Abstract
The objectives of this study were to evaluate the compatibility and the pharmacokinetic properties of combined amikacin and penicillin administration by intravenous regional limb perfusion (IVRLP) in horses. A tourniquet was applied proximal to the carpus of 7 clinically healthy adult horses and 2 g of amikacin and 10 × 106 IU of penicillin (100 mL total volume) were sequentially injected into the cephalic vein just distal to the tourniquet. Synovial samples were collected from the joint at several times after injection. All samples were analyzed for amikacin and penicillin concentration. The mean maximum concentration (Cmax) of both amikacin and penicillin was over 10-fold the relevant minimal inhibitory concentration (MIC) for all horses and remained above those MICs for at least 24 hours. The results of this study indicate that combining amikacin with penicillin during IVRLP in normal horses delivers high therapeutic synovial concentrations of both drugs.
Résumé
Pharmacocinétique de la perfusion régionale des membres en utilisant une combinaison d’amikacine et de pénicilline chez des chevaux debout. Les objectifs de cette étude consistaient à évaluer la compatibilité et les propriétés pharmacocinétiques de l’administration combinée d’amikacine et de pénicilline par perfusion intraveineuse régionale des membres (PIRM) chez les chevaux. Un tourniquet a été appliqué proximalement au carpe de sept chevaux adultes en bonne santé clinique et 2 g d’amikacine et 10 × 106 UI de pénicilline (volume total de 100 ml) ont été injectés en séquence dans la veine céphalique légèrement distale au tourniquet. Des échantillons synoviaux ont été prélevés de l’articulation plusieurs fois après l’injection. Tous les échantillons ont été analysés pour la concentration d’amikacine et de pénicilline. La concentration maximale moyenne (Cmax) de l’amikacine et de la pénicilline était plus de 10 fois supérieure à la concentration inhibitrice minimale (CIM) pertinente pour tous les chevaux et est demeurée au-dessus de ces CIM pendant au moins 24 heures. Les résultats de cette étude indiquent que la combinaison de l’amikacine avec la pénicilline durant la PIRM chez des chevaux en santé offre des concentrations synoviales thérapeutiques élevées des deux médicaments.
(Traduit par Isabelle Vallières)
Introduction
Intravenous regional limb perfusion (IVRLP) is a well-established method for delivering high antimicrobial concentrations to the equine distal limb for preventing and treating equine orthopedic and soft tissue infections (1,2). The IVRLP antimicrobial drug selection should ideally be based on culture and sensitivity results. It is, however, common practice to use an empirical broad-spectrum antibiotic for infection prevention early in the course of therapy, when microbial culture and susceptibility data are not available or when there is no positive bacterial culture (3). Broadening the antimicrobial spectrum by systemic administration of combined antimicrobial drugs was shown to improve treatment efficacy. Antibiotic combinations may be used to broaden the antimicrobial spectrum when mixed bacterial infections are known or suspected to be present, to help prevent or overcome resistance, or to improve efficacy through synergistic effects (4,5). Since similar antimicrobial drugs are used in IVRLP, and similar bacterial populations are targetted, it is reasonable to extrapolate from these data and predict that use of antimicrobial drug combinations in IVRLP will lead to similar advantages.
Penicillin belongs to the beta-lactam class of antimicrobials; it is effective mainly against Gram-positive bacteria and anaerobe bacteria by exerting a bactericidal effect. Penicillin has been the backbone of equine antibacterial drug therapy for many years and is still one of the most commonly used prophylactic antibiotic drugs (6). Although it is used clinically in IVRLP (1), a single IVRLP pharmacokinetic study using penicillin was only recently reported (7).
Amikacin belongs to the aminoglycoside class of antimicrobials; it has a broad spectrum of activity and is the most effective drug against commonly isolated bacteria causing orthopedic infections in horses (8). Moreover, amikacin is the most commonly used and studied antibiotic in IVRLP (1–3,7,9–14).
The beta-lactam-aminoglycoside combination is one of the most frequently used antimicrobial drug combination therapy, commonly used systemically (1,15). Clinically, this combination was used effectively as a local therapy both by IVRLP and by intra-synovial lavage (1,16). The beta-lactam-aminoglycoside combination has a synergistic effect that is exerted via bacterial cell wall destruction by beta-lactam antibiotics, facilitating the aminoglycoside transport into the bacterial cell, exerting its bactericidal effect by interfering with ribosomal protein synthesis (17). Although there is supportive evidence of synergism, the beta-lactam-aminoglycoside combination also proved to have a negative drug interaction in confined compartments in an in vitro study (18). Moreover, a recent equine study showed that the combination of amikacin and ticarcillin/clavulanate, used for IVRLP, resulted in reduced amikacin concentrations and antimicrobial activity in the synovial fluid (11).
