Abstract
Crotamine is a cationic, non-enzymatic, protein integrating a minor family of myotoxins, composed of 42 amino acid residues, described in Viperidae and Crotalidae snake’s families that has been used in neuroscience research, drug progressing and molecular diversity reports. Crotamine-like protein (CLP) from C.o.helleri venom was isolated in fraction 5 from 7 peaks obtained by sulfopropyl waters protein pak cationic exchange column. In tricine-SDS-PAGE under non-reduced conditions this CLP showed a single band of ~8 kDa molecular weight. CLP induced toxicity of K-562 cells with a CC50 of 11.09 µM. In mice adrenal gland after 24 h of CLP injection, cortical cells exhibited swollen mitochondria with scarce tubular cristae, some elements of smooth and rough endoplasmic reticula, widened nuclear envelope, slightly osmiophilic lipid droplets, and autophagic vacuoles. In some areas cortical cells plasma membrane and endothelial walls disappeared, which indicated a necrosis process. In other areas, endothelial cell cytoplasm did not present the normal caveolae and pinocytotic vesicles. To our knowledge, this is the first report on mice adrenal gland damages, caused by the injection of CLP from rattlesnakes. Our results propose that adrenal cortex lesions may be significant in the envenoming etiopathogenesis by CLP.
Keywords: Adrenal gland, crotamine-like protein, Crotalus oreganus helleri, cytotoxicity, leukemia k-562 cells
Introduction
Viperidae venomous snakes exhibit in their toxins, one of the most interesting animal adaptations of nature. These venoms have evolved as complex mixtures of organic and inorganic substances, which in contact with the tissues of other animals, produce severe local or systemic tissue alterations, allowing immobilization, capture and digestion of prey, as well as facilitating the survival and defences against natural enemies and predators. The manifestations of local tissue damage, such as hemorrhage and myonecrosis represent one of the most dramatic effects in envenoming by snakes of the Viperidae and Crotalidae family. The severity can range from mild local bleeding without necrosis, in less severe envenoming, to muscle necrosis and bleeding in distant structures such as endocrine organs, in the most serious cases.1–3
Crotamine, one of the important Crotalus venom constituents has caught significant consideration, because its high utility in neuroscience research, drug progressing, and molecular diversity reports. Crotamine is a cationic, non-enzymatic, protein integrating to a minor family of myotoxins composed of 42 amino acid residues, which contains 6 cysteine that form three disulfide bonds making up a tightly wound β-sheet core,4 found principally amid the Viperidae and Crotalidae families of snakes.
Nowadays, not many descriptions of the action of crotamine on endocrine organs, such as pituitary and/or adrenal gland have been published. The adrenal gland has morphological and biochemical characteristics that makes intensely vulnerable to the actions of different toxins. As with other endocrine organs studied in vivo, the adrenal gland is under the control of upstream organs (hypothalamic-pituitary system), habitually making it complicated to elucidate the mode of toxicity of a specific toxin. In the current work, it was supposed that there were damages on the adrenal glands, as a result of the experimental animal’s clinical manifestations, which showed similar pathophysiological symptoms as described in acute adrenal gland insufficiency.
Our results propose that adrenal cortex lesions may be significant in the etiopathogenesis of crotamine-like Protein (CLP) envenoming. Hence, this toxin could be inducing adrenal damages on envenomed patients. To our knowledge, this is the first report on mice adrenal gland damages, caused by the injection of CLP from any rattlesnakes.
Materials and methods
Snakes
The Southern Pacific rattlesnake (Crotalus oreganus helleri, Avid # 009–551–286) was obtained from California, Riverside County (USA). Snakes are located and maintained at the Natural Toxins Research Center, Texas A&M University-Kingsville, Kingsville, TX, USA.
Venom collection
Venom was obtained by letting the snake to bite into a para-film extended over a disposable plastic cup. The venom sample was centrifuged (500 × g for 10 min), and filtered through 0.45 μm filter under positive pressure. The venom was frozen at −90°C and then lyophilized.
