Abstract
The objectives of this study were to determine tissue depletion of fenbendazole in turkeys and estimate a withdrawal interval (WDI). Forty-eight 9-week-old turkeys were fed fenbendazole at 30 mg/kg of feed for 7 consecutive days. Three hens and 3 toms were sacrificed every 2 days from 2 to 16 days post-treatment, and tissues were collected to determine fenbendazole sulfone (FBZ-SO2) concentrations using mass spectrometry. At all timepoints, FBZ-SO2 concentrations in liver and skin-adherent fat were above the limit of quantification (1 ppb), with higher concentrations than those in kidney and muscle. Two turkeys had detectable FBZ-SO2 concentrations in kidney at 16 days. No detectable FBZ-SO2 concentrations were found in muscle at 14 and 16 days. Fenbendazole residues depleted very slowly from the liver and a WDI of at least 39 days should be observed under the conditions of this study, in order to comply with Canadian regulatory agencies.
Résumé
Déplétion du fenbendazole pour les résidus tissulaires après l’administration orale chez les dindons. Les objectifs de cette étude consistaient à déterminer la déplétion du fenbendazole dans les tissus chez les dindons et d’estimer un délai d’attente (DA). Du fenbendazole a été administré à quarante-huit dindons âgés de 9 semaines, à raison de 30 mg/kg d’aliments pendant 7 jours consécutifs. Trois dindes et 3 dindons ont été sacrifiés tous les deux jours pendant les jours 2 à 16 après le traitement et les tissus ont été prélevés pour déterminer les concentrations de fenbendazole sulfone (FBZ-SO2) en utilisant la spectrométrie de masse. À tous les moments de prélèvement, les concentrations de FBZ-SO2 dans le foie et le gras adhérent à la peau étaient supérieures à la limite de quantification (1 ppm), avec des concentrations supérieures à celles présentes dans les reins et les muscles. Deux dindes avaient des concentrations de FBZ-SO2 détectables dans les reins à 16 jours. Aucune concentration détectable de FBZ-SO2 n’a été trouvées dans les muscles à 14 et à 16 jours. Les résidus de fenbendazole se résorbaient très lentement du foie et un DA d’au moins 39 jours devrait être observé conformément aux conditions de cette étude afin de satisfaire aux exigences des agences réglementaires canadiennes.
(Traduit par Isabelle Vallières)
Introduction
Ascarids cause significant economic losses to poultry producers due to reduced feed intake, lower body weights, reduced egg production, and increased mortality following heavy infection (1–4). A number of anthelminthic compounds are available for the control of parasitic nematodes in food-producing animals; however, the options are limited in avian species including turkeys. Fenbendazole [FBZ; methyl-5-(phenylthio)-2-benzimidazole-carbamate], a benzimidazole, is a broad-spectrum veterinary anthelmintic that is widely used for treatment of gastrointestinal nematode infection in food-producing animals and has proven efficacy in poultry and game bird feeds (5–9). Fenbendazole exerts its effect by binding with greater affinity to parasite tubulin compared to that of mammalian and avian species and causing a disruption of the parasite tubulin-microtubule dynamic equilibrium, thus giving fenbendazole a wide margin of safety for use in food-producing animals (10,11). Because of this mechanism of action, the efficacy of benzimidazoles, including fenbendazole, correlates best with duration of therapy rather than dose. Therefore, the in-feed administration of this class of anthelminthic is preferable. Following its absorption, fenbendazole is predominantly metabolized in the liver through oxidation to oxfendazole (fenbendazole sulfoxide, FBZ-SO), a main active metabolite, and is subsequently metabolized to fenbendazole sulfone (FBZ-SO2), which is considered the marker residue for monitoring by North American regulatory agencies including the Canadian Food Inspection Agency (CFIA) (9,12).
