Abstract
Background
Steroids are known to play a role in the pathogenesis of glaucoma, but little is known about the effect of inhaled corticosteroids (ICS). The purpose of this study was to investigate whether there is a clinically significant association between inhalational steroids and intraocular pressure (IOP).
Methods
This was a cross-sectional, case–control study performed at St John’s Medical College, Bengaluru, India, from October 2013 to July 2015 of 200 patients using 800 mcg of budesonide or its equivalent dose of ICS and 200 healthy controls not using any form of steroids. Patients using ICS for a period of at least 6 months with no usage of oral or topical steroids within the last 3 months were included as cases. Age- and sex-matched controls were recruited from among the general patient population of the ophthalmological department. IOP and central corneal thickness (CCT) were analyzed. Cases were divided into two subgroups. Group 1 had IOP of <21 mm Hg and cup-to-disc ratio of <0.5. Group 2 had IOP of ≥21 mm Hg or cup-to-disc ratio of ≥0.5 or cup–disc asymmetry ≥0.2. These two subgroups were analyzed to determine whether there was an increased risk of developing ocular hypertension or glaucoma with extended use of ICS.
Results
A total of 400 subjects participated, with 200 in each group. The mean IOP of cases was 15.31 ± 3.27 mm Hg, statistically significantly higher than the mean of 13.39 mm Hg ± 1.95 in controls (P < 0.001). The mean CCT in cases was 522.02 ± 30.47 μm, lower than the mean of 528.73 ± 29.09 μm of the control group (P > 0.001). Of the 200 cases, 11 (5.5%) had ocular hypertension and 2 (1%) had open-angle glaucoma. There was no statistically significant correlation between duration of inhaled steroids usage and increase in IOP (P = 0.62). There was no development of ocular hypertension or glaucoma among the controls.
Conclusions
Our findings suggest a probable association between ICS and IOP and that it may be advisable to measure baseline IOPs and CCT and to follow patients on ICS at regular intervals.
Introduction
In today’s clinical spectrum obstructive airway diseases are mainly treated with steroids in various doses. Treatment protocols advise use of oral or inhalational steroids alone or in combination. It is well known that oral steroids cause several ocular side effects, most commonly cataract and glaucoma1; however, the ocular side effects of inhalational steroids are less well understood. Intraocular pressure (IOP) is the most common risk factor for progressive optic neuropathy in glaucoma and is affected by both topical and oral corticosteroids. Around 4% to 6% of the population are “steroid responders” who develop an increase in IOP of >15 mm Hg or with IOPs of >31 mm Hg after daily topical corticosteroid use for 4–6 weeks.2 Inhalational corticosteroids (ICS) are initiated in the management of obstructive airway disease, particularly asthma. These can be absorbed from the nasal mucosa and lungs, hence bypassing the degradation by the liver through first-pass metabolism and exposing the body to steroids for a longer duration.3 It is also possible that improper usage of inhalers can cause direct entry of the steroid into the eye.4
The exact mechanism of steroid-induced IOP increase is still unknown, although many theories have been postulated. The most commonly accepted hypothesis, proposed by Clark,2 is that steroid receptors are activated that alter the trabecular meshwork genes myocilin and optineurin, which in turn cause changes in the extracellular matrix. This change increases the resistance to aqueous outflow.1 Steroids can also reduce the phagocytic action of the trabecular meshwork endothelial cells.1
The ocular effects of different forms of steroids (oral, topical, intravitreal) and even the sub-Tenon’s route of administration have been extensively studied. However, ICS were not considered an important factor for IOP elevation. Garbe et al were the first to study the effect of inhalational steroids on IOP.3 Using the Quebec computerized health insurance database, they found an association between inhalational steroids and ocular hypertension. Although the study had several shortcomings in the method of glaucoma diagnosis, it was nonetheless a landmark study for recognizing inhalational steroids as a probable cause of raised IOP. Case reports have noted similar findings.5,6 Mitchell et al4 also showed a positive correlation between risk of glaucoma and use of inhaled steroids in patients with a family history of glaucoma. On the contrary, several prospective studies showed no effect of inhalational steroids on IOP after a period of 12–20 weeks.7,8 We designed a prospective, cross-sectional comparative study of IOP and central corneal thickness (CCT) in an Asian population of patients on long-term high-dose ICS.