Our goal was to evaluate the PK properties of the combination of penicillin G and amikacin sulfate delivered by IVRLP through the cephalic vein to standing horses and sampling the metacarpophalangeal (MCP) joint.
We hypothesized that the amikacin-penicillin combination delivered by IVRLP would yield maximal MCP synovial penicillin and amikacin concentrations (Cmax) well above the relevant minimal inhibitory concentration (MIC90) and that these concentrations would remain high for at least 24 h following the perfusion.
Materials and methods
Animals
Seven adult mixed breed horses participated in the study, including 5 geldings and 2 mares. The average age was 8 y (range: 4 to 15 y) and the average weight was 408 kg (range: 320 to 500 kg). Horses were clinically healthy according to physical evaluation, and all were sound when trotted. Horses were housed in separate stalls, had continuous access to fresh water, and were fed grass hay. The study design was approved by the University Animal Care and Use Committee.
Antimicrobial perfusion
Catheter application, horse sedation, and limb IVRLP treatments followed by tourniquet removal were performed as previously described (19). The antibiotic solutions were prepared just prior to performing the perfusion. Two grams of amikacin sulfate were diluted with isotonic saline in three 20-mL syringes (Amikacin–fresenius; Boden, Port Elizabeth, South Africa) and 10 × 106 IU benzylpenicillin sodium (Penicillin G Sodium; Sandoz GmbH, Kundl, Austria) were diluted with isotonic saline solution in two 20-mL syringes, to a total volume of 100 mL. The prepared solutions were administered sequentially, through the cephalic catheter, in five 20-mL syringes, over approximately 2 min.
Sample collection
Blood samples from the jugular catheter and synovial fluid samples from the metacarpophalangeal (MCP) joint of the treated limb were taken before IVRLP as described (19). Sedation with xylazine 0.5 mg/kg body weight (BW), IV was used as needed to prevent discomfort and movement during the sampling procedure.
Following aseptic preparation of the centesis site, aspiration of synovial fluid from the MCP joint was carried out using an approach described by Bassage et al (20). Blood sampling and treatment, acquisition of synovial fluid followed by phenylbutazone administration, amikacin sulfate instillation into each MCP joint, and bandaging were all as described (19). Horses were confined to a stall for physical examination, with an emphasis on the sites of perfusion and sampling along with a brief daily lameness evaluation during the study and for an additional 7 d after the last sampling.
Antibiotic analysis
Antibiotic analysis was based on established methodology in a previously reported PK study (14). Concentrations of amikacin and penicillin G in serum and in synovial fluid at each time point were determined by mass spectrometry [liquid chromatography/tandem mass spectrometry (LC/MS/MS)] and preparation of serum samples was as described (14). Concentrations above the upper limit of quantification were determined by diluting the samples with extracted drug-free serum/synovial fluid.
Liquid chromatography parameters for analysis of amikacin: A mixed-mode SiELC, Obelisc R column (5 μm, 100 × 2.1 mm, SiELC Technologies, Wheeling, Illinois, USA) was used for separation as previously described (19) except that the mobile phase consisted of 0.5% formic acid and acetonitrile at a flow rate of 0.6 mL/min. A gradient was applied by decreasing the acetonitrile concentration from 65% at 1 min to 5% at 3 min. This ratio was maintained for 0.5 min and then brought back to the initial conditions. The column compartment was kept at 30°C, and the injection volume was 10 μL.
Liquid chromatography parameters for analysis of penicillin-G: A C18 Symmetry column (3.5 μm, 100 × 2.1 mm, Waters, Ireland) was used for separation. The mobile phase consisted of 0.2% formic acid (Sigma-Aldrich, Israel) and acetonitrile at a flow rate of 0.35 mL/min. A gradient was applied by increasing the acetonitrile concentration from 5% at 0 min to 50% at 5 min. This ratio was maintained for 11 min and then brought back to the initial conditions. The column compartment was kept at 25°C, and the injection volume was 20 μL.