Fenbendazole medicated premix is approved for the control of nematodes in turkeys in the United States (US) with a zero-withdrawal time when it is fed at 16 ppm in complete feed for 6 d, with a tolerance limit of 6 ppm in the liver (target tissue), and 2 ppm in the muscle (9). The tolerance limit is the maximum allowable drug residue level in edible products of food-producing animals in the US and is equivalent to the maximum residue limit (MRL) used in Canada and the European Union. In Canada, although fenbendazole is approved for use in cattle and swine, it is not approved for use in turkeys, despite the fact that its use has proven effective when added to the feed. Additionally, while water additive fenbendazole products have been registered for use in chickens in Canada, Europe, and the US, none are currently approved for turkeys. The use of fenbendazole in turkeys in Canada therefore constitutes extra-label drug use (ELDU) and consequently, the Canadian Global Food Animal Residue Avoidance Databank (CgFARAD) has received numerous inquiries for the use of fenbendazole in growing turkeys for the prevention and treatment of ascarids.
Avoiding drug residue violations in edible tissues from treated animals is of importance for public health and consumer food safety. Maximum residue limits and average daily intakes are important regulatory parameters on which consumer food safety is based. Currently, there are no MRLs or withdrawal times established by the Veterinary Drugs Directorate (VDD) of Heath Canada for fenbendazole in the edible tissues of turkeys; thus, the detection of any fenbendazole residues in the edible tissues of turkeys by the CFIA is considered to be a regulatory violation. As a result, there is a need for a depletion study that will provide veterinarians with an evidence-based withdrawal interval (WDI) for fenbendazole in turkeys destined for human consumption, thereby ensuring food safety while also assisting producers and the industry with maintaining their productivity and animal welfare. The objectives of this depletion study were to assess residue depletion of fenbendazole in the edible tissues of growing turkeys and estimate a WDI following in-feed administration of fenbendazole using the most common dosing regimen according to historical CgFARAD requests from licensed Canadian veterinarians, which is 30 ppm (0.15 kg/metric ton) for 7 d.
Materials and methods
Forty-eight 2-day-old turkey poults (24 females and 24 males) were obtained from a commercial flock (Cuddy Farms, Strathroy, Ontario) and used in this study. Birds were raised at the Arkell Poultry Research Station at the University of Guelph, Guelph, Ontario, for the duration of the study according to standard procedures for poultry, with females and males being housed separately. Upon arrival, all birds were vaccinated against coccidia (Eimeria spp.) using Immucox for Turkeys (Ceva Animal Health, Cambridge, Ontario). Besides the test article, no other drugs were administered to the birds during the study. To be eligible for inclusion in the study, birds had to be healthy and free of disease; thus, the birds were monitored daily for general health. All birds were considered to be healthy at the start of the study based on physical examination. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Guelph, and conformed to standards set forth by the Canadian Council on Animal Care.
The experimental design, including the number of birds per sacrifice timepoint, was chosen based on current recommendations of the Veterinary International Conference on Harmonisation (VICH) guideline No. 48 for marker residue depletion studies to establish compound withdrawal periods (13). The guideline stipulates that the marker residue is the parent molecule, or metabolite, determined from total residue and metabolism studies in the target species to deplete in known relation to depletion of total residues. This guideline is currently used by the VDD of Health Canada and the US Food and Drug Administration-Center for Veterinary Medicine for regulatory approval of drugs in food-producing animals that require a withdrawal time. However, this depletion study was not conducted under good laboratory practices guidelines as would be required by Health Canada for pharmaceutical companies seeking veterinary drug approvals in Canada. All birds were reared on standard, commercial complete feeds containing no drugs, and provided water ad libitum. At 8 wk of age, birds were identified individually by wing tags. At 9 wk of age, all birds were switched to the test diet consisting of a commercial complete grower ration containing fenbendazole (Safe-Guard Premix 20%, 200 mg/g; Merck Animal Health, Intervet Canada, Kirkland, Quebec) at 30 mg of fenbendazole/kg of complete feed (0.15 kg/metric ton of feed) as the sole ration for 7 consecutive days. The diet was then switched to a commercial complete grower ration containing no fenbendazole, and birds were randomized to 1 of 8 sacrifice timepoints (2, 4, 6, 8, 10, 12, 14, or 16 d after the end of treatment with the test diet) with 6 birds (3 hens, 3 toms) allocated per sacrifice timepoint. All birds were sacrificed by carbon dioxide inhalation followed by exsanguination at the research station’s processing plant according to standard handling protocols. Following euthanasia, tissue samples, including skin-adherent fat (entire skin covering the breast muscle and underlying fat), breast muscle (minimum 100 g), liver (entire liver), and kidney (both kidneys) were collected in this order at each sacrifice timepoint for each bird. All samples were identified and stored individually at −80°C until they were analyzed.
