ABSTRACT
Cell polarity is fundamental to the function of most cells. The evolutionarily conserved molecular machinery that controls cell polarity is centered on a family of GTPases related to Cdc42. Cdc42 becomes activated and concentrated at polarity sites, but studies in yeast model systems led to controversy on the mechanisms of polarization. Here we review recent studies that have clarified how Cdc42 becomes polarized in yeast. On one hand, findings that appeared to support a key role for the actin cytoskeleton and vesicle traffic in polarity establishment now appear to reflect the action of stress response pathways induced by cytoskeletal perturbations. On the other hand, new findings strongly support hypotheses on the polarization mechanism whose origins date back to the mathematician Alan Turing. The key features of the polarity establishment mechanism in yeasts include a positive feedback pathway in which active Cdc42 recruits a Cdc42 activator to polarity sites, and differential mobility of polarity “activators” and “substrates.”
Keywords: Bem1, Cdc42, diffusion, GDI, GEF, PAK, polarity, positive feedback
Introduction
Cdc42 is a highly conserved Rho-family GTPase that regulates cell polarity in many eukaryotes.1-3 A key event in polarity establishment is the accumulation of GTP-Cdc42 at the cortical site that becomes the “front” of the cell. The location of the front can be regulated by a variety of internal or external spatial cues, depending on the cell type. However, elimination of known spatial cues in many systems has revealed that cells are nevertheless capable of polarizing toward apparently random sites, a process called symmetry breaking.4 These findings suggested that polarity pathways contain self-reinforcing positive feedback mechanisms capable of building a robust front starting from slight stochastic asymmetries in polarity protein concentrations. Here we review recent insights into the nature of positive feedback and the mechanism of Cdc42 accumulation gained from studies in yeast model systems.
Mechanism of positive feedback in yeast: GTP-Cdc42 recruits its GEF
Positive feedback requires a mechanism in which GTP-Cdc42 at one or more cortical sites can promote local accumulation of additional GTP-Cdc42 at those sites. Considerable evidence now supports a simple “local activation” mechanism in which cortical GTP-Cdc42 recruits a Cdc42-directed GEF from the cytoplasm to the cortex, where the GEF then activates neighboring molecules of Cdc42 (Fig. 1A).4
Figure 1.
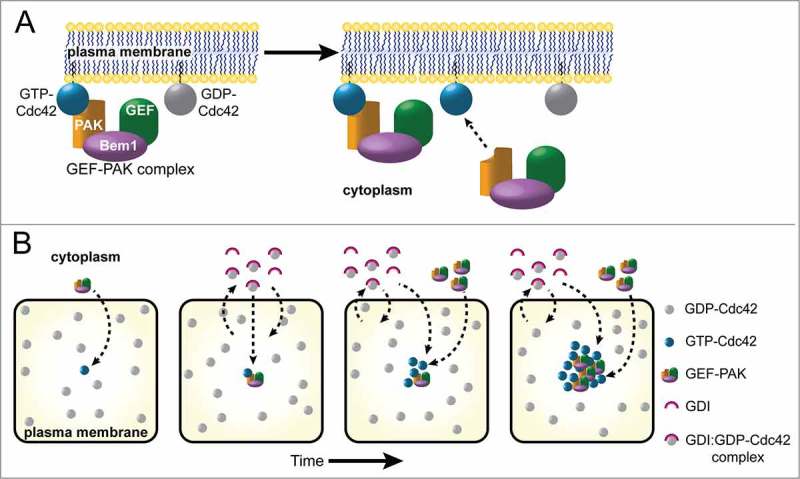
Positive Feedback and Differential Mobility Promote Cdc42 Polarization. (A) Activated, GTP-bound Cdc42 at the plasma membrane can recruit the GEF-PAK complex from the cytoplasm. In budding yeast the GEF-PAK complex includes a Cdc42-directed GEF, the scaffolding protein Bem1, and a PAK (p21-activated kinase) that binds GTP-Cdc42. Once at the membrane, the GEF can activate neighboring Cdc42. (B) Cartoon panels represent sequential snapshots of a small region at the plasma membrane viewed en face, where GDP-Cdc42 (gray circles) and GTP-Cdc42 (blue circles) diffuse slowly. A stochastically activated Cdc42 molecule (first panel) can recruit a GEF-PAK complex from the cytoplasm, activating neighboring Cdc42 to generate a small cluster of GTP-Cdc42. This in turn recruits more GEF-PAK complexes in a positive feedback loop. Meanwhile, GDP-Cdc42 rapidly exchanges between the plasma membrane and the cytoplasm via interaction with the GDI. GDP-Cdc42 deposited near the GEF-PAK cluster is rapidly converted to GTP-Cdc42, which can no longer be extracted by the GDI. Thus, as the cluster of GTP-Cdc42 grows, it accumulates both GEF-PAK complexes and Cdc42 supplied from the cytoplasm. The combination of positive feedback by the GEF-PAK complex and selective mobilization of GDP-Cdc42 by the GDI can amplify a small GTP-Cdc42 cluster into a bona fide polarity site.