Mice
Ultrastructural adrenal gland studies were carried out using NIH mice (18–22 g) from the Animal House of the National Institute of Hygiene “Rafael Rangel”, Caracas, Venezuela.21
Protein purification
Isolation of CLP was reached by one chromatographic step. Crotalus oreganus helleri venom sample (30 mg) was dissolved in 20 mM Tris-HCl pH 8.0 buffer, and run into a cationic exchange column (sulfopropyl waters protein pak 7.5 × 75 mm-10 μm, Milford, MA) equilibrated with 20 mM Tris-HCl, pH 8.0 buffer at a 1 mL/min flow rate. The eluting buffer was linearly integrated from 0% to 100% using a 20 mM Tris-HCl, pH 8.0 buffer containing 0.5 M NaCl. The proteins were eluted at a 1 mL/min flow rate over 90 min using a waters 1525 binary High Performance Liquid Chromatography (HPLC) system (Milford, MI, USA). A waters 2487 dual λ absorbance detector (Milford, MI, USA) to monitor absorbance at 280 nm, and Waters Breeze software were used to control the HPLC system and save the data. The active fractions were pooled, lyophilized, and stored at −20°C until used.
Protein concentration
As proposed by Stoscheck,5 protein concentration of CLP was spectrophotometrically expected by assuming that 1 unit of absorbance/cm of wavelength at 280 nm corresponds to 1 mg protein/mL.
SDS polyacrylamide gel electrophoresis
The CLP was subjected to electrophoresis by using a pre-cast 10–20% Tricine gel in an Xcell SureLock Mini-Cell (Invitrogen Life Technologies, USA). Gels were stained with 100 mL SimplyBlue SafeStain (Invitrogen Life Technologies, USA) for 1 h and distained overnight with 18 megaOhm water. SeeBlue Plus2 markers, ranging from 4 to 210 kDa, were used as standards.
Cytotoxicity assay
In order to test the cytotoxicity activity of CLP on human leukemia (K-562), cell lines were utilized by measuring the release of lactate dehydrogenase enzyme (LDH), in culture after CLP incubation, via the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, USA). The 50% cytotoxic concentration (CC50) of the sample was defined as the CLP concentration, which produced 50% of toxicity. Experiments were performed in triplicate.
Preparation of specimens for electron microscopy
Adult male NHI strain mice were divided into a control group, in which mice were injected intravenously (i.v.) with 0.1 mL of saline solution and the experimental group injected i.v. with 17 μg/mL of CLP in 100 μL of phosphate buffer saline (PBS). After 5 min and 24 h, three mice from each group were prepared for adrenal gland biopsies. The fragments were immediately obtained from control and experimental mice after to be euthanized by cervical dislocation. Samples were without delay in situ fixed with 3% glutaraldehyde and 1% OsO4 (both fixatives diluted in 320 mM phosphate buffer saline, pH 7.4), dehydrated in ethanol and embedded in LX-112 resin (Ladd Research Inc.). Ultrathin sections were stained with uranyl acetate and lead citrate and observed with a FEI, TecnaiSpirit 12G2 model transmission electron microscope with an accelerating voltage of 100 kV.
Results
Purification and isolation of CLP
CLP from C. o. helleri venom was isolated by sulfopropyl waters protein pak cationic exchange column, which produced 7 peaks (data not shown). The spastic inferior limbs paralysis activity (classical sign of crotamine presence) was detected only in fraction 5, named CLP.
SDS-polyacrylamide gel electrophoresis
Tricine-SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (10–20%) non-reduced conditions showed a single band of ~8 kDa molecular weight (Figure 1).
Figure 1.
