Abstract
Canine food allergies are the result of an immune-mediated hypersensitivity reaction to dietary proteins and can manifest as a variety of dermatologic and/or gastrointestinal clinical signs. Food elimination trials followed by provocation tests are used to diagnose food allergies; however, no research has been conducted to determine whether elimination trials and provocation tests are being properly implemented by pet owners. The objectives of this study were to determine the level of knowledge of dog owners regarding food allergies, and to investigate how dog owners approach diagnosis and treatment with their veterinarians. This information will provide veterinary teams with insight on how to work with dog owners to obtain successful diagnosis and treatment. The results indicate that appropriate diet selection for the food elimination trial, owner education on compliance during the trial, and re-challenging with the previous diet should be the focal points for veterinarians suspecting food allergies in a canine patient.
Résumé
Évaluation des connaissances des propriétaires de chiens portant sur le diagnostic et le traitement des allergies alimentaires canines. Les allergies alimentaires canines sont le résultat d’une réaction d’hypersensibilité à médiation immunitaire face aux protéines alimentaires et elles peuvent se manifester par divers signes cliniques dermatologiques et/ou gastro-intestinaux. Les essais d’élimination d’aliments suivis de tests de provocation sont utilisés pour diagnostiquer les allergies alimentaires. Cependant, aucune recherche n’a été réalisée pour déterminer si les essais d’élimination et les tests de provocation sont mis en place de façon adéquate par les propriétaires. Les objectifs de cette étude étaient de déterminer le niveau de connaissances des propriétaires de chiens concernant les allergies alimentaires et d’étudier la façon dont les propriétaires de chiens envisagent le diagnostic et le traitement avec leur médecin vétérinaire. Ces renseignements permettront aux équipes vétérinaires de constater comment travailler avec les propriétaires de chiens afin d’obtenir un diagnostic et un traitement réussi. Les résultats indiquent que le bon choix d’alimentation pour les essais d’élimination des aliments, l’éducation des propriétaires pour la conformité durant les essais et de nouveaux tests avec l’alimentation antérieure devraient être les principaux sujets pour les médecins vétérinaires soupçonnant des allergies alimentaires chez un patient canin.
(Traduit par Isabelle Vallières)
Introduction
Food allergies in dogs result from an immune-mediated hypersensitivity reaction to dietary proteins and manifest as various dermatologic and/or gastrointestinal clinical signs. Food allergies are one type of adverse food reaction (AFR) that occurs in dogs. Canine AFR can be categorized as either non-immune-mediated food reactions (e.g., lactose intolerance) or immune-mediated food reactions (e.g., food allergies); however, distinction between various types of AFR is difficult (1–5). Typical acute dermatological clinical signs may include erythema and pruritus, which may be intense. Lesions are typically localized to the paws, face, ears, abdomen, and/or perianal area (5,6). Chronic dermatological clinical signs may include recurrent ear infections, self-induced alopecia, excoriations, and lichenification (5,6). A median prevalence of 6% of all skin diseases in dogs and 20% of all allergic dermatological signs in dogs, have been attributed to AFR, although the exact pathogenesis has not been determined (7). Of the commonly reported gastrointestinal clinical signs in dogs, 10% to 15% are attributed to AFRs, including irregular bowel movements, halitosis, excessive gas, borborygymus, and nausea (5,6).
