Abstract
Case series
Patients: Male, 50 • Male, 3 • Female, 55
Final Diagnosis: Malposition of the array
Symptoms: Deafness
Medication: —
Clinical Procedure: Cochlear Implant 3D rendering
Specialty: Audiology
Objective:
Challenging differential diagnosis
Background:
The purpose of this study is to demonstrate the advantages of 3D volume rendering in postoperative control of implants placement compared to high-resolution computed tomography (HRCT).
Case Report:
We describe 3 patients who underwent HRCT study with and without 3D volume rendering after surgery for cochlear implantation. In 2 patients, the traditional HRCT showed a suspected malposition of the array, excluded only by the rendering reconstruction. In the other patient, thanks to the 3D rendering, we were able to identify the complete migration of the array out of the cochlea and the tip of the electrode near the opening of internal auditory canal, while the traditional images showed only that the array was not rolled up inside the cochlea.
Conclusions:
HRCT showed complex anatomic structures of the inner ear and contents of the middle ear cavity. The volume rendering, in the postoperative control, generates interactive 3D images of the cochlear implant, facilitating a clearer representation of the topographic complex of the cochlea, giving more detailed diagnostic information than the HRCT.
MeSH Keywords: Cochlear Implants; Ear, Inner; Imaging, Three-Dimensional; Multidetector Computed Tomography
Background
Increased use of cochlear implants (CI) and better availability of digital radiography and multislice CT allow medical imaging to play a key role in the preoperative period and following implantation [1]. In the preoperative period, medical imaging is required to determine the suitability of the ear to receive an implant, the choice of the ear to be operated upon, and to detect additional findings that may have a bearing on the surgery or subsequent patient management [2]. HRCT is recommended in all patients for pre-implant analysis of the temporal bone morphology, due to its reliability and wide availability. MRI is recommended in all cases of postmeningitic deafness and in others with doubtful CT findings.
In the postoperative period, HCTR allows clear depiction of the postoperative appearance position and insertion depth of CI. The modalities that are available in postoperative period include radiography with Stenvers’s view and CT, while MRI is restricted to pre-implantation assessment.
These modalities show the originally 3D objects in two-dimensional sections. Volume rendering based on HRCT temporal bone allows creating a dataset of images and animations visualizing the single structure of the human ear in 3 dimensions. We present 3 cases of CI candidates in which we performed volume rendering based on HRCT temporal bone, and discuss the advantages of this method in the postoperative period.
Case Report
Patient 1
In this 50-year-old patient who presented with a sensorineural hearing loss as a consequence of ototoxicity caused by chemotherapy, the use of volume rendering excluded the malposition of the array.
Preoperative CT imaging showed no abnormalities within the temporal bones or on the auditory pathway. The patient received a Cochlear® implant CI24RE. During the operation, the electrode array could be inserted smoothly. At 1 month after surgery, the postoperative speech perception scores, measured in a free-field condition, were poor.
In this patient with poor postoperative audiological performances, we suspected an extrusion because of extrusion or malposition of the electrode.
Postoperative HRCT was doubtful because of a suspicious extrusion of some of electrodes.
An interactive volume rendering based on HRCT was done. 3D imaging provided essential information because it excluded malposition of the electrode and so helped us to optimize the function of the CI (Figure 1).
Figure 1.
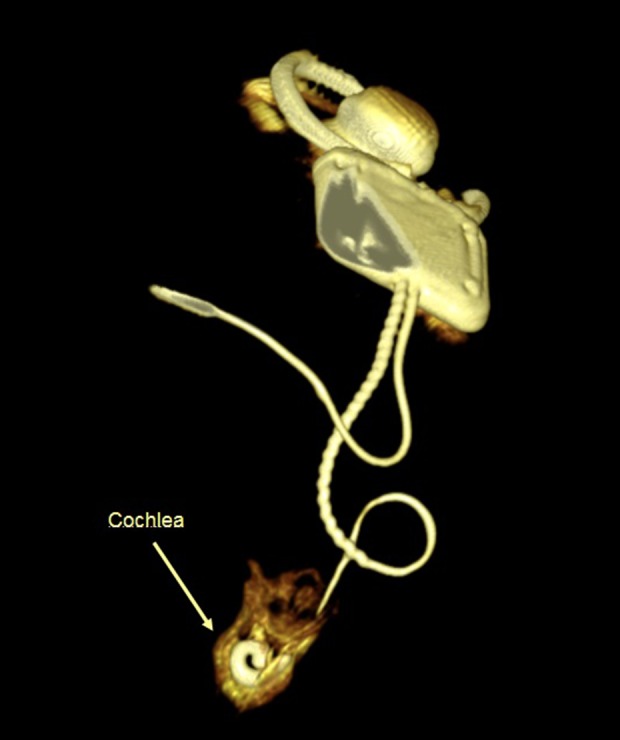
3D imaging excluded malposition of the electrode.