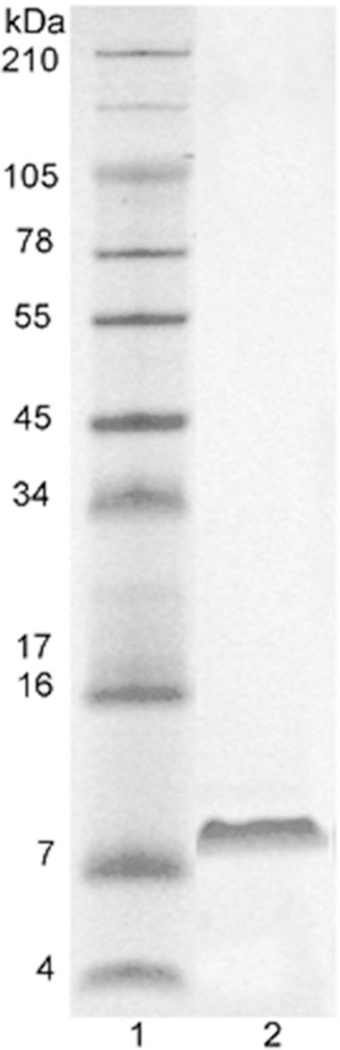
SDS polyacrylamide gel electrophoresis of crotamine. A total of 10 μg of crotamine was run on Tricine-SDS-PAGE (10– 20%) non-reduced conditions. Lane 1: molecular weight markers. Lane 2: crotamine in non-reduced conditions. The gel was stained with Coomassie blue.
Cytotoxicity assay
CLP induced toxicity of leukemia K-562 cells with a CC50 of 11.09 µM (Figure 2).
Figure 2.
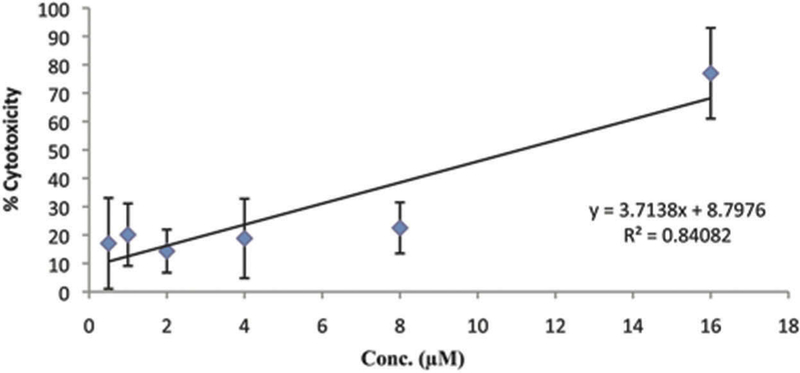
Cytotoxicity on leukemia K-562 cells by CRP. Crotamine-like protein induced toxicity of K-562 cells with a CC50 of 11.09 µM.
Adrenal gland changes induced by CLP analyzed by transmission electron microscopy
The cortex of the normal mouse adrenal gland at 24 h post-injection with saline showed mitochondria cortical cells with tubular cristae and some with dense granules, Golgi apparatus with short cisternae (saclike vesicles that comprise the endoplasmic reticulum), abundant smooth endoplasmic reticulum, lipofuscin granules, lipid droplets, narrow nuclear envelope space, numerous microvilli and capillaries with thin basement membrane and endothelial wall with fenestrae, as corresponding to a normal tissue (Figure 3(A–C). After 24 h of CRP injection, cortical cells exhibited swollen mitochondria with scarce tubular cristae, decrease of some element of smooth and rough endoplasmic reticulum, widened nuclear envelope, slightly osmiophilic lipid droplets and autophagic vacuoles. In some areas cortical cells plasma membrane and endothelial walls disappeared, which indicated necrosis. In other areas endothelial cell cytoplasm did not show normal caveolae and pinocytotic vesicles (Figure 4(D–G).
Figure 3.