Investigation of the genetic requirements for symmetry breaking in budding yeast identified a key role for the scaffold protein Bem1.5 Bem1 binds directly to several effectors of GTP-Cdc42 (in particular, 2 p21-activated kinases or PAKs called Cla4 and Ste20) and to the Cdc42-directed GEF itself.6-10 GTP-Cdc42 does not bind directly to its GEF, but the interactions mediated by Bem1 constitute an indirect recruitment pathway (Fig. 1A). Impairing the interactions between Bem1 and either the GEF or the Cdc42 effectors severely impaired polarization.5 Both Bem1 and the GEF accumulate together with GTP-Cdc42 at the polarity site, and blocking localization of either Bem1 or the GEF prevented polarization.11 Fusing the GEF directly to a PAK bypassed the need for Bem1, suggesting that the key function of Bem1 was simply to provide a physical linkage that can deliver the GEF to sites with GTP-Cdc42, thus providing positive feedback.12 Together, these findings strongly support the idea that positive feedback occurs by local activation of Cdc42 at sites that are enriched for GTP-Cdc42.
Computational modeling of polarization: The importance of differential mobility
Positive feedback is a key ingredient of mathematical models capable of generating spatial patterns starting from homogeneous initial conditions. These models date back to a classic paper by Alan Turing,13 who first recognized that another key ingredient is differential mobility: the idea that diffusion of different components occurs at different rates. A class of “activator-substrate” models, first discussed by Gierer and Meinhardt,14 are particularly relevant to polarization. In these models, an activator can locally convert substrate into more of the activator, yielding positive feedback. The activator has low mobility and tends to stay localized, while the substrate has high mobility. The behavior of Rho-family GTPases like Cdc42 is particularly well-suited to such a model, with GTP-Cdc42 as the activator and GDP-Cdc42 as the substrate.15-17
Cdc42 is tethered to membranes by C-terminal prenylation,18-22 but can be extracted to the cytoplasm by guanine nucleotide dissociation inhibitors (GDIs). GDIs bind selectively to GDP-Cdc42,23 and mask the prenyl moiety so that the GDI-GDP-Cdc42 complex diffuses freely in the cytoplasm. Because proteins in biologic membranes diffuse much more slowly than proteins in the cytoplasm, GTP-Cdc42 at the membrane would have much lower mobility than GDP-Cdc42 bound to GDI in the cytoplasm.
To illustrate how differential mobility can promote polarization, consider an unpolarized cell where most Cdc42 is inactive (GDP-Cdc42 either at the membrane or bound to GDI in the cytoplasm) and most of the GEF is in the cytoplasm (Fig. 1B). Fleeting random encounters between GEF and GDP-Cdc42 will lead to a low-level noisy distribution of GTP-Cdc42 around the membrane. A small local cluster of GTP-Cdc42 would recruit cytoplasmic GEF through Bem1, leading to activation of neighboring GDP-Cdc42. The low mobility of GTP-Cdc42 on the membrane retards the rate at which diffusion disperses the cluster, while the high mobility of GDP-Cdc42 (complexed with the GDI) in the cytoplasm allows rapid equilibration of GDP-Cdc42 throughout the cytoplasm, so that this polarity “substrate” is always available near the polarity site. Fresh GDP-Cdc42 deposited from cytoplasmic complexes into the growing membrane cluster becomes activated by the local GEF, further increasing the local concentration of GTP-Cdc42. This synergy between positive feedback and differential mobility underlies the growth of the polarity cluster.
As the cluster accumulates more and more GTP-Cdc42 and GEF, the cytoplasmic levels of GDP-Cdc42 and GEF fall, slowing GDP-Cdc42 and GEF recruitment into the cluster. Eventually, this “substrate depletion” limits further growth of the cluster. FRAP studies indicate that polarity proteins exchange in and out of the cluster on a ∼4 s timescale, whereas the cluster itself grows with a timescale of > 80 s.24-26 Thus, the cluster approaches a dynamic steady-state in which recruitment and loss of polarity proteins are balanced.