Subjects and Methods
The Institutional Review Board of St John’s Medical College, Bengaluru, India, approved this prospective, cross-sectional comparative study. Written informed consent was provided, and in those consenting, a detailed history was taken, including demographics, ocular disease, past medical illness, drug history, and personal history. Cases were recruited from the pulmonology department and were screened and evaluated from October 2013 to July 2015. Age- and sex-matched controls were selected from those visiting the primary care outpatient department during the same time period. Inclusion criteria were age >18 years and use of ICS of ≥800 μg equivalent of budesonide for at least 6 months. Patients who had preexisting glaucoma, family history of glaucoma, or who were taking any form of topical or nasal steroids were excluded. Patients with any cause of diminution of vision other than cataract, including retinal pathology, corneal pathology (including previous refractive surgeries), and uveitis, were also excluded, as were those who had received oral/parenteral corticosteroids for >2 weeks within the last 3 months. Patients with diabetes mellitus and hypertension were also excluded to avoid confounding bias. Controls did not have any respiratory disease and had never used corticosteroids in any form—topical, inhaled, or oral.
IOP was measured by Goldman applanation tonometry, with an average of 3 recordings. CCT was measured using the Tomey AL 1000 pachymeter (Tomey Corp, Nagoya Aichi, Japan); IOP was adjusted based on the CCT and recorded.9,10 If corrected, IOP was ≥21 mm Hg or disc changes (cup-to-disc ratio of ≥0.5 or cup–disc asymmetry of ≥0.2) then gonioscopy and visual fields testing were performed. Visual fields were measured using the SITA standard with the Humphrey Visual Field analyzer (Carl Zeiss Meditec, Dublin, CA). Glaucoma was diagnosed if there was increased IOP and disc changes or visual field changes. Significant visual field changes were defined as an abnormal Humphrey 30-2 glaucoma hemifield test plus one or more of the following field defects, which could not be explained by other ocular or neurologic causes: (1) arcuate or paracentral scotoma, (2) nasal step, or (3) advanced glaucomatous field loss.
Statistical Analysis
The sample size was calculated as 150 patients each of cases and controls to reach a 10% difference in the proportion of ocular hypertension between groups with a 5% level significance and 80% power. We took an excess number to account for potential dropouts.
Descriptive statistics were used to summarize the data. A t test was used to compare the mean IOP, adjusted IOP, and CCT between cases and controls. Cases were divided into two subgroups: group 1 had IOP of <21 mm Hg and cup-to-disc ratio of <0.5; group 2 had IOP of ≥21 mm Hg or a cup-to-disc ratio of ≥0.5 or cup–disc asymmetry of ≥0.2. Analysis within these two groups to find whether there was an increased risk of developing ocular hypertension/glaucoma with duration of inhaled steroid was performed using a nonparametric Mann-Whitney test, because they were not normally distributed.
Results
A total of 200 cases and 200 age- and sex-matched controls were included. The age range of participants was 18–89 years of age. The mean (± SD) age of controls was 54.21 ± 15.02 years; of cases, 53.65 ± 15.03 years (P = 0.78). The control population had 99 males (49.5%); the case population, 101 (50.5%; P = 0.67). The best-corrected visual acuity (BCVA) was not significantly different between groups (P = 0.93). In controls, mean BCVA was 0.18 ± 0.24 logMAR; in cases, 0.19 ± 0.29 in group 1 and 0.32 ± 0.54 in group 2.
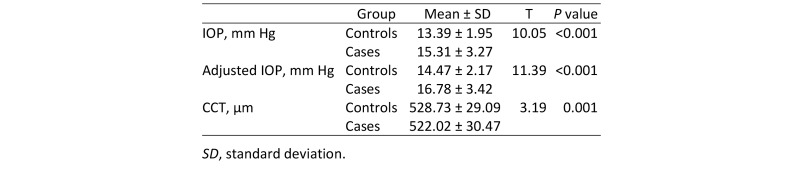
As shown in Table 1, the mean IOP among the cases was 15.31 ± 3.27 mm Hg, which was higher than the mean in controls (13.39 ± 1.95 mm Hg), and this difference was statistically significant (P < 0.001). The CCT of the cases was significantly lower than those of controls (P < 0.001).
Table 1.
