Abstract
This report describes an unusual presentation of multicentric B-cell lymphoma with central and peripheral nerve involvement in a horse that was presented with acute onset, severe, multiple limb lameness, and muscle atrophy. This case highlights the importance of including neoplasia in the differential list in horses presenting for severe limb lameness associated with muscle atrophy, muscle fasciculations, and weakness.
Résumé
Présentation atypique d’un lymphome à cellules B multisystémique chez un cheval. Ce rapport décrit la présentation inhabituelle d’un lymphome à cellules B multicentrique avec une implication du nerf central et périphérique chez un cheval qui avait été présenté avec une boiterie aiguë et grave de plusieurs membres et de l’atrophie musculaire. Ce cas souligne l’importance d’inclure la néoplasie dans la liste des diagnostics différentiels des chevaux présentés pour une boiterie grave d’un membre associée à l’atrophie musculaire, aux fasciculations musculaires et à la faiblesse.
(Traduit par Isabelle Vallières)
Lameness is one of the most prevalent veterinary complaints in the horse and is caused by trauma, congenital or acquired disorders, infections, metabolic disorders, or nervous and circulatory system disease. This report describes clinical and pathologic findings in a horse with acute onset left front and left hind limb lameness, an atypical gait, and mild muscle atrophy over the quadriceps region caused by a multicentric B-cell lymphoma. Upon treatment with corticosteroids and antibiotics, the attitude of the horse improved initially, but it continued to be severely lame. The horse developed pneumonia and died 1 mo after treatment. At necropsy the severe lameness was attributed to the neoplastic B-cell infiltrates of the dorsal nerve roots of the left sciatic nerve and to a lesser extent the ventral nerve roots at the level of the 7th cervical vertebra forming a poorly demarcated extradural mass.
Case description
A 20-year-old, 455-kg Quarter Horse gelding was presented to the William R. Pritchard Veterinary Medical Teaching Hospital of the University of California–Davis with a history of severe acute onset left hind limb lameness, suggestive of a long bone/appendicular fracture. The owner noticed a left-sided trembling during a barrel racing competition a week before presentation as well as left-sided muscle fasciculations and left hind lameness following moderate exercise 3 d before presentation. The horse also had mild obtundation. Due to progression of the lameness over the next 3 d, the attending veterinarian referred the horse for a possible left hind limb fracture. There was no recent history of trauma or injury, the horse was up-to-date on vaccinations and had been de-wormed 3 mo previously.
On admission, the horse was bright and alert, with an adequate body condition score of 4/9 and no evidence of external trauma. The initial physical examination showed mild tachycardia (52 beats/min) and tachypnea (24 breaths/min), but the remainder of the examination was unremarkable. On the lateral aspect of the left front hoof there was a hoof crack extending 3 cm distally from the coronary band. Muscle atrophy was noticed in the cervical region, more pronounced on the left side of the neck, together with mild atrophy of the left quadriceps musculature. The horse was grade 4/5 lame on the left front limb and left hind limb. Although he was able to bear weight on both limbs, he had an unstable left carpus — characterized by knuckling. In addition, he collapsed in his left stifle and hock (Video Clip S1 available on request from the corresponding author).
An orthopedic examination revealed no response to hoof testers in the left front limb or pain elicited on palpation of the quarter crack. An abaxial sesamoid nerve block did not significantly change the lameness. No pain was elicited on palpation of the soft tissue and boney structures. Radiographs of the left elbow and left stifle showed no evidence of boney abnormalities. No lesions were identified on ultrasound examination of the left pelvis, and a rectal examination was unremarkable. On standing cervical spine radiographs a mild osteoarthritis of the C6–7 cervical facet joint was noted.
A complete blood (cell) count (CBC) revealed a normal white blood cell count [6.4 × 103 cells/μL; reference range (RR): 5 to 11.6 × 103 cells/μL] with a mild lymphopenia (1.5 × 103 cells/μL; RR: 1.6 to 5.8 × 103 cells/μL). Biochemistry analysis identified a mild hyperglycemia (6.6 mmol/L; RR: 2.8 to 5.9 mmol/L), hyperproteinemia (81 g/L; RR: 58 to 77 g/L) characterized by a hyperglobulinemia (51 g/L; RR: 16 to 50 g/L), mildly elevated creatine kinase (CK, 495 IU/L; RR: 119 to 287 IU/L), mild hypertriglyceridemia (0.46 mmol/L; RR: 0.02 to 0.50 mmol/L), and mild hyperbilirubinemia (77.0 μmol/L; RR: 8.6 to 39.3 μmol/L).