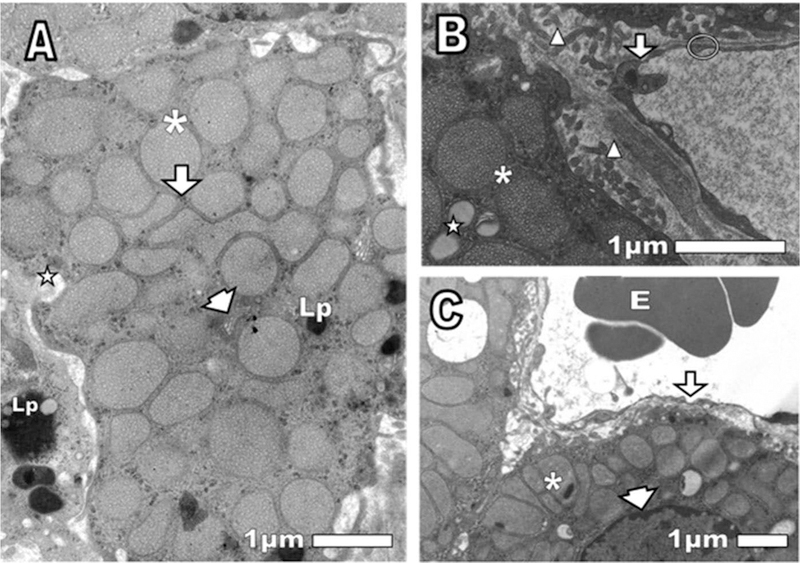
(A, B, and C) Normal control cortex adrenal gland. After 24 of saline intravenously injection was observed (A) asterisk: mitochondria with tubular cristae. Arrowhead: Golgi apparatus. Arrow: smooth endoplasmic reticulum. Lp: lipofuscin granules in the central cell and a neighboring cell. Star: partially extracted lipid droplet. (B): Triangle: microvilli of cortical cells (way to increase the secretory activity). Arrow: endothelial fenestrae, thin endothelial wall not thickened, as befits a normal capillary. Circle: thin basal membrane. (C): Asterisk: mitochondria with osmiophilics granules. Arrow: endothelium fenestrae not thickened. E: erythrocyte. Arrowhead: the narrow space nuclear envelope, as suitable for a normal cell.
Figure 4.
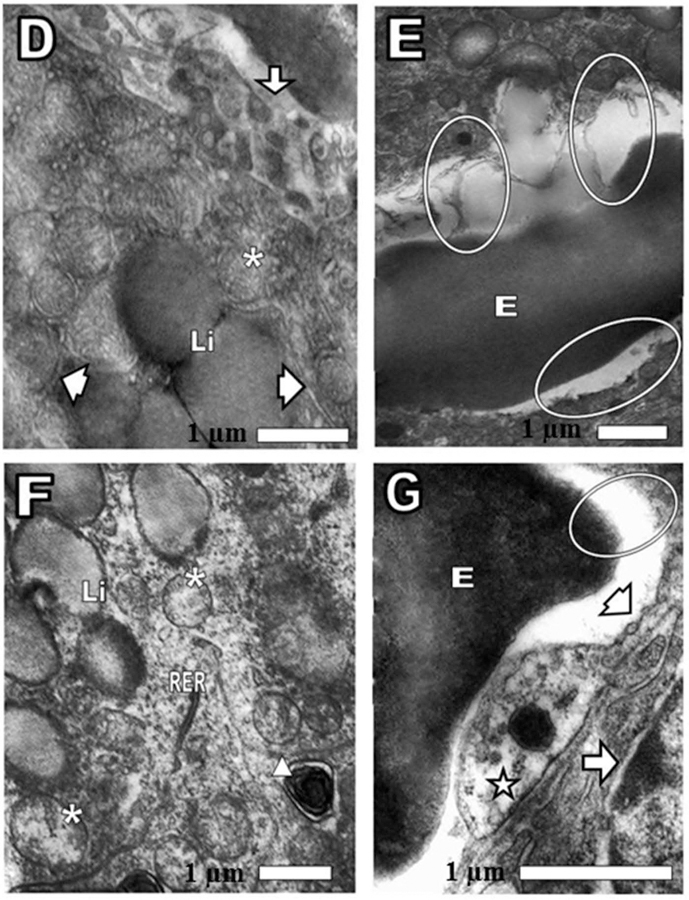
(D, E, F, and G) Pathological changes in cortex adrenal gland. After 24 of CRP intravenously injection was observed (D) Li: lipid drops, slightly osmiophilics or electrondense. Arrow: continuity of capillary endothelial wall was lost. Arrowhead: smooth endoplasmic reticulum (SER). Asterisk: mitochondria (E) oval: loss of the endothelium and the plasma membrane of cortical cell. On the lower oval, only the cortical cell surface without the plasma membrane was observed. E: erythrocyte. (F) Asterisk: mitochondria swollen and tubular cristae loss. SER was not observed. Triangle: autophagic vacuole. Li: lipid droplets. RER: rough endoplasmic reticulum. (G) Arrow head: fenestrae. Oval: disappearance of the endothelial wall and the surface of the cortical cell without plasma membrane. E: erythrocyte. Star: note the absence of caveolae and pinocytotic vesicles. Arrow: nuclear envelope of cortical cell showing the outer and inner membranes enclosing the perinuclear cistern and a connection site, where the outer membranes of the nuclear envelope looks swollen and is continuous with those of the rough endoplasmic reticulum.
