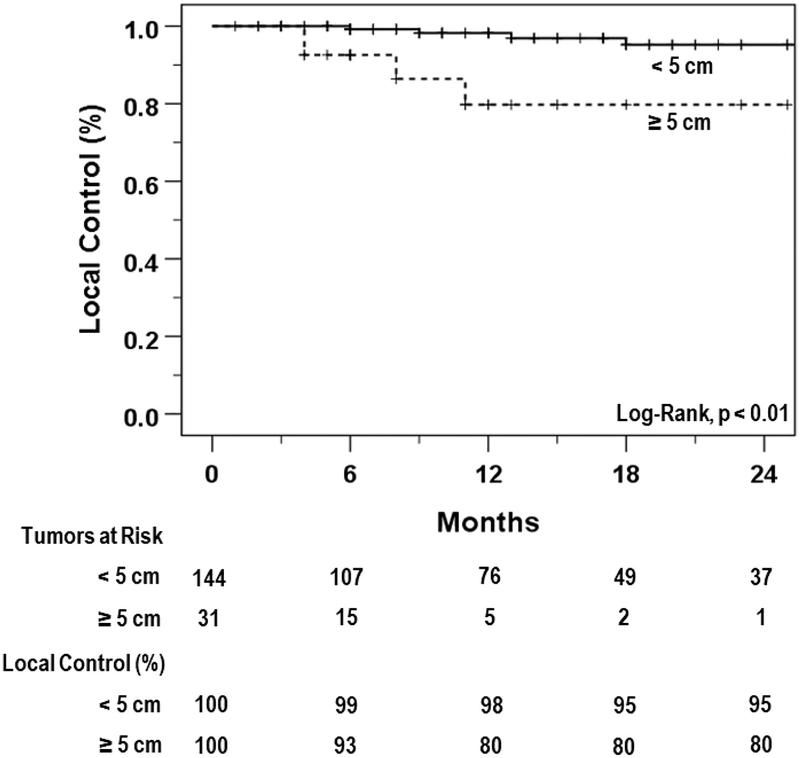
Among patients with early stage lung cancer, there was no difference in LC for adenocarcinoma compared with squamous cell carcinoma histology (93.5% vs 93.9%, respectively; P = .93). The 1-year LC rate for the 31 tumors (17.7%) >5 cm in diameter was 79.8%, which was significantly lower than the 98.2% 1-year LC rate for tumors <5 cm in diameter (Fig 2; P < .01).
Figure 2.
Kaplan-Meier plots showing local control between small (<5 cm; solid) and large (≥5 cm; dashed) tumors.
Survival analysis
At the time of the last follow-up, 103 patients (58.9%) were alive. The median OS time for all patients was 31.0 months (95% confidence interval [CI], 22.7–39.3 months). The cumulative proportion of patients surviving at 6, 12, 18, 24, 36 months was 94.3%, 77.8%, 64.6%, 59.5%, and 48.0% using Kaplan-Meier survival analysis (Fig 1).
For patients with primary tumors, the median OS time was 40.0 months (95% CI, 26.3–53.8 months), and for patients with metastatic tumors, the median OS was 20.0 months (95% CI, 7.6–32.4 months). The median DFS time for patients with primary tumors was 25.0 months (Fig 1; 95% CI, 15.6–34.4 months), and PFS for patients with metastatic tumors was 7.0 months (Fig 1; 95% CI, 4.7–9.4 months).
Toxicity analysis
Overall, RP of any grade occurred in 98 patients (56.7%) with 82 (47.4%), 12 (6.9%), and 4 (2.3%) developing grades 1, 2, and 3 RP, respectively. There was no observed grade 4 or 5 RP. The median time until onset of symptomatic RP was 3.5 months. The univariate analysis results in Table 3 show that all ipsilateral lung parameters, most total lung parameters, and the heart Dmax were all associated with symptomatic RP. There was no difference in the rate of RP among patients receiving concurrent systemic therapy (4.5% vs 9.5%; P = .415). The results of the recursive partitioning analysis to identify optimal dose-volume constraints for these parameters is shown in Table 4.
Table 3.