The failure of detection in this patient was attributed to unsuccessful rehabilitation. Six months after implant surgery, the women’s oral language skills were good.
Patient 2
The second case was a 3-year-old boy with profound bilateral sensorineural hearing loss caused by mutations in the gene encoding the gap junction protein connexin 26, GJB2 [3]. Use of volume rendering allowed us to diagnose a slight displacement of the receiver, and 3D reconstruction avoided revision surgery. Preoperative HRCT revealed a bilateral malformation of the inner ear, described as a common cavity.
In this condition there is no differentiation between cochlea and vestibule, which together form a cystic cavity. This occurs due to developmental arrest in the fourth week of gestation when the cochlea and vestibule are not yet formed.
3D reconstruction helped us to identify and evaluate the characteristics of these anomalies, which need to be assessed and understood in detail in order to plan a correct surgical approach (Figure 2).
Figure 2.
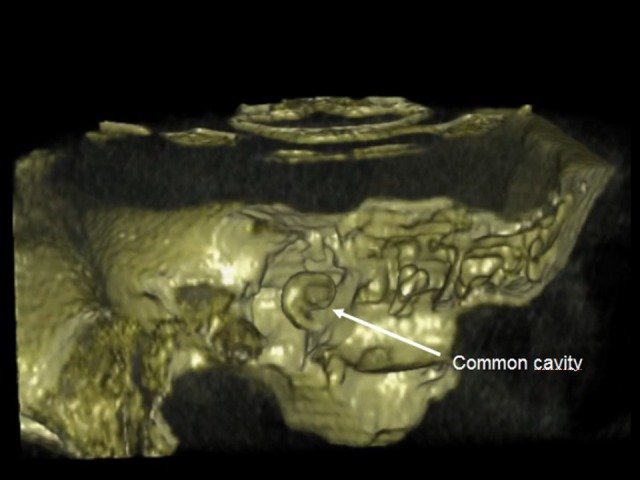
Common cavity: cochlea and vestibule together form a cystic cavity.
With this technique, we were able to obtain better resolution of anomalies compared to traditional CT imaging.
In common cavity malformations, the exact location and amount of neural tissue are unclear, and the surgeon can use full-banded implants rather than the half-banded ones oriented towards the modiolus. In such cases, the use of a pre-curved electrode may help avoid the risk of the electrode entering the internal auditory canal (IAC) [4]. This patient was considered a candidate for CI. He received a Cochlear® implant CI 512 Contour Advance without problems. Postoperative volume rendering based on HCRT temporal bone was made. It confirmed the correct insertion of electrode array into the common cavity, as well as a partial receiver-stimulator displacement.
Several conditions of this type may require revision surgery but, in this case, while the conventional radiological images suggested we should perform revision surgery, volume rendering 3D help us to find a minimal receiver-stimulator displacement (Figure 3), which did not justify a revision surgery.
Figure 3.
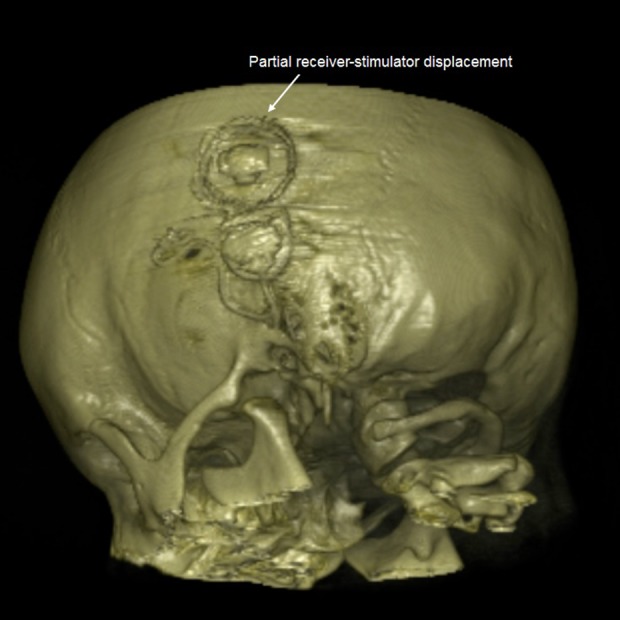
Minimal receiver-stimulator displacement that does not justify a revision surgery.