Based on the aforementioned findings, with presence of significant gait abnormalities despite normal radiographic and ultrasound images, the list of differential diagnoses consisted of neurologic diseases rather than orthopedic, and included multifocal spinal cord trauma, myelitis with predominance of lower motor neuron involvement (infectious and non-infectious), equine motor neuron disease (EMND), equine protozoal myeloencephalitis, West Nile virus, and neoplasia.
The patient was medicated with flunixin meglumine (Intervet, Madison, New Jersey, USA), 0.5 mg/kg body weight (BW) IV, q12h, and morphine sulfate (Westward, Cherry Hill, New Jersey, USA), 0.1 mg/kg BW, IM, q12h, to reduce possible inflammation and pain, and was placed on stall rest. The horse remained recumbent frequently for prolonged periods of time. It was able to rise without assistance, but with some difficulty such as prolonged time to rise and increased fasciculations. The gelding maintained a good appetite, adequate gastrointestinal borborygmi in all 4 quadrants, and urination and defecation appeared normal during hospitalization.
A neurologic examination was performed by a Board-certified neurologist (MA) 2 d later during hospitalization; the findings included mild obtundation and normal cranial nerve responses, reactions, and reflexes. Segmental spinal reflexes, including cervicofacial, cutaneous trunci, anal and perineal, were within normal limits. Flexor (withdrawal) reflexes were reduced in all limbs especially in the left hind limb. Cutaneous sensation, tail and anal tone were normal. Proprioceptive deficits were observed in all limbs. Upon gait evaluation, tetraparesis, and weakness of all limbs were noticed, with hind limbs being more affected than front limbs; the left hind limb was most severely affected and had a dropped stifle and hock in the walk as well as a reduced toe extension (Video Clip S1). The horse had mild to moderate multifocal to diffuse asymmetrical muscle atrophy of cervical, thoraco-lumbar, gluteal, and limb muscles; the latter were most prominent on the left hind limb. Generalized muscle fasciculations predominantly in the left triceps muscle, were apparent standing at rest. The findings indicated multifocal processes in the central and peripheral nervous systems, with predominant lower motor neuron involvement, and left femoral and sciatic nerve deficits.
An equine protozoal myeloencephalopathy (EPM) indirect immunofluorescent antibody (IFA) blood test for Sarcocystis neurona had a titer of 80 and was negative for Neospora hughesii. An IgM antibody capture enzyme-linked immunosorbent assay (ELISA) blood test for West Nile virus (WNV) was negative. Although not suspected based on neuroanatomical localization (unlikely distribution and clinical signs), a nasal swab was tested by polymerase chain reaction (PCR) for EHV-1; the result was negative.
Cytology of cerebrospinal fluid (CSF) obtained from the lumbosacral space revealed xanthochromic fluid with a total nucleated cell count of 28 cells/μL, which included 2% neutrophils, 60% small mononuclear cells, and 38% large mononuclear cells based on a 100-cell count. Protein in the CSF was elevated at 3.7 g/L (RR: 0.2 to 0.8 g/L). An IFA on CSF was negative for both S. neurona and N. hughesii. Although muscle atrophy was presumed to be of neurogenic origin, presenting clinical signs made EMND less likely, and muscle biopsies to further explore this assumption were offered, but declined due to financial constraints.
In the absence of a specific diagnosis, the horse was started on broad-spectrum oral antibiotics (trimethoprim-sulfamethoxazole; Aurobindo Pharma, Dayton, New Jersey, USA), 30 mg/kg BW, PO, q12h, and supportive neuroprotective and anti-inflammatory treatments, which included α-tocopherol (Stuart Products, Bedford, Texas, USA), 10 IU/kg BW, PO, q24h, dimethyl sulfoxide (DMSO; Valhoma Corporation, Tulsa, Oklahoma, USA), 1 mg/kg BW, IV, q12h for 2 d, and phenylbutazone (MWI, Boise, Idaho, USA), 2.2 mg/kg BW, IV, q12h. Based on the negative EPM result, a single high dose of dexamethasone (MWI, Boise, Idaho, USA), 0.088 mg/kg BW, IM was administered, followed by a tapering dose in order to reduce nervous system inflammatory response. After initiation of therapy the attitude of the horse improved substantially over a period of 4 d, but he continued to display a markedly altered gait.
