Abstract
Purpose
Understanding patients’ attitudes toward novel therapeutic options can help guide providers in personalizing treatment regimens for glaucoma patients. This study aimed to determine factors associated with acceptance of new drug delivery options among glaucoma patients.
Methods
A total of 199 patient volunteers participated in an interviewer-administered survey from June to August 2016 at the Glaucoma Service of Massachusetts Eye and Ear. The questionnaire was designed to determine acceptance of 6 drug delivery approaches: (1) triple combination eye drop, (2) microdose eye spray, (3) drug-eluting contact lens, (4) drug-eluting periocular ring insert, (5) injectable subconjunctival drug insert, and (6) injectable anterior chamber implant. Other factors analyzed included self-reported demographics, disease severity, and prior ocular history.
Results
The average respondent age was 63.2 ± 15.1 years; 48% were female. For approaches 1–6 listed above, overall acceptance rates were, respectively, 85%, 54%, 31%, 43%, 32%, and 30%. Patients with greater disease severity and prior incisional glaucoma surgery were more likely to pursue alternatives to traditional eye drops.
Conclusions
There is limited acceptance of alternatives to traditional eye drop medications among glaucoma patients. Understanding motivating factors and potential barriers to patient acceptance of novel drug delivery approaches is important in how providers will incorporate these glaucoma treatment options into practice.
Introduction
Glaucoma is the leading cause of irreversible blindness in the world.1 Progressive visual loss can be slowed by lowering intraocular pressure (IOP) through medications, laser treatment, or incisional surgery. Although self-administered eye drops are often first-line treatments for glaucoma, self-reported data and pharmacy claims data indicate poor adherence, especially with increasingly complex medication regimens.2,3 In addition, patients’ eye drop instillation techniques can be poor, which may also explain suboptimal IOP control despite self-reports of good adherence to medication regimens.4
In response to these problems, many novel drug delivery approaches are in development, aimed at improving treatment efficacy by eliminating issues with traditional self-administered drops. We explore patient attitudes toward 6 new medical treatment options, many of which are in trials or have demonstrated efficacy in delivering medications to the eye. Although combination eye drops containing two medications already exist, we explore patient acceptance of a triple combination eye drop containing three different medications, applied topically twice daily, similar to currently available drops.5 The second intervention surveyed is a nebulized medication that is applied as a precision microdroplet spray to the ocular surface. This device allows a smaller dose to be administered to minimize potential side effects of medications, and it may be easier for some patients to self-administer. Its efficacy in administering dilating drops has been demonstrated.6 The third device is a contact lens that delivers sustained-release latanoprost, which has been shown to lower intraocular pressures effectively in monkeys for at least 8 days of continuous wear.7 The fourth device is a topical periocular medication ring insert composed of bimatoprost incorporated within a silicone matrix placed in the upper and lower fornices by a physician in the office. A recent study suggested IOP lowering can be maintained for up to 6 months.8 The fifth device is a subconjunctival injection of liposomal latanoprost that has been shown to have sustained IOP lowering effects for up to 3 months in a series of 6 patients.9 The sixth device is an intracameral implant that delivers bimatoprost with IOP-lowering effects up to 6 months after one injection into the anterior chamber.10
A previous study by SooHoo et al found that 55% of surveyed patients preferred staying on daily eye drops to switching to more invasive alternatives, including punctal plugs, refillable medication depots, or injections.11 Chan et al12 found higher acceptance rates (57%–62%) for devices using punctal plug, subconjunctival, and intracameral routes among Chinese individuals in Singapore. In that study, more than 70% of those patients were willing to pay equal to or more than what they currently pay for topical medications. The goal of the current study was to determine the acceptance of a wider range of drug delivery approaches that have clinical promise in a sample of glaucoma patients in the United States. Self-reported demographics and clinical factors are explored to help identify potential factors influencing acceptance. Understanding patient attitudes toward these treatment modalities can help direct development of future devices and allow providers to better individualize treatment regimens for glaucoma.