Patient 3
This was a 55-year-old woman with a mastoid cavity. Volume rendering allowed us to diagnose the migration of the electrode out of the cochlea and we removed the implant. CT imaging did not allow correct reconstruction of the anatomy of the ear due to the previous open tympanoplasty surgery due to cholesteatoma. She was implanted with the Nucleus Cochlear CI24RE. One week after surgery, she arrived in our department with headaches, tinnitus [5], vertigo [6], and facial paresthesia. CT imaging showed that the array was not rolled up inside the cochlea.
Volume rendering 3D allowed us to navigate from the posterior cranial fossa. The multielectrode wire was progressively followed. Volume rendering made it possible to verify migration of the array, which did not follow the cochlea’s turns. Furthermore, the tip of the electrode was bent near the inner ear canal (Figure 4). The implant was removed without complications, although the patient was at risk of infections or facial twitching.
Figure 4.
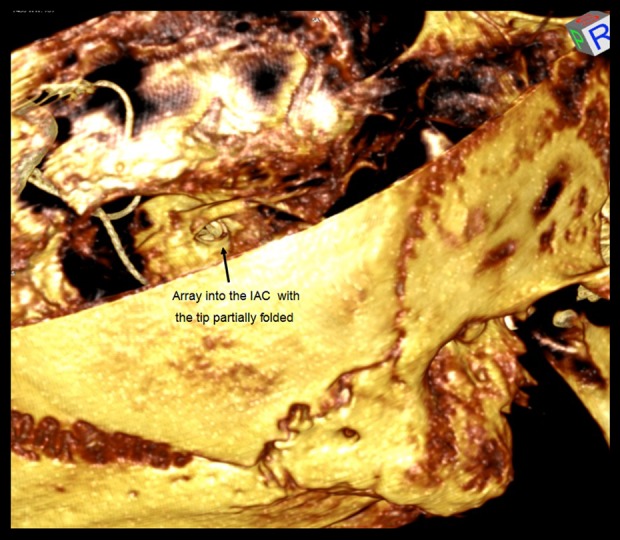
Migration of the array, which does not follow the cochlea’s turns. The tip of the electrode is bent near the inner ear canal.
Discussion
CI is recognized as a standard treatment for profound deafness that may have congenital and acquired causes. The major risk factors for congenital HL include consanguinity for genetic mutations [7] or intrauterine infections such as maternal rubella or cytomegalovirus (CMV) [8], which cause bilateral deafness in children. The etiological factors of acquired MPSHL are varied. A previously reported analysis of 310 adult cases included meningitis, viruses, vascular diseases, idiopathic sudden sensorineural hearing loss, chronic suppurative otitis media, advanced malignant external otitis [9,10], trauma, ototoxic medications, and advanced otosclerosis [11,12]. For the rendering process, we used VOLVIEW 2.0 graphic software. Volume management processing involves image acquisition, resampling, and editing. Acquisition quality is better with the newer CT scanners, resulting in larger volumes of high-resolution data. These high-resolution data allow high-quality 3D rendering, achieving high-definition imaging. The workstation automatically resamples the volume data by 2 separate operations. Axial sections are first scaled in the x and y axes, decreasing the volume dataset size. A second operation resamples in the z axis, calibrating in units similar to those of the x and y axes. The other step of the algorithm involves distinction of the bones, ligaments, and muscles from the CT image. Editing is performed for each axial image, outlining the relevant structure and erasing the surrounding structures.