The horse was discharged from the hospital 4 d after presentation due to financial constraints of the owner and the owner’s preference to continue the treatment at home. Discharge instructions included: administration of phenylbutazone (MWI), 2.2 mg/kg BW, PO, q24h for 7 d, trimethoprim-sulfamethoxazole (Aurobindo Pharma), 30 mg/kg BW, PO, q12h for 7 d, and dexamethasone (MWI), 0.06 mg/kg BW, IM q24h for 3 d, followed by 0.04 mg/kg BW, IM, q24h for 4 more days.
At the follow-up examination 1 wk after discharge, the horse showed significant improvement of his demeanor and gait. Although muscle atrophy was still apparent, the horse was walking much more comfortably. At this stage the treatment with dexamethasone (MWI) was discontinued and the phenylbutazone (MWI) and trimethoprim sulfamethoxazole (Aurobindo Pharma) were continued for 5 more days.
Three weeks later the horse was referred to the VMTH again because of worsening of his condition. He had been found down in the stall by his owner that afternoon with difficulty breathing. Upon arrival at the clinic the horse was found dead in the trailer.
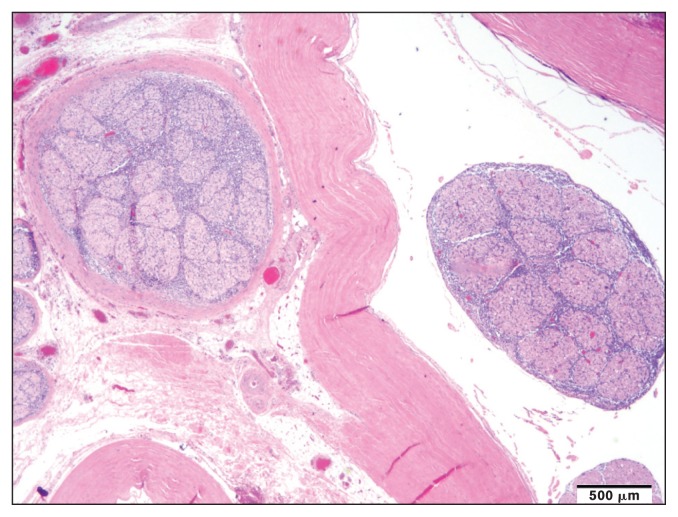
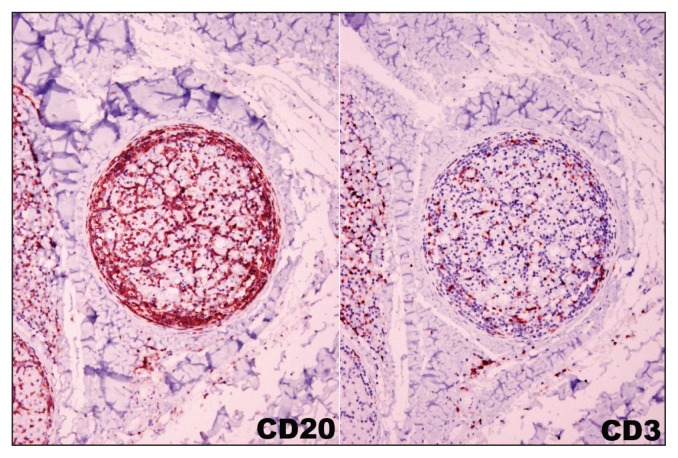
Gross and histopathologic examination revealed multicentric B-cell lymphoma in numerous organs and body systems, including the intestinal tract, the central and peripheral nervous system, and the heart. Grossly, the duodenum, jejunum, and less severely the cecum and colonic mucosa were disrupted by numerous black to purple variably ulcerated nodules, overlain with small amounts of yellow fibrinous material. Mesenteric lymph nodes were variably enlarged. Two raised, dark red nodules were identified on the heart, one was adjacent to the left ventricular papillary muscle and another was expanding the septal leaflet of the aortic valve. Duodenum, jejunum, cecum, colon, mesenteric lymph node, heart, left sciatic nerve, meninges of the cerebrum, cerebellum, and midbrain, as well as the cervical spinal cord (C5, C6, C7) were variably infiltrated by a mono-morphic population of round cells (Figure 1). Intermediate-sized nuclei and a small to moderate amount of cytoplasm suggested a neoplastic population of lymphoid origin. The cells consistently had strong positive membranous immunoreactivity for CD20, but were negative for CD3 (Figure 2). These CD20 positive neoplastic B-lymphocytes were identified in the meninges of the cerebrum, cerebellum, midbrain, and spinal cord as well as dorsal nerve roots and to a lesser extent the ventral nerve roots at the level of the 7th cervical vertebra (Figure 2). A poorly demarcated extradural mass of neoplastic B-cells was noted at the level of the 7th cervical vertebra (ventral and lateral). Admixed with the dense B-cell population there were dispersed small CD3+ T-cells, interpreted as a reactive T-cell population.