Subjects and Methods
This is a cross-sectional study of patients from the glaucoma subspecialty clinic at Massachusetts Eye and Ear, Boston, Massachusetts, who participated in an interviewer-administered survey. Ethical approval was obtained from the Institutional Review Board of Massachusetts Eye and Ear. Consecutive patients were recruited from June to August 2016. Patients age 18–85 who were either being monitored or treated for all types of glaucoma were included. All patients provided written informed consent. The questionnaire was developed by discussion among the authors about which new drug delivery devices had recent publications or presentations at national glaucoma meetings and by modifying previously published questionnaires to address their shortcomings, such as lack of inclusion of certain drug delivery devices.11,12 See Appendix.
Study Variables
The survey questionnaire was divided into four sections: acceptance of new treatments, attitudes about glaucoma, self-reported measures of adherence, and demographics. The drug delivery approaches included (1) a triple combination eye drop self-applied daily, (2) microdose eye spray self-applied twice daily, (3) drug-eluting contact lens to be self-applied monthly, (4) drug-eluting periocular ring insert applied by a provider every 6 months, (5) injectable subconjunctival drug insert placed in the office every 3 months, and (6) an injectable anterior chamber drug implant injected in the office every 6 months.
For all approaches other than triple combination drops, if respondents were willing to accept the device, they were further asked if they would be willing to use the device if it cost more than what they currently pay for topical medications. We presented theoretical costs for each drug delivery system at multiples of current medication costs based on duration of action for each treatment.
Demographics were self-reported, and basic clinical data were extracted from the patients’ electronic medical records. Clinical factors recorded included severity of glaucoma, number of medications being used, prior medication intolerances, prior laser procedures, and prior ocular surgery.
Statistical Analysis
Categorical variables were analyzed using chi-square test. Parametric continuous variables were analyzed using independent samples t test. A P value of <0.05 was considered statistically significant.
Results
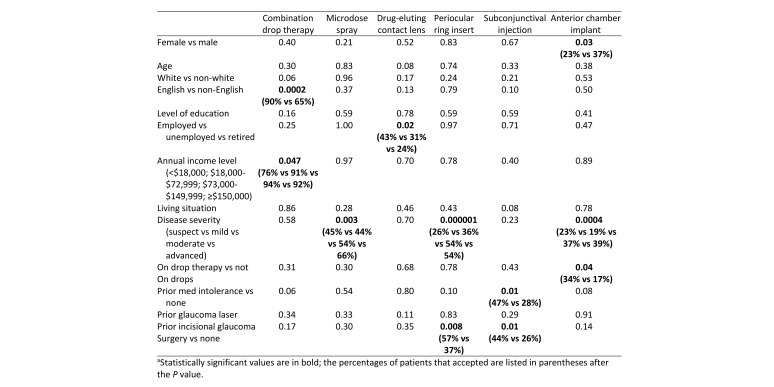
Participant characteristics are displayed in Table 1. Of 199 respondents who completed the survey, 48% were female, and average respondent age was 63.2 ± 15.1 years. Glaucoma severity ranged widely, and 78% of patients were currently taking eye drops. Overall patient acceptance rates were 85% for triple combination eye drop, 54% for microdose eye spray, 31% for drug-eluting contact lens, 43% for drug-eluting periocular ring insert, 32% for injectable subconjunctival drug insert, and 30% for injectable anterior chamber implant. Table 2 shows P values from analyses of whether specific factors were associated with increased willingness to try new drug delivery approaches.
Table 1.
Study participant demographics and characteristics
Table 2.