Food allergies are predominantly mediated through acute, intermediate, or late-phase immunoglobulin E (IgE) mechanisms, and as a result, a wide range of serum IgE levels are reported during serum allergy testing in dogs (1,2,4,8,9). Food allergies have also been found to be mediated through delayed immune mechanisms, known as a type III or IV hypersensitivity, although the exact pathogenesis is unclear (2,4,8). Olivry and Mueller (7) reviewed serum testing as a method of diagnosing food allergies in dogs and reported that the accuracy of serum IgE testing ranges from 58% to 87%, with positive predictable values ranging from 15% to 100%, which means serum IgE testing cannot accurately predict food allergies. Also, other laboratory assays, skin biopsies, saliva testing, as well as intradermal testing, and endoscopic provocation tests are considered unreliable and are not suitable as screening tests for AFR in dogs (2,10). The gold standard to accurately diagnose food allergies is a food elimination trial (3,8,9), although this gives no information about the underlying immunologic mechanism and cannot discriminate between non-immune-mediated food reactions and food allergy. A food elimination trial consists of 3 steps: i) elimination of the offending food allergen (i.e., dietary protein); ii) a trial period with a strict elimination diet containing either a novel or hydrolyzed protein source; and iii) a re-challenge period (i.e., provocation testing) with previous food to observe for recurrence of clinical signs. Selecting a novel protein diet relies on the ability of the veterinary team to obtain a lifetime nutritional history from the dog owner to ensure the protein is in fact novel. When considering novel ingredient selection, the most common allergenic animal-based protein sources identified for dogs include beef, dairy products, chicken, and lamb, with other proteins being reported less often (e.g., eggs, fish, and pork) (2,5). While less common than animal-based proteins, the most common allergenic plant-based protein for dogs is wheat, with other plant proteins being reported less often (e.g., soy, rice, and corn) (2,5). Commercially available diets with hydrolyzed proteins are typically soy- or chicken-based and vary in how extensively the proteins have been hydrolyzed.
The objectives of this survey were to i) determine the level of knowledge of dog owners regarding canine food allergies, and to ii) investigate how dog owners approach diagnosis and treatment with their veterinarians. The aim was to use this information to provide veterinary teams with insights on how to work with dog owners to obtain successful diagnosis and treatment of food allergies. To our knowledge, a survey of this scope has not been previously done, and may highlight areas for improvement of veterinarian-owner communication on diagnosis and treatment of food allergies in dogs.
Materials and methods
Study population
Dog owners were recruited throughout Ontario, Canada. Participation was voluntary, and participants were able to withdraw from the study at any point. Inclusion criteria for participation included: owners 18 y of age or older, with a dog 1 y of age or older that had suspected or confirmed food allergies. The dogs were divided by size into small (0.0 to 10.0 kg), medium (10.1 to 35.0 kg), and large (> 35 kg) breeds. The survey focused on an adult population as only few elimination diets are commercially available for growing dogs. Owners were instructed that the survey must be filled out before their first visit with a veterinary dermatologist. Owners provided their dog’s previous nutritional history, current nutrition plan (including how the current food was selected), and the method of diagnosing their dog’s food allergies.
Survey design and distribution
The Canine Food Allergies Survey (available upon request from the corresponding author), created and distributed online using LimeSurvey (LimeSurvey, Hamburg, Germany), consisted of 57 questions in multiple choice, multiple response, Likert, close-ended, and open-ended format and was divided into 9 sections: i) general information about your dog; ii) medical information for your dog; iii) current commercial dog food(s); iv) previous commercial dog food(s); v) current homemade diet recipe(s); vi) treats and human foods; vii) medications/supplements; viii) personal beliefs/knowledge; and ix) owner demographics. The study was approved by the University of Guelph Research Ethics Board (REB Approval #14AP035, June 3, 2014).
The survey was open from June 2014 to December 2015 and was distributed by e-mail to veterinary practices in Ontario, social media, and postcard distribution at 3 local veterinary hospitals and at local veterinary conferences.
Statistical analysis
All categorical data were analyzed using SAS software (version 9.2; SAS institute, Cary, North Carolina, USA). The parametric survey data were analyzed using a Chi-squared test to compare the predicted values to the actual values for each survey question. Significance was declared at P < 0.05.
Results
When the online survey was closed, 166 online responses were recorded. Due to a lack of responses, sections vii, viii, and ix were removed from analysis. The results presented are based upon the data collected from 93 respondents completing sections I to VI. Variation in the total number of responses per question is due to questions that may not have been applicable to all respondents.
Canine demographics
Owner-reported demographic characteristics of the dog population are available upon request. Clients reported a wide variation in the means by which they obtained their pets [breeder — 42%; shelter/rescue — 30.1%; other (e.g., family friend or previous owners), 25.8%]. Of all dogs included in the study, 17% were small breed, 28% medium breed, and 55% large breed. Owners reported the dogs as 48% male and 52% female, with ages ranging from 1 to 13 y, and a median age of 5 y. Most dogs were owner-reported as spayed/neutered (85%) as opposed to intact (15%).