Liquid chromatography-tandem mass spectrometry analysis
Determination of FBZ-SO2 residues in edible tissues of turkeys was conducted by the University of Guelph Laboratory Services Division, Guelph, Ontario. Tissue extracts were assayed for FBZ-SO2 concentrations in each tissue matrix type using a liquid chromatography-tandem mass spectrometry system consisting of an Agilent 1200 series pump, column heater, and autosampler (Agilent Technologies Canada, Mississauga, Ontario) coupled to a triple quadrupole mass spectrometer (QTRAP 5500; AB SCIEX, Concord, Ontario). Tissue samples were extracted and analyzed following the addition of a deuterated internal standard. Tissues were homogenized and extracted into acetonitrile using a Geno/Grinder with salting out. Sample extracts were further refined prior to analysis using centrifugation (5 min at 4000 × g at 15°C) followed by dispersive solid phase extraction (C18) to remove potential interferences from co-extractive compounds. Isocratic chromatographic separation was carried out using a Poroshell 120EC-C18 column (50 mm × 4.6 mm I.D., 2.7 μm; Agilent Technologies, Santa Clara, California, USA) and a mobile phase of methanol-water-formic acid (60:40:0.1, v/v/v), which was delivered at a flow rate of 0.5 mL/min. The column temperature was maintained at 35°C. The sample injection volume was 2 μL and the run time was 6 min. The target ion transitions for FBZ-SO2 and deuterated FBZ-SO2 (d3) were 332.0 to 299.9 and 335.0 to 299.9, respectively. The qualifying ion transitions for FBZ-SO2 and deuterated FBZ-SO2 were 332.0 to 159.0 and 335.0 to 158.8, respectively. Analysis was performed in positive ion mode (MRM) with a declustering potential of 31 V, collision energy of 30 V and ion spray voltage of 4500 V. Quantitation was conducted using a matrix matched standard curve and deuterated FBZ-SO2. The validated limit of quantification (LOQ) and limit of detection (LOD) for FBZ-SO2 calibration curves were 1 part per billion (ppb) and 0.3 ppb, respectively, for the edible tissues skin-adherent fat, muscle, liver, and kidney. The calibration curves (1 to 600 ppb) were evenly distributed with a coefficient of determination (r2) > 0.99 for each curve. Calibration curves were run at the beginning and end of each day’s sample testing runs with additional standards interspersed throughout the run. The highly specific nature of LC-MS/MS detection was verified by including the injection of both solvent and matrix blanks following the highest calibration standards (600 ppb) analyzed. Results demonstrated no residue carry over between injections and no interfering co-extractives were present in any of the tissues tested. Intra-day accuracy and precision (percent recovery, n = 3) for the 5 ppb fortified quality control samples for FBZ-SO2 for skin/fat, kidney, liver, and muscle were 92% ± 2.5, 101% ± 2.0, 95% ± 6.7, and 100% ± 3.0, respectively, and for the 50 ppb fortified quality control samples for FBZ-SO2 for skin/fat, kidney, liver, and muscle were 91% ± 0.7, 95% ± 1.0, 94% ± 0.4, and 86% ± 1.2, respectively. Inter-day accuracy and precision (percent recovery, n = 3) for the 5 ppb fortified quality control samples for FBZ-SO2 for skin/fat, kidney, liver, and muscle were 96% ± 3.0, 92% ± 5.3, 92% ± 3.5, and 80% ± 4.2, respectively, and for the 50 ppb fortified quality control samples for FBZ-SO2 for skin/fat, kidney, liver, and muscle were 92% ± 1.3, 82% ± 3.2, 82% ± 3.2, and 82% ± 3.5, respectively. The percent coefficient of variation for reference curve FBZ-SO2 concentrations in all matrices, including the LOQ, was < 15%. Quality control samples (5 ppb and 50 ppb) were included in all test sample runs and were within a tolerance of ± 15% of the nominal value.