Turbo ion spray ESI/MS/MS in positive ion mode was operated at a temperature of 550°C and 450°C for amikacin and penicillin-G, respectively. Multiple reactions monitoring (MRM) was applied. The precursor ion was 586.3 m/z for amikacin and 357 for penicillin G. The product quantifier ions were 163.3 and 197.9 m/z for amikacin and penicillin G, respectively. The qualifier ions were 425.3 and 181.9 m/z for amikacin and penicillin G, respectively. Drug-free serum/synovial fluids were fortified with amikacin and penicillin G to obtain calibration curves in the range of 0.5 to 250 μg/mL in synovial fluid and 0.25 to 25 μg/mL in serum. Three replicates of calibration samples were prepared on 3 different days. The accuracy and intra-day and inter-day precision were calculated for 0.25 μg/mL, 2.5 μg/mL, 25 μg/mL in serum and 0.5 μg/mL, 25 μg/mL, 250 μg/mL in synovial fluid. These concentrations represented the lower level of quantification, the mid-level of quantification, and the high level of quantification.
The calibration curves for each of the antibiotics in both serum and in synovial fluid were linear over the tested range with a correlation coefficient of 0.991 (serum) and 0.963 (synovial) for penicillin-G and 0.981 (serum) and 0.978 (synovial) for amikacin. Accuracy was 95% to 120% (serum) and 90% to 113% (synovial) for penicillin-G and 98% to 112% (serum), 96% to 109% (synovial) for amikacin. Precision was 8% to 27% (serum) and 9% to 14% (synovial) for penicillin-G and 18% to 20% (serum), 14% to 18% (synovial) for amikacin.
Pharmacokinetic analysis
Amikacin and penicillin in the serum and the synovial fluid were assessed using a pharmacokinetic program (PK solutions 2.0; Summit Research Services Montrose, Colorado, USA), which computed (area under the curve) AUC0–36 using the trapezoidal rule. Serum and synovial fluid maximum synovial fluid concentration (Cmax) of both drugs and the time of maximum synovial fluid concentration of both drugs (Tmax) were determined by viewing graphs of the time course of each drug in synovial fluid. To evaluate the predicted efficacy of penicillin, we recorded the duration of time that the drug remained above the MIC90 (T > MIC90) and calculated the AUC0–36/MIC90 ratio. To evaluate the predicted efficacy of amikacin Cmax/MIC90 ratio and AUC0–36/MIC90 ratio were calculated. Amikacin and penicillin MIC90 for common susceptible equine bacterial pathogens were defined as 16 μg /mL and 1 μg /mL respectively, (21,22).
Statistical analysis
Statistical analyses were performed with computerized software (Statistix 8 Student edition, Analytical Software, Tallahassee, Florida, USA). A repeated measures analysis of variance (ANOVA) test followed by Tukey’s HSD all-pairwise comparisons test were used to compare the differences in penicillin or amikacin concentrations in the different time points after regional limb perfusion. These tests were also used to compare concentrations in the plasma and synovial fluid. Comparisons of Cmax, Tmax, and AUC between the plasma and the synovial fluid were made with Wilcoxon signed-rank test. Differences were considered significant at P ≤ 0.05. Unless otherwise noted, results are presented as median (1st quartile, 25%; 3rd quartile, 75%), or as mean ± standard error of the mean (SEM).
Results
Other than 1 horse with local edema at the cephalic catheter site and temporary lameness, none of the horses showed clinically noticeable adverse effects during the study. That 1 horse moved following injection of the entire perfusate, dislodging the cephalic catheter which resulted in immediate swelling at the site of venipuncture. In spite of this incident, the tourniquet remained in place and all synovial and blood samples were taken from this horse according to the protocol. The horse was mildly lame at a walk and had significant edema of the affected leg. After the last sample, the horse was treated with phenylbutazone, 2.2 mg/kg BW, q12h, PO for 5 d and application of 1% diclofenac sodium liposomal cream to the venipuncture site (Voltaren Emulgel 1%; Novartis Consumer Health SA, Nyon, Switzerland), q12h for 5 d and the limb was bandaged. Both lameness and edema resolved after 1 wk. One horse was excluded from the study because of aggressive behavior during the injection, which led to tourniquet release resulting in low synovial antibiotic concentrations.
Synovial fluid was successfully obtained from the MCP joint of all horses at all time points.