Suspicion and diagnosis of food allergies
When dog owners were asked who suspected a potential food allergy first, most owners reported themselves (60%), followed by the family veterinarian (35%) (Table 1; P > 0.05). The most common age range reported for the onset of clinical signs that led to a suspicion of food allergy was 1 to 6 y (56%), followed by 6 mo to 1 y (25%) (Table 1; P = 0.0467).
Table 1.
Suspicion and diagnosis of canine food allergies.
| Question | Frequency | Chi-squared | P-value |
|---|---|---|---|
| Who first suspected your dog’s food allergy? (n = 93) | |||
| A. Veterinarian | 35.48 | ||
| B. Myself (dog owner) | 60.22 | 5.11 | 0.7458 |
| C. Family/Friend | 2.15 | NS | |
| D. Nutritionist | 1.08 | ||
| E. Groomer | 1.08 | ||
| How old was your dog when the concern of food allergies was FIRST suspected? (n = 93) | |||
| A. Less than 6 months of age | 11.83 | 12.78 | 0.0467 |
| B. 6 months to 1 year of age | 24.73 | ||
| C. 1 year to 6 years of age | 55.91 | ||
| D. Older than 6 years of age | 7.53 | ||
| Were any of the following tests done to diagnose your dog with food allergies? (n = 93) | |||
| A. Blood testing for food allergies | 37.93 | 6.78 | 0.5606 |
| B. Muscle strength testing for food allergies (i.e., kinesiology) | 0.00 | NS | |
| C. Saliva testing for food allergies | 3.45 | ||
| D. Other: | 58.62 | ||
| Including: Food elimination trial | 32.97 |
Significance declared at P < 0.05. NS — not significant at P > 0.05.
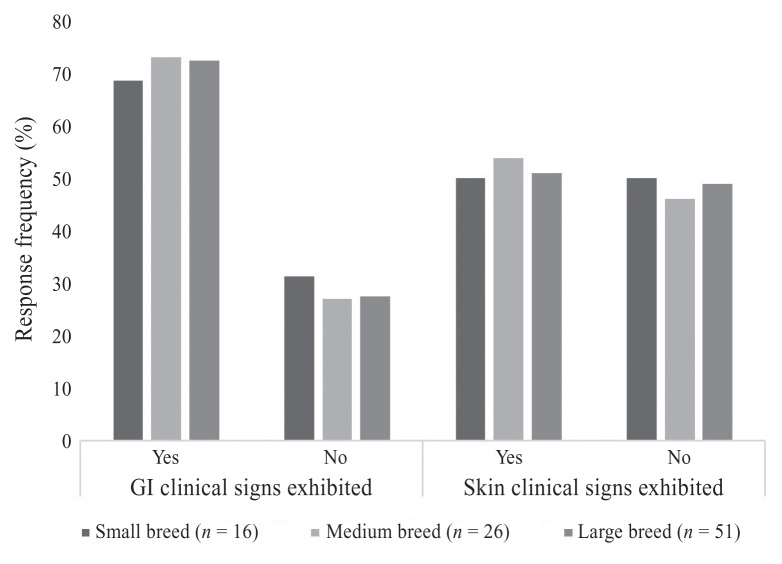
The frequency of dermatological signs of food allergy reported by the owners, is presented in Figure 1. The 3 most frequent dermatological variables found were: licking and chewing their paws (72%), bilateral ear infections (48%), and diagnosed skin infections (40%). Paw licking/chewing was the most common dermatological sign for all breed sizes; however, there seemed to be variation in breed size in regard to the second and third most common dermatological signs.
Figure 1.
Owner-reported gastrointestinal (GI) and dermatological (skin) clinical signs in their dogs with suspected or confirmed food allergies, reported by breed size.
The frequency of gastrointestinal signs of food allergies reported by the owners, is presented in Figure 1. The most common gastrointestinal signs were: excessive gas (46%), soft stools (44%), and vomiting (38%).
Most owners reported that they opted for an alternative testing type (other, 59%) compared with using blood serum testing (38%) or saliva testing (3%) for the diagnosis of food allergies (Table 1; P > 0.05). The most common response for “other” was food elimination trial or single ingredient elimination trial, with additional responses being skin biopsies and intradermal testing.
