Abstract
Diffusion MRI studies characterizing the changes in white matter (WM) due to vascular cognitive impairment, which includes all forms of small vessel disease are reviewed. We reviewed the usefulness of diffusion methods in discriminating the affected WM regions and its relation to cognitive impairment. These studies were categorized based on the diffusion MRI techniques used. The most common method was the diffusion tensor imaging, whereas other methods included diffusion weighted imaging, diffusion kurtosis imaging, intravoxel incoherent motion, and studies based on diffusion tractography. The diffusion measures showed correlation with cognitive scores and disease progression, with mean diffusivity being the most robust parameter. Future studies should focus on incorporating multi-compartment and higher order diffusion models, which can handle the presence of multiple and crossing fibers inside a voxel.
Keywords: White Matter diseases, Vascular cognitive imapairment and dementia, Cerebral small vessel diseases, Diffusion MRI
1. Introduction
In the late 1800s, Otto Binswanger was the first to describe pathological changes in the white matter (WM) in patients with dementia [1]. This contrasted with the neuronal damage in the cortex described around the same time by Alois Alzheimer. Interest in the WM pathology waned with few reports published on Binswanger’s disease until the invention of modern neuroimaging methods. This contrasted to a growing interest in Alzheimer’s disease (AD), which was observed pathologically in patients with dementia. With the invention of tomographic methods that were first applied to x-rays in computed tomography (CT) and shortly afterwards to magnetic resonance imaging (MRI), visualization of WM became routine in neurological diagnosis, and Binswanger’s disease was more frequently diagnosed [2, 3], resulting in an “epidemic” of the disease by 1981, according to the medical journal the Lancet.[4]. Pathological changes in the subcortical regions of the brain were now recognized as a major contributor to dementia, resulting in a dramatic rise in interest vascular cognitive impairment and dementia [5]. As a result of the revival of interest in the WM, one hundred years after its original description, our understanding of the optimal ways to image the WM and the underlying pathophysiology of chronic vascular disease has greatly expanded, culminating in the development of diffusion-based methods to determine the integrity of the WM. The ability to determine the pathological state of the WM during life has led to dramatic advances in our understanding of the role of the WM in dementia [6]. Advances in imaging methods related to diffusion have grown so rapidly that they had out distanced the clinical conditions. This review will focus on MRI methods to assess changes in WM that through imaging reveal aspects of the underlying pathobiology.
Diffusion imaging is able to more accurately define the condition of the WM fiber tracts than conventional Fluid Attenuated Inversion Recovery (FLAIR) sequences used in routine clinical studies. Elderly individuals have a high incidence of WM hyperintensities (WMHs) on MRI [7]. While WMHs tend to correlate with cognitive decline, a significant number of healthy elderly have WMHs that increase in size with age [8]. Separating the pathological WM changes from those in normal individuals can be difficult with FLAIR MRI. This is the challenge faced by diffusion MRI, which more clearly delineates the pathological changes in WM. The problem is that there are many diffusion methods and the optimal ones are still being uncovered. Because the field is in flux, this review will describe a number of studies without attempting to choose the optimal method. In the past several years, a number of novel methods diffusion imaging have been tested and are now ready to enter use in clinical studies. This review will contrast and compare various diffusion MRI methods related to chronic WM diseases.
1.1. Diagnosis of patients with chronic WM disease
In the last ten years the MRI methods used to study cerebral small vessel disease (SVD) have been reviewed by multiple groups [9–16]. The MRI biomarkers used to study SVD are similar to those used for studying other neurodegenerative diseases, such as AD. The two diseases SVD and AD can coexist with aging to cause mixed dementia (MX). The terminology used in classifying different forms of dementia and the terminology of the MRI methods across different studies has been previously reviewed to encourage more standard data reporting [16]. In this review, while reporting on different studies, we retain the terminology used by the respective authors. Multiple infarcts (MI) are patients with large cerebrovascular infarcts either due to large vessel thrombosis or embolus. Since these patients often have atherosclerosis of the carotid arteries or atrial fibrillation, they have strokes at random intervals, making it difficult to determine the natural history. There are a smaller number of patients with MI. We have excluded the studies related to this group from the review unless they are a part of a SVD study.
There is a spectrum of diseases that result in changes in the WM, creating a challenge to the clinician to identify those patients with axonal injury that are at risk of progression. At one end of the spectrum are WM changes of aging without pathological changes in the WM often associated with long-standing hypertension. When the WMHs on FLAIR are extensive without a clear underlying pathological process, the term leukoaraiosis (LA) can be used [17]. Pathological injury with loss of myelin can be detected by MRI with proton magnetic resonance spectroscopy (1H-MRS) with low levels of N-ascetylaspartate (NAA) in regions with damaged axons. Low values for NAA can be used as a surrogate marker for WM injury [18]. Diffusion provides another measure of WM injury as shown by elevated mean diffusivity (MD) and reduced fractional anisotropy (FA). Subcortical ischemic vascular disease (SIVD) is used to describe large WM lesions secondary to SVD, which can be identified by either reduced NAA or elevated MD. Finally, there are patients with WMHs that have both AD probably secondary to neuronal loss and vascular disease similar to SIVD, which are called MX. Both AD and MX patients have reduced amyloid-β1–42 /amyloid- β1–40 ratio and elevated phospho-tau181 in the CSF, and have deficits in memory function. Those with pure AD lack WMHs and have no neurological findings.
Patients with the diagnosis of SIVD generally have vascular risk factors with SVD secondary to hypertension, diabetes, hyperlipidemia, and sleep apnea. They often have asymmetric hyperreflexia and impaired balance with a tendency to fall. There may be lacunar strokes in the WM, and progression tends to be slow with gradual enlargement of the WM lesions. Cerebrospinal fluid (CSF) shows an increase in albumin index (ratio of albumin in CSF to that in blood), and amyloid-β1–42 and phosphoTau in the CSF are normal. MX is a term used to describe patients that have evidence of AD as shown in reduced amyloid-β1–42/amyloid-β1–40 ratio in the CSF along with elevated phospho-tau181. In addition, they have abnormal diffusion in the WM, indicating that that they have both AD and SIVD. Clinically, they have reduced memory function similar to AD and increased albumin ratio similar to SIVD. LA patients have large WMHs on FLAIR. However, diffusion is generally normal. The neuropsychological findings show normal function or mild cognitive impairment. This is an unstable group and can progress over time to either SIVD or MX.
2. MRI methods in VCI
Several MRI biomarkers have been used to study WM disease. They range from biomarkers implemented on standard clinical scanners which can be used across multiple sites and advanced methods requiring special techniques. Clinically applicable MRI biomarkers include small subcortical infarctions, lacunes of presumed vascular origins, WMHs of presumed vascular origin, perivascular spaces, cerebral microbleeds, and brain atrophy [15, 16]. The more advanced MRI biomarkers include blood-brain barrier permeability, magnetic resonance spectroscopy, diffusion MRI (dMRI) cerebral blood flow, cerebral vascular reactivity, and brain connectivity as measured by functional MRI (fMRI) and dMRI [9, 11, 12].
WMHs as a marker of WM damage are common across all studies of SVD. WMHs are easily observed in FLAIR images and software programs are available for automatic segmentation of WMH, making it possible to quantify them and follow their changes longitudinally [19]. WMHs to some extent are present in all cases of SVD. The disadvantage of WMHs as a biomarker is that it is not specific to SVD, because it can occur with normal aging without causing any cognitive decline. WMHs of presumed vascular origin affect the periventricular/deep cerebral WM, basal ganglia, and pons. There is some discussion whether WMHs in subcortical gray matter should be included. If subcortical WMHs are included then the recommendation is that it should be explicitly stated [16]. Studies from 1966 to 2009 have been reviewed with meta-analysis for the clinical importance of WMHs [20]. In 99 subjects with ages in the range of 75–89 years, the increase in WMHs was associated with decline in cognition, mobility, and increase in depressive symptoms [21]. There has been a large European multicenter Leukoaraiosis and Disability (LADIS) study with 639 Patients in the range of 65–84 years of age. The results of the LADIS study have been previously reviewed [22]. Subjects were selected in this study with WMH classified in the ranges of mild, moderate, and severe based on a revised Fazekas scale [23]. Subjects with highest severity of WMHs at baseline already had functional impairment as measured by a forty item Disability assessment for dementia. The same scale also showed declining performance with increased WMHs.
WMHs are a binary indicator of WM damage: they indicate which regions have changes, but neither provides information about possible damage in normal-appearing WM (NAWM) on FLAIR nor information of the severity of damage within the WMHs. This ambiguity has contributed to the search for newer MRI methods, such as diffusion imaging to better characterize WM damage [24, 25]. The LADIS group also evaluated the value of diffusion imaging in conjunction with WMHs [26]. Here we review the different ways dMRI can be used to probe WM integrity as related to SVD.