In addition to limiting growth of the main polarity cluster, the depletion of cytoplasmic species changes the fate of any new small cluster of GTP-Cdc42 that may occur at the membrane. With lower amounts of cytoplasmic substrates, it becomes harder for a new cluster to grow, as counteracting events (GAP activity that causes GTP hydrolysis, extraction of GDP-Cdc42 from the membrane by GDI, or unbinding of Bem1-GEF from GTP-Cdc42) now outpace the rate at which new Cdc42 or Bem1-GEF join the cluster from the cytoplasm. This substrate depletion effect can operate even if the cytoplasmic concentrations are not drastically reduced, because near the steady-state there is a fine balance between recruitment of proteins from the cytoplasm to the cluster and loss of proteins from the cluster back to the cytoplasm. Simple computational models have demonstrated that a combination of positive feedback, differential mobility, and substrate depletion has great potential to produce spontaneous polarization.16,17
Mechanisms of differential mobility: A tale of 2 yeasts
The computational models predicted that differential mobility of GDP-Cdc42 and GTP-Cdc42 would be critical for polarization of Cdc42. The only mechanism known to provide such differential mobility was the GDP-selective binding and extraction of Cdc42 to the cytoplasm by the GDI.23 However, the GDI turned out to be non-essential for polarization of Cdc42 in both budding and fission yeasts,24,27 presenting an apparent conundrum.
In budding yeast, exchange of Cdc42 between membrane and cytoplasm occurred, albeit at a reduced rates, even in cells lacking the GDI.28 It is unclear whether this exchange is mediated by some uncharacterized GDI-like factor, or simply reflects the imperfect tethering of Cdc42 to the membrane, as biochemical experiments suggest that Cdc42 can hop on and off membranes without aid from other proteins.23 Moreover, membrane-cytoplasm exchange of a constitutively active mutant, Cdc42Q61L, was significantly reduced compared with that of wild-type Cdc42, even in the absence of the GDI.28 Thus, a GDI-independent mechanism still preferentially extracts (and hence mobilizes) GDP-Cdc42 to a greater degree than GTP-Cdc42. Computational modeling indicated that even though the GDI-independent exchange was considerably slower than that mediated by the GDI, it would still provide sufficient GDP-Cdc42 mobilization to polarize Cdc42.28
In fission yeast, analyses of Cdc42 mobility revealed that diffusion of GDP-Cdc42 at the membrane was much more rapid than that of GTP-Cdc42.27 Thus, in this case extraction to the cytoplasm was not required to yield differential mobility of Cdc42 species. The basis for the differential diffusion (in fission yeast) or extraction (in budding yeast) of GDP-Cdc42 compared with GTP-Cdc42 remains unknown. As effectors bind selectively to GTP-Cdc42, it is possible that interactions with effectors slow the mobility of the GTP-Cdc42-effector complexes. However, known Cdc42 effectors are cytoplasmic or peripheral membrane proteins that would not be expected to dramatically affect Cdc42 mobility at the membrane. Low-mobility integral membrane proteins could selectively slow the movement of GTP-Cdc42 if they only bound to active Cdc42. Alternatively, conformational changes caused by GTP binding could strengthen the interaction of GTP-Cdc42 with the membrane.
To investigate whether differential mobility of Cdc42 was required for polarization, Bendezu et al. (2015) replaced the prenyl-tethered wild-type Cdc42 with a version containing a transmembrane domain, drastically reducing the mobility of both GDP-Cdc42 and GTP-Cdc42.27 This eliminated the enrichment of total Cdc42 at polarity sites, consistent with the notion that differential mobility of Cdc42 is responsible for its concentration at polarity sites.27
The strange case of overexpressed Cdc42Q61L
All of the studies discussed above are consistent with a polarity establishment mechanism that combines positive feedback via local recruitment of GEF complexes to sites enriched for GTP-Cdc42 with differential mobility of active species (low mobility GTP-Cdc42 and bound GEF complexes) and polarity “substrates” (high mobility GDP-Cdc42 and free cytoplasmic GEF complexes). However, there is one experimental finding that cannot fit this paradigm and instead sparked an entirely different line of thinking.
When constitutively active Cdc42Q61L was overexpressed in budding yeast that are arrested in G1 phase of the cell cycle (when they would not normally polarize), the mutant protein became concentrated at one or sometimes 2 or 3 sites on the cortex.29 Given the concentration of Cdc42Q61L at specific cortical sites, this process initially seemed likely to reflect a normal cell polarity pathway. Because it does not require the Cdc42-directed GEF to become activated, Cdc42Q61L cannot be clustered by the GEF-dependent positive feedback pathway described above. Moreover, because there is only one form of the protein (GTP-bound), there is no obvious way that differential mobility could explain its concentration at specific cortical sites. How, then, does Cdc42Q61L become clustered at the cortex?