Discussion
The adrenal gland is an endocrine organ that is affected by natural toxins, which induced severe lesions.6–8 Damages produced by toxins and toxic agents in the adrenal cortex are more common in the zona fasciculata and reticularis than in the zona glomerulosa. The adrenal cortex is in charge of producing steroid hormones with a 17-carbon nucleus, after cycles of hydroxylation reactions that are developed in the mitochondria and endoplasmic reticulum. Several toxic agents that influence the adrenal cortex comprise natural and synthetic steroids, amphiphilic chemicals, aliphatic compounds of short-chain and compounds that affect hydroxylation.9
The cytotoxic assays were carried out to demonstrate the cytotoxic capability of CLP on cells. We choose human leukemia (K-562) cell lines because in previous experience in our laboratory (data not shown), we have seen how crotamine reduces the viability of these tumor cells. The natural activities of CLP have been assayed in mice by intraperitoneal injection using sub-lethal doses corresponding to 2.5 mg of toxin/kg body weight, which cause the hind limb paralysis and the necrosis of the mice muscle cells.10 Elevated concentrations of crotamine endorse the discharge of lysosomal hydrolytic enzymes, which can induce cell death. Hayashi et al.11 estimated that crotamine at higher concentrations (>1 mM), provoke significant tumor cell death by increasing lysosomal membrane permeability, by the action of lysosomal hydrolytic enzymes (cathepsins, into the cytosol of these cells).
Morphologic evaluation of cortical lesions offers insight into the sites of inhibition of steroidogenesis, the damages in the adrenal cortex are diverse, we have found decreased elements at smooth endoplasmic reticulum (critical for normal steroid secretion process); vacuolar and granular degeneration, the hemorrhage and necrosis process are mainly found in the acute event as observed after 24 h of CLP injection (Figure 4(D–G). Differently, chronic damages are usually atrophy, nodular hyperplasia and fibrotic processes being the induced proliferative lesions uncommon. In the adrenal cortex are produced many hormones such as the steroid hormone aldosterone, responsible for rising sodium reabsorption and stimulating at the kidneys the potassium excretion and regulating extracellular liquid quantity. Some of the lesion that we observed were in the zona fasciculata, which is the relatively dense zone representing around of the 70% of the cortex. Here the cells are composed of pillars of secretory cells, which produces glucocorticoids and in mice small amounts of androgens, estrogens, and progestins,12 divided by notorious capillaries and the cells normally have many intracellular lipid droplets. In the (Figure 4(F)) we observed the lipid drops, slightly osmiophilics or electrondense and diminished in number. In early works, Dietrich13 had observed the diminution in size and peripheral (juxtavascular) arrangement in the cell of the lipoid granules, as the first stage of lipoid loss accompanying infected war wounds, almost certainly caused by bacterial endotoxins. In contrast, fatal wounds non-infected were consistently associated with a large amount and uniform distribution of lipoid granules in the cell. The typical lipoids in the cells of the cortex, which are existent even in whole fasting quickly, vanish in severe pyogenic infections as shown both by experimental revisions and by human autopsy conclusions. These pathological variations advise contribution on the part of the adrenal glands in the fight to infection.14
We found mitochondrial swelling and loss of cristae. Structural abnormalities of mitochondria caused by toxins, which have been already described with other natural toxins6–8,15 these anomalies consisted not only of the mitochondria swelling increase, but also of abnormal shape, and variations in the number and particular patterns of cristae (Figure 4(F)). The functional consequences of these mitochondrial abnormalities may be chronic and systemic evolution, caused by the common underlying deterioration of oxidative phosphorylation.