Figure 1.
Neoplastic lymphocytes infiltrating the dorsal nerve root at the level of the 7th cervical vertebra.
Figure 2.
Numerous CD20 positive neoplastic B-lymphocytes surround and infiltrate the nerve root. Admixed with the tumor cell population are dispersed CD3 positive reactive T-lymphocytes.
Discussion
This case report describes an unusual presentation of multicentric B-cell lymphoma with central and peripheral nerve involvement in a horse that was presented with acute onset of severe unilateral multiple limb lameness consistent with a fractured limb and muscle atrophy that have not been previously described in the horse. The horse was referred for assessment of acute onset left hind limb lameness with suspicion of bone fracture and required an exhaustive examination to diagnose neoplasia as the primary cause. On evaluation, a multiple limb gait abnormality was confirmed, together with mild atrophy of the left quadriceps musculature. No boney abnormalities were observed in radiographs or on ultrasound examinations. Thorough anamnesis and physical examination, combined with full hematological and serum biochemistry, and an abaxial sesamoid nerve block were pursued to eliminate possible systemic disease or pain as the cause of the tremors observed in the left front limb. Once these causes were deemed unlikely, neurologic diseases were considered. The presence of an altered mental status (mild obtundation) in the absence of other systemic pathologic findings (such as EPM or EHV-1) and lack of significant improvement despite administration of analgesic drugs, suggested a possible multifocal neurologic component (central and peripheral) with predominant lower motor neuron involvement, and left femoral and sciatic deficits. The abnormal gait, tetraparesis, weakness (knuckling/collapse) upon weight-bearing, muscle fasciculations/tremor and muscle atrophy were all consistent with lower motor neuron (LMN) paresis. Most common causes of lameness such as trauma, compressive injury, EPM, EMND, and vertebral disease were already discarded, but neoplasia was still a possibility. Neoplasia can cause nerve damage through compression or infiltration (1). Although uncommonly reported, lymphoma is the most common neoplasia that can affect nerves in horses. In 1970, Bruere et al (2) described a subtle “shifting lameness” with no obvious external pathological changes caused by lymphoma involving the bone marrow. Neurolymphomatosis is a rare manifestation of lymphoma characterized by neoplastic infiltration of cranial and spinal nerves, and nerve roots (3–4).