Pharmacokinetic analyses
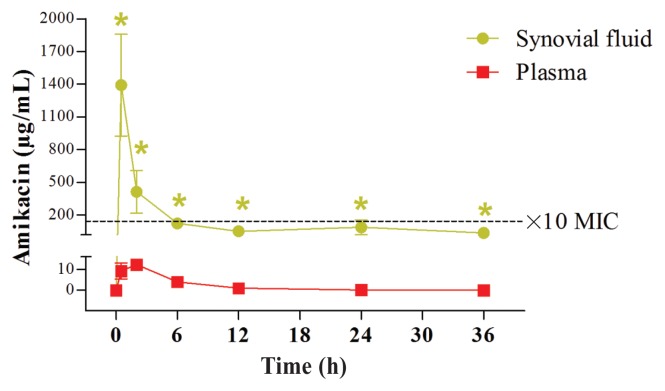
Amikacin and penicillin were not detected in the serum or in the synovial fluid before perfusion. Median and percentiles of AUC and mean, median, and percentiles of Cmax of amikacin and penicillin measured in the serum and synovial fluid of the MCP joint are summarized in Table 1. The concentrations of amikacin and penicillin over time in the synovial fluid and in the serum are presented in Figures 1 and 2, respectively. The amikacin concentration in the synovial fluid of the MCP was well above the MIC of most susceptible pathogens and remained above the MIC in all horses for at least 24 h. The Cmax/MIC90 ratio for amikacin in the synovial fluid was > 10 for all horses.
Table 1.
Pharmacokinetic values, mean, median (50th), 25th and 75th percentiles for amikacin and penicillin in serum and in synovial fluid in the MCP joint after regional limb perfusion. Cmax = maximal concentration (μg/mL), AUC0–36 = area under the concentration time curve from 0 to 36 h (μg·h/mL).
| Antibiotic | Dose | Sample source | Cmax Percentiles | AUC0–36 Percentiles | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mean (95% CI) | 25th | Median 50th | 75th | Mean (95% CI) | 25th | Median 50th | 75th | |||
| Amikacin | 2 g | Plasma | 15 (7 to 23) | 7 | 15 | 20 | 74 (43 to 104) | 49 | 72 | 92 |
| Joint | 1391 (192 to 2590) | 209 | 1306 | 2623 | 2127 (381 to 3874) | 817 | 3350 | 4586 | ||
| Penicillin | 10 MU | Plasma | 8 (2 to 14) | 3 | 7 | 12 | 23 (2 to 44) | 13 | 16 | 30 |
| Joint | 507 (224 to 791) | 252 | 464 | 777 | 1374 (418 to 2330) | 572 | 1155 | 2202 | ||
Figure 1.
Amikacin concentrations over time in the synovial fluid and in the serum after IV-RLP with the combination of penicillin and amikacin. Over time, amikacin concentrations (Mean ± SEM) in the synovial fluid were significantly higher compared with the pre-treatment levels, up to at least 36 h after injection (marked by green star; P < 0.05, Repeated measures ANOVA). Furthermore, Amikacin concentrations after the IV-RLP were significantly higher in the synovial fluid than in the serum, in all time points up to 36 h (P < 0.05). The black dotted line represents the ×10 MIC concentration (160 μg/mL).
Figure 2.
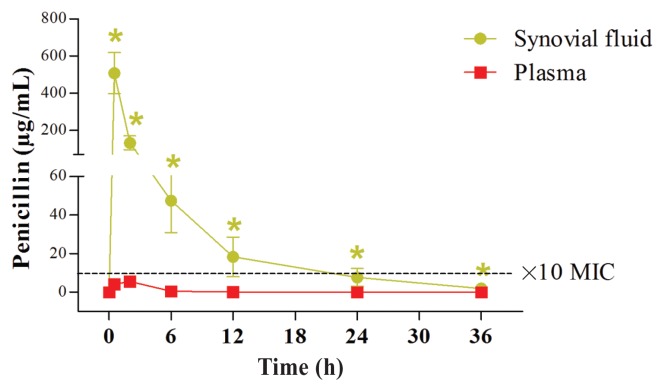
Penicillin concentrations over time in the synovial fluid and in the serum after IV-RLP with the combination of penicillin and amikacin. Over time, penicillin concentrations (Mean ± SEM) in the synovial fluid were significantly higher as compared to the pre-treatment levels, up to at least 36 h post injection (marked by green star; P < 0.05, Repeated measures ANOVA). Furthermore, penicillin concentrations after the IV-RLP were significantly higher in the synovial fluid than in the serum, in all time points up to 36 h (P < 0.05). The black dotted line represents the ×10 MIC concentration (10 μg/mL).
The Tmax for both amikacin and penicillin in the synovial fluid was at the time of the tourniquet release (0.5 h). Tmax for amikacin and penicillin in the serum was 1.92 h and 1.5 h, respectively. The concentration of penicillin in the synovial fluid of the MCP joint was initially greater than the minimal inhibitory concentration (MIC) of most susceptible pathogens and the time greater than the MIC (T > MIC) was at least 24 h. The AUC0–36/MIC90 ratios for penicillin in the synovial fluid were > 125 for all horses.