Treatment of food allergies
When suspecting food allergies, dog owners, family veterinarians, or veterinary specialists (other than dermatologists) responded by changing the dog’s food. The type of food selected depended on who changed the food (Table 2; P < 0.0001). If the dog owner changed the food on their own, 47% chose to switch to an over-the-counter (OTC) brand (e.g., pet store brand or wholesale brand), 33% switched to a raw or homemade diet, and 20% changed to a veterinary diet (Table 2). When the veterinarian was responsible for changing the diet, 85% switched the dog to a veterinary diet, 7% chose to switch to an OTC brand, and 7% chose to switch to a raw or homemade diet (Table 2). Finally, when a veterinary specialist switched the dogs’ food, 57% chose to change to a veterinary diet, 29% chose to switch to a raw or homemade diet, with the remaining 14% switching to an OTC diet (Table 2).
Table 2.
Dietary selection, duration for amelioration of clinical signs, and re-challenging with the previous diet to confirm food allergies.
| Question | Frequency | Chi-squared | P-value |
|---|---|---|---|
| Which statement below BEST reflects how your dog was treated/is being treated for food allergies? (n = 93) | |||
| I changed my dog’s food on my own: | 34.75 | < 0.0001 | |
| A. To a grocery store brand of food | 0.00 | ||
| B. To a homemade diet recipe | 2.22 | ||
| C. To a pet store brand of food | 42.22 | ||
| D. To a veterinary brand of food | 20.00 | ||
| E. To a wholesale brand of food | 4.44 | ||
| F. Other | 31.12 | ||
| My family veterinarian changed my dog’s food: | |||
| A. To a grocery store brand of food | 0.00 | ||
| B. To a homemade diet recipe | 2.43 | ||
| C. To a pet store brand of food | 7.32 | ||
| D. To a veterinary brand of food | 85.37 | ||
| E. To a wholesale brand of food | 0.00 | ||
| F. Other | 4.88 | ||
| A veterinary specialist changed my dog’s food: | |||
| A. To a grocery store brand of food | 0.00 | ||
| B. To a homemade diet recipe | 28.57 | ||
| C. To a pet store brand of food | 14.29 | ||
| D. To a veterinary brand of food | 57.14 | ||
| E. To a wholesale brand of food | 0.00 | ||
| F. Other | 0.00 | ||
| How long after the last dietary change did it take for your dog’s skin signs to improve? (n = 77) | |||
| A. Less than 2 weeks | 16.88 | 13.2522 | 0.1035 |
| B. From 2 weeks to 1 month | 33.77 | NS | |
| C. From 1 month to 3 months | 31.17 | ||
| D. Greater than 3 months | 9.09 | ||
| E. My dog did not have skin signs | 9.09 | ||
| How long after the last dietary change did it take for your dog’s gastrointestinal signs to improve? (n = 77) | |||
| A. Less than 2 weeks | 42.86 | 11.0217 | 0.2005 |
| B. From 2 weeks to 1 month | 18.18 | NS | |
| C. From 1 month to 3 months | 12.99 | ||
| D. Greater than 3 months | 5.19 | ||
| E. My dog did not have gastrointestinal signs | 20.78 | ||
| AFTER your dog’s skin and/or gastrointestinal signs improved did you feed your dog the diet he/she originally had skin and/or gastrointestinal signs on? (n = 77) | |||
| A. Yes | 10.39 | 1.9939 | 0.3690 |
| B. No | 89.61 | NS | |
| If yes, how quickly did the skin/gastrointestinal signs reoccur after feeding the original diet? (n = 8) | |||
| A. Less than 24 hours after | 12.50 | 3.7838 | 0.1508 |
| B. 1 to 3 days after | 37.50 | NS | |
| C. 4 to 7 days after | 25.00 | ||
| D. 8 to 14 days after | 25.00 | ||
| E. Do not recall time frame | 0.00 | ||
| F. Skin/gastrointestinal signs did not reoccur | 0.00 |
Significance declared at P < 0.05. NS — not significant at P > 0.05.