Univariate comparison of symptomatic RP positive and negative groups
| Parameter | RP (−) N = 159 Median (IQR) |
RP (+) N = 16 Median (IQR) |
Absolute difference | Percentage difference | P-value |
|---|---|---|---|---|---|
| Tumor size and dose | |||||
| Tumor diameter (cm) | 2.8 (2.0–4.2) | 3.1 (2.1–5.0) | +0.3 | 8.5 | .48 |
| GTV diameter (cm) | 2.2 (1.6–3.1) | 2.5 (1.6–4.0) | +0.3 | 12.8 | .40 |
| GTV volume (cm3) | 5.8 (2.1–15.0) | 8.5 (2.1–34.2) | +2.7 | 37.2 | .41 |
| PTV diameter (cm) | 3.9 (3.2–5.0) | 4.4 (3.3–5.4) | +0.5 | 12.0 | .35 |
| PTV volume (cm3) | 31.5 (17.5–62.0) | 44.9 (19.7–84.8) | +13.4 | 35.1 | .37 |
| Prescription BED3 | 240.0 (216.7–258.3) | 257.5 (216.7–258.3) | +17.5 | 7.0 | .50 |
| PTV-to-GTV ratio | 5.5 (3.6–9.0) | 4.9 (2.8–8.7) | −0.6 | 11.6 | .86 |
| PTV Dmax (Gy) | 62.5 (56.3–62.5) | 62.5 (62.5–68.4) | 0.0 | 0.0 | .06 |
| Total Lung | |||||
| MLD (Gy) | 3.0 (2.2–4.0) | 4.0 (3.1–5.4) | +1.0 | 27.3 | .02 |
| V2.5 Gy (%) | 22.8 (15.4–30.3) | 28.3 (21.9–36.8) | +5.5 | 21.6 | .06 |
| V5 Gy (%) | 13.6 (9.4–18.2) | 16.2 (12.7–22.4) | +2.6 | 17.2 | .09 |
| V10 Gy (%) | 8.3 (5.6–12.2) | 10.7 (8.0–16.1) | +2.5 | 25.9 | .04 |
| V13 Gy (%) | 6.3 (4.1–9.0) | 8.9 (5.9–12.7) | +2.6 | 33.6 | .03 |
| V20 Gy (%) | 3.6 (2.2–5.3) | 5.8 (3.2–8.5) | +2.2 | 47.0 | .02 |
| V30 Gy (%) | 1.8 (1.1–2.9) | 3.3 (1.7–4.4) | +1.5 | 60.0 | .02 |
| V40 Gy (%) | 1.0 (0.7–1.6) | 1.7 (1.0–2.6) | +0.7 | 49.3 | .03 |
| V50 Gy (%) | 0.5 (0.4–0.9) | 0.8 (0.6–1.4) | +0.2 | 32.6 | .03 |
| VP Gy (%) | 0.6 (0.4–0.9) | 0.7 (0.5–1.4) | +0.1 | 22.2 | .05 |
| Ipsilateral lung | |||||
| MLD (Gy) | 5.3 (3.7–7.2) | 8.0 (5.4–10.4) | +2.8 | 42.3 | <.01 |
| V2.5 Gy (%) | 31.8 (23.3–42.8) | 39.7 (35.3–50.9) | +7.9 | 22.0 | <.01 |
| V5 Gy (%) | 24.6 (18.1–34.1) | 33.8 (27.6–44.1) | +9.3 | 31.8 | <.01 |
| V10 Gy (%) | 16.6 (10.8–23.8) | 24.3 (17.0–32.3) | +7.7 | 37.5 | .01 |
| V13 Gy (%) | 12.8 (8.1–19.0) | 20.4 (13.2–26.8) | +7.5 | 45.5 | .01 |
| V20 Gy (%) | 7.3 (4.3–11.4) | 13.4 (7.5–18.1) | +6.1 | 58.5 | .01 |
| V30 Gy (%) | 3.8 (2.3–6.8) | 7.5 (3.6–10.8) | +3.7 | 65.4 | .02 |
| V40 Gy (%) | 2.4 (1.4–4.2) | 4.2 (2.2–6.7) | +1.8 | 53.8 | .02 |
| V50 Gy (%) | 1.5 (0.9–2.7) | 2.5 (1.5–4.1) | +1.1 | 53.3 | .03 |
| VP Gy(%) | 1.4 (0.9–2.6) | 2.1 (1.5–4.1) | +0.7 | 39.1 | .05 |
| Heart | |||||
| Mean dose (Gy) | 0.4 (0.1–2.1) | 1.2 (0.4–3.3) | +0.7 | 89.3 | .10 |
| Max dose (Gy) | 6.0 (0.5–17.4) | 12.5 (1.6–32.2) | +6.5 | 69.9 | .03 |
| Airway | |||||
| Mean dose (Gy) | 3.1 (1.0–6.2) | 4.9 (2.3–8.4) | +1.9 | 46.6 | .13 |
| Max dose (Gy) | 12.4 (6.3–20.4) | 17.9 (9.6–25.4) | +5.4 | 35.9 | .10 |
Abbreviations: BED3 = biologically effective dose at an alpha:beta ratio of 3 Gy; GTV = gross tumor volume; IQR = interquartile range; MLD = mean lung dose; PTV = planning target volume; RP = radiation pneumonitis.