3. Diffusion MRI (dMRI) Methods in VCI
There have been several reviews of dMRI [27–31] and an excellent book by Callaghan [32]. Here we give a short summary to introduce the terminology and the methods used in dMRI. The reader is referred to the previous reviews for more detailed explanations. The most common diffusion imaging experiment is the pulsed gradient spin echo (PGSE) [33], which effectively consists of a bipolar gradient pulse for encoding random motion followed by a spin-echo echo planar imaging sequence. If the water motion can be described by a Gaussian process then the signal in a voxel is given byS =S e0−bd, where b is an experimental parameter depending on the diffusion sensitizing gradients.
Isotropic diffusion occurs when there is equal diffusion in all directions. If a preferred direction of diffusion exists, such as diffusion in cylindrical tubes, then we have anisotropic diffusion. A Gaussian anisotropic diffusion is described by a diffusion tensor D (symmetric 3×3 matrix with 6 unknowns), and the dMRI signal at a voxel is given by , where n is a normal vector along the gradient direction [34]. The diffusion tensor imaging (DTI) is the most widely used diffusion experiment. It assumes Gaussian translational motion of water molecules which leads to mono-exponential signal decay. The typical parameters calculated from DTI are mean diffusivity (MD) and fractional anisotropy (FA), which is a number from 0 to 1, and is a measure of the difference in the diffusion along the three directions. FA is equal to 0 for isotropic diffusion and 1 for anisotropic diffusion when the diffusion is only in one direction.
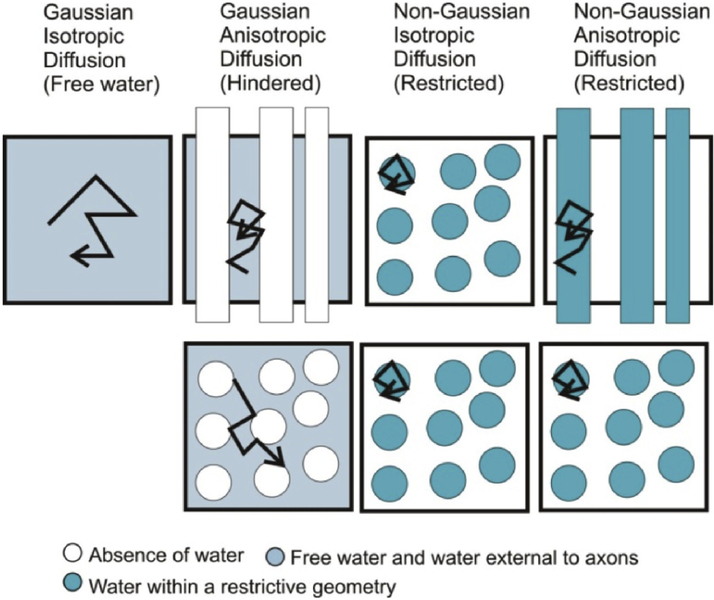
Figure 1 shows when the diffusion is Gaussian and non-Gaussian and when it is isotropic and anisotropic with examples of simple geometries. In geometries with restriction and barriers the diffusion within the restricted geometries is non-Gaussian (also called restricted diffusion) and the diffusion in water outside the restricted spaces is Gaussian (also called hindered diffusion). If no restrictions are present the diffusion is isotropic (also called as free water diffusion). The Gaussian diffusion can be described by a single exponential while non-Gaussian is not mono-exponential. Diffusion weighted imaging (DWI) gives a measure of mean diffusivity by making diffusion measurement in three orthogonal directions and taking the mean of the diffusion coefficient in the three directions. This is a short simple experiment and was the precursor to DTI. The DTI experiment is currently the most widely used diffusion experiment. It has been useful to characterize the microstructure based on anisotropic Gaussian diffusion.
Figure 1.
The figure shows examples when the diffusion is Gaussian and non-Gaussian and when it is isotropic and anisotropic. Isotropic diffusion occurs in fluids without barriers or spherically symmetric barriers. Anisotropic diffusion occurs when there is a preferential direction for diffusion. In a geometry with a bundle of cylindrical tubes, diffusion in the fluid outside the tubes and the diffusion of water in the tubes is both anisotropic because the diffusion along the length of the tubes is greater because it is unrestricted as compared to the diffusion perpendicular to the tubes axis. The diffusion in the space outside the tubes can be approximated by Gaussian diffusion while the diffusion within the tubes is bounded by the tube walls and is non-Gaussian. The diffusion in the space external to the tubes is also hindered and the diffusion within the tubes restricted diffusion.
Gray matter consists of neuronal cell bodies, dendrites, myelinated and unmyelinated axons, astrocytes and oligodendrocytes. White matter consists of bundles myelinated axons connecting gray matter regions. In a typical diffusion MRI voxel of the size 2mm3, the tissue properties can be inhomogeneous. Several approached have been studied to model the tissue heterogeneity and the signal decay not being adequately defined by a single exponential. One approach to model tissue heterogeneity has been with multiple-compartment models. The taxonomy of different diffusion models and their relative merits have been reviewed [35, 36]. IVIM [37] is a diffusion technique which measures the microvascular perfusion and parenchymal tissue properties with the use of a 2-compartment diffusion model [38]. Few studies employed IVIM in SVD subjects to investigate the microstructural integrity and perfusion simultaneously [39, 40]. Other multicompartment models were developed to explain the partial volume effects of the large diffusion voxel (2mm3). This includes the two-compartment free-water model to include the presence of CSF or edema in a voxel [41], the multi-compartment CHARMED model [42] which has hindered and restricted diffusion, and the three compartment NODDI model [43] which includes a free-water compartment, a hindered diffusion compartment and a restricted diffusion compartment. An alternative method to model deviation from the single exponential signal decay is the method of diffusion kurtosis imaging (DKI) based on the cumulant expansion of the diffusion signal [44]. This takes into account the non-Gaussian nature of diffusion of water molecules [45]. While there are have been no studies reported in VCI related disorders using CHARMED or NODDI, there have been a few studies utilizing free-water and DKI in VCI subjects [46–48].
Diffusion based tractography is a separate analysis category [49], which use local information of the orientation distribution of the white matter tracts and connects adjacent voxels to map white matter tracts from one gray matter region to another. A structural connectivity matrix, connecting different brain regions, can be calculated from the orientation distribution function at each voxel. Parameters based on network analysis, such as network efficiency, strength, path length and density can be further used in group analysis.
4. Diffusion MRI Studies
We reviewed the literature on diffusion MRI for chronic WM disease studies up to July 2018 in PubMed. The search query, “(diffusion mri OR diffusion magnetic resonance imaging OR DTI OR DWI OR DKI OR IVIM) AND (VCI OR vascular cognitive impairment OR SVD OR cerebral small vessel disease OR Binswanger’s disease OR SIVD OR subcortical ischemic vascular disease OR leukoaraiosis OR mixed dementia)” was searched in keywords, title and abstract. References were searched to include any relevant article that was missed in the online database search. A total of 55 journal articles were finally selected for review.
The review focuses on various aspects such as diffusion acquisition protocols, the models used for data analysis and nature of WM disease studied. Diffusion acquisition protocols varied from employing single b-value shell to multi-shell acquisitions to cover a range of b-values and gradient directions. Diffusion biomarkers depended on the diffusion model and the type of analysis used in the studies. As an example, studies involving DTI used MD and FA as the parameters and performed whole brain or region based statistical analysis to report on the significance of such biomarkers in detecting the disease. In this review, the relevant studies are compared and categorized based on these different analysis methods. The diffusion methods include DWI, DTI, advanced multi-compartment diffusion models, tractography analysis, and connectivity analysis. Diseases primarily include LA, VCI, SIVD and SVD. Moreover, the analyses done by prominent study cohorts (LADIS, RUN DMC and SCANS) aimed at exploring biomarkers for vascular cognitive impairment are reviewed. Categorizing the articles based on the diseases studied yields a majority of them belonging to SVD with totally 33 articles out of 55 and rest of them are, 16 in LA, 5 in VCI and 1 reporting SIVD. Similarly, the number of studies categorized based on diffusion methods varies from 31 in DTI, 12 in tractography, 7 in DWI and 5 using other advanced diffusion models. Most of the works belong to one of the study cohorts, LADIS, RUNDMC or SCANS where 12 reported by RUN DMC, 9 by SCANS group and 3 were LADIS.
4.1. Diffusion Weighted Imaging (DWI)
Earlier studies used trace/apparent diffusion coefficient (ADC) images calculated from DWI to characterize abnormal WM regions in vascular cognitive impairment. The studies reviewed [50–56] are summarized in Table 1. All the reports studied the LA subject group, except for [56], who studied the SVD group. Among the studies reviewed there are two longitudinal studies. The main analysis method consisted of calculating the mean ADC, the histogram peak height, or its mode over either the WM, the whole brain region excluding the CSF, or the normally appearing brain tissue.