Lymphoma is the most common hematopoietic neoplasm encountered in horses (5) and can occur at any age (6). The lymphoma can be classified into multicentric, generalized, alimentary or intestinal, splenic, mediastinal, thymic, and cutaneous (6). Lymphoma arises from lymphoid tissue that includes lymph nodes, spleen, and GI tract-associated lymphoid tissue (5,7,8). Multicentric lymphoma is the most common form and consists of widespread involvement of lymph nodes, most likely through distribution of neoplastic lymphocytes via lymphatic circulation (5). Other locations include the liver, kidney, bone marrow (leukemic lymphoma), the upper airway and lungs, heart, adrenal glands, retro-orbital, skeletal muscle, and central nervous system (brain, meninges, spinal cord) (9–17). Metastasis can occur to other organs (6). When lymphoid tissue other than lymph nodes is involved, the lymphoma is classified as generalized, and is considered end-stage (6). Current literature describes lymphoma as a cause of neurologic alterations, lameness, osteolysis, and pathologic fractures (18–25). On 2 surveys of postmortem examinations, prevalence of lymphoma in the horse was estimated to be 2% to 5% (26,27). Zeman et al (25) described a case of multicentric lymphoma with involvement of vertebral bone that resulted in hind limb paresis. A recent report found that lymphoma was amongst the most common malignant neoplasms in older horses, with none of the 19 affected animals having secondary nervous system disease (28). In another study, only 2% of 203 horses with lymphoma examined at necropsy had dissemination to the central nervous system, and none were presented with signs of primary peripheral nerve involvement (29). However, peripheral nerve involvement was documented in multicentric and central nervous system lymphoma in the horse (30–31). Infiltration of peripheral nerves with neoplastic lymphoid cells (neurolymphomatosis) causing chronic unilateral hind limb lameness was described in another 2 horses by Lehmbecker (3), and primary peripheral nerve lymphoma was also described recently in another horse (23). In a case report by Adolf et al (30), the clinical signs included severe unilateral thoracic limb lameness with marked muscle atrophy and severe pain. In another case report a horse was also presented with unilateral hind limb gait abnormality at the walk, with normal cranial nerve responses, weakness in all 4 limbs, intermittent muscle fasciculations, and increased recumbency times (23). The degree of pain observed in 1 of these horses prompted euthanasia, but pain was not observed in our horse. Histologic examination in those 2 horses revealed lymphocytic infiltration of thoracic nerves (proximal radial nerve and ventral branch of the 8th cervical spinal nerve in one horse, and both brachial plexi in the second one). The latter also had dense perineural and endoneural infiltrates with different size lymphocytes in both sciatic nerves. It is unknown whether painless neuropathy may reflect an earlier stage in the disease course (23) and potentially, infiltrating cells dissecting into and around nerve fibers and bundles could explain the initiation of neuropathic pain.
Unfortunately, clinical signs of lymphoma for most affected horses are typically nonspecific, and diagnosis is made late in the course of the disease, after it has progressed to end-stage, at which time clinical signs reflect the organ(s) involved (5). As observed in this case, results of CBC and serum biochemistry are often not helpful with diagnosis of lymphoma (5). The typical leukogram of horses with lymphoma includes mild to moderate leukocytosis due to mature neutrophilia (25). Anemia, hyperfibrinogenemia, hyperproteinemia, and hypoalbuminemia were not observed, but a normal CBC with only mild hyperproteinemia (81 g/L) secondary to hyperglobulinemia (51 g/L) occurred. Results of the cerebrospinal fluid (CSF) analysis showed xanthochromia with a dramatically increased protein of 3.7 g/L. The cytologic examination of the fluid showed a moderate lymphocytic inflammation.
Gross and microscopic examination demonstrated neoplastic lymphocytes scattered throughout numerous organs, including the central and peripheral nervous system, and could explain the altered mentation in this case (32). Histopathology revealed neoplastic lymphocytes infiltrating the central and peripheral nervous system. Neoplastic infiltrates were most concentrated within the extradural space, nerve roots, and meninges at the level of the 7th cervical vertebral body. Additionally, high numbers of neoplastic lymphocytes were identified within the left sciatic nerve. Lower numbers of neoplastic cells were scattered throughout the cerebral and cerebellar meninges, as well as the perivascular spaces with mild infiltration of the adjacent neuropil. The widespread infiltration of central and peripheral nervous system by neoplastic B-lymphocytes explains the altered mentation and gait abnormalities.
Medical management of the patient did result in an initial improvement of demeanor although the lameness persisted. Finally, the horse died due to a complication with severe bronchoalveolar pneumonia, with intralesional bacteria, likely an opportunistic infection attributable to his compromised immune system secondary to the lymphoma and treatment with steroids. Recently, 1 case of EHV-5 infection was associated with lymphoma in the horse (33); however, analysis for EHV-5 was not performed in the present case. No respiratory signs were apparent on physical examination in previous visits. Lymphoma has been associated with reduced immune capacity by causing T-cell deficiency not evaluated here (34). This could have been further compromised by possible immune-suppressive effects of steroidal drugs.
Antemortem confirmation of lymphoma could not be made in this horse. Lack of definitive clinical signs has hampered antemortem diagnosis of lymphoma in the past (25). In retrospect, a full abdominal and thoracic ultrasound could have been performed on this horse and potentially would have helped identify the presence of masses to be sampled (5,35). However, no abnormal lung sounds were auscultated while the horse was hospitalized and there were no clinical signs referable to the thoracic and gastrointestinal systems such as weight loss, diarrhea, colic, or coughing.