A combination high-resolution CT and MRI allows good surgical planning, both in the presence of normal and pathological ears, and 3D-volume rendering of the cochlea and CI enables the determination of key features of temporal bone morphology and the control of array placement that may escape detection with routine computed tomographic scanning [13,14]. Misplacement of the CI electrode is a major complication of CI. CT is currently the method of choice for diagnosis and management planning. Electrode misplacement can result in poor outcome and carries the risk of injury to important adjacent structures. Reported sites of severe electrode misplacement detected by CT include the superior and horizontal semicircular canals, the vestibule, the Eustachian tube, the internal carotid artery, and the internal auditory canal [15]. The techniques of navigation within the structures and/or rotation of the areas have provided highly reliable data, especially when the two-dimensional images are equivocal.
During the preoperative period, 3D images can contribute to planning the surgical approach, and can orient and modify surgical strategy allow examining the anatomic relationships of the various temporal structures with the ability to predict or avoid intraoperative complications. This feature helped us even more in the case of malformed ears, in which the normal anatomy was severely impaired [16,17].
In the postoperative period, 3D images helped us to evaluate the correct positioning of the processor and the array, especially when the patients had poor performance or in the cases of malformations (such as a common cavity). In these cases, we can decide on revision surgery. Three-dimensional reconstruction of the inner ear provides a more accurate image of the relationship of the electrode within the cochlear canal, with direct demonstration of electrode insertion depth in the cochlea, which is not possible with X-ray plain film [18].
In future studies, we will evaluate the benefit of 3D volume rendering using the Nucleus audio processor equipped with a data recording system that can record in real time the use of CI in different acoustic environments and in various categories of volume levels [19]. Radiological evaluation plays a key role in CI surgery, but we must always keep in mind that temperament plays a role in the adaptation to CI and certain character traits may be risk or protective factors for surgery and rehabilitation outcomes [20–22].
Conclusions
Volume rendering allows a 3D reconstruction of the anatomy of the ear, which can be very useful for academic and didactic purposes. HRCT shows complex anatomic structures of the inner ear and contents of the middle ear cavity. Volume rendering, in the postoperative control, generates interactive 3D images of the cochlear implant, providing a clearer representation of the topographic complex of the cochlea and giving more detailed diagnostic information than with HRCT.
Acknowledgments
We thank Demetrio Milardi for 3D image reconstruction.
Footnotes
Conflicts of interest
None.
References:
- 1.Phelps PD, Annis JA, Robinson PJ. Imaging for cochlear implants. Br J Radiol. 1990;63(751):512–16. doi: 10.1259/0007-1285-63-751-512. [DOI] [PubMed] [Google Scholar]
- 2.Gleeson TG, Lacy PD, Bresnihan M, et al. High resolution computed tomography and magnetic resonance imaging in the pre-operative assessment of cochlear implant patients. J Laryngol Otol. 2003;117(9):692–95. doi: 10.1258/002221503322334495. [DOI] [PubMed] [Google Scholar]
- 3.Amorini M, Romeo P, Bruno R, et al. Prevalence of deafness-associated connexin-26 (GJB2) and connexin-30 (GJB6) pathogenic alleles in a large patient cohort from Eastern Sicily. Ann Hum Genet. 2015;79(5):341–49. doi: 10.1111/ahg.12120. [DOI] [PubMed] [Google Scholar]
- 4.McElveen JT, Jr, Carrasco VN, Miyamoto RT, Linthicum FH. Cochlear implantation in common cavity malformations using a transmastoid labyrinthotomy approach. Laryngoscope. 1997;107:1032–36. doi: 10.1097/00005537-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Trovato M, Ruggeri RM, Guzzo E, et al. Expression of P53 and isoforms in beningn and malignant lesions of the head and neck. Histol Histopathol. 2017;32(4):371–77. doi: 10.14670/HH-11-802. [DOI] [PubMed] [Google Scholar]