Actin-containing structures also became enriched at sites of Cdc42Q61L clustering,30 and Cdc42Q61L clustering was prevented by mutations or treatments that blocked actin polymerization or actin-dependent vesicle traffic.29 These findings led to the idea that Cdc42Q61L itself might be delivered to specific sites on the membrane by secretory vesicles traveling on polarized actin cables. As Cdc42 effectors promote actin cable nucleation, this could lead to a trafficking-based “local delivery” positive feedback mechanism: a small cluster of Cdc42Q61L could nucleate an actin cable, leading to delivery of vesicles containing more Cdc42Q61L, and hence nucleation of more cables, delivery of more vesicles, etc. Once established, Cdc42Q61L clusters were quite stable, so delivery of fresh Cdc42Q61L to the plasma membrane would need to be balanced by retrieval of Cdc42Q61L, presumably by endocytosis. Recycling of the protein back to an internal compartment would once again allow it to be loaded onto secretory vesicles for delivery to the plasma membrane (Fig. 2A).31
Figure 2.
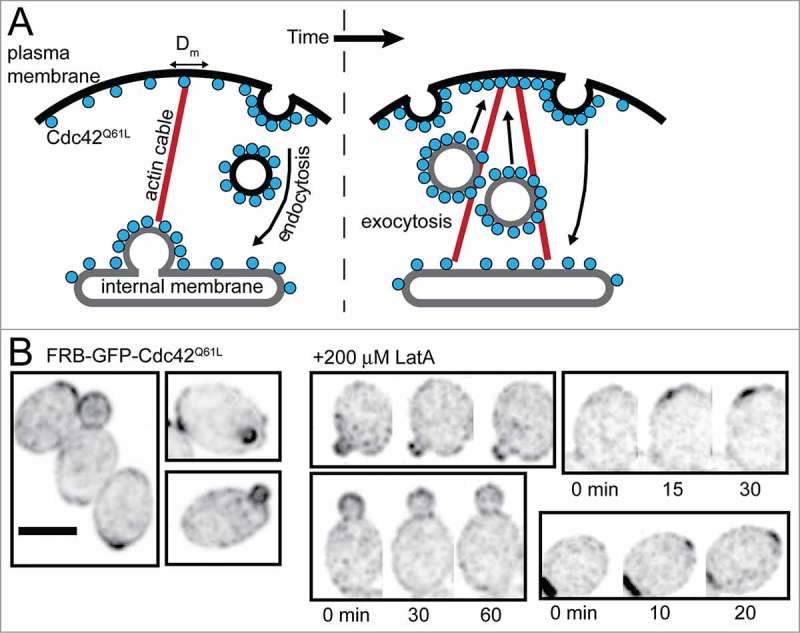
Clustering of Cdc42Q61L does not require F-actin. (A) When overexpressed, GTP-locked Cdc42Q61L accumulates in localized clusters at the plasma membrane. This was hypothesized to be mediated by a local delivery positive feedback loop in which Cdc42Q61L orients actin cables, along which secretory vesicles deliver additional Cdc42Q61L, which generates more actin cables, enhancing Cdc42Q61L delivery. In order for Cdc42Q61L to become stably polarized, its delivery must be balanced by retrieval through endocytosis to re-supply the internal membrane pool. (B) Inverted, cropped maximum projection images of wild-type cells expressing GFP-tagged Cdc42Q61L as well as endogenous, wild-type Cdc42. Left: diploid cells show polarized GFP- Cdc42Q61L. Right: Cdc42Q61L remains polarized (and also accumulates at new polarity sites) in the presence of 200 µM Lat A, sufficient to depolymerize F-actin. Scale bar, 5 µm. Time is in min.
The idea that clustering of overexpressed Cdc42Q61L represents a normal polarity pathway has been challenged on several grounds. Most strikingly, Cdc42Q61L does not form clusters in G1 cells if it is not overexpressed, and Cdc42Q61L cannot rescue polarization in cycling cells deprived of wild-type Cdc425. If Cdc42Q61L clustering reflects a normal polarization pathway, why does it not occur with more physiologic levels of Cdc42?
A different interpretation of the Cdc42Q61L overexpression data was suggested by the observation that such overexpression leads to cell lysis.5,30 Lysis reflects weaknesses in the yeast cell wall, raising the possibility that Cdc42Q61L overexpression causes wall defects. Cell wall defects trigger a “wound-healing” response involving polarization of actin structures toward the wound site.32 Thus, one possibility is that Cdc42Q61L overexpression causes cell wall defects, which in turn recruit repair machinery, including actin and perhaps also Cdc42, to the wound site(s). This could explain why more than one cluster can form (because there is more than one wound), and why lower levels of Cdc42Q61L (which do not cause wounds) are unable to cluster in otherwise similar circumstances.
Does actin or vesicle traffic contribute to Cdc42 polarization?
The polarity-like effects of Cdc42Q61L overexpression prompted an extended investigation into whether a local delivery-style positive feedback mechanism might contribute to polarization of wild-type Cdc42. As Cdc42 can polarize even in the absence of polymerized actin,5 such a pathway would presumably act in parallel with the actin-independent local activation pathway described above.