Based on this case report, lymphoma should be included in the differential diagnosis for adult horses presenting for severe single or multiple limb lameness with associated signs such as muscle atrophy, muscle fasciculations, and weakness. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Smith MO, George LW. Localization and differentiation of neurologic diseases. In: Smith BP, editor. Large Animal Internal Medicine. 4th ed. St Louis, Missouri: Mosby Elsevier; 2009. pp. 117–146. [Google Scholar]
- 2.Bruere AN, Sutton RJ, Davis GB. Observations on a case of equine lymphosarcoma. N Z Vet J. 1970;18:244–252. doi: 10.1080/00480169.1970.33915. [DOI] [PubMed] [Google Scholar]
- 3.Lehmbecker A, Liebing J, Barthel Y, et al. Neurolymphomatosis in three horses with multicentric T-cell-rich B-cell lymphoma. J Comp Pathol. 2014;151:181–185. doi: 10.1016/j.jcpa.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Johnson AL. Differential diagnosis of muscle tremor and paresis. In: Furr M, Reed S, editors. Equine Neurology. 2nd ed. Ames, Iowa: Blackwell; 2015. pp. 149–156. [Google Scholar]
- 5.Taintor J, Schleis S. Equine lymphoma. Equine Vet Educ. 2011;23:205–213. [Google Scholar]
- 6.Aleman M, Watson JL. Diseases of the hematopoietic and hemolymphatic systems. In: Smith BP, editor. Large Animal Internal Medicine. 5th ed. St. Louis, Missouri: Elsevier; 2015. pp. 1044–1083. [Google Scholar]
- 7.Browning AP. Splenic lymphosarcoma in a stallion association with an acute abdominal crisis. Vet Rec. 1986;119:178–179. doi: 10.1136/vr.119.8.178. [DOI] [PubMed] [Google Scholar]
- 8.Burba D, Jann H, Confer A. Surgical reduction of a laryngeal lymphosarcoma mass causing dyspnea in a horse. Vet Clin North Am Equine Pract. 1991;13:14–18. [Google Scholar]
- 9.Allen BV, Wannop CC, Wright IM. Multicentric lymphosarcoma with lymphoblastic leukaemia in a young horse. Vet Rec. 1984;115:130–131. doi: 10.1136/vr.115.6.130. [DOI] [PubMed] [Google Scholar]
- 10.Freeman SL, England GC, Bjornson S, Smith RK. Uterine T cell lymphoma in a mare, with multicentric involvement. Vet Rec. 1997;141:391–393. doi: 10.1136/vr.141.15.391. [DOI] [PubMed] [Google Scholar]
- 11.Germann SE, Richter M, Schwarzwald CC, Wimmershoff J, Spiess BM. Ocular and multicentric lymphoma in a young racehorse. Vet Ophthalmol. 2008;11:51–56. doi: 10.1111/j.1463-5224.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 12.Held JP, McCracken MD, Toal R, Latimer F. Epididymal swelling attributable to generalized lymphosarcoma in a stallion. J Am Vet Med Assoc. 1992;201:1913–1915. [PubMed] [Google Scholar]
- 13.Labelle P, De Cock HE. Metastatic tumors to the adrenal glands in domestic animals. Vet Pathol. 2005;42:52–58. doi: 10.1354/vp.42-1-52. [DOI] [PubMed] [Google Scholar]
- 14.Morrison LR, Freel K, Henderson I, Hahn C, Smith SH. Lymphoproliferative disease with features of lymphoma in the central nervous system of a horse. J Comp Pathol. 2008;139:256–261. doi: 10.1016/j.jcpa.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Bentz BG, VanWinkle TJ, Bernard WV. Lymphosarcoma involving the brainstem and spinal cord in a horse. Equine Vet Educ. 1995;7:124–126. [Google Scholar]
- 16.Neufeld JL. Lymphosarcoma in the horse: A review. Can Vet J. 1973;14:129–135. [PMC free article] [PubMed] [Google Scholar]
- 17.Neufeld JL. Lymphosarcoma in a mare and review of cases at the Ontario Veterinary College. Can Vet J. 1973;14:149–153. [PMC free article] [PubMed] [Google Scholar]