Discussion
We demonstrated that amikacin and penicillin administered sequentially by IVRLP resulted in a high synovial fluid concentration of both drugs. Although we did not administer amikacin alone in this study, our amikacin Cmax concentrations in the synovial fluid, using a combination of amikacin-penicillin, were higher than in a recent similar study (7). In that study identical doses of the amikacin-penicillin combination were used but the synovial amikacin concentration turned out to be an order of magnitude lower than in the current study. In addition, 12 h after perfusion, penicillin was detectable in only 1 horse, compared to all horses in the current study. These differences may be partially related to the fact that in our study a higher volume of perfusate was used (100 mL compared to 60 mL). Two recent studies, using significantly different experimental setups, have both shown that using a higher volume in IVRLP is associated with significantly higher antibiotic concentrations than with lower volumes (13,14). However, 2 other studies found no significant effect of perfusate volume on synovial antibiotic concentrations and the lower volumes had a trend towards higher concentrations (12,23). Another potential explanation for the discrepancy between the 2 studies, is that in Nieto et al (7) the tourniquet may have failed to completely prevent blood flow and thus some of the perfusate may have leaked into the systemic circulation while the tourniquet was still on. This is consistent with the fact that not only the penicillin but also the amikacin concentration appeared markedly lower in that study compared to the current one. Also, the absence of systemic antibiotic concentration measurements that prevented assessment of tourniquet failure was stated by these authors as a limitation (7). Finally, Nieto at al (7) mixed both antibiotics together in the same syringe while in vitro studies have demonstrated that aminoglycosides and penicillin can have mutual antagonist effects (18,24). All the above may explain our significantly higher antibiotic concentrations.
The amikacin-penicillin combination in the present study also yielded seemingly markedly higher amikacin synovial PK variables compared with previously reported synovial fluid amikacin IVRLP PK studies in which amikacin was used as the sole drug (10,12–14). For example, current results include a mean Cmax of 1391 (95% CI: 192 to 2590) versus 277 and 579 μg/mL and a mean AUC of 2127 (95% CI: 381 to 3874) versus 499 and 1042 μg·h/mL in 2 of these previous PK studies (10,14). Moreover, the synovial amikacin PK/PD most critical parameter, Cmax/MIC90 ratio, was > 10 not just on an average but in each individual horse, and that is associated with an optimal bactericidal effect and with a reduced selection for resistance (25).
A comparison to other independent studies may have limitations. Variables such as the strength of the applied tourniquet by different persons, the different breeds, and thus different volumes of the distal limbs, different temperaments of the horses, and different environmental conditions, could all potentially reduce the reliability of the comparison. Nevertheless, due to the markedly positive PK results demonstrated in this study, it may be concluded that the sequential delivery of the combination therapy: amikacin and penicillin, during IVRLP, has a potential therapeutic advantage. Adding penicillin to the amikacin IVRLP does not seem to have a negative effect on amikacin concentrations in the MCP joint and may even be associated with an increase in synovial fluid amikacin concentrations in the MCP joint after cephalic IVRLP.
One horse in this study developed regional swelling around the perfusion site accompanied by lameness. According to our clinical and research experience, this reaction is very unusual in IVRLP with amikacin alone and is likely associated with the use of penicillin. Thus, when using IVRLP with penicillin, this potential of local adverse reaction should be taken into consideration. In our clinical use, edema continued to be observed when penicillin was diluted with 40 mL; however, after increasing the dilution volume to 60 mL for penicillin in over 20 additional cases, no edema was observed.
Alhough systemic antimicrobial combinations have been used for many years in equine medicine (15), very few studies evaluated antimicrobial drugs combinations in IVRLP (7,11,19). While one IVRLP PK study found reduced antimicrobial concentration of amikacin when combined with ticarcillin/clavulanate (11), another IVRLP PK study reported a higher imipenem concentration when it was combined with marbofloxacin (19). In both of these studies, the two antibiotic drugs were injected sequentially, each antibiotic drug in separate syringes. Thus, each antimicrobial combination should be tested in its relevant setting (e.g., IVRLP) and the PK achieved by systemic combined drug administration cannot be automatically assumed to be similar when IVRLP is used.