Table 1:
Characteristics of DWI Studies
| Study/Subject | dMRI Protocol | Analysis | Findings |
|---|---|---|---|
|
Helenius et al., 2001 [50] LA=85, HC=22, IS=10 |
Siemens 1.5T b=1000, D=3 |
Cross-sectional Regional | High ADC in WMHs and NAWM of severe LA than mild LA |
|
Viana-Baptista et al., 2008 [52] LA=29 |
GE 1.5T b=1000, D=6 |
Cross- sectional Regional | Positive correlation of ADC with Age, BP, VSS and negative correlation with attention |
|
Ropele et al., 2009 [53] LA=340 |
1.5T b=900–1000, D=3 |
Cross- sectional Histogram | ADC and not magnetization transfer ratio was related to WMH severity |
|
Viana-Baptista et al., 2011 [54] LA=29 |
GE 1.5T b=1000, D=6 |
Cross- sectional Regional | Significant negative correlations between ADC and motor function |
|
Oztoprak et al., 2015 [56] SVD=50, MS=35, HC=85 |
Siemens 1.5T b=1000, D=3 |
Cross- sectional Regional | High ADC in thalamus of S VD subjects than in MS and HC |
|
Mascalchi et al., 2002 [51] LA=10 |
Philips 1.5T b=1000, D=3 |
Longitudinal Histogram | ADC increases, brain volume index decreases with disease progression; No change in GS, MMSE |
|
Jokinen et al., 2013 [55] LA=340 |
1.5T b=900–1000, D=3 |
Longitudinal Histogram | ADC correlates with cognitive decline and disease progression. |
The main observations in the cross-sectional studies [50, 53, 55] was of increased ADC values with WMHs severity. ADC measures were correlated with other VCI markers such as MRI visual rating scale, blood pressure, gait score, neuropsychological tests scores and magnetization transfer ratio (MTR) maps. Age, blood pressure and visual scores were positively correlated with mean ADC values in NAWM and WMHs [52]. On the other hand, MTR metrics, attention and motor function scores negatively correlated with ADC [52–54]. The significance of ADC was reported to outperform other markers in detection of WM pathology. Longitudinal studies [51, 55] showed increased diffusion with progression of WMH severity. Correlations between changes in ADC values with changes in other markers such as gait score, neuropsychological tests scores and brain volume index were also reported in these studies. While gait and MMSE score did not significantly change between time points of disease progression [51], ADC correlated with decline in cognitive measures such as speed and motor control, executive functions, and memory tests scores [55]. The main advantage of the ADC measure was its potential to differentiate acute and chronic lesions in LA. Memory, intelligence, attention, and executive function cognitive scores were strongly correlated with ADC in LA subjects. Irrespective of these benefits of ADC, the limitation of DWI model to represent anisotropic diffusion in neuronal tissues lead to DTI model in later SVD studies.
4.2. Diffusion Tensor Imaging (DTI)
Thirty two DTI studies were included for this review [47, 57–86]. We grouped the studies based on cross-sectional or longitudinal analysis. Seven of these reports studied LA subjects and others studied subgroups such as SVD, VCI and SIVD. We separately grouped the studies based on cross-sectional or longitudinal analysis. Studies on LA were entirely cross-sectional [47, 57, 62] whereas studies related to VCI were predominantly longitudinal. Longitudinal studies [63, 67, 70, 78–81, 83–86] focused on characterizing the disease progression using DTI measures.
4.2.1. Cross-sectional DTI Studies
The characteristics of cross-sectional DTI studies in LA subjects are tabulated in Table 2 and those for other disease groups (SVD, SIVD, and VCI) are summarized in Table 3. Except for [61], which is based on whole brain histogram and VBA analysis, the other 6 studies did regional analysis of mean MD and FA calculated on NAWM and WMH. These studies demonstrated low FA and high MD values in WMHs and NAWM of LA subjects as compared to controls. Studies [59, 61] which employed MRI FLAIR or T1 weighted volumes computed total brain volume, WMH volume, and parenchymal volume as conventional MRI markers. These studies showed that DTI had better correlation with cognitive scores than WMHs or other regional brain volumes.
Table 2.
Characteristics of Cross-sectional DTI studies in LA Subjects
| Study/Subject | dMRI Protocol | Analysis | Findings |
|---|---|---|---|
|
Jones et al., 1998 [57] LA=9, HC=10 |
GE 1.5T b=0-614 D=7 |
Regional | Low FA and High MD in NAWM of LA subjects as compared to HC |
| O’Sullivan et al., 2001 [58] LA=30, HC=17 | GE 1.5T |
Regional | Low FA and High MD in LA than in HC; MMSE and WCST correlated with MD and FA |
| O’Sullivan et al., 2004 [59] LA=36, HC=19 | GE 1.5T |
Regional | MD and FA differences between LA and HC in WMHs and in NAWM; No correlations between WMH volume and CS but significant correlations between FA and MD of NAWM and CS |
|
Nitkunan et al., 2006 [60] LA=25 |
GE 1.5T b=1000 D=12 |
Regional | Positive correlation between mean NAA and FA, and negative correlation with MD; Positive correlation between WMH volume and MD, and negative correlation with FA |
|
Nave et al., 2007 [61] LA=36 |
GE 1.5T b=1000 D=32 |
VBA, Histogram | No correlations between GM/WM volume and CS Significant correlations between MD and FA with CS |
|
Wang et al., 2017 [62] LA=42, HC=42 |
Siemens 3T b=1000 D=20 |
Regional | MD and FA differences between LA and HC in WMHs and in NAWM; FA positively correlated with CS but no correlation with MD |
|
Zhong et al., 2017 [47] LA=75 |
GE 3T b=1000 D=30 |
Regional | MD and FA are significantly correlated with CS and their correlation is higher than the correlation of CBF with CS |
Table 3.
Characteristics of Cross-sectional studies reporting DTI findings in SVD, SIVD and VCID
| Study/Subject | dMRI Protocol | Analysis | Findings |
|---|---|---|---|
|
Nitkunan et al., 2008 [64] SVD=29, HT=63, NT=42 |
GE 1.5T b=1000 |
Regional | High WMH volume in SVD; Higher MD, lower FA and lower NAA with disease progression; |
|
Zhou et al., 2008 [65] VCI=19, PS=19, HC=19 |
Philips 3T b=800 D=15 |
Histogram | Lower FA in VCI and PS than HC; MMSE correlated with FA in VCI, whereas MMSE correlated with MD in PS and HC |
|
Kim et al., 2011 [66] MCI=27, Dementia=34 |
GE 3T b=600 D=45 |
VBM | MD and FA are significantly correlated with cognitive and motor deficits |
|
Huang et al., 2014 [68] SVD=12, HC=6 |
GE 1.5T b=1000 |
Regional | Significant difference between SVD and HC for MD but not for NAA, and weak but not significant differences in FA |
|
Thong et al., 2014 [69] Mild VCI=25, Moderately severe VCI=30, AD=20 HC=25 |
Siemens 3T b=1150 D=61 |
VBA, Regional | Thinner cortex and lower volumes in all disease groups as compared to HC; Higher MD and lower FA in moderately severe VCI and AD than in HC; Only higher MD in mild VCI than HC; |
|
Croall et al., 2017 [71] SVD=199 |
3T b=1000 D=32 |
Histogram | Significant correlations between DTI and number of lacunar infarcts with CS, but none between WMH volume and CS |
|
Tu et al., 2017 [72] SIVD=35, AD=40, HC=33 |
GE 3T b=1000 D=20 |
Regional | Higher Fazekas scale and MD and lower FA and FABS in SIVD than in AD |
Some studies used other MRI biomarkers, such as magnetic resonance spectroscopy [60] and cerebral blood flow (CBF) measured by arterial spin labelling (ASL) [47]. MRS markers included N-acetyl aspartate (NAA), total cholines (Cho), combined glutamate and glutamine (Glx) and myo-inositol (mI). CBF was calculated in WMH, normal appearing WM (NAWM) and cortex. These measures were correlated with cognitive scores. Among these other MRI markers DTI showed the strongest correlation with cognitive scores. Table 2 describes the group analysis and correlation results of each study. The characteristics of cross-sectional DTI studies VCI, SIVD and SVD (Table 3) were similar to the LA subjects. Regional and histogram based analysis were used. Parameters such as median, histogram peal height and histogram mode were computed. The decreased anisotropy and increased diffusion were clinically correlated to the axonal loss, demyelination and gliosis observed in VCI subjects. The correlation between MD and FA were consistent with cognitive decline whereas correlations for other MRI markers were weaker. Despite these advantages, the pathogenesis underlying DTI changes is not clearly understood.