Careful examination of Cdc42 polarization in budding yeast treated with drugs that depolymerize actin indicated that loss of F-actin could lower the frequency with which cells polarized Cdc42, as well as the stability of the polarized cluster over time.25,26,33,34 In fission yeast, the effects were considerably more dramatic, with almost complete disassembly of polarity sites from the cell tips, and development of transitory weak polarity clusters at abnormal locations.27,35 These findings supported a role for F-actin in Cdc42 polarization, but recent studies indicate that this role is actually indirect.
In budding yeast, mutations that delay formation of polarized actin cables had no effect whatsoever on Cdc42 polarization.28 And while complete actin depolymerization using drugs did reduce the polarization efficiency, much of that effect could be traced to altered cell-cycle progression, so that fewer cells entered the cell cycle phase where they would normally polarize.28 Actin depolymerization triggered stress response pathways,36 which could impact polarization indirectly. Remarkably, a new study found that in fission yeast, all of the dramatic effects of actin depolymerization on Cdc42 were due to a stress response involving the MAPK Sty137. When STY1 was deleted, cells maintained robust Cdc42 clusters at the cell tips, and even continued polarized growth in the complete absence of F-actin.37 These recent studies suggest a re-interpretation of prior work: F-actin is only important for robust polarity because when actin filaments are disassembled, stress responses are activated that disrupt polarity.
In parallel with the analysis of actin requirements, other work addressed the plausibility of the local delivery mechanism as a way to polarize Cdc42. If Cdc42 delivery on vesicles is to enhance the local Cdc42 concentration at a polarity site, then Cdc42 must be at least as concentrated on secretory vesicles as it is at the polarity site.38 If it were not, then vesicle fusion would lead to dilution, rather than concentration, of Cdc42. Similarly, if endocytosis is to recycle Cdc42 so as to maintain a steady-state level of Cdc42 at the plasma membrane, then Cdc42 must be as concentrated on endocytic vesicles as it is on exocytic vesicles.38 Otherwise, vesicle traffic would lead to a continual transfer of Cdc42 from internal membranes to the plasma membrane. However, Cdc42 on secretory vesicles appears to be about 3-fold less concentrated than it is at the polarity site,39 and it appears that GFP-Cdc42 is largely excluded from endocytic vesicles.39,40 Thus, instead of promoting polarization, vesicle trafficking may actually perturb polarization by diluting Cdc42.38,41 Consistent with that hypothesis, there are circumstances (e.g. during yeast mating) when the polarity site is unstable and can move around the cortex, and in those instances it appears that actin-mediated vesicle traffic provides a negative feedback that contributes to the instability of the polarity site.42-45
In aggregate, the data on wild-type Cdc42 support a critical role for differential mobility of GDP-Cdc42 and GTP-Cdc42, rather than actin or vesicle traffic, as the driver for Cdc42 polarization in yeast.27,28,39 But differential mobility of GDP/GTP forms cannot explain how Cdc42Q61L becomes concentrated. Although at non-lethal expression levels Cdc42Q61L did not polarize on its own, it did accumulate at bud tips of cells that also contained wild-type Cdc42 (Fig. 2B). This provided an opportunity to ask whether or not actin is required for the concentration of Cdc42Q61L. We found that following complete actin depolymerization Cdc42Q61L remained at bud tips, and even accumulated at new polarity sites (Fig. 2B). Thus, Cdc42Q61L localization does not require actin-mediated trafficking. The simplest way to explain Cdc42Q61L concentration in these cells is that polarity sites built by wild-type Cdc42 accumulate many proteins that can bind GTP-Cdc42. Transient interactions with these proteins may reduce Cdc42Q61L mobility at the polarity site, compared with its diffusion at other parts of the membrane, resulting in local enrichment of Cdc42Q61L.
Conclusion and implications for other systems
Early work on polarization in budding yeast led to conflicting views on the mechanisms of Cdc42 polarization, but recent work has clarified the mechanism. Effects of overexpressing an activated Cdc42 mutant now appear to reflect pathological events in dying cells rather than physiologic polarization, and the effects of perturbing actin on polarization now appear to reflect the action of stress response pathways. These realizations undermine the hypothesis that actin-mediated vesicle traffic serves to polarize Cdc42.