Statistical analysis
Data analyses were conducted with the assistance of biostatisticians (Steven Radecki, Petoskey, Michigan, USA; William Sears, Department of Population Medicine, University of Guelph, Guelph, Ontario). The FBZ-SO2 concentrations in tissues at each sacrifice timepoint were compared by analysis of variance (ANOVA) for repeated measures (Version 9.1.3; SAS Institute, Cary, North Carolina, USA). Prior to analysis of data for the WDI estimation, FBZ-SO2 concentrations were transformed using the natural logarithm (Ln). Least square means and 95% confidence intervals were reported along with back-transformed values. The statistical significance level was set at P ≤ 0.05. Statistical analyses were begun with a full factorial model that included all interactions (i.e., gender, tissue, and time) with terms being removed if P-values were > 0.05. Regression analyses were performed on tissue FBZ-SO2 concentrations to determine the time required for FBZ-SO2 levels to reach a predetermined tissue concentration, i.e., the LOQ for the analyte in each matrix. As there is no approved MRL for fenbendazole in edible tissues of turkeys in Canada, the allowable levels are 0 ppb of the analyte, which was set at the current CFIA-validated LOD for FBZ-SO2 of 1 ppb (Dr. Joseph Boison, Adjunct Professor of Chemistry, Chemistry Department, University of Saskatchewan, Saskatoon, Saskatchewan, personal communication, 2016). Initial regression analysis including all sacrifice timepoints was influenced by extreme values on the first sacrifice timepoint (day 2). Removal of the first sacrifice timepoint (day 2) in its entirety yielded a better statistical fit of the data. Additionally, due to the fact that most, if not all, values at the final sacrifice timepoint were above the CFIAs LOD, analyses of FBZ-SO2 tissue residue data for WDI determination by a statistical method that determined the statistical tolerance limit for the central 99% of the population, as used by Health Canada and the FDA for regulatory submissions, was not possible in the current study. Instead, regression analysis with 95% CI was used to estimate the WDI. The WDI was estimated as the point at which the upper bound of this confidence interval crossed the CFIAs LOD of 1 ppb.
Results
All diets used in the study, including the test diet, were mixed as standard complete turkey feeds by Floradale Feed Mill, Floradale, Ontario, and shipped in bags to the Arkell Poultry Research Station. Certificates of analysis (Merck Pharmaceutical Laboratory, Lawrence, Kansas, USA) were completed on repeated (n = 4) sampling of the single batch of test diet. Assay values ranged from 26.2 to 26.9 ppm fenbendazole (as-is-basis) and were all within the accepted permissible assay value of 80% to 120% labeled claim (30 ppm fenbendazole on an as-is-basis).