4.2.2. Longitudinal DTI Studies
Longitudinal studies are important because they answer questions related to disease progression and identify early markers of the disease. The results from various longitudinal studies ranging from 1 to 8 years of follow-up are reported in Tables 4–6. Two longitudinal studies from individual groups are reported in Table 4 [63, 67]. DTI whole brain histogram features showed changes towards increased MD and decreased FA in a span of 1 year period [63], whereas significant changes in DTI over thalamus and putamen correlating with WMHs accrual over a period of 4 years shown in another study [67]. Two other studies have reported on the significance of a single DTI measure calculated from DTI image [70, 85]. These include the peak width of skeletonized MD (PSMD) and a novel DTI segmentation angular measure. These markers characterized the SVD related brain changes in both cross-sectional and longitudinal studies. RUN DMC (Radboud University Nijmegen Diffusion tensor and Magnetic resonance imaging Cohort) study [87] summarized in Table 5 and SCANS (St George’s Cognition and Neuroimaging in Stroke) study [83] summarized in Table 6 were the 2 large cohort studies with 8 and 3 years follow-up period respectively reporting SVD related biomarkers.
Table 4.
Characteristics of longitudinal studies reporting DTI findings
| Study/Subject | dMRI Protocol | Analysis | Findings |
|---|---|---|---|
|
Nitkunan et al., 2008 [63] LA, at baseline=35, LA at one year=27 |
GE 1.5T b=1000 D=12 | Histogram | Higher MD and lower FA, but no changes in WMH volume, brain volume, and CS between time points |
|
Cavallari et al., 2014 [67] SVD=56 |
Siemens 3T b=1000 D=12 |
Regional | Baseline: Thalamus and putamen MD correlated with WMH volume; Follow-up: Thalamus FA correlated with annual WMH accrual rate over 4 years |
Table 6:
Characteristics of studies belonging to SCANS study
| Study | Analysis | Findings |
|---|---|---|
| Lawrence et al., 2013 [82] | Cross-sectional Histogram | DTI and number of lacunar infarcts correlated with CS decline; DTI suggested ischemic demyelination in SVD |
| Benjamin et al., 2016 [83] | Longitudinal Histogram | DTI and WMH volume had greater significance in predicting disease progression than CS |
| Zeestraten et al., 2016 [84] | Longitudinal Histogram | Histogram peak height of MD was the most sensitive marker for disease progression among all other DTI metrics |
| Williams et al., 2017 [85] | Longitudinal Histogram | A novel DTI segmentation method predicted cognitive decline over a 3 year period |
| Zeestraten et al., 2017 [86] | Longitudinal Regional | DTI markers and WMH volume correlated significantly with CS decline and predicted dementia over 3 years. |
Table 5:
Characteristics of RUN DMC studies
| Study | Analysis | Findings |
|---|---|---|
| Gons et al., 2010 [73] | Cross- sectional Regional | BP correlated with WMHs volume; High BP correlated with low FA and high MD; Lower FA and higher MD in HT than in NT |
| van Norden et al., 2012 [74] | Cross- sectional Regional | DTI correlated with CS in mild, moderate and severe WMHs; DTI had limited additional value as compared to WMHs volume with respect to correlations with cognitive decline |
| van der Hoist et al., 2013 [77] | Cross- sectional Regional, TBSS | DTI correlated with CS and hippocampal integrity; Higher MD associated with 5 year dementia risk |
| Tuladhar et al., 2015 [88] | Cross- sectional Regional, TBSS | DTI of genu and splenium correlated with CS; DTI of various WM tracts related to CS |
| van Uden Hoist et al., 2015 [78] | Longitudinal Regional | No significant relation between DTI and CS decline after adjusting for confounders; Higher MD associated with 5 year dementia risk |
| van Leijsen et al., 2018 [81] | Longitudinal Regional | WM damage can be detected by DTI before the appearance of WMH lesions. |
RUN DMC group has published results both on cross-sectional and longitudinal analysis with 8 years follow-up period. DTI, MRI, neuropsychological tests scores and dementia risk score were collected as part of this study. Nearly 500 SVD subjects were included with 8 years follow-up scans and the diffusion data was collected using a 1.5 T Siemens scanner with b-value of 900 s/mm2 in 30 gradient directions. Cross-sectional studies [73–77, 88] showed DTI outperforming conventional MRI markers such as WMH volume, hippocampal volume and number of lacunar infarcts. Moreover, DTI measures were strongly correlated with hippocampal integrity in diseased subjects. The longitudinal analysis demonstrated DTI changes with disease progression significant correlations with risk of dementia and mortality risk. DTI changes were observed in NAWM and hippocampus which makes DTI, a good predictor of conversion to dementia and risk of death. Unlike RUN DMC, SCANS reports were predominantly longitudinal (Table 6), and aimed at analyzing the histogram features and estimating sample size required for those features. The study cohort includes nearly 100 SVD subjects with 3 year follow-up scans collected using 1.5 T GE scanner with b-value of 1000 s/mm2 in 25 gradient directions. One cross-sectional study [82] detailed on the significance of histogram derived features from entire DTI maps such as MD, FA, AxD and RD. The diffusion observations in NAWM correlated with cognitive decline. On the other hand, longitudinal analyses [83–86] proved histogram peak height of MD to be better than any other measure in predicting disease progression with a smaller sample size estimate.
4.2.3. DTI measures in VCI Subjects
Mean values of MD and FA in NAWM collected from the reviewed studies are summarized in Table 7. MD is higher in patients as compared to healthy controls. Among the patients, MD is least in AD and highest in SIVD. FA shows an inverse trend to that of MD across the subgroups. Interestingly, the MD for NAWM is higher in patients than for HCs, indicating that white matter damage is seen in NAWM, which is classified as normal by FLAIR.
Table 7.
Mean values of MD and FA in NAWM reported for healthy controls (HC), Leukoaraiosis (LA), Alzheimer’s disease (AD), cerebral small vessel disease (SVD) and subcortical ischemic vascular disease (SIVD) collated from reviewed papers
| Subject Group | HC | LA | AD | SVD | SIVD |
|---|---|---|---|---|---|
| Mean MD | 0.8±0.05 | 0.87±.04 | 0.82±0.17 | 0.92±0.12 | 1.12±0.4 |
| Mean FA | 0.4±0.07 | 0.34±0.06 | 0.39±0.09 | 0.31±0.06 | 0.32±12 |
4.3. Studies using Other Diffusion Models
DKI can model non-Gaussian diffusion and various DKI metrics such as mean kurtosis, axial and radial kurtosis (in addition to DTI metrics) were helpful in disease diagnosis. Two DKI studies [46, 47] showed increased sensitivity of DKI measures which could differentiate NAWM between mild and severe LA subjects and better correlated with cognitive decline as compared to cerebral blood flow. The IVIM model simultaneously offers microstructural and microvascular perfusion of WM and GM. SVD studies using IVIM [39, 40] showed increased parenchymal diffusivity and decreased perfusion in SVD patients, with cognitive decline correlations in WM lesions. A recent study [48] published the significance of free water diffusion model in characterizing SVD. This model separates the free water fraction from the anisotropic diffusion in WM based on a two compartment model. Increased extracellular fluid was suggested to be the main cause behind diffusion alterations in SVD rather than degeneration of WM tracts. More studies in future are required with advanced models to resolve the pathogenesis underlying diffusion MRI changes in VCI.
4.4. Structural Connectivity
Structural connectivity in brain denotes the connectivity between brain regions established by tracts running between any 2 regions. Tracts are generated by fiber tracking algorithms which could be deterministic or probabilistic [28, 89]. Measures extracted from these tracts are of significance in differentiating disease from normal tissues. Extending this, network based connectivity analysis involve incorporating network measurements from connectivity matrices [90]. The relevant studies included in this review are summarized in Table 8. Most of the studies performed tractography based on deterministic fiber tracking [91, 92] except [93, 94] where probabilistic tracking based on constrained spherical deconvolution (CSD) model [95] was followed. Network measures such as network efficiency and connectivity strength were used widely except [94, 96, 97] where the DTI values were analyzed on WM tracts.