Table 4.
Optimal cutoff values for significant predictors of symptomatic RP
| Parameter | Cutoff value | Symptomatic RP | |
|---|---|---|---|
| ≤ Cutoff (%) | ≥ Cutoff (%) | ||
| Total lung | |||
| MLD | 5.1 Gy | 6.1 | 26.9 |
| V10 Gy | 8.6% | 4.7 | 13.8 |
| V20 Gy | 6.7% | 5.6 | 25.8 |
| V30 Gy | 3.8% | 5.5 | 29.6 |
| V40 Gy | 2.2% | 6.7 | 25.9 |
| Ipsilateral lung | |||
| MLD | 8.6 Gy | 5.6 | 26.7 |
| V5 Gy | 27.1% | 3.3 | 16.3 |
| V10 Gy | 23.2% | 4.9 | 20.0 |
| V20 Gy | 14.9% | 5.6 | 27.6 |
| V30 Gy | 8.1% | 5.7 | 25.0 |
| V40 Gy | 5.7% | 6.6 | 28.0 |
Abbreviations: MLD = mean lung dose; RP = radiation pneumonitis.
Discussion
This study explored the impact of lung tumor size on LC and toxicity after SBRT. We found that LC was associated with larger tumor size and smaller PTV-to-GTV ratio. Tumor size did not directly impact RP, but may in some cases be associated with higher ipsilateral lung doses, which was most strongly correlated with symptomatic RP. In full, our findings provide greater insight into balancing treatment planning objectives to maximize tumor control and minimize toxicity for lung SBRT.
Several studies have evaluated the impact of tumor size on LC after lung SBRT. Dunlap et al. reported decreased LC with increasing tumor size,9 whereas other retrospective series evaluating lung tumors >5 cm have shown comparable LC rates to those achieved for smaller lung tumors.6–8 In the largest reported series assessing SBRT for 92 lung tumors >5 cm in size, Verma et al. reported 1-and 2-year LC rates of 95.7% and 73.2%, respectively.7 Interestingly, a smaller PTV-to-GTV ratio was also associated with LC in our study. This ratio reflects several factors, including the extent of tumor motion with respiration and PTV margin size; overall, it takes into account the importance of adequate tumor margins, motion assessment, and image guidance in delivering an effective treatment.
Dosimetric parameters like BED10 are known to affect outcomes after lung SBRT.26–28 We did not observe an effect of BED10 in our study, but this is most likely because the majority of the tumors received a relatively high dose, and there were a relatively low number of LC events within the follow-up period of the study. Overall, our 2-year LC rate of 93% was comparable with the 3-year LC rates of 88% to 92% demonstrated in other prospective studies that evaluated SBRT for early stage primary lung tumors.1–5
There are several potential explanations to account for the impact of tumor size on LC, including the possibility of increased microscopic extension of larger tumors, more hypoxic regions in larger tumors, or the greater likelihood that a larger tumor will be in closer proximity to an organ at risk of toxicity, necessitating some dosimetric compromises with somewhat lower PTV coverage. Assuming that an adequate dose can be safely prescribed and adequate margins around the gross disease can be used to minimize the risk of geographic/marginal miss, any association between tumor size and LC is likely to be relatively weak. However, achieving such an ideal treatment plan for a larger tumor may not always be possible.