Participants reported that 82% of dogs suffering with dermatologic signs improved within 3 mo of changing the diet, while dogs with gastrointestinal clinical signs had a shorter recovery time, with 61% improving within 1 mo (Table 2; P > 0.05). Discussion with a veterinarian on importance of re-challenging with the previous diet to confirm a diagnosis of food allergies (i.e., provocation testing) occurred for only 33% of dog owners (Figure 2); however, 17% of dog owners were unable to recall this information. Only 10% of dog owners reported re-challenging their dog with the previous diet to confirm the diagnosis of a food allergy (Table 2; P > 0.05). Of the 8 pet owners who re-challenged, both dermatological and gastrointestinal clinical signs returned at variable times after re-challenging (< 24 h, 12.5%; 1 to 3 d, 37.5%; 4 to 7 d, 25%; 8 to 14 d, 25%; Table 2; P > 0.05). Half of the participants revealed that their veterinarian did not inquire about diet at every visit, and only 1/3 of participants stated that their veterinarian recommended their current diet (Figure 2).
Figure 2.
Owner-reported responses to inquiries from their veterinarian.
Dietary indiscretions during the food elimination trial was reported by 75% of dog owners to include human foods such as fruits and vegetables, meats, dairy products, egg products, breads and cereals, and other (Table 3; P > 0.05). Provision of additional food sources during the trial was also reported by 51% of dog owners to include: dental chews, rawhides, flavored bones, jerky treats, meaty bones, animal-based treats (e.g., pigs’ ears, pizzle sticks), and toys infused with flavors (Table 3; P > 0.05). Participants reported that 25% of dogs had access to unmonitored food sources including cat litter boxes, prey that was hunted/killed, and garbage (Table 3; P > 0.05).
Table 3.
Owner-reported exposure of their dogs to additional foods and unmonitored food sources during food elimination trial.
| Question | Frequency | Chi-squared | P-value |
|---|---|---|---|
| Are you giving any of the following to your dog? (check all that apply) (n = 62) | |||
| A. Dental chews | 22.58 | 5.1162 | 0.2756 |
| B. Rawhides | 7.53 | NS | |
| C. Flavored bones | 9.68 | ||
| D. Jerky treats | 9.68 | ||
| E. Meaty bones | 17.20 | ||
| F. Pigs ears/pizzle sticks/other animal-based treats | 11.83 | ||
| G. Toys infused with flavors | 2.15 | ||
| Are you feeding any human foods or table scraps to your dog as treats? (check all that apply) (n = 62) | |||
| A. Vegetables | 52.69 | 5.5556 | 0.4748 |
| B. Fruits | 33.33 | NS | |
| C. Breads/cereals | 9.68 | ||
| D. Meats | 29.03 | ||
| E. Eggs | 8.60 | ||
| F. Dairy products | 7.53 | ||
| G. Other | 7.53 | ||
| Does your pet have access to unmonitored food sources? (n = 93) | |||
| A. Yes | 24.73 | 0.4127 | 0.8135 |
| B. No | 75.27 | NS |
Significance declared at P < 0.05. NS — not significant at P > 0.05.
Discussion
This survey revealed a lack of routine nutritional assessment during veterinary consultations and a lack of owner compliance with proper food elimination trial protocols (e.g., provision of additional foods and access to unmonitored food sources). In addition, it is clear from the survey data that re-challenging with the previous diet was a limiting step to confirm the diagnosis of food allergy as half of the study participants did not discuss re-challenging with their veterinarian. The survey, however, did not clarify whether owners would have been willing to do a re-challenge using the previous diet.
As per recommendation of the World Small Animal Veterinary Association (WSAVA) and the American Animal Hospital Association (AAHA), an extended nutritional assessment should be performed for any animal suspected/at risk of a nutrition-related problem (11,12). However, many owners reported that their veterinarian was not aware and/or did not recommend their dog’s current diet. This is an area that deserves more focus in veterinary practice in order to communicate the importance of an appropriate elimination diet (e.g., novel protein or hydrolyzed protein) in diagnosing canine food allergies. Providing this information to the dog owner when food allergies are first suspected ensures a diagnosis is made using the gold standard, rather than subjecting the dog to reported ineffective testing methods for food allergies such as serum, saliva, hair, or intradermal testing (3,10,13–15). Not only are these tests ineffective for diagnosing food allergies, they are costly and in the authors’ experience they may actually impede diagnosis of the true food allergies once the dog owner has these test results. Although allergy testing performed by intradermal testing or allergen-specific IgE serology testing is not recommended as a screening test, it is an effective tool for confirming environmental allergies (16). Also, flea combing, skin scraping, hair plucking, cytological examination of skin, ear smears, and skin biopsies are useful to rule out etiologies of skin disease other than food allergies (16).