Table 8:
Characteristics of studies using tractography and diffusion based connectivity analysis
| Study/Subjects | Diffusion Protocol | Analysis | Conclusions |
|---|---|---|---|
|
Correia et al., 2008 [100] VCI=14, HC = 18 |
Siemens 1.5T DTI b=1000, D=12 |
Cross-sectional Connectivity Network Analysis | Significant correlations between network measures and cognitive scores |
|
Lawrence et al., 2014 [101] LA = 115, HC=50 |
GE 1.5T DTI b=1000, D=25 |
Cross-sectional Connectivity Histograms | Reduced network connectivity in SVD. Network measures have stronger correlation with cognitive scores than with WMH volume |
|
Kim et al., 2015 [102] VCI = 232 |
Phillips 3T DTI b=1000, D=45 |
Cross-sectional Connectivity Whole Brain | Network segregation correlated with cognitive scores and WMHV |
|
Reijmer et al., 2016 [93] VCI = 232 |
Siemens 3T b=700 |
Cross-sectional Connectivity Whole Brain | FA correlated with cognitive scores in central brain regions |
|
Tuladhar et al., 2016 [103] SVD = 436 |
Siemens 1.5T DTI b=900, D=30 |
Cross-sectional Connectivity Whole Brain | WMH volume and MD of WM correlates with cognitive scores |
|
Tuladhar et al., 2016 [104] SVD = 436 |
Siemens 1.5T DTI b=900, D=30 |
Longitudinal Connectivity Whole Brain | Network efficiency predicts dementia |
|
Metoki et al., 2017 [96] SVD = 106 |
GE 1.5T DTI b=1000, D=25 |
Cross-sectional Cingulum Uncinate fasciculus | Lower memory scores in SVD than HC MD correlated with memory scores FA only correlated with working memory |
|
Lawrence et al., 2018 [105] SVD=26, HC=19 |
Siemens 3T DTI b=1000, D=63 |
Cross-sectional Connectivity Whole Brain ROIs | Lower network measures in SVD than HC Similar fMRI network measures in SVD andHC |
|
Biesbroek et al., 2018 [94] VBI=159 |
Phillips 3T DTI b=1200, D=45 |
Cross-sectional Connectivity Whole Brain ROIs | Specific white matter tracts are strongly correlated with specific cognitive scores |
|
D’Souza et al., 2018 [97] SVD=30 |
Siemens 3T DTI b=1000, D=30 |
Cross-sectional Connectivity Whole Brain ROIs | MD and FA are correlated with cognitive scores and no correlation with WMH volume |
|
Lawrence et al., 2018 [106] SVD=97 |
GE 3T DTI b=1000, D=30 |
Longitudinal Network Connectivity Histogram | Network global efficiency during follow up correlated with increased dementia |
|
Lisiecka-Ford et al., 2018 [107] SVD=114 |
GE 3T DTI b=1000, D=30 |
Cross-sectional Connectivity Whole Brain ROIs | Network efficiency of reward network negatively correlated with apathy scores. |
All network measures indicated network disruption in tracts passing through NAWM and WMHs regions. Longitudinal analysis showed network disruption in dementia subjects as compared non-dementia in the follow-up period. Tractography analysis is attractive as compared to regional or histogram methods because it potentially identifies tracts related to specific cognitive decline. In future, more studies are anticipated with advanced CSD model based probabilistic tractography analysis [98]. Moreover, fixel based analysis [99] which computes metrics on more than one fiber population present within a voxel is potentially another significant method for predictions in SVD.
5. Conclusion
DTI with parameters MD and FA is currently the most widely used diffusion method to study SVD subjects. Several groups have been studying the relationship between MD, FA and the NAWM in the FLAIR image. The general trend is that in damaged white matter MD increases and FA goes down because of breakdown of axon walls and fluid accumulation. These results collected across various studies shows that even the NAWM appears to be damaged in the diffusion image. Newer diffusion imaging methods are being developed to probe the microstructure more closely. These methods include building biophysical models so that tissue structure can be parsimoniously defined by fewer parameters that reflect tissue properties. The NODDI model discussed earlier is in this class of methods. More importantly alternative methods of data acquisitions are also being developed which employ more complex diffusion encoding (other than bipolar gradients of the PGSE experiment) to decode the tissue heterogeneity within a voxel. These methods are sensitive to the variability of the mean diffusivity within a voxel (size variance) and to the variability of the fiber orientation within a voxel (micro anisotropy) [108].
Highlights.
We review diffusion MRI studies in chronic white matter diseases.
DTI with parameters MD and FA is currently the most widely used diffusion method to study SVD subjects.
In damaged white matter MD increases and FA goes down because of breakdown of axon walls and fluid accumulation.
Various studies reveal that even the NAWM appears to be damaged in the diffusion images.
Future studies should be aimed at multi-compartment and higher order diffusion models.
Abbreviations
Patient Groups
- AD
Alzheimer’s disease
- CADASIL
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy
- HC
Healthy Controls
- MCI
Mild cognitive impairment
- MS
Multiple sclerosis
- SIVD
Subcortical ischemic vascular disease
- SVD
Cerebral small vessel disease
- VCI
Vascular cognitive impairment
- VCID
Vascular cognitive impairment and dementia
MRI Units
- b
b-value in s/mm2
- D
Gradient directions
- T
Tesla
MRI methods
- dMRI
Diffusion MRI
- DTI
Diffusion Tensor Imaging
- DWI
Diffusion weighted MRI
- FLAIR
Fluid Attenuated Inversion Recovery
- fMRI
Functional MRI
- IVIM
Intravoxel incoherent motion imaging
- MRS
Magnetic resonance spectroscopy
MRI measures
- ADC
Apparent diffusion coefficient
- CBF
Cerebral blood flow
- FA
Fractional Anisotropy
- FS
Fazekas Scale
- FW
Freewater fraction
- GM
Gray Matter
- MD
Mean diffusivity
- MTR
Magnetization transfer ratio
- NAA
N-acetyl aspartate
- NAWM
Normal appearing White Matter
- WM
White Matter
- WMH
WM Hyperintensity
- VBM
Voxel based morphometry
Cognitive and Other Measures
- BP
Blood pressure
- CS
Cognitive Score
- FABS
Total score of Frontal Assessment Battery
- GS
Gait Score
- MMSE
Mini mental state examination
- VSS
Visual scale score
- WCST
Wisconsin Card Sorting Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Olszewski J, Subcortical arteriosclerotic encephalopathy: review of the literature on the so-called Binswanger’s disease and presentation of two cases, World Neurol 3 (1965) 359–373. [PubMed] [Google Scholar]
- [2].Caplan LR, Schoene WC, Clinical features of subcortical arteriosclerotic encephalopathy (Binswanger disease), Neurology 28 (1978) 1206–1206. [DOI] [PubMed] [Google Scholar]
- [3].Rosenberg GA, Kornfeld M, Stovring J, Bicknell JM, Subcortical arteriosclerotic encephalopathy (Binswanger) computerized tomography, Neurology 29 (1979) 1102–1102. [DOI] [PubMed] [Google Scholar]
- [4].Binswanger’s encephalopathy, Lancet. 1 (1981) 923. [PubMed] [Google Scholar]
- [5].Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease, Alzheimer’s & Dementia 11 (2015) 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Iadecola C, The pathobiology of vascular dementia, Neuron 80 (2013) 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, DeCarli C, Carmichael OT, White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain, Stroke 45 (2014) 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hunt A, Orrison W, Yeo R, Haaland K, Rhyne R, Garry P, Rosenberg G, Clinical significance of MRI white matter lesions in the elderly, Neurology 39 (1989) 1470–1470. [DOI] [PubMed] [Google Scholar]
- [9].Alves GS, de Carvalho LA, Sudo FK, Briand L, Laks J, Engelhardt E, A panel of clinical and neuropathological features of cerebrovascular disease through the novel neuroimaging methods, Dementia & neuropsychologia 11 (2017) 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Banerjee G, Wilson D, Jager HR, Werring DJ, Novel imaging techniques in cerebral small vessel diseases and vascular cognitive impairment, Biochimica et biophysica acta 1862 (2016) 926–938. [DOI] [PubMed] [Google Scholar]
- [11].Blair GW, Hernandez MV, Thrippleton MJ, Doubal FN, Wardlaw JM, Advanced Neuroimaging of Cerebral Small Vessel Disease, Current treatment options in cardiovascular medicine 19 (2017) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smith EE, Beaudin AE, New insights into cerebral small vessel disease and vascular cognitive impairment from MRI, Current opinion in neurology 31 (2018) 36–43. [DOI] [PubMed] [Google Scholar]