Recent data for both budding and fission yeasts strongly support a mechanism that relies on actin-independent positive feedback by local activation of Cdc42: GTP-Cdc42 recruits GEF complexes from the cytoplasm to activate neighboring GDP-Cdc42.12,27,28 The efficacy of this mechanism is enhanced by the differential mobility of GDP-Cdc42 and GTP-Cdc42. GTP-Cdc42 appears to diffuse more slowly and be associated with the plasma membrane more tightly than GDP-Cdc42. As a consequence, local conversion of GDP-Cdc42 to GTP-Cdc42 allows accumulation of local GTP-Cdc42, while the depleted GDP-Cdc42 is replenished rapidly thanks to its faster mobility. Similarly, membrane bound GEF accumulates without local depletion of the highly mobile cytoplasmic GEF. Together, local activation and differential mobility enable the concentration of GTP-Cdc42 at polarity sites. This process eventually leads to some global depletion of the polarity “substrates,” GDP-Cdc42 and cytoplasmic GEF complexes. Substrate depletion limits growth of the polarity site, and also limits growth of any newly arising GTP-Cdc42 clusters.46
The mechanism described above is well supported for both budding and fission yeasts, and may function similarly in other systems. Local activation involves concentration of the relevant GEF at polarity sites, and there is some evidence that this occurs in both plants and animals. In Arabidopsis xylem cells, patterning of the plant cell wall is controlled by ROP11 (Rho of plants), which is co-localized with its GEF ROPGEF4.47 In migrating astrocytes, the GEF βPIX accumulates at the leading edge together with activated Cdc42.48 The yeast positive feedback mechanism requires effector-GEF complexes that can target GEFs to sites with active GTPases, and such complexes are known to occur in animals, where the αPIX and βPIX GEFs bind directly to PAKs, generating a yeast-like GEF-PAK complex.49,50 Differential mobility of GTP- and GDP-bound forms of the GTPase is conferred (though not exclusively) by GDIs, which are also conserved in plants and animals.51 Thus, the ingredients for the simple yeast polarity mechanism are widely present in metazoans. Future studies will be required to assess whether they cooperate in the same manner to promote polarity.
Methods
Construction of the yeast strain used in this study is described.28 To depolymerize F-actin, cells were grown in complete synthetic media with 2% dextrose (CSMD) to log phase, then mounted onto CSMD + agarose slabs containing 200 µM Lat A and imaged. For more experimental details on Lat A treatment and microscopy, see Woods et al., 2016.28
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Stefano Di Talia, Amy Gladfelter, Helen Lai, and Jian-geng Chiou for comments on the manuscript.
Funding
This work was supported by NIH/NIGMS grant GM62300 to D.J.L.
References
- [1].Park HO, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev 2007; 71:48-96; PMID:17347519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Etienne-Manneville S. Cdc42 - the centre of polarity. J Cell Sci 2004; 117:1291-300; PMID:15020669 [DOI] [PubMed] [Google Scholar]
- [3].Wu CF, Lew DJ. Beyond symmetry-breaking: competition and negative feedback in GTPase regulation. Trends Cell Biol 2013; 23:476-83; PMID:23731999; http://dx.doi.org/ 10.1016/j.tcb.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnson JM, Jin M, Lew DJ. Symmetry breaking and the establishment of cell polarity in budding yeast. Curr Opin Genet Dev 2011; 21:740-6; PMID:21955794; http://dx.doi.org/ 10.1016/j.gde.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Irazoqui JE, Gladfelter AS, Lew DJ. Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol 2003; 5:1062-70; PMID:14625559 [DOI] [PubMed] [Google Scholar]
- [6].Peterson J, Zheng Y, Bender L, Myers A, Cerione R, Bender A. Interactions between the bud emergence proteins Bem1p and Bem2p and Rho- type GTPases in yeast. J Cell Biol 1994; 127:1395-406; PMID:7962098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ito T, Matsui Y, Ago T, Ota K, Sumimoto H. Novel modular domain PB1 recognizes PC motif to mediate functional protein-protein interactions. EMBO J 2001; 20:3938-46; PMID:11483497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Endo M, Shirouzu M, Yokoyama S. The Cdc42 binding and scaffolding activities of the fission yeast adaptor protein Scd2. J Biol Chem 2003; 278:843-52; PMID:12409291 [DOI] [PubMed] [Google Scholar]
- [9].Bose I, Irazoqui JE, Moskow JJ, Bardes ES, Zyla TR, Lew DJ. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J Biol Chem 2001; 276:7176-86; PMID:11113154 [DOI] [PubMed] [Google Scholar]