Food elimination trials begin with elimination of the offending food and selection of a new diet containing novel or hydrolyzed protein. When reviewing who was responsible for changing the diet after suspicion of a food allergy, most owners switched to an OTC diet on their own, while veterinarians and veterinary specialists were more likely to change the diet to a veterinary diet formulated for diagnosing and treating food allergies. Several recent studies have found when comparing the ingredient list to the actual ingredients within an OTC diet, there have been many incidences of ingredient cross-contamination. Raditic et al (17) analyzed 4 OTC diets, all of which contained venison as a protein source. Enzyme-linked immunosorbent assay (ELISA) testing revealed that 3 of the diets tested positive for soy proteins, which were not part of the ingredient lists. Willis-Mahn et al (18) used ELISA testing to analyze 4 OTC diets that had “no soy” claims and found that 3 of these diets tested positive for soy proteins. Two of the diets tested had > 25 ppm for soy, which was above the upper limit of detection (18). These studies reflect the variability and higher potential for cross-contamination with protein sources in OTC diets. It is important to point out that OTC diets are not intended to diagnose or treat diseases, such as food allergies, and cross-contamination is not a concern for healthy animals. Therefore, the selection of an OTC diet for a food elimination trial is not recommended and may actually preclude the diagnosis of food allergies. Veterinary diets have been found to have minimal incidence of cross-contamination, but still should be carefully selected based on the previous diet history of the dog for a food elimination trial diet (17). A veterinary diet formulated to treat or diagnose food allergies, or a complete and balanced homemade diet should be used. The veterinary diet can be either novel protein (based on the dog’s lifetime nutritional history) or hydrolyzed protein, whereas a homemade diet will need to be formulated with novel protein and carbohydrate sources by a Board-certified veterinary clinical nutritionist. The homemade diet recipe must contain multivitamin and mineral supplements to avoid nutritional deficiencies. There are pros and cons to both dietary approaches, but those are beyond the scope of this paper. These recommendations are in agreement with those made by WSAVA, stating that diet choices should be restricted to formulations created for disease-associated nutritional disorders of the animal, rather than providing OTC diets (11,12).
For dermatological signs, most owners reported that the time for amelioration of clinical signs was approximately 12 wk from the beginning the food elimination trial. The rate of cell turnover for skin tissue rejuvenation can vary among breeds, due to differences in the dogs, their protein and lipid turnover, and the environment in which they live (7,19). The longest duration of cell turnover of healthy skin in dogs is 20 to 21 d, with damaged skin or skin infections taking a longer time to heal (19,20). This is measured by the duration it takes for 1 cell layer to move from the stratum basale to the stratum corneum (19,20). In comparison to humans with an epithelial layer of 10 to 15 cells, a dog only possesses an epithelial layer 3 to 5 cells thick. With this turnover rate, a food elimination trial duration of a minimum of 8 wk for complete amelioration of dermatological symptoms is consistent with the findings of the current study, though various breeds may need longer for complete amelioration of all clinical signs (3,10,19,20). Olivry et al (21) described complete remission of dermatologic signs of food allergies in 90% of dogs when the elimination trial was a minimum of 8 wk long.
For gastrointestinal signs, the duration of amelioration reported by owners was much more rapid, with recovery times varying from < 2 wk at minimum, and most clinical signs ameliorated by 4 wk. This is most likely due to the high cell turnover rate. Newly generated intestinal epithelial cells migrate from the base of the crypts toward the villus tip region, where loss of senescent epithelial cells occurs. Complete renewal of the villus epithelium takes 2 to 6 d in most mammals (20,22). Due to more rapid amelioration of gastrointestinal signs, compared with dermatological signs, a food elimination trial duration for complete resolution of gastrointestinal signs is recommended to be 6 to 8 wk (10,20).