- [13].Norrving B, Evolving Concept of Small Vessel Disease through Advanced Brain Imaging, Journal of stroke 17 (2015) 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Patel B, Markus HS, Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker, International journal of stroke : official journal of the International Stroke Society 6 (2011) 47–59. [DOI] [PubMed] [Google Scholar]
- [15].Shibuya M, Leite CDC, Lucato LT, Neuroimaging in cerebral small vessel disease: Update and new concepts, Dementia & neuropsychologia 11 (2017) 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M, S.T.f.R.V.c.o. nEuroimaging, Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration, Lancet Neurol 12 (2013) 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hachinski VC, Potter P, Merskey H, Leuko-araiosis, Arch Neurol 44 (1987) 21–23. [DOI] [PubMed] [Google Scholar]
- [18].Gasparovic C, Prestopnik J, Thompson J, Taheri S, Huisa B, Schrader R, Adair JC, Rosenberg GA, 1H-MR spectroscopy metabolite levels correlate with executive function in vascular cognitive impairment, Journal of neurology, neurosurgery, and psychiatry 84 (2013) 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dadar M, Pascoal TA, Manitsirikul S, Misquitta K, Fonov VS, Tartaglia MC, Breitner J, RosaNeto P, Carmichael OT, Decarli C, Collins DL, Validation of a Regression Technique for Segmentation of White Matter Hyperintensities in Alzheimer’s Disease, IEEE transactions on medical imaging 36 (2017) 1758–1768. [DOI] [PubMed] [Google Scholar]
- [20].Debette S, Markus HS, The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis, Bmj 341 (2010) c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wolfson L, Wakefield DB, Moscufo N, Kaplan RF, Hall CB, Schmidt JA, Guttmann CR, White WB, Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons, J Gerontol A Biol Sci Med Sci 68 (2013) 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].The LSG, Poggesi A, Pantoni L, Inzitari D, Fazekas F, Ferro J, O’Brien J, Hennerici M, Scheltens P, Erkinjuntti T, Visser M, Langhorne P, Chabriat H, Waldemar G, Wallin A, Wahlund A, 2001–2011: A Decade of the LADIS (Leukoaraiosis And DISability) Study: What Have We Learned about White Matter Changes and Small-Vessel Disease?, Cerebrovascular diseases (Basel, Switzerland) 32 (2011) 577–588. [DOI] [PubMed] [Google Scholar]
- [23].Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA, MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging, AJR Am J Roentgenol 149 (1987) 351–356. [DOI] [PubMed] [Google Scholar]
- [24].Pasi M, van Uden IW, Tuladhar AM, de Leeuw FE, Pantoni L, White Matter Microstructural Damage on Diffusion Tensor Imaging in Cerebral Small Vessel Disease: Clinical Consequences, Stroke 47 (2016) 1679–1684. [DOI] [PubMed] [Google Scholar]
- [25].Lyoubi-Idrissi AL, Jouvent E, Poupon C, Chabriat H, Diffusion magnetic resonance imaging in cerebral small vessel disease, Revue neurologique 173 (2017) 201–210. [DOI] [PubMed] [Google Scholar]
- [26].Schmidt R, Ropele S, Ferro J, Madureira S, Verdelho A, Petrovic K, Gouw A, van der Flier WM, Enzinger C, Pantoni L, Inzitari D, Erkinjuntti T, Scheltens P, Wahlund LO, Waldemar G, Rostrup E, Wallin A, Barkhof F, Fazekas F, L.s. group, Diffusion-weighted imaging and cognition in the leukoariosis and disability in the elderly study, Stroke 41 (2010) e402–408. [DOI] [PubMed] [Google Scholar]
- [27].Mori S, Zhang J, Principles of diffusion tensor imaging and its applications to basic neuroscience research, Neuron 51 (2006) 527–539. [DOI] [PubMed] [Google Scholar]
- [28].Assaf Y, Pasternak O, Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review, Journal of molecular neuroscience : MN 34 (2008) 51–61. [DOI] [PubMed] [Google Scholar]
- [29].Beaulieu C, The basis of anisotropic water diffusion in the nervous system - a technical review, NMR Biomed 15 (2002) 435–455. [DOI] [PubMed] [Google Scholar]
- [30].Nilsson M, van Westen D, Stahlberg F, Sundgren PC, Latt J, The role of tissue microstructure and water exchange in biophysical modelling of diffusion in white matter, MAGMA 26 (2013) 345–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Le Bihan D, Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure, Radiology 268 (2013) 318–322. [DOI] [PubMed] [Google Scholar]
- [32].Callaghan PT, Translational dynamics and magnetic resonance : principles of pulsed gradient spin echo NMR, Oxford University Press, Oxford: etc., 2011, XVII, [1], 547 s., [541] k. tabl. pp. [Google Scholar]
- [33].Stejskal EO, Tanner JE, Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient., J. Chem. Phys 42 (1965) 288–292. [Google Scholar]
- [34].Basser PJ, Mattiello J, LeBihan D, Estimation of the effective self-diffusion tensor from the NMR spin echo, J Magn Reson B 103 (1994) 247–254. [DOI] [PubMed] [Google Scholar]
- [35].Panagiotaki E, Schneider T, Siow B, Hall MG, Lythgoe MF, Alexander DC, Compartment models of the diffusion MR signal in brain white matter: a taxonomy and comparison, Neuroimage 59 (2012) 22412254. [DOI] [PubMed] [Google Scholar]
- [36].Ferizi U, Schneider T, Panagiotaki E, Nedjati-Gilani G, Zhang H, Wheeler-Kingshott CA, Alexander DC, A ranking of diffusion MRI compartment models with in vivo human brain data, Magn Reson Med 72 (2014) 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Le Bihan D, Intravoxel incoherent motion perfusion MR imaging: a wake-up call, Radiology 249 (2008) 748–752. [DOI] [PubMed] [Google Scholar]
- [38].Le Bihan D, Breton E, Lallemand D, Aubin M, Vignaud J, Laval-Jeantet M, Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging, Radiology 168 (1988) 497–505. [DOI] [PubMed] [Google Scholar]
- [39].Wong SM, Zhang CE, van Bussel FC, Staals J, Jeukens CR, Hofman PA, van Oostenbrugge RJ, Backes WH, Jansen JF, Simultaneous investigation of microvasculature and parenchyma in cerebral small vessel disease using intravoxel incoherent motion imaging, NeuroImage. Clinical 14 (2017) 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang CE, Wong SM, Uiterwijk R, Staals J, Backes WH, Hoff EI, Schreuder T, Jeukens CR, Jansen JF, van Oostenbrugge RJ, Intravoxel Incoherent Motion Imaging in Small Vessel Disease: Microstructural Integrity and Microvascular Perfusion Related to Cognition, Stroke 48 (2017) 658–663. [DOI] [PubMed] [Google Scholar]
- [41].Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y, Free water elimination and mapping from diffusion MRI, Magn Reson Med 62 (2009) 717–730. [DOI] [PubMed] [Google Scholar]
- [42].Assaf Y, Basser PJ, Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain, Neuroimage 27 (2005) 48–58. [DOI] [PubMed] [Google Scholar]
- [43].Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC, NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain, Neuroimage 61 (2012) 1000–1016. [DOI] [PubMed] [Google Scholar]
- [44].Jensen JH, Helpern J.a., Ramani A, Lu H, Kaczynski K, Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging., Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 53 (2005) 1432–1440. [DOI] [PubMed] [Google Scholar]
- [45].Lu H, Jensen JH, Ramani A, Helpern JA, Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging, NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo 19 (2006) 236–247. [DOI] [PubMed] [Google Scholar]
- [46].Xu S, Ye D, Lian T, Cai J, Xiao Z, Huang Y, Chen X, Zhang Z, Assessment of severity of leukoaraiosis: a diffusional kurtosis imaging study, Clinical imaging 40 (2016) 732–738. [DOI] [PubMed] [Google Scholar]
- [47].Zhong G, Zhang R, Jiaerken Y, Yu X, Zhou Y, Liu C, Lin L, Tong L, Lou M, Better Correlation of Cognitive Function to White Matter Integrity than to Blood Supply in Subjects with Leukoaraiosis, Frontiers in aging neuroscience 9 (2017) 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Duering M, Finsterwalder S, Baykara E, Tuladhar AM, Gesierich B, Konieczny MJ, Malik R, Franzmeier N, Ewers M, Jouvent E, Biessels GJ, Schmidt R, de Leeuw FE, Pasternak O, Dichgans M, Free water determines diffusion alterations and clinical status in cerebral small vessel disease, Alzheimer’s & dementia : the journal of the Alzheimer’s Association 14 (2018) 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tournier JD, Mori S, Leemans A, Diffusion tensor imaging and beyond, Magn Reson Med 65 (2011) 1532–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Johanna Helenius MLS MD; Salonen Oili MD, PhD; Kaste Markku MD, PhD; Tatlisumak Turgut MD, Leukoaraiosis, Ischemic Stroke, and Normal White Matter on Diffusion-Weighted MRI, (2001). [DOI] [PubMed] [Google Scholar]