Additionally, we observed under CLP activity a decreased of the elements of the smooth endoplasmic reticulum, which is a system necessary for lipids synthesis, calcium homeostasis and detoxification reactions for the conversion of water insoluble substances in water-soluble compounds suitable for kidney excretion.16 In Figure 4(F) was noticed the presence of autophagic vacuoles (autophagosomes), which represent an enhanced process during cellular injury, long regarded as a nonspecific process, but can be selective, involving autophagic adaptor proteins.15 A dilatation of the nuclear envelope of cortical cell was noticed (Figure 4(G)), death in tumor cells is characterized by variably swollen mitochondria and dilatation of the nuclear envelope. Therefore, dying tumor cells had structures that are either classically apoptotic or necrotic. Crotamine has also been described as molecule producing muscle injuries by authors,17,18 who had previously remarked that crotamine in skeletal muscle produced lesions consisting of necrosis of the muscle fibers. It is usually assumed that necrosis triggers substantial inflammatory response while apoptosis does not. Our ultrastructural study extends these observations showing association of how our adrenal cells died. Frequent ruptures of cortical cell plasma membrane, conducting to necrosis, obviously no related to apoptosis and it explains inflammation. Another significant finding was the destruction of the endothelial wall of capillaries by the CLP, comparing these results with the control. After 24 of CLP injection, the continuity of capillary endothelial wall was lost (Figure 4(D)).
The disappearance of the endothelial wall, accompanied by a cortical cell without plasma membrane and the absence of pinocytotic vesicles and caveolae were observed; pinocytic vesicles are a particular type of plasma membrane invaginations in many cell varieties, especially in endothelial cells, responsible for the transport through the cellular cytoplasm. At the present time, solid confirmation has now been advanced that caveolae are static fixed domains and are not involved in endocytosis.19 Nevertheless, it has been demonstrated that the SV40 virus is taken in by caveolae and that these structures can be stimulated from the fixed state by signals derived from the virus.20 Their disappearance, as well as the pinocytotic vesicles, under CLP action is a clear pathological sign.
Acknowledgments
We would also like to thank, Nora Diaz De Leon and Mark HockMüller (NNTRC Serpentarium curator) and all the NNTRC and Immunochemistry and Ultrastructural Laboratory (UCV) personnel.
Funding
Funding for this project was provided by Grant from the Science and Technology Fund (FONACIT) programs (PEI 201400352) (Universidad Central de Venezuela, Dr. Rodrguez-Acosta); NCRR/BMRG, Viper Resource Grant #s 8P40OD01960-10 and 3P40OD01096-10S1 (National Natural Toxins Research Center (NNTRC), Texas A&M University-Kingsville, Dr. E.E. Snchez), Special Programs, and the Robert A. Welch Foundation Department, grant # AC-0006 (TAMUK-Department of Chemistry).
Footnotes
Declaration of interest
The authors affirm that they have no conflicts of interest to declare.
Ethical statement
Expert personnel did all the experimental events regarding the use of live animals. The applicable regulations as well as institutional guidelines, according to protocols approved by the National Natural Toxins Research Center at Texas A&M University-Kingsville in compliance with IUCUC guidelines, (IACUC-NNTRC # 12-09-A3-2015), the ethical regulations of the Anatomic Institute of the Universidad Central de Venezuela and the norms obtained from the guidelines for the care and use of laboratory animals, published by the US National Institute of Health (NIH, 1985)21 were followed.