- 18.Kannegieter NJ, Alley MR. Ataxia due to lymphosarcoma in a young horse. Aust Vet J. 1987;64:377–379. doi: 10.1111/j.1751-0813.1987.tb09608.x. [DOI] [PubMed] [Google Scholar]
- 19.Lester GD, MacKay RJ, Smith-Meyer B. Primary meningeal lymphoma in a horse. J Am Vet Med Assoc. 1992;201:1219–1221. [PubMed] [Google Scholar]
- 20.Moore BR, Weisbrode SE, Biller DS, Williams J. Metacarpal fracture associated with lymphosarcoma-induced osteolysis in a horse. J Am Vet Med Assoc. 1995;207:208–210. [PubMed] [Google Scholar]
- 21.Rousseaux CG, Doige CE, Tuddenham TJ. Epidural lymphosarcoma with myelomalacia in a seven-year-old Arabian gelding. Can Vet J. 1989;30:751–753. [PMC free article] [PubMed] [Google Scholar]
- 22.Schnabel LV, Njaa BL, Gold JR, Meseck EK. Primary alimentary lymphoma with metastasis to the liver causing encephalopathy in a horse. J Vet Intern Med. 2006;20:204–206. doi: 10.1892/0891-6640(2006)20[204:palwmt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Westerman TL, Poulsen KP, Schlipf JW, Jr, Valentine BA. Neurotropic T-cell-rich B-cell lymphoma in a 14-year-old Morgan gelding. Can Vet J. 2014;55:379–382. [PMC free article] [PubMed] [Google Scholar]
- 24.Williams MA, Welles EG, Gailor RJ, et al. Lymphosarcoma associated with neurological signs and abnormal cerebrospinal fluid in two horses. Prog Vet Neurol. 1992;3:51–56. [Google Scholar]
- 25.Zeman DH, Snider TG, III, McClure JJ. Vertebral lymphosarcoma as the cause of hind limb paresis in a horse. J Vet Diagn Invest. 1989;1:187–188. doi: 10.1177/104063878900100222. [DOI] [PubMed] [Google Scholar]
- 26.Baker JR, Ellis CE. A survey of post mortem findings in 480 horses 1958 to 1980: (1) causes of death. Equine Vet J. 1981;13:43–46. doi: 10.1111/j.2042-3306.1981.tb03448.x. [DOI] [PubMed] [Google Scholar]
- 27.Kerr KM, Alden CL. Equine neoplasia: A ten-year survey. Proc Am Assoc Vet Lab Diagn. 1974;17:183–187. [Google Scholar]
- 28.Miller MA, Moore GE, Bertin FR, Kritchevsky JE. What’s new in old horses? Postmortem diagnoses in mature and aged equids. Vet Pathol. 2016;53:390–398. doi: 10.1177/0300985815608674. [DOI] [PubMed] [Google Scholar]
- 29.Durham AC, Pillitteri CA, San Myint M, Valli VE. Two hundred three cases of equine lymphoma classified according to the World Health Organization (WHO) classification criteria. Vet Pathol. 2013;50:86–93. doi: 10.1177/0300985812451603. [DOI] [PubMed] [Google Scholar]
- 30.Adolf JE, Perkins GA, Ainsworth DM, de Lahunta A. Lymphoma of the central nervous system in horses. Compend Contin Educ Pract Vet. 2001;23:194–202. [Google Scholar]
- 31.Frankhauser R, Bestetti G, Fatzer R, Straub R, von Tscharner C. Lymphosarcoma of the horse with involvement of the peripheral nerves. Dtsch Tierarztl Wochenschr. 1977;84:85–89. [PubMed] [Google Scholar]
- 32.Schalm OW. Lymphosarcoma in the horse. Vet Clin North Am Equine Pract. 1981;3:23–26. [Google Scholar]
- 33.Vander Werf K, Davis E. Disease remission in a horse with EHV-5-associated lymphoma. J Vet Intern Med. 2013;27:387–389. doi: 10.1111/jvim.12050. [DOI] [PubMed] [Google Scholar]
- 34.de Lahunta A, Glass E. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. St. Louis, Missouri: WB Saunders; 2009. The neurologic examination; pp. 487–501. [Google Scholar]
- 35.Sherlock C, Dawson L, Mair T. Ultrasound as a diagnostic tool in the investigation of a pony with intestinal lymphoma. Equine Vet Educ. 2017;29:78–81. [Google Scholar]