Traditionally, concentration-dependent antimicrobials are considered more suitable for use in IVRLP. The reason is that the PK of this group, with the emphasis being on a high peak concentration as opposed to a sustained concentration of drugs, best matches the technique. For the sake of the animal’s welfare the technique involves sedation of the horse to ameliorate the pain elicited from the tourniquet. Thus, when considering the potential risk of frequent sedation (26), IVRLP is typically used once a day in a clinical setting (1,9). However, rather than systemic administration, the results presented here suggest that the use of time-dependent penicillin antimicrobials for IVRLP may be justified because this technique delivers much higher therapeutic concentrations of the antimicrobial in infected ischemic tissues for a longer period (27). In addition, after the tourniquet is released the high antimicrobial concentrations diffuse from the surrounding tissues which serve as a depot (3). The reason is that the pharmacokinetic/pharmacodynamic (PK/PD) relations of this group correlate well with the therapeutic efficacy of the various beta-lactam antibiotics including penicillin, especially the duration of time that the drug concentration is greater than the relevant MIC (T > MIC) (28). Our high synovial penicillin concentrations were greater than the 1 μg/mL MIC break point for its susceptible pathogens (22) and remained greater for 24 h. The other critical PK/PD parameter for beta-lactam antibiotics is AUC0–36/MIC90 and in certain situations, such as osteomyelitis or other isolated infections, the Cmax/MIC90 is also a valuable predictor of efficacy (29). The penicillin AUC0–36/MIC90 ratio was > 125, and the Cmax/MIC90 ratio was > 10; these values are indicative of highly effective bacterial killing that leads to minimal development of bacterial resistance (29). These results justify the once-a-day use of penicillin by IVRLP in the clinical setting. Although previous studies also enabled the goal of Cmax/MIC90 > 10 to be reached, we believe the higher amikacin concentration in the synovial fluid achieved in the current study, along with the combination of amikacin-penicillin, may possess a clinical advantage. It was recently shown in vitro, that some isolates of methicillin-resistant Staphylococcus aureus (MRSA) have greater MIC values for amikacin, some of them exceeding the upper limit of the E-test strips (256 μg/mL) (30). As MRSA is a common and problematic pathogen in equine practice (31), reaching greater concentrations (> 256 μg/mL) in the joint during IVRLP may be helpful, and even crucial in some clinical cases. Also, according to a thorough recent in vitro study on septicemic human neonatal blood (32), the combination of aminoglycosides-beta-lactams resulted in marked synergism against resistant coagulase negative Staphylococci and can be used effectively to treat neonatal septicemia. Thus, using the combination reported herein has the potential to effectively combat MRSA and other resistant bacteria in horses as well.
Our results contradict the Zantingh et al (11) recommendation that the combination of aminoglycoside-beta-lactams should not be used in IVRLP. This contradiction indicates that each different drug combination should be evaluated for its own PK properties. The PK data acquired in the present study support our working hypothesis that the amikacin-penicillin combination delivered through IVRLP is potentially efficacious for horses with bacterial infection in the distal portion of the limb. One major drawback in this study design is the lack of IVRLP with amikacin and IVRLP with penicillin separate groups. This would have enabled a direct comparison between the IVRLP with each antibiotic compared to the other and to both combined. The small study group is another limitation of this study, one that is common to most equine research studies. However, in this case the most important PK/PD values (Cmax/MIC and AUC/MIC) of amikacin and penicillin were uniformly above the ideal threshold in all tested horses. Thus, due to these unequivocal consistent results, the small study group was not an obstacle to the power of statistics.
Another limitation is the use of healthy horses and the lack of a biological assay to assess the activity of the antimicrobial drugs and not only their synovial concentrations. In addition, the lack of published PK data regarding penicillin use in IVRLP did not enable us to compare our penicillin PK values with another study in which penicillin was used as a sole IVRLP antibiotic. Nevertheless, in a similar study setting we did perform a preliminary study (unpublished), conducted on 2 healthy adult horses, using solely 10 × 106 MU benzylpenicillin sodium. In these 2 horses we found lower penicillin concentrations in the MCP joint (mean: Cmax 67 μg/mL) than in the current combination study (mean: Cmax 556 μg/mL). Thus, the amikacin in the combined perfusion did not seem to inhibit the accumulation of penicillin in the joint, it may have facilitated penicillin accumulation, but that is yet to be elucidated in future studies.
In summary, our results indicate that properly combining penicillin and amikacin during IV-IVRLP in healthy horses delivered high therapeutic concentrations of both drugs. Therefore, this drug combination should be considered for use in IVRLP in order to broaden the antimicrobial coverage when dealing with treatment or prevention of distal limb infections in horses. CVJ
Footnotes
This study was performed at the Kimron Veterinary Institute, Bet Dagan, Israel and funded by a grant from the Koret School of Veterinary Medicine Veterinary Teaching Hospital.