The 9% of patients who developed symptomatic RP in our series was comparable to the range of 9% to 28% reported in past studies.10–21 Optimal dose-volume constraints to prevent symptomatic RP are not clearly defined, but our data support a stronger correlation between the ipsilateral lung doses and RP rather than the entire lung. Our optimal mean ipsilateral lung dose cutoff of 8.6 Gy is comparable with a cutoff of 9.1 Gy, as reported by Chang et al.29
Our cutoff values in Table 4 for the volume of lung receiving 5 Gy to 40 Gy is also of some value to dosimetrists in treatment planning.18,29,30 Interestingly, PTV and internal target volume size has been reported to be associated with the development of symptomatic RP in other studies, but we did not find any association with the PTV-to-GTV ratio in our data.13,14,21,31 Tumor size did not correlate with risk of RP, most likely owing to the impact of tumor location because some tumors are surrounded by more normal lung tissue than others. Interestingly, the heart Dmax was associated with the development of RP in our patients, which has been described after conventionally fractionated radiation for locally advanced tumors but not SBRT for early stage tumors.32 Whether the heart dose is directly related to RP is unclear because this may also reflect the impact of other dosimetric factors or the volume of nearby normal lung tissue.
The main limitations of this study are its retrospective nature and the single institution analyzed, which may lead to some bias and reduce the generalizability of our findings. Furthermore, although RP typically occurs within 1 year of SBRT and would have been captured for most patients in this study, a longer follow-up time would have been valuable to capture more local recurrences and increase confidence in our reported long-term LC rates.10,14,15,17
Ideally we would have reported multivariate analyses for LC and RP; however, because of the relatively small number of LC and RP events during the follow-up period of this study, the Cox and binary logistic regression analyses we attempted did not identify any independent associations. A longer follow up of our population would have improved the yield of these tests.
On the other hand, a strength of the study is the large range of tumor sizes represented, which makes our primary assessment of the impact of tumor size on LC and RP more likely to be accurate.
Conclusions
Our findings support tumor size and PTV-to-GTV ratio as important predictors of LC after lung SBRT. This should be balanced with minimizing ipsilateral lung doses to minimize pulmonary morbidity from treatment.
Acknowledgments
The authors acknowledge the West Virginia University Initiation to Research Opportunities Summer Research Program, which provided salary support to Mr. Sean Parker.
Sources of support: The West Virginia University, Initiation to Research Opportunities Summer Research Program is supported by a grant from the National Institute of General Medical Sciences (2U54GM104942–02). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303: 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68:72–77. [DOI] [PubMed] [Google Scholar]
- 3.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small cell lung carcinoma: 4-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. [DOI] [PubMed] [Google Scholar]
- 4.Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiation therapy for early-stage non-small cell lung cancer: Results of a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:1343–1349. [DOI] [PubMed] [Google Scholar]
- 5.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small cell lung cancer patients treated with stereotactic body radiation therapy. J Clin Oncol. 2009;27:3290–3296. [DOI] [PubMed] [Google Scholar]
- 6.Woody NM, Stephans KL, Marwaha G, Djemil T, Videtic GM. Stereotactic body radiation therapy for non-small cell lung cancer tumors greater than 5 cm: Safety and efficacy. Int J Radiat Oncol Biol Phys. 2015;92:325–331. [DOI] [PubMed] [Google Scholar]
- 7.Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiation therapy for large (≥5 centimeters) non-small cell lung tumors. Cancer. 2017;123:688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson J, Niles C, Patel A, et al. Stereotactic body radiation therapy for large (>5 cm) non-small cell lung cancer. Clin Lung Cancer. 2017;18:396–400. [DOI] [PubMed] [Google Scholar]