The final step of the elimination trial is the re-challenge with the previous diet, to observe for the reoccurrence of clinical signs and to confirm the diagnosis of food allergies. Clinical signs typically occur within minutes to hours of re-feeding, with the longest duration up to 14 d (23). Although this is an important step to confirm the diagnosis of food allergies, many owners reported that they did not recall any discussion with their veterinarian regarding re-challenging their dog with the previous diet. It is important to inform owners that the previous diet is not re-fed until the symptoms reoccur with the same severity as at the beginning of the trial, but only until the first appearance of clinical signs (2). At that point, the diagnosis of food allergies is confirmed and the dog owner must be instructed to stop feeding the offending food. Owner compliance with a strict food elimination trial is essential to guarantee a successful diagnosis of food allergies by ensuring diet consistency and no provision of confounding food sources, as well as at the step of re-challenging. Diet consistency during the trials was shown to be poor, as many owners still provided human food or treats, or their dogs had access to unmonitored food sources, such as the litterbox or garbage. Owner compliance was reported by Bethlehem et al (3) as the limiting factor for diagnosis of food allergies.
The authors acknowledge that this survey had several limitations. First, the sample size was small, which did not allow statistical analyses to assess differences between small, medium, and large breeds. Moreover, dog owners were asked to recall information on nutrition history for their own dogs, which could have been from many years ago, and as such, may not have been accurate. Dog owners were also asked to recall conversations that took place with their veterinarians. Their recall may not have been accurate or their interpretation of the conversations may not have been the same as what their veterinarian intended. Additionally, it was not possible to separate suspected food allergic dogs from confirmed food allergic dogs without obtaining medical records for individual dogs; which was not possible with anonymous survey data. Future research should include review of medical records to verify information provided by dog owners. Lastly, not all dog owners completed the survey or responded to all questions. which could have biased the results.
After determining the level of owner knowledge regarding food allergies, and the method by which diagnosis and treatment were carried out, the findings of this survey indicate that a strict food elimination trial often did not occur or was not performed appropriately. During client communication, avoidance of potential confounders such as commercial treats and unmonitored food sources during the trial, and the importance of re-challenging with the previous food at the end of the trial should be highlighted. Full compliance at each step in the trial should result in improved accuracy of diagnosis and treatment of food allergies in dogs. CVJ
Footnotes
Disclosure: Dr. Jacqueline M. Parr is a paid employee of Royal Canin, Canada and Dr. Adronie Verbrugghe is the Royal Canin Veterinary Diets Endowed Chair in Canine and Feline Clinical Nutrition at the Ontario Veterinary College, University of Guelph.
All authors have no conflicts of interest.
This study was funded by a gift with no restrictions by Royal Canin Canada.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Kennis RA. Food allergies: Update of pathogenesis, diagnoses, and management. Vet Clin North Am Small Anim Pract. 2006;36:175–184. doi: 10.1016/j.cvsm.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Verlinden A, Hesta M, Millet S, Janssens GPJ. Food allergy in dogs and cats: A review. Crit Rev Food Sci. 2006;46:259–273. doi: 10.1080/10408390591001117. [DOI] [PubMed] [Google Scholar]
- 3.Bethlehem S, Bexley J, Mueller RS. Patch testing and allergen-specific serum IgE and IgG antibodies in the diagnosis of canine adverse food reactions. Vet Immunol Immunopathol. 2012;145:582–589. doi: 10.1016/j.vetimm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Kawano K, Oumi K, Ashida Y, Horiuchi Y, Mizuno T. The prevalence of dogs with lymphocyte proliferative responses to food allergens in canine allergic dermatitis. J Vet Sci. 2013;16:735–739. doi: 10.2478/pjvs-2013-0104. [DOI] [PubMed] [Google Scholar]