- [51].Mascalchi MM PhD; Moretti M MD; Nave R. Della MD; Lolli F MD, PhD; Tessa C MD; Carlucci G MD; Bartolini L; Pracucci G MD; Pantoni L MD, PhD; Filippi M MD; and Inzitari D MD, Longitudinal evaluation of leukoaraiosis with whole brain ADC histograms, (2002). [DOI] [PubMed] [Google Scholar]
- [52].Viana-Baptista M, Bugalho P, Jordao C, Ferreira N, Ferreira A, Forjaz Secca M, Esperanca-Pina JA, Ferro JM, Cognitive function correlates with frontal white matter apparent diffusion coefficients in patients with leukoaraiosis, Journal of neurology 255 (2008) 360–366. [DOI] [PubMed] [Google Scholar]
- [53].Ropele S, Seewann A, Gouw AA, van der Flier WM, Schmidt R, Pantoni L, Inzitari D, Erkinjuntti T, Scheltens P, Wahlund LO, Waldemar G, Chabriat H, Ferro J, Hennerici M, O’Brien J, Wallin A, Langhorne P, Visser MC, Barkhof F, Fazekas F, L.s. group, Quantitation of brain tissue changes associated with white matter hyperintensities by diffusion-weighted and magnetization transfer imaging: the LADIS (Leukoaraiosis and Disability in the Elderly) study, J Magn Reson Imaging 29 (2009) 268–274. [DOI] [PubMed] [Google Scholar]
- [54].Viana-Baptista M, Bugalho P, Jordao C, Ribeiro O, Esperanca-Pina JA, Ferro J, Motor dysfunction correlates with frontal white matter ischemic changes in patients with leukoaraiosis, Journal of aging research 2011 (2011) 950341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jokinen H, Schmidt R, Ropele S, Fazekas F, Gouw AA, Barkhof F, Scheltens P, Madureira S, Verdelho A, Ferro JM, Wallin A, Poggesi A, Inzitari D, Pantoni L, Erkinjuntti T, Group LS, Diffusion changes predict cognitive and functional outcome: the LADIS study, Annals of neurology 73 (2013) 576583. [DOI] [PubMed] [Google Scholar]
- [56].Oztoprak B, Oztoprak I, Topalkara K, Erkoc MF, Salk I, Role of thalamic diffusion for disease differentiation between multiple sclerosis and ischemic cerebral small vessel disease, Neuroradiology 57 (2015) 339–347. [DOI] [PubMed] [Google Scholar]
- [57].L. MD Jones Derek K PhD; Horsfield Mark A. PhD; Simmons Andrew PhD; Williams Steve C.R. PhD; Markus Hugh S. DM, Characterization of White Matter Damage in Ischemic Leukoaraiosis with Diffusion Tensor MRI, (1998). [DOI] [PubMed] [Google Scholar]
- [58].O’Sullivan MPESM PhD; Jones DK PhD; Jarosz JM FRCR; Williams SCR PhD; and Markus HS FRCP, Normal-appearing white matter in ischemic leukoaraiosis: A diffusion tensor MRI study, (2001). [DOI] [PubMed] [Google Scholar]
- [59].O’Sullivan M, Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis, Journal of Neurology, Neurosurgery & Psychiatry 75 (2004) 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nitkunan A, McIntyre DJ, Barrick TR, O’Sullivan M, Shen Y, Clark CA, Howe FA, Markus HS, Correlations between MRS and DTI in cerebral small vessel disease, NMR in biomedicine 19 (2006) 610616. [DOI] [PubMed] [Google Scholar]
- [61].Della Nave R, Foresti S, Pratesi A, Ginestroni A, Inzitari M, Salvadori E, Giannelli M, Diciotti S, Inzitari D, Mascalchi M, Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: correlation with motor and cognitive impairment, AJNR. American journal of neuroradiology 28 (2007) 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang S, Yuan J, Guo X, Teng L, Jiang H, Gu H, Hu W, Jiang T, Correlation between prefrontalstriatal pathway impairment and cognitive impairment in patients with leukoaraiosis, Medicine 96 (2017) e6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nitkunan A, Barrick TR, Charlton RA, Clark CA, Markus HS, Multimodal MRI in cerebral small vessel disease: its relationship with cognition and sensitivity to change over time, Stroke 39 (2008) 19992005. [DOI] [PubMed] [Google Scholar]
- [64].Nitkunan A, Charlton RA, McIntyre DJ, Barrick TR, Howe FA, Markus HS, Diffusion tensor imaging and MR spectroscopy in hypertension and presumed cerebral small vessel disease, Magn Reson Med 59 (2008) 528–534. [DOI] [PubMed] [Google Scholar]
- [65].Zhou Y, Lin FC, Zhu J, Zhuang ZG, Li YS, Tao J, Qian LJ, Xu JR, Lei H, Whole brain diffusion tensor imaging histogram analysis in vascular cognitive impairment, J Neurol Sci 268 (2008) 60–64. [DOI] [PubMed] [Google Scholar]
- [66].Kim SH, Park JS, Ahn HJ, Seo SW, Lee JM, Kim ST, Han SH, Na DL, Voxel-based analysis of diffusion tensor imaging in patients with subcortical vascular cognitive impairment: correlates with cognitive and motor deficits, Journal of neuroimaging : official journal of the American Society of Neuroimaging 21 (2011) 317–324. [DOI] [PubMed] [Google Scholar]
- [67].Cavallari M, Moscufo N, Meier D, Skudlarski P, Pearlson GD, White WB, Wolfson L, Guttmann CR, Thalamic fractional anisotropy predicts accrual of cerebral white matter damage in older subjects with small-vessel disease, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34 (2014) 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Huang LA, Ling XY, Li C, Zhang SJ, Chi GB, Xu AD, Study of white matter at the centrum semiovale level with magnetic resonance spectroscopy and diffusion tensor imaging in cerebral small vessel disease, Genetics and molecular research : GMR 13 (2014) 2683–2690. [DOI] [PubMed] [Google Scholar]
- [69].Thong JY, Du J, Ratnarajah N, Dong Y, Soon HW, Saini M, Tan MZ, Ta AT, Chen C, Qiu A, Abnormalities of cortical thickness, subcortical shapes, and white matter integrity in subcortical vascular cognitive impairment, Human brain mapping 35 (2014) 2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Baykara E, Gesierich B, Adam R, Tuladhar AM, Biesbroek JM, Koek HL, Ropele S, Jouvent E, I. Alzheimer’s Disease Neuroimaging, Chabriat H, Ertl-Wagner B, Ewers M, Schmidt R, de Leeuw FE, Biessels GJ, Dichgans M, Duering M, A Novel Imaging Marker for Small Vessel Disease Based on Skeletonization of White Matter Tracts and Diffusion Histograms, Annals of neurology 80 (2016) 581–592. [DOI] [PubMed] [Google Scholar]
- [71].Croall ID, Lohner V, Moynihan B, Khan U, Hassan A, O’Brien JT, Morris RG, Tozer DJ, Cambridge VC, Harkness K, Werring DJ, Blamire AM, Ford GA, Barrick TR, Markus HS, Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies, Clinical science 131 (2017) 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tu MC, Lo CP, Huang CF, Hsu YH, Huang WH, Deng JF, Lee YC, Effectiveness of diffusion tensor imaging in differentiating early-stage subcortical ischemic vascular disease, Alzheimer’s disease and normal ageing, PloS one 12 (2017) e0175143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gons RA, de Laat KF, van Norden AG, van Oudheusden LJ, van Uden IW, Norris DG, Zwiers MP, de Leeuw FE, Hypertension and cerebral diffusion tensor imaging in small vessel disease, Stroke 41 (2010) 2801–2806. [DOI] [PubMed] [Google Scholar]
- [74].van Norden AG, de Laat KF, Fick I, van Uden IW, van Oudheusden LJ, Gons RA, Norris DG, Zwiers MP, Kessels RP, de Leeuw FE, Diffusion tensor imaging of the hippocampus and verbal memory performance: the RUN DMC study, Human brain mapping 33 (2012) 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].van Norden AG, de Laat KF, van Dijk EJ, van Uden IW, van Oudheusden LJ, Gons RA, Norris DG, Zwiers MP, de Leeuw FE, Diffusion tensor imaging and cognition in cerebral small vessel disease: the RUN DMC study, Biochimica et biophysica acta 1822 (2012) 401–407. [DOI] [PubMed] [Google Scholar]
- [76].van Norden AG, van Uden IW, de Laat KF, van Dijk EJ, de Leeuw FE, Cognitive function in small vessel disease: the additional value of diffusion tensor imaging to conventional magnetic resonance imaging: the RUN DMC study, Journal of Alzheimer’s disease : JAD 32 (2012) 667–676. [DOI] [PubMed] [Google Scholar]
- [77].van der Holst HM, Tuladhar AM, van Norden AG, de Laat KF, van Uden IW, van Oudheusden LJ, Zwiers MP, Norris DG, Kessels RP, de Leeuw FE, Microstructural integrity of the cingulum is related to verbal memory performance in elderly with cerebral small vessel disease: the RUN DMC study, NeuroImage 65 (2013) 416–423. [DOI] [PubMed] [Google Scholar]