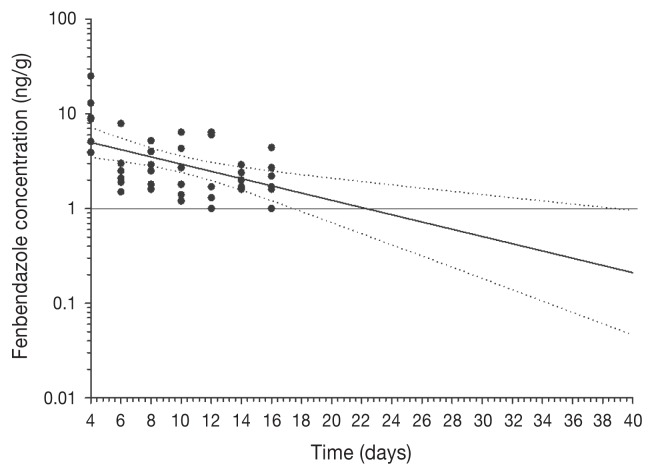
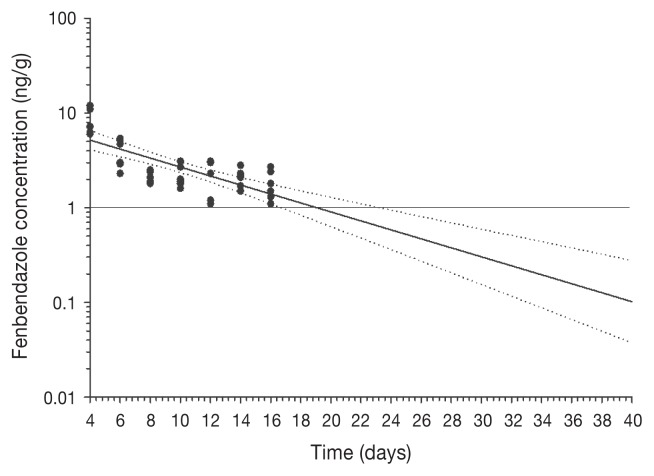
All birds remained healthy throughout the study period and no observable adverse effects were noted in any birds while on the test diet. There were no statistically significant gender-related differences in the depletion of FBZ-SO2 in the present study. The FBZ-SO2 concentrations in the liver and skin-fat of all birds were above the assay LOQ (1 ppb or 1 ng/g) at all study sacrifice timepoints, with residues being higher than those in the kidneys and breast muscle tissues at each respective study sacrifice timepoint (P < 0.0001, Table 1), with the exception of kidney tissues at 2 d post-treatment, where mean FBZ-SO2 concentrations were higher than that obtained in skin-fat (P = 0.0189). The FBZ-SO2 concentration in kidneys in all birds was above the LOQ at 2 and 4 d post-treatment, while at 6 d post-treatment and thereafter it was above the LOQ in some individual birds at each sacrifice timepoint (Table 1). The FBZ-SO2 concentration in the breast muscle in all birds was above the LOQ at 2 d post-treatment, while at 4 to 12 d post-treatment it was above the LOQ in some individual birds at each sacrifice timepoint, and at 14 d and 16 d post-treatment it was not detectable in any of the birds (Table 1). Regression of the depletion data, excluding the day 2 sacrifice timepoints, to the CFIA-validated LOD of FBZ-SO2 of 1 ppb with 95% CI, was possible in the liver and skin-adherent fat matrices only. The upper 95% CI from the analyses was below 1 ppb at 39 d and 23 d in the liver and skin-adherent fat, respectively, suggesting that these would be the minimum WDIs for each of these tissues (Figures 1 and 2, respectively) using the current data set.
Table 1.