References
- 1.Bjarnason JB, Fox JW. Hemorrhagic metalloproteinases from snake venoms. Pharmacol Ther 1994;62:325–372. [DOI] [PubMed] [Google Scholar]
- 2.Rengifo C, Rodriguez-Acosta A. Serpientes Veneno Y Tratamiento Médico En Venezuela Caracas: Fondo Editorial de la Facultad de Medicina Universidad Central de Venezuela, Caracas, Venezuela; 2004:1–120. [Google Scholar]
- 3.Nanjaraj AN, Ramakrishnan C, Joshi V, et al. Progressive hemorrhage and myotoxicity induced by Echis carinatus venom in murine model: Neutralization by inhibitor cocktail of N,N,N’,N’-tetrakis (2-pyridylmethyl) ethane-1,2-diamine and silymarin. Quintas LEM. PLoS ONE 2015;10:e0135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laure CJ. Die primarstruktur des crotaminins Hoppe-Seyler’s Z. Physiol Chem 1975;356:213–215. [PubMed] [Google Scholar]
- 5.Stoscheck CM. Quantitation of proteins. Meths Enzymol 1990;182:50–68. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Acosta A, Strauss M, Pulido-Mendez M, Finol HJ. Adrenal gland ultrastructural changes in inoculated mice with Tityus discrepans (Buthidae) venom. Rev Cient (Maracaibo) 2000;10:480–485. [Google Scholar]
- 7.Rodriguez-Acosta A, Vega J, Finol HJ, Pulido-Mendez M. Ultrastructural alterations in adrenal gland cortex caused by the toxic effect of bee (Apis mellifera) venom. J Sub Cytol Pathol 2003;35:309–314. [PubMed] [Google Scholar]
- 8.Rodríguez-Acosta A, Peña L, Pulido-Mendez M, Finol HJ. Cellular and subcellular changes in muscle, nerves and neuromuscular junctions caused by bee (Apis mellifera) venom. J Sub Cytol Pathol 2004;36:91–96. [PubMed] [Google Scholar]
- 9.Rosol TJ, Yarrington JT, Latendresse J, Capen CC. Adrenal gland: Structure, function, and mechanisms of toxicity. Toxicol Pathol 2001;29:41–48. [DOI] [PubMed] [Google Scholar]
- 10.Gonçalves JM, Arantes EG. Estudos sobre venenos de serpentes brasileiras III—determinacao quantitativa de crotamina no veneno de cascavel Brasileira. An Acad Bras Cienc 1956;28:369–371. [Google Scholar]
- 11.Hayashi M, Nascimento F, Kerkis A, et al. Cytotoxic effects of crotamine are mediated through lysosomal membrane permeabilization. Toxicon 2008;52:508–517. [DOI] [PubMed] [Google Scholar]
- 12.Hinson JP, Raven PW. Adrenal toxicology. In: Harvey PW, Rush KC, Cockburn A, eds. Endocrine and Hormonal Toxicology New York: John Wiley & Sons, New York, USA; 2000:67. [Google Scholar]
- 13.Dietrich A Die Nebennieren bei den Wundinfektionskrankheiten. Centr Allg Path U Path Anat 1918;29:169. [Google Scholar]
- 14.Scott WJM. The influence of the adrenal glands on resistance: ii. The toxic effect of killed bacteria in adrenalectomized rats. J Exp Med 1924;39:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavelka M, Roth J. Functional Ultrastructure: Atlas of Tissue Biology and Pathology Springer, Berlin, Germany; 2015. [Google Scholar]
- 16.Chen S, Novick P, Ferro-Novick S. ER structure and function. Curr Opin Cell Biol 2013;25:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron DL, Tu AT. Characterization of myotoxin A from the venom of prairie rattlesnake (Crotalus viridis viridis). Biochemistry 1977;16:2546–2553. [DOI] [PubMed] [Google Scholar]
- 18.Tu AT, Morita M. Attachment of rattlesnake venom myotoxin a to a sarcoplasmic reticulum: Peroxidase conjugated method. Br J Exp Pathol 1983;64:633–637. [PMC free article] [PubMed] [Google Scholar]
- 19.Thompsen P, Roepstorff K, Stahlhut M, Van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytis trafficking. Mol Biol Cell 2002;13:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002;296:535–539. [DOI] [PubMed] [Google Scholar]
- 21.NIH. Principles of laboratory animal care. Nat Inst Health USA 1985;85(23):1–112. [Google Scholar]