Comparison of intraocular pressure (IOP), adjusted IOP, and central corneal thickness (CCT) between controls and cases
Of the 200 cases, 187 (93.5%) had normal IOP (<21 mm Hg) and optic nerves (cup-to-disc ratio of <0.5). These 187 cases formed subgroup 1. There was above-normal IOP (≥21 mm Hg) or optic nerve changes (cup-to-disc ratio of ≥0.5 or cup–disc asymmetry of ≥0.2) in 13/200 (9.5%). These 13 cases formed subgroup 2. Subgroup 2 were considered at risk for glaucoma. None of the controls had increased IOP or optic nerve changes. A two-sample proportion test comparing the occurrence of increased IOP in cases and controls revealed a statistically significant difference between groups (P < 0.0001).
The mean duration of ICS use among the cases was 2.76 ± 3.03 years: 3.06 ± 3.08 years in subgroup 2 and 2.76 years in subgroup 1 (the difference was not statistically significant between subgroups [P = 0.62]).
Perimetry was performed for patients in subgroup 2 only. Of the 13 patients, 2 showed early glaucomatous field changes in the form of bilateral arcuate scotomas.
Discussion
Steroids have a wide array of unwanted side effects on various organs and body systems; the eye is a frequent target. Two of the most significant ocular side effects of steroids are cataract formation and glaucoma. Both of these are major causes of blindness, with glaucoma estimated to be the cause of blindness in 80 million people by the year 2020.11 In southern India, statistics show that glaucoma contributes to 10.2% of blindness.12 With approximately 17.23 million people affected by asthma in India and a majority of them being treated with steroids, the potential risks are great.13 It is thus imperative to identify all possible risk factors for glaucoma and to manage cases at an early stage.
Our study provides evidence that ICS use may result in IOP increase. This result agrees with the findings of Opatowsky et al and Dreyer.5,6 Although we identified 11 cases (5.5%) of ocular hypertension and 2 (1%) of primary open-angle glaucoma, we found no correlation with the duration of steroid use. A limitation of our study was that it was cross sectional; hence, we cannot conclusively attribute the IOP difference solely to inhalational steroids. Also, we did not examine subgroups for the effect of dosage and duration of steroids. This analysis was, however, performed in a multicenter, four-armed study by Duh et al7 in which the effects of budesonide over the dosage range of 100–800 mcg and above for a period of 12–20 weeks were studied, and no significant elevation in IOP was observed. Nath el al14 also examined the effect of different ICS doses (1–1000 mcg) on IOP and found that higher dosage and duration of steroids was associated with increased prevalence of glaucoma (42.8%). Their study found a 3.92% prevalence of glaucoma.
A strength of our study is that it is one of the few Indian studies that examine this relationship. The careful IOP measurements and glaucoma diagnosis with a relatively large sample size add to its strength.
Steroid responders can present at any time during the course of treatment, from days to months; hence, a prospective study that includes all of these patients is desirable. This may be the reason why follow-up studies of 12–20 weeks did not find inhalational steroids to cause an increased risk of glaucoma.7 A recent randomized controlled trial studied 22 patients with ocular hypertension and well-controlled primary open-angle glaucoma on ICS for 6 weeks and found no significant increase in mean IOP from baseline.15 The main limitation of this study was the short treatment duration and the fact that the study model might have detected only moderate responders. Our study excluded patients with a definite family history of glaucoma. However, there is concern about the validity of no family history among the patients with increased IOP. This is because there has been positive correlation between risk of glaucoma and a family history in patients on inhaled steroids in studies by Garbe et al and Mitchell et al.3,4
The normal prevalence of ocular hypertension worldwide ranges from 1.1% to 13% according to many population studies.16 Among the southern Indian population of Vellore the prevalence was reported as 3%17; the Andhra Pradesh Eye Disease Study reported a prevalence of 0.32%.18 Data from the Ocular Hypertension Treatment Study has shown that patients with ocular hypertension have an average estimated risk of 10% of developing glaucoma over 5 years.19 Hence, our study has important clinical implications. Although a positive correlation between the inhaled steroids duration and the development of glaucoma was not found, it may be advisable to record a baseline IOP prior to commencement of treatment and to follow patients on inhaled corticosteroids regularly in order to detect ocular hypertension effectively.
Acknowledgments
The authors thank Dr. Mary Joseph, Dr. Sonika Porwal, and Dr. Puneeth Isloor for their valuable help and suggestions.