- 6.Galletti B, Mannella VK, Santoro R, et al. Ear, nose and throat (ENT) involvement in zoonotic diseases: A systematic review. J Infect Dev Ctries. 2014;8(1):17–23. doi: 10.3855/jidc.4206. [DOI] [PubMed] [Google Scholar]
- 7.Galletti F, Cammaroto G, Galletti B, et al. Technetium-99m (99mTc)-labelled sulesomab in the management of malignant external otitis: Is there any role? Eur Arch Otorhinolaryngol. 2015;272(6):1377–82. doi: 10.1007/s00405-014-2938-1. [DOI] [PubMed] [Google Scholar]
- 8.Galletti B, Mannella VK, Santoro R, et al. Malignant external otitis. A case series from an Italian Tertiary-Care Hospital. Acta Medica Mediterranea. 2014;30(6):1317–23. [Google Scholar]
- 9.Freni F, Mannella VK, Cammaroto G, et al. Classic and reversal steps stapedotomy performed with CO2 laser: A comparative analysis. Eur Arch Otorhinolaryngol. 2014;271(5):981–86. doi: 10.1007/s00405-013-2500-6. [DOI] [PubMed] [Google Scholar]
- 10.Catalano N, Cammaroto G, Galletti B, et al. The role of cVEMPs and vHIT in the evaluation of otosclerosis and its eventual vestibular impairment: Preliminary findings. B-ENT. 2017;13(1 Suppl. 27):31–36. [PubMed] [Google Scholar]
- 11.Ciodaro F, Mannella VK, Cammaroto G, et al. Oral gabapentin and intradermal injection of lidocaine: Is there any role in the treatment of moderate/severe tinnitus? Eur Arch Otorhinolaryngol. 2015;272(10):2825–30. doi: 10.1007/s00405-014-3304-z. [DOI] [PubMed] [Google Scholar]
- 12.Ciodaro F, Mannella VK, Nicita RA, et al. Therapeutic efficacy of the Galletti-Contrino manoeuvre for benign paroxysmal positional vertigo of vertical semicircular canals in overweight subjects. Eur Arch Otorhinolaryngol. 2018;275(10):2449–55. doi: 10.1007/s00405-018-5086-1. [DOI] [PubMed] [Google Scholar]
- 13.Lui CC, Peng JP, Li JH, et al. Detection of receiver location and migration after cochlear implantation using 3D rendering of computed tomography. Otol Neurotol. 2013;34(7):1299–304. doi: 10.1097/MAO.0b013e318298aac5. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Feng Y, Chen D, et al. [The application of 3D reconstruction of CT scans for the observation of the cochlear implanted electrode location] Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2006;20(13):597–99. [in Chinese] [PubMed] [Google Scholar]
- 15.Ying YL, Lin JW, Oghalai JS, et al. Cochlear implant electrode misplacement: Incidence, evaluation, and management. Laryngoscope. 2013;123:757–66. doi: 10.1002/lary.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes JPP, Veloso JRC, Altemani AMAM, et al. Three-dimensional volume imaging to increase the accuracy of surgical management in a case of recurrent chordoma of the clivus. Am J Case Rep. 2018;19:1168–74. doi: 10.12659/AJCR.911592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsini AY, Ibrahim A. Pulsating tonsil due to medial displacement of the internal carotid artery. Am J Case Rep. 2017;18:502–6. doi: 10.12659/AJCR.902915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong WJ, Cheng HM, Ma H, et al. Evaluation of the implanted cochlear implant electrode by CT scanning with three-dimensional reconstruction. Acta Otolaryngol. 2012;132(2):116–22. doi: 10.3109/00016489.2011.626794. [DOI] [PubMed] [Google Scholar]
- 19.Cristofari E, Cuda D, Martini A, et al. A Multicenter clinical evaluation of data logging in cochlear implant recipients using automated scene classification technologies. Audiol Neurootol. 2017;22(4–5):226–35. doi: 10.1159/000484078. [DOI] [PubMed] [Google Scholar]
- 20.Leo A, Naro A, Cannavò A, et al. Could autonomic system assessment be helpful in disorders of consciousness diagnosis? A neurophysiological study. Exp Brain Res. 2016;234(8):2189–99. doi: 10.1007/s00221-016-4622-8. [DOI] [PubMed] [Google Scholar]
- 21.De Salvo S, Bonanno L, Giorgianni R, et al. Functional MRI and laser-evoked potentials evaluation in Charcot-Marie-Tooth syndrome. Neurol Sci. 2018;39(7):1185–89. doi: 10.1007/s10072-018-3401-7. [DOI] [PubMed] [Google Scholar]
- 22.Mento C, Galletti F, Freni F, et al. The role of temperament in traumatic hearing loss: A single case study of a cochlear-implanted patient. Int J Adolesc Med Health. 2016;28(1):107–13. doi: 10.1515/ijamh-2014-0075. [DOI] [PubMed] [Google Scholar]