Fenbendazole sulfone concentrations (ng/g) in 48 turkeys following in feed administration at 30 mg/kg of feed for 7 consecutive days.
| Days post-treatment | Liver | Skin-adherent fat | Kidney | Muscle |
|---|---|---|---|---|
| 2 | 252.67 ± 342.76a,b | 56.17 ± 56.42a | 70.33 ± 98.13 | 24.73 ± 36.98 |
| 4 | 10.82 ± 7.66a,b | 9.08 ± 2.88a | 3.25 ± 2.35 | 1.22 ± 1.06 (4/6) |
| 6 | 3.15 ± 2.38a | 3.92 ± 1.34a | < LOQ (1/6) | < LOQ (1/6) |
| 8 | 3.0 ± 1.38a | 2.13 ± 0.27a | < LOQ (3/6) | < LOQ (1/6) |
| 10 | 2.97 ± 2.03a | 2.18 ± 0.58a | < LOQ (2/6) | < LOQ (2/6) |
| 12 | 3.78 ± 2.97a | 2.17 ± 0.86a | 1.23 ± 1.35 (3/6) | < LOQ (2/6) |
| 14 | 2.1 ± 0.48a | 2.10 ± 0.46a | < LOQ (1/6) | ND (0/6) |
| 16 | 2.27 ± 1.19a | 1.8 ± 0.63a | < LOQ (2/6) | ND (0/6) |
Data are expressed as mean ± SD.
versus kidney and muscle.
versus skin-adherent fat within a timepoint (P < 0.05).
Numbers in parentheses denote number of individual birds with FBZ sulfone concentrations above LOQ. LOQ — limit of quantification = 1 ng/g; ND — not detected; n = 6, number of birds per sacrifice timepoint.
Figure 1.
Liver concentration-time profile across sacrifice timepoints (2, 4, 6, 8, 10, 12, 14, and 16 d post-treatment) for fenbendazole sulfone following in-feed administration at 30 mg/kg for 7 consecutive days in 42 turkeys (n = 6 birds per sacrifice timepoint). Values are plotted on a log scale. The horizontal line at 1 ng/g, the limit of assay quantification (LOQ). Dotted lines, upper and lower 95% CI.
Figure 2.
Skin-adherent fat concentration-time profile across sacrifice timepoints (2, 4, 6, 8, 10, 12, 14, and 16 d post-treatment) for fenbendazole sulfone following in-feed administration at 30 mg/kg for 7 consecutive days in 42 turkeys (n = 6 birds per sacrifice timepoint). Values are plotted on a log scale. The horizontal line at 1 ng/g, the limit of assay quantification (LOQ). Dotted lines, upper and lower 95% CI.
Discussion
No mortality or signs of morbidity were noted in this study, supporting the safety of fenbendazole fed to turkeys at 30 mg/kg of feed for 7 d. Anthelminthic compounds are used extensively for the prevention and treatment of parasitic nematodes in poultry, and fenbendazole has been found to be effective against this type of infection (5–9). There are currently no fenbendazole products approved by Health Canada for use in turkeys. The use of fenbendazole in this species, therefore, is considered extra-label, with no (zero) allowable levels of fenbendazole in edible tissues. Edible tissues containing drug residues can pose health hazards to the consumer including increased drug resistance, allergic reactions, and possible direct toxic effects. Thus, a safe withdrawal interval must be provided by the prescribing veterinarian, such that the drug will not represent a public health concern, or be out of regulatory compliance, when fenbendazole is used in turkeys. The CgFARAD is frequently contacted for fenbendazole withdrawal guidance after in-feed administration to turkeys to control parasitic nematodes. Given the lack of tissue residue profiles following in-feed administration of fenbendazole to turkeys, and to estimate an appropriate WDI that assures consumer safety, the study reported here was conducted by the CgFARAD.