This study was presented in part in 2015 at the 50th Annual Summit of the American College of Veterinary Surgeons in Nashville Tennessee, USA and in 2016 at the 25th Annual Scientific Meeting of the European College of Veterinary Surgeons in Lisbon, Portugal.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Rubio-Martinez LM, Elmas CR, Black B, Monteith G. Clinical use of antimicrobial regional limb perfusion in horses: 174 cases (1999–2009) J Am Vet Med Assoc. 2012;241:1650–1658. doi: 10.2460/javma.241.12.1650. [DOI] [PubMed] [Google Scholar]
- 2.Kelmer G. Regional limb perfusion in horses. Vet Rec. 2016;178:581–584. doi: 10.1136/vr.i3082. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Martínez LM, Cruz AM. Antimicrobial regional limb perfusion in horses. J Am Vet Med Assoc. 2006;228:706–712. doi: 10.2460/javma.228.5.706. [DOI] [PubMed] [Google Scholar]
- 4.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Anti Agent and Chem. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahal JJ. Antibiotic combinations: The clinical relevance of synergy and antagonism. Medicine. 1978;57:179. [PubMed] [Google Scholar]
- 6.Hardefeldt LY, Browning GF, Thursky KA, et al. Antimicrobials used for surgical prophylaxis by equine veterinary practitioners in Australia. Equine Vet J. 2018;50:65–72. doi: 10.1111/evj.12709. [DOI] [PubMed] [Google Scholar]
- 7.Nieto JE, Trela J, Stanley SD, Yamout S, Snyder JR. Pharmacokinetics of a combination of amikacin sulfate and penicillin G sodium for intravenous regional limb perfusion in adult horses. Can J Vet Res. 2016;80:230–235. [PMC free article] [PubMed] [Google Scholar]
- 8.Moore RM, Schneider RK, Kowalski J, Bramlage LR, Mecklenburg LM, Kohn CW. Antimicrobial susceptibility of bacterial isolates from 233 horses with musculoskeletal infection during 1979–1989. Equine Vet J. 1992;24:450–456. doi: 10.1111/j.2042-3306.1992.tb02875.x. [DOI] [PubMed] [Google Scholar]
- 9.Kelmer G, Tatz A, Bdolah-Abram T. Cephalic or saphenous vein catheter use for regional limb perfusion in 44 horses with synovial injury involving the distal aspect of the limb. Vet Surg. 2012;41:938–943. doi: 10.1111/j.1532-950X.2012.01006.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelmer G, Bell GC, Martin-Jimenez T, et al. Evaluation of regional limb perfusion with amikacin using the saphenous, cephalic, and palmar digital veins in standing horses. J Vet Pharmacol Ther. 2013;36:236–240. doi: 10.1111/j.1365-2885.2012.01414.x. [DOI] [PubMed] [Google Scholar]
- 11.Zantingh AJ, Schwark WS, Fubini SL, Watts AE. Accumulation of amikacin in synovial fluid after regional limb perfusion of amikacin sulfate alone and in combination with ticarcillin/clavulanate in horses. Vet Surg. 2014;43:282–288. doi: 10.1111/j.1532-950X.2014.12119.x. [DOI] [PubMed] [Google Scholar]
- 12.Moser DK, Schoonover MJ, Holbrook TC, Payton ME. Effect of regional intravenous limb perfusate volume on synovial fluid concentration of amikacin and local venous blood pressure in the horse. Vet Surg. 2016;45:851–858. doi: 10.1111/vsu.12521. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey JL, Hardy J, Cohen ND. Effects of regional limb perfusion volume on concentrations of amikacin sulfate in synovial and interstitial fluid samples from anesthetized horses. Am J Vet Res. 2016;77:582–588. doi: 10.2460/ajvr.77.6.582. [DOI] [PubMed] [Google Scholar]
- 14.Oreff GL, Dahan R, Tatz AJ, Raz T, Britzi M, Kelmer G. The effect of perfusate volume on amikacin concentration in the metacarpophalangeal joint following cephalic regional limb perfusion in standing horses. Vet Surg. 2016;45:625–630. doi: 10.1111/vsu.12490. [DOI] [PubMed] [Google Scholar]
- 15.Durward-Akhurst SA, Mair TS, Boston R, Dunkel B. A comparison of two antimicrobial regimens on the prevalence of incisional infections after colic surgery. Vet Rec. 2013;172:287–291. doi: 10.1136/vr.101186. [DOI] [PubMed] [Google Scholar]
