ABSTRACT
Rho family GTPase signaling regulates the actin cytoskeleton and is critical for behaviors that range from the cell to tissue-scale. A theme in Rho GTPase biology is that there are many more regulators, such as guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), than GTPases themselves. Here, we review different, modular cases where GEFs and GAPs function together to elicit precise spatial and temporal control of signaling. We focus on examples from metazoan development, where precise regulation of Rho GTPases is critical for proper tissue form and function.
KEYWORDS: Bcr, C-GAP, Cdc42, Ect-2, morphogenesis, Rac, RGA-3, RGA-4, RhoA, RhoGEF2, Spv-1
Introduction
Rho GTPases are highly conserved signaling proteins that affect basic cellular processes, like cytoskeletal dynamics, cell division, adhesion, migration, and vesicle trafficking.14,40 Because of this, Rho GTPases are critical to regulate highly complex, coordinated processes in multicellular organisms.14,42 The prevalence and importance of Rho GTPase-regulated pathways indicates that Rho GTPases themselves require precise and often sophisticated regulation. On the protein/signaling level, most Rho GTPases cycle between a GTP-bound, active state that is promoted by GEFs (guanine nucleotide exchange factors), and a GDP-bound, inactive state that is promoted by GAPs (GTPase activating proteins). In addition, GDIs (GTPase dissociation inhibitors) sequester Rho GTPases in the inactive state in the cytosol. The number of predicted RhoGEFs and RhoGAPs outweighs the number of Rho GTPases, in humans (∼80 GEFs, 70 GAPs, and 20 Rho GTPases) and in other organisms (e.g. Drosophila: ∼29 GEFs, 23 GAPs, and 6 Rho GTPases), implying complexity of Rho GTPase regulation.3,22 The Rho family of Ras-like GTPases are often described as “molecular switches,” with GEF activity necessary to activate signaling and GAP activity required for subsequent signal depletion. However, there has long been evidence that Rho GTPase regulation is not restricted to this simple scheme. For instance, inhibiting RhoGAP led to RhoA signal activation, similar to activation by RhoGEFs.56 This suggested that simultaneous GEF and GAP activity could be required to set the appropriate signaling level for a Rho-family GTPase. The oncogenic potential of a RhoGEF, which activates the Cdc42 GTPase, was recapitulated by mutants that increased the intrinsic GDP to GTP exchange rate.24 In contrast, a hydrolysis-deficient Cdc42 mutant only partially recapitulated the oncogenic potential.36 These results indicated that Rho GTPases are more than just molecular switches and that cycling of the GTPase is essential for proper Rho signaling activity. Since these pioneering studies, increasing numbers of complex interactions between RhoGAPs and RhoGEFs have been observed in various organisms. Therefore, it is important to understand the design principles governing different modes of regulation. Importantly, we need to move beyond the functions of individual molecules and understand how modular combinations of GEFs and GAPs influence GTPase function.18 This mini-review aims to categorize various regulatory modules that have been observed to operate in metazoan development. Several other excellent papers and reviews have discussed the modular regulation of GTPase signaling in polarity establishment, in general, or in yeasts.6,27 Here, we discuss modules that have been shown to serve as building blocks of complex regulatory systems that function in metazoan growth and form. We hope to create a framework for thinking about Rho GTPase signaling and its roles in multicellular development.
Categories of regulation models
Dynamic processes, such as the activity or localization of molecules in a cell, can always be described in terms of 2 principal components: space and time. Therefore, the relationship between the activities of 2 molcules, such as a RhoGEF and a RhoGAP can roughly broken down into 4 categories: 1) same place and same time; 2) same place and different time; 3) different place and same time; 4) different place and different time. The last category indicates largely independent activities and is of less interest to us because there would not be a functionally interacting GEF/GAP module within a single cell.
Additionally, GEF/GAP relationships differ and therefore can be categorized by their outcome in terms of effect on Rho activity. The functional outcome is not necessarily uniform among GEF/GAP pairs that have similar spatiotemporal relationships, which emphasizes the complexity and versatility of Rho regulation modules.
We start by discussing the simplest case, case 3, in which a GEF/GAP pair is active at the same time, but at different places within a cell.
Compartmentalization of regulators creates defined Rho activity zones
Rho GTPases as regulators of the actin cytoskeleton have very broad function, but need to fulfill highly specialized tasks. Therefore, restriction of Rho activity to defined areas within the cell is essential to coordinate cell functions. Spatial confinement of Rho GTPase signaling activity can be achieved in a simple way: If inhibitors/GAPs and activators/GEFs are active at the same time, but occur in different locations within the cell, the Rho GTPase signal will be restricted to the zone where positive regulators are present and inhibitors are absent (Fig. 1).
Figure 1.