The results of this study showed that concentrations of the marker residue FBZ-SO2 were above the assay LOQ, and therefore violative in all birds, in the breast muscle at 2 d and in the kidneys at 2 d and 4 d post-treatment. Furthermore, some birds had FBZ-SO2 concentrations above the LOQ for up to 12 d post-treatment in the breast muscle, and across all sacrifice timepoints in the kidneys. Considering that the same dose of fenbendazole was administered in-feed to birds of the same age and being kept under the same environmental conditions (e.g., lighting, temperature), these findings may indicate individual variation in FBZ-SO2 depletion from the muscle and kidneys of turkeys (14). The FBZ-SO2 concentrations in the liver and skin-adherent fat remained above the LOQ in all birds, at all sacrifice timepoints, with concentrations being significantly higher in these tissues than in corresponding kidney and breast muscle. The liver is the slowest depleting organ for many veterinary drugs undergoing hepatic metabolism. The degree of metabolism for a drug is affected by the drugs lipophilicity. In this study, fenbendazole also accumulated in the skin-adherent fat. Previous residue depletion and metabolism studies with fenbendazole in turkeys showed that most of the fenbendazole was eliminated in the feces by 24 h post-dosing (9). In this study, FBZ-SO2 concentrations were detected in all edible tissues studied beginning at 2 d post-treatment, with mean FBZ-SO2 concentrations at the day 2 and day 4 sacrifice timepoints following the rank order: liver > skin-adherent fat > kidneys and breast muscle. This trend in the accumulation of FBZ-SO2 in turkey tissues is similar to that reported after fenbendazole administration to pheasants at 100 ppm for 7 d without feed withdrawal (15). Additionally, beginning at 6 d post-treatment and thereafter, the mean FBZ-SO2 concentrations in the edible tissues followed the rank order: liver and skin-adherent fat > kidneys and breast muscle.
In the US, fenbendazole is currently approved for use in complete turkey feeds at 16 ppm to be fed for 6 consecutive days with no withdrawal time required and FDA approved tolerances of 6 ppm and 2 ppm in liver and muscle, respectively (9). In Canada, the target tissue for fenbendazole that is monitored by the CFIA is the liver and the marker residue that is monitored is FBZ-SO2. In Canada, as there are no MRLs approved for fenbendazole in edible tissues of turkeys, the detection of any amount by the CFIA at processing constitutes a residue violation and a regulatory compliance concern. Unfortunately, the depletion data set obtained from this study did not allow us to calculate the WDIs for fenbendazole in the collected tissues using a statistical tolerance limit of 99%, as is required with sponsor submissions for regulatory approval of veterinary drugs for use in food-producing animals. Although our study design was based on the VICH guidelines, a pilot study was not feasible to establish a range of sacrifice days that would have provided raw data points to the LOQ for the assay (i.e., 1 ppb). Our choice of sacrifice timepoints was based on limited data obtained from a New Animal Drug Application (NADA) 131-675-July 3, 2000 for Fenbendazole for grower turkeys that was designed to evaluate fenbendazole depletion to a tolerance level of 6 ppm in the liver and 2 ppm in the muscle (9). As such, analysis of the data and determination of a WDI for liver and skin-adherent fat relied on extrapolation of the data from the last sacrifice timepoint to the LOQ of the assay and did not include raw data points at the assay LOQ of 1 ppb. Initial regression analysis including all sacrifice timepoints was influenced by extreme values on the first sacrifice time point (day 2). A pilot study would have allowed us to re-design the sacrifice timepoints to focus on later timepoints closer to the assay LOQ, while removing earlier ones. Modeling fenbendazole depletion data to the current CFIA LOD for FBZ-SO2 of 1 ppb resulted in estimations of WDIs of at least 39 and 23 d for the liver and skin-adherent fat, respectively. Given that the liver is considered the target tissue for fenbendazole residues testing by the CFIA following its extra-label use in food-producing animals, a WDI estimation of at least 39 d following the last treatment with fenbendazole should be observed under the conditions of this study. It is important to note that the estimation of a WDI for FBZ in this study does not represent an official WDI, which may only be issued by Health Canada.