P values for differences in rate of acceptance of novel glaucoma treatments according to demographic and clinical factorsa
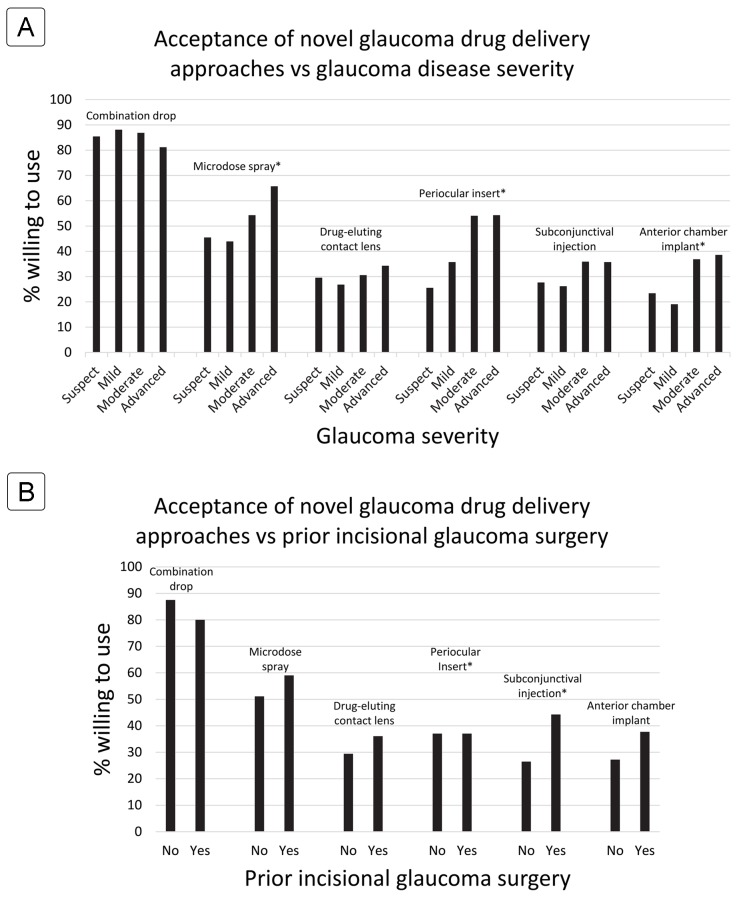
Figure 1 illustrates that more advanced glaucoma severity and prior incisional glaucoma surgery were consistently associated with greater likelihood of acceptance of novel drug devices. These differences in acceptance were statistically significant for 3 of 6 treatments in the disease severity analysis, and 2 of 6 treatments in the incisional glaucoma surgery analysis.
Figure 1.
Acceptance rates of novel glaucoma drug delivery approaches at varying glaucoma disease severity levels (A) and in relation to prior incisional glaucoma surgery (B). The asterisk accompanying drug delivery approach indicates the difference among groups was statistically significant at P < 0.05 level.
For triple combination eye drop therapy, being an English-speaker and having greater income increased the likelihood of acceptance (90% vs 65% [P = 0.0002]; 76% for annual income <$18,000 vs 91%, 94%, and 92% for $18,000–$72,999, $73,000–$149,999, and ≥$150,000, resp. [P = 0.047]).
For microdose spray medication, patients with more severe cases of glaucoma were more likely to accept this treatment modality (45% for glaucoma suspect, 44% for mild glaucoma, 54% for moderate, 66% for advanced [P = 0.003]).
For drug-eluting contact lenses, employed patients had greater acceptance than those who were unemployed or retired (43%, 31%, and 24%, resp. [P = 0.02]).
For drug-eluting periocular ring inserts, patients with a history of prior incisional glaucoma surgery tended to have a higher acceptance (57% vs 37% [P = 0.008]). In addition, patients with more severe glaucoma had greater acceptance than those with milder severity of disease (26% for glaucoma suspect, 36% for mild glaucoma, 54% for moderate, 54% for advanced [P = 0.000001]).
For injectable subconjunctival implants, patients who had a prior incisional glaucoma surgery and prior medication intolerance were more likely to accept this device (44% vs 26% [P = 0.01] and 47% vs 28% [P = 0.01], resp.).
For injectable drug implants, males (37% vs 23% [P = 0.03]), patients with more severe disease (23% for glaucoma suspect, 19% for mild glaucoma, 37% for moderate, 39% for advanced P = 0.0004), and those already on drops (34% vs 17% [P = 0.04]) were significantly more likely to accept the injectable drug implant.
When asked if they were still willing to use microdose spray and drug-eluting contact lens if they had to pay twice the cost of traditional drops, 59% and 66%, respectively, of patients who accepted the novel treatments were willing. For both periocular ring inserts and injectable subconjunctival implants, 68% were willing to pay three times the cost of traditional drops. Lastly, for injectable drug implants, 60% were willing to pay four times the cost of traditional drops.