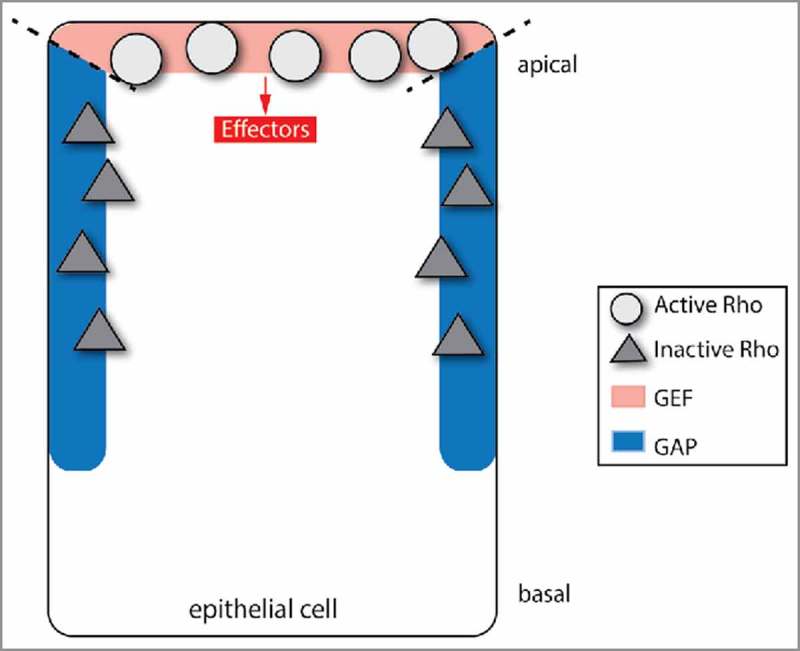
Compartmentalized activator and inhibitor lead to a defined Rho activity zone. When GAP and GEF are spatially segregated, Rho GTPase molecules will be activated in the area that is populated by the GEF, and inactivated in the parts of the cell that are populated by the GAP. This simple way of spatially restricting Rho activity is used during Drosophila spiracle cell development.
One example in which this type of GEF/GAP relationship was found is during Drosophila spiracle formation. Spiracles are formed by a tissue folding event that is initiated by constriction on the apical sides of cells. This apical constriction requires apical-specific activation of myosin 2 by the RhoA GTPase, Drosophila Rho1. Apical constriction of spiracle cells depends on the coordinated action of a RhoGAP, Crossveinless-c (Cv-c), and 2 GEFs, RhoGEF64C and RhoGEF2.47 During spiracle cell invagination, the GEFs are apically localized, and the GAP is localized to the lateral sides of cells. These distinct GEF and GAP localizations appear to restrict Rho signaling activity to the apical domain of the cell.
A similar restriction of Rho signaling was also observed during Drosophila gastrulation.29 During gastrulation, apical constriction of epithelial cells also causes cell internalization and the formation of tissue layers.50 In this case, a Rho GAP, called Cumberland-GAP (C-GAP), is partly localized to lateral junctions and is required to restrict myosin 2 activity to the apical domain of the cell. One difference, in this case, is that C-GAP is also clearly localized to the cell apex. However, these studies lend support to the existence of a signaling module where differential localization of GAP and GEF can lead to a zone of Rho GTPase signaling.
Cycles of GTPase activation and inactivation tune Rho activity level
Next, we discuss examples of case 1, where a GEF and GAP acting on the same GTPase are present at the same cellular location at the same time and are sometimes even in the same complex. Interactions of this category initially appear counter-intuitive: Every event in which a Rho GTPase is activated by the GEF could coincide with immediate deactivation by the GAP—seemingly an energy-consuming, zero-sum game. However, this process would lead to cycling of Rho GTPases between the GTP-bound, ON and GDP-bound, OFF states. From this cycling arise interesting properties, which can be used by the organism to create precise regulation of Rho GTPases.2,15
A simplified view is that an increase in the GTP-bound form positively influences GTPase function, whereas a decrease in the amount of GTP-bound GTPase will negatively affect its function. However, negative effects of over-activation of a signal are well-documented in many systems, most prominently in oncogenes such as Ras. Loss of a Ras GAP leads to abnormal Ras signaling.8 Therefore, it is likely that other systems, such as the Rho family GTPases, use GAPs and GEFs to tune Rho signaling activity to an optimal level, avoiding either hyper- or hypoactivation (Fig. 2A).28,37
Figure 2.