Acknowledgments
The authors thank Dr. Mike Joyce (private poultry practitioner, Hillsburgh, Ontario) and Dr. Joseph Boison (Adjunct Professor of Chemistry, Chemistry Department, University of Saskatchewan, Saskatoon, Saskatchewan) for their assistance with study design, and Steven Radecki (Petoskey, Michigan, USA) and William Sears (Department of Population Medicine, University of Guelph) for their assistance with the statistical analysis. The certificate of analysis of fenbendazole in the test diet was provided by Merck Pharmaceutical Laboratory, Lawrence, Kansas, USA. This study was funded by Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) and the Turkey Farmers of Ontario. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Permin A, Christensen JP, Bisgaard M. Consequences of concurrent Ascaridia galli and Escherichia coli infections in chickens. Acta Vet Scand. 2006;47:43–54. doi: 10.1186/1751-0147-47-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikeme MM. Observations on the pathogenicity and pathology of Ascaridia galli. Parasitol. 1971;63:169–179. doi: 10.1017/s003118200007949x. [DOI] [PubMed] [Google Scholar]
- 3.Matta SC, Ahluwalia SS. Studies on effect of Ascaridia galli infection on growth rates of chicks. Indian J Poult Sci. 1980;15:1–4. [Google Scholar]
- 4.Hemsley RV. Fourth stage Ascaridia spp. larvae associated with high mortality in turkeys. Can Vet J. 1971;12:147–149. [PMC free article] [PubMed] [Google Scholar]
- 5.Norton RA, Yazwinski TA, Johnson Z. Use of fenbendazole for the treatment of turkeys with experimentally induced nematode infections. Poult Sci. 1991;70:1835–1837. doi: 10.3382/ps.0701835. [DOI] [PubMed] [Google Scholar]
- 6.Yazwinski TA, Andrews P, Holtzen H, Presson B, Wood N, Johnson Z. Dose-titration of fenbendazole in the treatment of poultry nematodiasis. Avian Dis. 1986;30:716–718. [PubMed] [Google Scholar]
- 7.Kirsch R. Treatment of nematodiasis in poultry and game birds with fenbendazole. Avian Dis. 1984;28:311–318. [PubMed] [Google Scholar]
- 8.Yazwinski TA, Tucker CA, Reynolds J, Johnson Z, Pyle D. Efficacies of fenbendazole and levamisole in the treatment of commercial turkeys for Ascaridia dissimilis infections. J Appl Poult Res. 2009;18:318–324. [Google Scholar]
- 9.New Animal Drug Application (NADA) 131-675-July 3, 2000. Freedom of Information Summary. Supplemental new animal drug application. SAFE-GUARD® (Fenbendazole) For Growing Turkeys. [Last accessed December 15, 2018]. Available from: https://www.fda.gov.
- 10.Lacey E, Gill JH. Biochemistry of benzimidazole resistance. Acta Trop. 1994;56:245–262. doi: 10.1016/0001-706x(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 11.Gozalo AS, Schwiebert RS, Lawson GW. Mortality associated with fenbendazole administration in pigeons (Columba livia) J Am Assoc Lab Anim Sci. 2006;45:63–66. [PubMed] [Google Scholar]
- 12.Short CR, Flory W, Hsieh LC, Barker SA. The oxidative metabolism of fenbendazole: A comparative study. J Vet Pharmacol Ther. 1988;11:50–55. doi: 10.1111/j.1365-2885.1988.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 13.VICH GL48 (MRK) Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food-Producing Animals: Marker Residue Depletion Studies to Establish Product Withdrawal Periods. [Last accessed December 14, 2018]. Available from: https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM207941.pdf.
- 14.Short CR, Barker SA, Hsieh LC, et al. The elimination of fenbendazole and its metabolites in the chicken, turkey and duck. J Vet Pharmacol Ther. 1988;11:204–209. doi: 10.1111/j.1365-2885.1988.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 15.Griffith R, Yaeger M, Hostetter S, et al. Safety of fenbendazole in Chinese ring-necked pheasants (Phasianus colchicus) Avian Dis. 2014;58:8–15. doi: 10.1637/10479-010213-Reg.1. [DOI] [PubMed] [Google Scholar]