References
- 1.Jobling AI, Augusteyn RC. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin Exp Optom. 2002;85:61–75. doi: 10.1111/j.1444-0938.2002.tb03011.x. [DOI] [PubMed] [Google Scholar]
- 2.Clark AF. Steroids, ocular hypertension, and glaucoma. J Glaucoma. 1995;4:354–69. [PubMed] [Google Scholar]
- 3.Garbe E, LeLorier J, Bolvin JE, Suissa S. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucoma. JAMA. 1997;277:722–7. [PubMed] [Google Scholar]
- 4.Mitchell P, Cumming RG, Mackey DA. Inhaled corticosteroids, family history, and risk of glaucoma. Ophthalmology. 1999;106:2301–6. doi: 10.1016/S0161-6420(99)90530-4. [DOI] [PubMed] [Google Scholar]
- 5.Opatowsky I, Feldman RM, Gross R, et al. Intraocular pressure elevation associated with inhalation and nasal corticosteroids. Ophthalmology. 1995;102:177–9. doi: 10.1016/s0161-6420(95)31039-1. [DOI] [PubMed] [Google Scholar]
- 6.Dreyer EB. Inhaled steroid use and glaucoma [letter]. N Engl J Med. 1993;329:1822. doi: 10.1056/nejm199312093292420. [DOI] [PubMed] [Google Scholar]
- 7.Duh MS, Walker AM, Lindmark B, Laties AM. Association between intraocular pressure and budesonide inhalation therapy in asthmatic patients. Ann Allergy Asthma Immunol. 2000;85:356–61. doi: 10.1016/S1081-1206(10)62545-8. [DOI] [PubMed] [Google Scholar]
- 8.Samiy N, Walton DS, Dreyer EB. Inhaled steroids: effect on intraocular pressure in patients without glaucoma. Can J Ophthalmol. 1996;31:120–3. [PubMed] [Google Scholar]
- 9.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol. 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhan A, Browning AC, Shah S, et al. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol Vis Sci. 2002;43:1389–92. [PubMed] [Google Scholar]
- 11.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thulasiraj RD, Nirmalan PK, Ramakrishnan R, et al. Blindness and vision impairment in a rural south Indian population: the Aravind Comphrensive Eye Survey. Ophthalmology. 2003;110:1491–8. doi: 10.1016/S0161-6420(03)00565-7. [DOI] [PubMed] [Google Scholar]
- 13.Jindal SK, Aggarwal AN, Gupta D, et al. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH). Int J Tuberc Lung Dis. 2012;16:1270–7. doi: 10.5588/ijtld.12.0005. [DOI] [PubMed] [Google Scholar]
- 14.Nath T, Roy SS, Kumar H, Agrawal R, Kumar S, Satsangi SK. Prevalence of steroid-induced cataract and glaucoma in chronic obstructive pulmonary disease patients attending a tertiary care center in India. Asia-Pacific J Ophthalmol. 2017;6:28–32. doi: 10.22608/APO.201616. [DOI] [PubMed] [Google Scholar]
- 15.Moss EB, Buys YM, Low SA, Yuen D, Jin YP, Chapman KR, Trope GE. A randomized controlled trial to determine the effect of inhaled corticosteroid on intraocular pressure in open-angle glaucoma and ocular hypertension: the ICOUGH Study. J Glaucoma. 2016;26:182–6. doi: 10.1097/IJG.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 16.Thomas R, Parikh R, George R, Kumar RS, Muliyil J. Five-year risk of progression of ocular hypertension to primary open angle glaucoma: a population-based study. Indian J Ophthalmol. 2003;51:329–33. [PubMed] [Google Scholar]
- 17.Jacob A, Thomas R, Braganza A, Muliyil J, Koshi SP. Prevalence of primary glaucoma in an urban South Indian population. Indian J Ophthalmol. 1998;46:81–5. [PubMed] [Google Scholar]
- 18.Dandona L, Dandona R, Srinivas M, et al. Open-angle glaucoma in an urban population in southern India: the Andhra Pradesh Eye Disease Study. Ophthalmology. 2000;107:1702–9. doi: 10.1016/s0161-6420(00)00275-x. [DOI] [PubMed] [Google Scholar]
- 19.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]