- [10].Winters MJ, Pryciak PM. Interaction with the SH3 domain protein Bem1 regulates signaling by the Saccharomyces cerevisiae p21-activated kinase Ste20. Mol Cell Biol 2005; 25:2177-90; PMID:15743816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Woods B, Kuo CC, Wu CF, Zyla TR, Lew DJ. Polarity establishment requires localized activation of Cdc42. J Cell Biol 2015; 211:19-26; PMID:26459595; http://dx.doi.org/ 10.1083/jcb.201506108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kozubowski L, Saito K, Johnson JM, Howell AS, Zyla TR, Lew DJ. Symmetry-Breaking Polarization Driven by a Cdc42p GEF-PAK Complex. Curr Biol 2008; 18:1719-26; PMID:19013066; http://dx.doi.org/ 10.1016/j.cub.2008.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Turing A. The chemical basis of morphogenesis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 1952; 237:37-72. [Google Scholar]
- [14].Gierer A, Meinhardt H. A theory of biological pattern formation. Kybernetik 1972; 12:30-39; PMID:4663624 [DOI] [PubMed] [Google Scholar]
- [15].Otsuji M, Ishihara S, Co C, Kaibuchi K, Mochizuki A, Kuroda S. A mass conserved reaction-diffusion system captures properties of cell polarity. PLoS Comput Biol 2007; 3:e108; PMID:17559299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mori Y, Jilkine A, Edelstein-Keshet L. Wave-pinning and cell polarity from a bistable reaction-diffusion system. Biophys J 2008; 94:3684-97; PMID:18212014; http://dx.doi.org/ 10.1529/biophysj.107.120824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goryachev AB, Pokhilko AV. Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett 2008; 582:1437-43; PMID:18381072 [DOI] [PubMed] [Google Scholar]
- [18].Johnson DI. Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev 1999; 63:54-105; PMID:10066831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ziman M, Preuss D, Mulholland J, O'Brien JM, Botstein D, Johnson DI. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP- binding protein involved in the control of cell polarity. Mol Biol Cell 1993; 4:1307-1316; PMID:8167411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Boivin D, Beliveau R. Subcellular distribution and membrane association of Rho-related small GTP-binding proteins in kidney cortex. Am J Physiol 1995; 269:F180-9; PMID:7653591 [DOI] [PubMed] [Google Scholar]
- [21].Finegold AA, Johnson DI, Farnsworth CC, Gelb MH, Judd SR, Glomset JA, Tamanoi F. Protein geranylgeranyltransferase of Saccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires the CDC43 gene product but not the DPR1 gene product. Proc Natl Acad Sci U S A 1991; 88:4448-52; PMID:2034682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maltese WA, Sheridan KM. Isoprenoid modification of G25K (Gp), a low molecular mass GTP-binding protein distinct from p21ras. J Biol Chem 1990; 265:17883-90; PMID:2120220 [PubMed] [Google Scholar]
- [23].Johnson JL, Erickson JW, Cerione RA. New insights into how the Rho guanine nucleotide dissociation inhibitor regulates the interaction of Cdc42 with membranes. J Biol Chem 2009; 284:23860-71; PMID:19581296; http://dx.doi.org/ 10.1074/jbc.M109.031815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Slaughter BD, Das A, Schwartz JW, Rubinstein B, Li R. Dual modes of cdc42 recycling fine-tune polarized morphogenesis. Dev Cell 2009; 17:823-35; PMID:20059952; http://dx.doi.org/S1534-5807(09)00445-6 [pii] 10.1016/j.devcel.2009.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Howell AS, Jin M, Wu CF, Zyla TR, Elston TC, Lew DJ. Negative feedback enhances robustness in the yeast polarity establishment circuit. Cell 2012; 149:322-33; PMID:22500799; http://dx.doi.org/ 10.1016/j.cell.2012.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Freisinger T, Klünder B, Johnson J, Müller N, Pichler G, Beck G, Costanzo M, Boone C, Cerione RA, Frey E, et al.. Establishment of a robust single axis of cell polarity by coupling multiple positive feedback loops. Nat Commun 2013; 4:1807; PMID:23651995; http://dx.doi.org/ 10.1038/ncomms2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bendezu FO, Vincenzetti V, Vavylonis D, Wyss R, Vogel H, Martin SG. Spontaneous Cdc42 polarization independent of GDI-mediated extraction and actin-based trafficking. PLoS Biol 2015; 13:e1002097; PMID:25837586; http://dx.doi.org/ 10.1371/journal.pbio.1002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Woods B, Lai H, Wu CF, Zyla TR, Savage NS, Lew DJ. Parallel actin-independent recycling pathways polarize Cdc42 in budding yeast. Curr Biol 2016; 26:2114-26; PMID:27476596; http://dx.doi.org/ 10.1016/j.cub.2016.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science (New York, N.Y 2003; 299:1231-5; PMID:12560471 [DOI] [PubMed] [Google Scholar]
- [30].Gulli MP, Jaquenoud M, Shimada Y, Niederhäuser G, Wiget P, Peter M. Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol Cell 2000; 6:1155-67; PMID:11106754 [DOI] [PubMed] [Google Scholar]