- 5.Mueller RS, Olivry TT, Prélaud P. Critically appraised topic on adverse food reactions of companion animals (2): Common food allergen sources in dogs and cats. BMC Vet Res. 2016;12:1–4. doi: 10.1186/s12917-016-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenox C. Food Trials Clinician’s Brief. 2013:31–32. [Google Scholar]
- 7.Olivry T, Mueller RS. Critically appraised topic on adverse food reactions of companion animals (3): Prevalence of cutaneous adverse food reactions in dogs and cats. BMC Vet Res. 2017;13:51. doi: 10.1186/s12917-017-0973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaschen FP, Merchant SR. Adverse food reactions in dogs and cats. Vet Clin North Am Small Anim Pract. 2011;41:361–379. doi: 10.1016/j.cvsm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Suto A, Suto Y, Onohara N, et al. Food allergens inducing a lymphocyte-mediated immunological reaction in canine atopic-like dermatitis. J Vet Med Sci. 2015;77:251–254. doi: 10.1292/jvms.14-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller RS, Olivry TT. Critically appraised topic on adverse food reactions of companion animals (4): Can we diagnose adverse food reactions in dogs and cats with in vivo or in vitro tests? BMC Vet Res. 2017;13:275. doi: 10.1186/s12917-017-1142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldwin K, Bartges J, Buffington T, et al. AAHA Nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc. 2010;46:285–296. doi: 10.5326/0460285. [DOI] [PubMed] [Google Scholar]
- 12.WSAVA Nutritional Assessment Guidelines Task Force Members. Freeman L, Becvarova I, Cave N, et al. WSAVA Nutritional Assessment Guidelines. J Small Anim Pract. 2011;52:385–396. doi: 10.1111/j.1748-5827.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- 13.Foster AP, Knowles TG, Hotston Moore A, Cousins PDG, Day MJ, Hall EJ. Serum IgE and IgG responses to food antigens in normal and atopic dogs, and dogs with gastrointestinal disease. Vet Immunol Immunopathol. 2003;92:113–124. doi: 10.1016/s0165-2427(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 14.Hagen-Plantinga EA, Leistra MH, Sinke JD, Vroom MW, Savelkoul HF, Hendriks WH. Measurement of allergen-specific IgG in serum is of limited value for the management of dogs diagnosed with cutaneous adverse food reactions. Vet J. 2017;220:111–116. doi: 10.1016/j.tvjl.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Coyner K, Schick A. Inaccuracy of a hair and saliva test for allergies in dogs. [Last accessed December 3, 2018];Vet Dermatol. 2016 27(Suppl 1):68. Available from: https://dermvetolympia.com/wp-content/uploads/2015/08/Allergy-saliva-testposter.pdf. [Google Scholar]
- 16.Hensel P, Santoro D, Favrot C, Hill P, Griffin C. Canine atopic dermatitis: Detailed guidelines for diagnosis and allergen identification. BMC Vet Res. 2015;11:196. doi: 10.1186/s12917-015-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raditic DM, Remillard RL, Tater KC. ELISA testing for common food antigens in four dry dog foods used in dietary elimination trials. J Anim Physiol Anim Nutr. 2010;95:90–97. doi: 10.1111/j.1439-0396.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- 18.Willis-Mahn C, Remillard R, Tater K. ELISA testing for soy antigens in dry dog foods used in dietary elimination trials. J Am Anim Hosp Assoc. 2014;50:383–9. doi: 10.5326/JAAHA-MS-6063. [DOI] [PubMed] [Google Scholar]
- 19.Miller W, Griffin C, Campbell K. Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis, Missouri: Elsevier; 2013. pp. 2–108.pp. 685–689. [Google Scholar]
- 20.Moriello KA, Kahn CM, Line S. The Merck Veterinary Manual. Whitehouse Station, New Jersey: Merck & Co; 2010. pp. 649–852. [Google Scholar]
- 21.Olivry T, Mueller RS, Prélaud P. Critically appraised topic on adverse food reactions of companion animals (1): Duration of elimination diets. BMC Vet Res. 2015;11:225. doi: 10.1186/s12917-015-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JM, Duckworth CA, Burkitt MD, Watson AJM, Campbell BJ, Pritchard DM. Epithelial cell shedding and barrier function. A matter of life and death at the small intestinal villus tip. Vet Pathol. 2015;52:445–455. doi: 10.1177/0300985814559404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffers JG, Meyer EK, Sosis EJ. Responses of dogs with food allergies to single-ingredient dietary provocation. J Am Vet Med Assoc. 1996;209:608–611. [PubMed] [Google Scholar]