- [78].van Uden IW, van der Holst HM, Schaapsmeerders P, Tuladhar AM, van Norden AG, de Laat KF, Norris DG, Claassen JA, van Dijk EJ, Richard E, Kessels RP, de Leeuw FE, Baseline white matter microstructural integrity is not related to cognitive decline after 5 years: The RUN DMC study, BBA clinical 4 (2015) 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].van der Holst HM, van Uden IW, Tuladhar AM, de Laat KF, van Norden AG, Norris DG, van Dijk EJ, Rutten-Jacobs LC, de Leeuw FE, Factors Associated With 8-Year Mortality in Older Patients With Cerebral Small Vessel Disease: The Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study, JAMA Neurol 73 (2016) 402–409. [DOI] [PubMed] [Google Scholar]
- [80].van Uden IW, Tuladhar AM, van der Holst HM, van Leijsen EM, van Norden AG, de Laat KF, Rutten-Jacobs LC, Norris DG, Claassen JA, van Dijk EJ, Kessels RP, de Leeuw FE, Diffusion tensor imaging of the hippocampus predicts the risk of dementia; the RUN DMC study, Human brain mapping 37 (2016) 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].van Leijsen EMC, Bergkamp MI, van Uden IWM, Ghafoorian M, van der Holst HM, Norris DG, Platel B, Tuladhar AM, de Leeuw FE, Progression of White Matter Hyperintensities Preceded by Heterogeneous Decline of Microstructural Integrity, Stroke 49 (2018) 1386–1393. [DOI] [PubMed] [Google Scholar]
- [82].Lawrence AJ, Patel B, Morris RG, MacKinnon AD, Rich PM, Barrick TR, Markus HS, Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George’s cognition and neuroimaging in stroke (SCANS) study, PloS one 8 (2013) e61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Benjamin P, Zeestraten E, Lambert C, Ster IC, Williams OA, Lawrence AJ, Patel B, MacKinnon AD, Barrick TR, Markus HS, Progression of MRI markers in cerebral small vessel disease: Sample size considerations for clinical trials, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 36 (2016) 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zeestraten EA, Benjamin P, Lambert C, Lawrence AJ, Williams OA, Morris RG, Barrick TR, Markus HS, Application of Diffusion Tensor Imaging Parameters to Detect Change in Longitudinal Studies in Cerebral Small Vessel Disease, PLoS One 11 (2016) e0147836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Williams OA, Zeestraten EA, Benjamin P, Lambert C, Lawrence AJ, Mackinnon AD, Morris RG, Markus HS, Charlton RA, Barrick TR, Diffusion tensor image segmentation of the cerebrum provides a single measure of cerebral small vessel disease severity related to cognitive change, Neuroimage Clin 16 (2017) 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zeestraten E, Change in multimodal MRI markers predicts dementia risk in cerebral small vessel disease, Neurology 89 (2017) 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].van Norden AG, de Laat KF, Gons RA, van Uden IW, van Dijk EJ, van Oudheusden LJ, Esselink RA, Bloem BR, van Engelen BG, Zwarts MJ, Tendolkar I, Olde-Rikkert MG, van der Vlugt MJ, Zwiers MP, Norris DG, de Leeuw FE, Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol, BMC neurology 11 (2011) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tuladhar AM, van Norden AG, de Laat KF, Zwiers MP, van Dijk EJ, Norris DG, de Leeuw FE, White matter integrity in small vessel disease is related to cognition, NeuroImage. Clinical 7 (2015) 518524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mori S, van Zijl PC, Fiber tracking: principles and strategies - a technical review, NMR in biomedicine 15 (2002) 468–480. [DOI] [PubMed] [Google Scholar]
- [90].Hagmann P, Cammoun L, Gigandet X, Gerhard S, Grant PE, Wedeen V, Meuli R, Thiran JP, Honey CJ, Sporns O, MR connectomics: Principles and challenges, Journal of neuroscience methods 194 (2010) 34–45. [DOI] [PubMed] [Google Scholar]
- [91].P. S Basser Peter J.. 2 Carlo Pierpaoli,1 Jeffrey Duda,1 and Akram Aldroubi3, In Vivo Fiber Tractography Using DT-MRI Data, (2000). [DOI] [PubMed] [Google Scholar]
- [92].Susumu Mori P, Crain Barbara J. MD, PhD, Chacko VP PhD, and van Zijl Peter C. M. PhD, ThreeDimensional Tracking of Axonal Projections in the Brain by Magnetic Resonance Imaging, (1999). [DOI] [PubMed] [Google Scholar]
- [93].Reijmer YD, Fotiadis P, Piantoni G, Boulouis G, Kelly KE, Gurol ME, Leemans A, O’Sullivan MJ, Greenberg SM, Viswanathan A, Small vessel disease and cognitive impairment: The relevance of central network connections, Human brain mapping 37 (2016) 2446–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Biesbroek JM, Leemans A, den Bakker H, Duering M, Gesierich B, Koek HL, van den Berg E, Postma A, Biessels GJ, g. on behalf of the Utrecht Vascular Cognitive Impairment study, Microstructure of Strategic White Matter Tracts and Cognition in Memory Clinic Patients with Vascular Brain Injury, Dement Geriatr Cogn Disord 44 (2018) 268–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Jeurissen B, Leemans A, Jones DK, Tournier JD, Sijbers J, Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution, Human brain mapping 32 (2011) 461–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Metoki A, Brookes Rebecca L., Zeestraten Eva, Lawrence Andrew J., Morris Robin G., Barrick Thomas R., Markus Hugh S., and Charlton Rebecca A., Mnemonic function in small vessel disease and associations with white matter tract microstructure, Neuropsychologia 104 (2017) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].D’Souza MM, Gorthi SP, Vadwala K, Trivedi R, Vijayakumar C, Kaur P, Khushu S, Diffusion tensor tractography in cerebral small vessel disease: correlation with cognitive function, The neuroradiology journal 31 (2018) 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J, Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data, NeuroImage 103 (2014) 411–426. [DOI] [PubMed] [Google Scholar]
- [99].Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, Connelly A, Investigating white matter fibre density and morphology using fixel-based analysis, NeuroImage 144 (2017) 58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Correia S, Lee SY, Voorn T, Tate DF, Paul RH, Zhang S, Salloway SP, Malloy PF, Laidlaw DH, Quantitative tractography metrics of white matter integrity in diffusion-tensor MRI, NeuroImage 42 (2008) 568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lawrence AJ, Chung Ai Wern, Morris Robin G., Markus Hugh S., and Barrick Thomas R.., Structural network efficiency is associated with cognitive impairment in small-vessel disease, Neurology 83 (2014) 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kim HJ, Im K, Kwon H, Lee JM, Ye BS, Kim YJ, Cho H, Choe YS, Lee KH, Kim ST, Kim JS, Lee JH, Na DL, Seo SW, Effects of amyloid and small vessel disease on white matter network disruption, Journal of Alzheimer’s disease : JAD 44 (2015) 963–975. [DOI] [PubMed] [Google Scholar]
- [103].Tuladhar AM, van Dijk E, Zwiers MP, van Norden AG, de Laat KF, Shumskaya E, Norris DG, de Leeuw FE, Structural network connectivity and cognition in cerebral small vessel disease, Human brain mapping 37 (2016) 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].v.U. I Tuladhar AM, Rutten-Jacobs LC, Lawrence A, van der Holst H, van Norden A, de Laat K, van Dijk E, Claassen JA, Kessels RP, Markus HS, Norris DG, de Leeuw FE, Structural network efficiency predicts conversion to dementia, Neurology 86 (2016) 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].T. DJ Lawrencea Andrew J., Stamatakis Emmanuel A., Markus Hugh S., A comparison of functional and tractography based networks in cerebral small vessel disease, NeuroImage. Clinical 18 (2018) 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lawrence AJ, Zeestraten Eva A., Benjamin Philip, Lambert Christian P., Morris Robin G., Barrick Thomas R., and Markus Hugh S., Longitudinal decline in structural networks predicts dementia in cerebral small vessel disease, Neurology 90 (2018) e1898–e1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lisiecka-Ford DM, Tozer Daniel J., Morris Robin G., Lawrence Andrew J., Barrick Thomas R., and Markus Hugh S., Involvement of the reward network is associated with apathy in cerebral small vessel disease, Journal of affective disorders 232 (2018) 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Westin CF, Knutsson H, Pasternak O, Szczepankiewicz F, Ozarslan E, van Westen D, Mattisson C, Bogren M, O’Donnell LJ, Kubicki M, Topgaard D, Nilsson M, Q-space trajectory imaging for multidimensional diffusion MRI of the human brain, Neuroimage 135 (2016) 345–362. [DOI] [PMC free article] [PubMed] [Google Scholar]