- [31].Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell 2007; 129:411-22; PMID:17448998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kono K, Saeki Y, Yoshida S, Tanaka K, Pellman D. Proteasomal degradation resolves competition between cell polarization and cellular wound healing. Cell 2012; 150:151-164; PMID:22727045; http://dx.doi.org/ 10.1016/j.cell.2012.05.030 [DOI] [PubMed] [Google Scholar]
- [33].Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J Cell Biol 2004; 166:889-900; PMID:15353546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Okada S, Leda M, Hanna J, Savage NS, Bi E, Goryachev AB. Daughter cell identity emerges from the interplay of Cdc42, septins, and exocytosis. Dev Cell 2013; 26:148-61; PMID:23906065; http://dx.doi.org/ 10.1016/j.devcel.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bendezu FO, Martin SG. Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol Biol Cell 2011; 22:44-53; PMID:21148300; http://dx.doi.org/ 10.1091/mbc.E10-08-0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Harrison JC, Bardes ES, Ohya Y, Lew DJ. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat Cell Biol 2001; 3:417-20; PMID:11283616 [DOI] [PubMed] [Google Scholar]
- [37].Mutavchiev DR, Leda M, Sawin KE. Remodeling of the fission yeast Cdc42 cell-polarity module via the Sty1 p38 stress-activated protein kinase pathway. Curr Biol 2016; 26:2921-8; PMID:27746023; http://dx.doi.org/ 10.1016/j.cub.2016.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Layton AT, Savage NS, Howell AS, Carroll SY, Drubin DG, Lew DJ. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr Biol 2011; 21:184-94; PMID:21277209; http://dx.doi.org/ 10.1016/j.cub.2011.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Watson LJ, Rossi G, Brennwald P. Quantitative analysis of membrane trafficking in regulation of cdc42 polarity. Traffic 2014; 15:1330-43; PMID:25158298; http://dx.doi.org/ 10.1111/tra.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Slaughter BD, Unruh JR, Das A, Smith SE, Rubinstein B, Li R. Non-uniform membrane diffusion enables steady-state cell polarization via vesicular trafficking. Nat Commun 2013; 4:1380; PMID:23340420; http://dx.doi.org/ 10.1038/ncomms2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Savage NS, Layton AT, Lew DJ. Mechanistic mathematical model of polarity in yeast. Mol Biol Cell 2012; 23:1998-2013; PMID:22438587; http://dx.doi.org/ 10.1091/mbc.E11-10-0837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ozbudak EM, Becskei A, van Oudenaarden A. A System of Counteracting Feedback Loops Regulates Cdc42p Activity during Spontaneous Cell Polarization. Dev Cell 2005; 9:565-71; PMID:16198298 [DOI] [PubMed] [Google Scholar]
- [43].Dyer JM, Savage NS, Jin M, Zyla TR, Elston TC, Lew DJ. Tracking shallow chemical gradients by actin-driven wandering of the polarization site. Curr Biol 2013; 23:32-41; PMID:23200992; http://dx.doi.org/ 10.1016/j.cub.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McClure AW, Minakova M, Dyer JM, Zyla TR, Elston TC, Lew DJ. Role of polarized g protein signaling in tracking pheromone gradients. Dev Cell 2015; 35:471-82; PMID:26609960; http://dx.doi.org/ 10.1016/j.devcel.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hegemann B, Unger M, Lee SS, Stoffel-Studer I, van den Heuvel J, Pelet S, Koeppl H, Peter M. A cellular system for spatial signal decoding in chemical gradients. Dev Cell 2015; 35:458-70; PMID:26585298; http://dx.doi.org/ 10.1016/j.devcel.2015.10.013 [DOI] [PubMed] [Google Scholar]
- [46].Wu CF, Chiou JG, Minakova M, Woods B, Tsygankov D, Zyla TR, Savage NS, Elston TC, Lew DJ. Role of competition between polarity sites in establishing a unique front. eLife 2015; 4:e11611; PMID:26523396; http://dx.doi.org/ 10.7554/eLife.11611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oda Y, Fukuda H. Secondary cell wall patterning during xylem differentiation. Curr Opin Plant Biol 2012; 15:38-44; PMID:22078063; http://dx.doi.org/ 10.1016/j.pbi.2011.10.005 [DOI] [PubMed] [Google Scholar]
- [48].Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol 2006; 16:2395-405; PMID:17081755; http://dx.doi.org/ 10.1016/j.cub.2006.10.026 [DOI] [PubMed] [Google Scholar]
- [49].Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the Cool/Pix proteins: key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J Biol Chem 2002; 277:5644-50; PMID:11741931 [DOI] [PubMed] [Google Scholar]
- [50].Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, et al.. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 2003; 114:215-27; PMID:12887923 [DOI] [PubMed] [Google Scholar]
- [51].Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 2011; 12:493-504; PMID:21779026; http://dx.doi.org/ 10.1038/nrm3153 [DOI] [PMC free article] [PubMed] [Google Scholar]