- 9.Dunlap NE, Larner JM, Read PW, et al. Size matters: A comparison of T1 and T2 peripheral non-small cell lung cancers treated with stereotactic body radiation therapy (SBRT). J Thorac Cardiovasc Surg. 2010;140:583–589. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita H, Nakagawa K, Nakamura N, et al. Exceptionally high incidence of symptomatic grade 2 to 5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol. 2007;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita H, Kobayashi-Shibata S, Terahara A, et al. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiation therapy. Radiat Oncol. 2010;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stauder MC, Macdonald OK, Olivier KR, et al. Early pulmonary toxicity following lung stereotactic body radiation therapy delivered in consecutive daily fractions. Radiother Oncol. 2011;99:166–171. [DOI] [PubMed] [Google Scholar]
- 13.Ong CL, Palma D, Verbakel WF, Sltoman BJ, Senan S. Treatment of large stage I-II lung tumors using stereotactic body radiation therapy (SBRT): Planning considerations and early toxicity. Radiother Oncol. 2010;97:431–436. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo Y, Shibuya K, Nakamura M, et al. Dose-volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2012;83: e545–e549. [DOI] [PubMed] [Google Scholar]
- 15.Kanemoto A, Matsumoto Y, Sugita T. Timing and characteristics of radiation pneumonitis after stereotactic body radiation therapy for peripherally located stage I lung cancer. Int J Clin Oncol. 2015;20: 680–685. [DOI] [PubMed] [Google Scholar]
- 16.Jo IY, Kay CS, Kim JY, et al. Significance of low-dose radiation distribution in development of radiation pneumonitis after helical-tomotherapy-based hypofractionated radiation therapy for pulmonary metastases. J Radiat Res. 2014;55:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guckenberger M, Baier K, Polat B, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiation therapy. Radiother Oncol. 2010;97:65–70. [DOI] [PubMed] [Google Scholar]
- 18.Guckenberger M, Allgauer M, Appold S, et al. Safety and efficacy of stereotactic body radiation therapy for stage 1 non-small cell lung cancer in routine clinical practice: A patterns-of-care and outcome analysis. J Thorac Oncol. 2013;8:1050–1058. [DOI] [PubMed] [Google Scholar]
- 19.Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol. 2009;91:307–313. [DOI] [PubMed] [Google Scholar]
- 20.Barriger RB, Forquer JA, Brabham JG, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:457–462. [DOI] [PubMed] [Google Scholar]
- 21.Baker R, Han G, Sarangkasiri S, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys. 2013;85:190–195. [DOI] [PubMed] [Google Scholar]
- 22.Kong FM, Galvin J, Haken RT, Machtay M, Bradley J. Contouring Atlas. RTOG Foundation Inc; 2017. [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute, U.S. Department of Health and Human Services. National Cancer Institute Common Toxicity Criteria for Adverse Events v. 4.0. Rockville, Maryland: National Institutes of Health; 2009. [Google Scholar]
- 25.Stone C. Classification and regression trees. Belmont, California: Wadsworth International Group; 1984. [Google Scholar]
- 26.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiation therapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–S100. [DOI] [PubMed] [Google Scholar]
- 27.Kong FM, Zhao J, Wang J, Faivre-Finn C. Radiation dose effect in locally advanced non-small cell lung cancer. J Thorac Dis. 2014;6: 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guckenberger M, Wulf J, Mueller G, et al. Dose-response relationship for image guided stereotactic body radiation therapy of pulmonary tumors: Relevance of 4D dose calculation. Int J Radiat Oncol Biol Phys. 2009;74:47–54. [DOI] [PubMed] [Google Scholar]
- 29.Chang JY, Liu H, Balter P, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiation therapy for stage I non-small cell lung cancer. Radiat Oncol. 2012;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricardi U, Filippi AR, Guarneri A, et al. Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta Oncol. 2009;48:571–577. [DOI] [PubMed] [Google Scholar]
- 31.Bongers EM, Botticella A, Palma DA, et al. Predictive parameters of symptomatic radiation pneumonitis following stereotactic or hypofractionated radiation therapy delivered using volumetric modulated arcs. Radiother Oncol. 2013;109:95–99. [DOI] [PubMed] [Google Scholar]
- 32.Huang EX, Hope AJ, Lindsay PE, et al. Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol. 2011;50:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]