Patient self-reported adherence was also surveyed, with 95.0% of respondents saying they always take their medications as prescribed. Additionally, 95.8% of respondents reported they missed eye drops 0–5 times in the past 30 days, and 98.8% said they regularly use their drops continually for at least 2 weeks without missing a dose.
Discussion
In our cohort, we found acceptance rates of 30%–54% for drug delivery approaches other than traditional eye drops, which is lower than the 57%–63% acceptance rates reported by Chan et al12 from Singapore. SooHoo et al11 reported 45% overall acceptance in another US cohort, but interventions surveyed included laser and invasive surgery. Consistent with prior studies, our cohort tended to prefer less invasive routes such as combination eye drop therapy, microdose spray, and drug-eluting periocular ring insert. It has been previously noted that a significant barrier to accepting newer drug delivery methods was patient perception of discomfort with more invasive procedures.11 Ocular surface disease and discomfort have also been associated with traditional eye drops and are recognized as another reason for poor adherence with traditional glaucoma eye drops.13,14 Patient discomfort continues to be a significant barrier that could be addressed in the development of novel medication interventions and in patient education about newer drug delivery devices. Overall, more than half of patients willing to try new drug delivery approaches were willing to do so at a higher cost, suggesting that convenience and ease of use are valued by patients.
Patient-reported acceptance may differ from actual utilization once a treatment becomes more mainstream in clinical practice. Intravitreal injection for macular degeneration and diabetic macular edema is an example of a commonly performed ophthalmic office procedure that is significantly more invasive than eye drops. Many patients tolerate the intervention every month for up to years at a time. With antivascular endothelial growth factor injections, some patients experience symptomatic improvement in their vision after treatment. Thus from the patient’s perspective, this perceived benefit may outweigh the risks or discomfort associated with the procedure. This may not be the case with glaucoma medications, where lowering of IOP likely will not result in any symptomatic improvement in vision. Additionally, there are more medical and laser options to treat glaucoma, and the threshold to accept invasive glaucoma drug delivery approaches may be higher.
Patients that accepted more invasive interventions in our study tended to have greater glaucoma severity and prior incisional glaucoma surgery. This may be because they feel their situation is dire enough to accept a greater level of risk in order to prevent permanent blindness. Another possible explanation is that patients may perceive their disease stage or need for surgery as a result of failure of current treatments, making them more willing to try an alternative intervention that may prove more efficacious. Drug delivery platforms that can deliver more than one medication, and therapeutic approaches that would be compatible with filtering blebs or glaucoma drainage devices would be particularly suited for patients with more advanced disease.
A limitation of this study is the abridged description given to patients regarding the efficacy and side effects of the various drug delivery approaches. With only preliminary data available on most of these drug delivery systems, patient education regarding their application was kept brief and theoretical. A more detailed conversation with their primary eye care provider about risks and benefits may result in different acceptance rates. The actual length of time between treatments for the extended release drug delivery systems might also alter patients’ decisions to accept the treatments. Furthermore, the study questionnaire, while modeled after those administered in previous publications on similar topics, was not formally validated.
Though drug-eluting punctal plugs are in development for glaucoma treatment, this treatment method was not assessed. Non-drug-eluting punctal plugs are already in relatively wide use in ophthalmology and optometry clinics, and including them would have added additional time and complexity to the survey. However, we do acknowledge that this modality has potential to affect glaucoma treatment in the near future.
Our findings may also not be generalizable to other populations. This cohort had a very high self-reported adherence rate (98%), which we believe may be over-inflated based on other studies on self-reported adherence rates.2 This may have been due to reluctance to admit directly to the survey administrator that they were not adherent to their medication regimens. Additionally, the patients we surveyed were relatively well-educated (with only 8% who had not completed high school) and had good social support (74% live with family). This may contribute to better adherence to medications and hence lower acceptance of alternative methods to deliver glaucoma medications.