- 16.Stewart AA, Goodrich LR, Byron CR, Evans RB, Stewart MC. Antimicrobial delivery by intrasynovial catheterisation with systemic administration for equine synovial trauma and sepsis. Aust Vet J. 2010;88:115–123. doi: 10.1111/j.1751-0813.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- 17.Prescott JF, Nicholson VM. The effects of combinations of selected antibiotics on the growth of Corynebacterium equi. J Vet Pharmacol Ther. 1984;7:61–64. doi: 10.1111/j.1365-2885.1984.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 18.Wallace SM, Chan LY. In vitro interaction of aminoglycosides with beta-lactam penicillins. Antimicrob Agents Chemother. 1985;28:274–281. doi: 10.1128/aac.28.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahan R, Britzy G, Sutton GA, Shir S, Kelmer G. Evaluation of the pharmacokinetic properties of a combination of marbofloxacin and imipenem administered by regional limb perfusion to standing horses. J Equine Vet Sci. 2017;53:1–7. [Google Scholar]
- 20.Bassage Ii LH, Ross MW. Diagnostic analgesia. In: Ross MW, Dyson SJ, editors. Diagnosis and Management of Lameness in the Horse. 2nd ed. St. Louis, Missouri: WB Saunders; 2003. pp. 93–124. [Google Scholar]
- 21.Jacks SS, Giguère S, Nguyen A. In vitro susceptibilities of Rhodococcus equi and other common equine pathogens to azithromycin, clarithromycin, and 20 other antimicrobials. Antimicrob Agents Chemother. 2003;47:1742–1745. doi: 10.1128/AAC.47.5.1742-1745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamson PJ, Wilson WD, Hirsh DC, Baggot JD, Martin LD. Susceptibility of equine bacterial isolates to antimicrobial agents. Am J Vet Res. 1985;46:447–450. [PubMed] [Google Scholar]
- 23.Hyde M, Lynch TM, Clark CK, Slone DE, Hughes FE. The influence of perfusate volume on antimicrobial concentration in synovial fluid following intravenous regional limb perfusion in the standing horse. Can Vet J. 2013;54:363–367. [PMC free article] [PubMed] [Google Scholar]
- 24.Thauvin C, Eliopoulos GM, Wennersten C, Moellering RC. Antagonistic effect of penicillin-amikacin combinations against Enterococci. Antimicrob Agents Chemother. 1985;28:78–83. doi: 10.1128/aac.28.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKellar QA, Sanchez Bruni SF, Jones DG. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J Vet Pharmacol Ther. 2004;27:503–514. doi: 10.1111/j.1365-2885.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- 26.Sutton D, Preston T, Christley R, Cohen N, Love S, Roussel A. The effects of xylazine, detomidine, acepromazine and butorphanol on equine solid phase gastric emptying rate. Equine Vet J. 2002;34:486–492. doi: 10.2746/042516402776117818. [DOI] [PubMed] [Google Scholar]
- 27.Pille F, Baere S, Ceelen L, et al. Synovial fluid and plasma concentrations of ceftiofur after regional intravenous perfusion in the horse. Vet Surg. 2005;34:610–617. doi: 10.1111/j.1532-950X.2005.00095.x. [DOI] [PubMed] [Google Scholar]
- 28.Craig WA. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 29.Gunderson BW, Ross GH, Ibrahim KH, Rotschafer JC. What do we really know about antibiotic pharmacodynamics? Pharmacotherapy. 2001;21:302S–318S. doi: 10.1592/phco.21.18.302s.33905. [DOI] [PubMed] [Google Scholar]
- 30.Caron JP, Bolin CA, Hauptman JG, Johnston KA. Minimum inhibitory concentration and postantibiotic effect of amikacin for equine isolates of methicillin-resistant Staphylococcus aureus in vitro. Vet Surg. 2009;38:664–669. doi: 10.1111/j.1532-950X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- 31.Weese JS, Rousseau J, Willey BM, Archambault M, McGeer A, Low DE. Methicillin-resistant Staphylococcus aureus in horses at a veterinary teaching hospital: Frequency, characterization, and association with clinical disease. J Vet Intern Med. 2006;20:182–186. doi: 10.1892/0891-6640(2006)20[182:msaiha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Brilene T, Soeorg H, Kiis M, et al. In vitro synergy of oxacillin and gentamicin against coagulase-negative staphylococci from blood cultures of neonates with late-onset sepsis. APMIS. 2013;121:859–864. doi: 10.1111/apm.12048. [DOI] [PubMed] [Google Scholar]