There appears to be a discrepancy between physician acceptance and patient acceptance of these approaches. A survey of ophthalmologists showed greater than 80% acceptance among providers in trying novel drug delivery approaches for glaucoma.15 It is unclear if the promise of these novel interventions is overestimated by providers or if patients are underestimating the benefits of these nontraditional approaches to drug delivery for glaucoma management. Ultimately, careful study of the risks and benefits of these approaches, and taking account the patient’s perspective, will help shape the role of these therapeutic options in future practice.
Despite the added convenience that novel drug delivery approaches may offer, only 30%–54% of patients reported that they would opt for them. We found that greater glaucoma severity and prior incisional glaucoma surgery were associated with greater willingness to try new approaches. Physicians should take these findings into account when counseling patients regarding these treatments.
Acknowledgments
The authors acknowledge the Harvard Catalyst Biostatistical Consulting group for their assistance with the statistical analysis, and Lisa A. Cowan, MD, PhD, for providing a template to help us design our Excel analysis datasheet.
Appendix: Patient Questionnaire
References
- 1.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887. [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995;26:233–6. [PubMed] [Google Scholar]
- 3.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48:5052–7. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Patil B, Shah BM, Bali SJ, Mishra SK, Dada T. Evaluating eye drop instillation technique in glaucoma patients. J Glaucoma. 2012;21:189–92. doi: 10.1097/IJG.0b013e31820bd2e1. [DOI] [PubMed] [Google Scholar]
- 5. ClinicalTrials.gov Identifier NCT01216943. https://clinicaltrials.gov/ct2/show/results/NCT01216943?sect=X4370156#othr.
- 6.Ianchulev T, Chayet A, Kahook M, Packer M, Pasquale L, Weinreb RN. Pharmacodynamic profile of mydriatic agents delivered by ocular piezo-ejection microdosing compared with conventional eyedropper. Ther Deliv. 2016;7:751–60. doi: 10.4155/tde-2016-0061. [DOI] [PubMed] [Google Scholar]
- 7.Ciolino JB, Ross AE, Tulsan R, et al. Latanoprost-eluting contact lenses in glaucomatous monkeys. Ophthalmology. 2016;123:2085–92. doi: 10.1016/j.ophtha.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt JD, Sall K, DuBiner H, et al. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase ii randomized controlled study. Ophthalmology. 2016;123:1685–94. doi: 10.1016/j.ophtha.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Wong TT, Novack GD, Natarajan JV, Ho CL, Htoon HM, Venkatraman SS. Nanomedicine for glaucoma: sustained release latanoprost offers a new therapeutic option with substantial benefits over eyedrops. Drug Deliv Transl Res. 2014;4:303–9. doi: 10.1007/s13346-014-0196-9. [DOI] [PubMed] [Google Scholar]
- 10.Lewis RA, Christie WC, Day DG, et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am J Ophthalmol. 2017;175:137–47. doi: 10.1016/j.ajo.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 11.SooHoo JR, Golas L, Marando CM, et al. Glaucoma patient treatment preferences. Ophthalmology. 2016;123:1621–2. doi: 10.1016/j.ophtha.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Chan HH, Wong TT, Lamoureux E, Perera S. A survey on the preference of sustained glaucoma drug delivery systems by Singaporean Chinese Patients: a comparison between subconjunctival, intracameral, and punctal plug routes. J Glaucoma. 2015;24:485–92. doi: 10.1097/IJG.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 13.Janz NK, Wren PA, Lichter PR, Musch DC, Gillespie BW, Guire KE. Quality of life in newly diagnosed glaucoma patients: the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2001;108:887–97. doi: 10.1016/s0161-6420(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 14.Ghate D, Edelhauser HF. Barriers to glaucoma drug delivery. J Glaucoma. 2008;17:147–56. doi: 10.1097/IJG.0b013e31814b990d. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi EV, Kalout P, Pasquale LR, Kohane DS, Ciolino JB. Clinicians’ perspectives on the use of drug-eluting contact lenses for the treatment of glaucoma. Ther Deliv. 2014;5:1077–83. doi: 10.4155/tde.14.76. [DOI] [PMC free article] [PubMed] [Google Scholar]