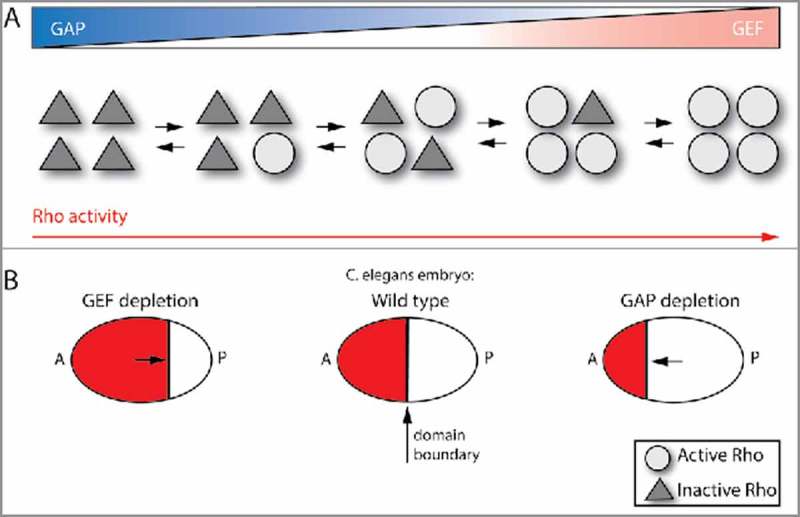
Coordinated GEF and GAP tune Rho activity level. (A) Simultaneous, co-localized activator (GEF) and deactivator (GAP) activities create constant cycling between Rho GTPase activation and inactivation. As opposed to Rho GTPases exclusively being activated by a GEF (right case) or just inactivated by a GAP (left case), this GEF/GAP coordination can create a moderate level of Rho GTPase signaling activity (middle cases), which is essential for some Rho GTPase functions. (B) Example, C. elegans embryo: Rho activity needs to be optimized to correctly position the boundary between the anterior, contractile cortex (A) and the posterior, non-contractile cortex (P) (middle case). Depletion of GAP activity leads to excessive contractility and a boundary that is too far to the anterior (A) (right case), whereas depletion of the GEF has the opposite effect (left case).
One system in which the level of contractile function appears to be tuned is polarity establishment in the cortex of the C. elegans single cell embryo. In the C. elegans embryo, polarity establishment involves the assembly of a cortical actin and myosin 2 (actomyosin) network that contracts and flows toward the anterior side of the embryo.33 This contraction sets the position of the boundary between the anterior contractile cortex and posterior non-contractile cortex, where asymmetrically distributed partitioning defective (Par) proteins also accumulate (Fig. 2B). Importantly, the actomyosin network reaches a steady-state occupying the anterior half of the embryo. This contraction is regulated by the C. elegans RhoA homolog, Rho-1, and its GEF, Ect-2.31,45 Evidence that this is a tuned contraction and not simply an “on” switch comes from studies that identified Rga-3 and Rga-4, 2 Rho-1 GAPs, as being required for proper positioning of the contractile network.43,44 Thus, proper positioning of the anterior/posterior boundary requires both the Ect-2 GEF and the Rga-3/4 GAPs. Loss of the Rga-3/4 GAPs results in over-contraction that results in the boundary to be positioned too far to the anterior44 (Fig. 2B). This effect is rescued by the co-depletion of both Rga-3/4 and Ect-2, suggesting that GAP and GEF activities need to be balanced for proper Rho-1 function. This balance calibrates the position of the boundary between anterior and posterior domains (Fig. 2B). Interestingly, Rga-3/4 and Ect-2 both localize to the anterior, contractile cortex, suggesting that they operate in the same domain to modulate the levels of active Rho-1.44
Such a tuning of GTPase activity extends beyond RhoA functionality. Balanced activation and inactivation of Rac-1 promotes spine and synapse formation in rodent neurons.53 In these neurons, the Rac-1 GEF, Tiam1, and the Rac-1 GAP, Bcr, are present not only in the same place, but are in the same protein complex. Loss of the GAP, Bcr, leads to increased Rac-1 activation and excessive spine growth, which can be rescued by Tiam1 inhibition. This result indicates that, again, balance between GEF and GAP activities is key to achieve optimal Rac-1 activation and synaptogenesis. In this system, the existence of this physical complex of GEF and GAP implies strong spatiotemporal coupling of their activities.
These examples show how coinciding GEF and GAP activities can tune functional levels of Rho GTPase signaling activity. This type of module may have the advantage of establishing a greater dynamic range in GTPase activity, such that the optimal level of Rho signaling activity can be adjusted to a setpoint that is needed by the cell.
A question that will be important to understand is how GEFs and GAPs in this type of module can be regulated. How is the GTPase signaling activity held at an optimal level, or adjusted if necessary? One idea is that, within a complex, GAP and GEF activities could be modulated independently by varying the components of the complex.12 However, to our knowledge, this model is yet to be verified experimentally.
Cycles of GTPase activation and inactivation create Rho signaling zones
Rather than simply tuning activity level, a paired GEF/GAP in the same place can also affect the spatial distribution of the signal. Many cellular functions, from the most basic to the highly specialized, depend on compartmentalization and polarity. There are several ways in which spatial restriction can be achieved.2 One such mechanism emerges from simultaneous, co-localized, spatially restricted activity of positive (GEF) and negative (GAP) regulators. In the cell region in which the regulators are active, Rho GTPase molecules are activated by GEFs and then either bound by local effectors or inactivated by the GAP. On the one hand, binding of the GAP to active, unoccupied Rho GTPase molecules could have an anchoring effect. In addition, this GEF/GAP relationship creates constant GTPase flux between active and inactive states. Therefore, the fraction of Rho GTPase molecules that is active and not bound by effectors will likely be deactivated and not move to other parts of the cell. This interaction could then create a stable, restricted zone of active Rho (Fig. 3).
Figure 3.
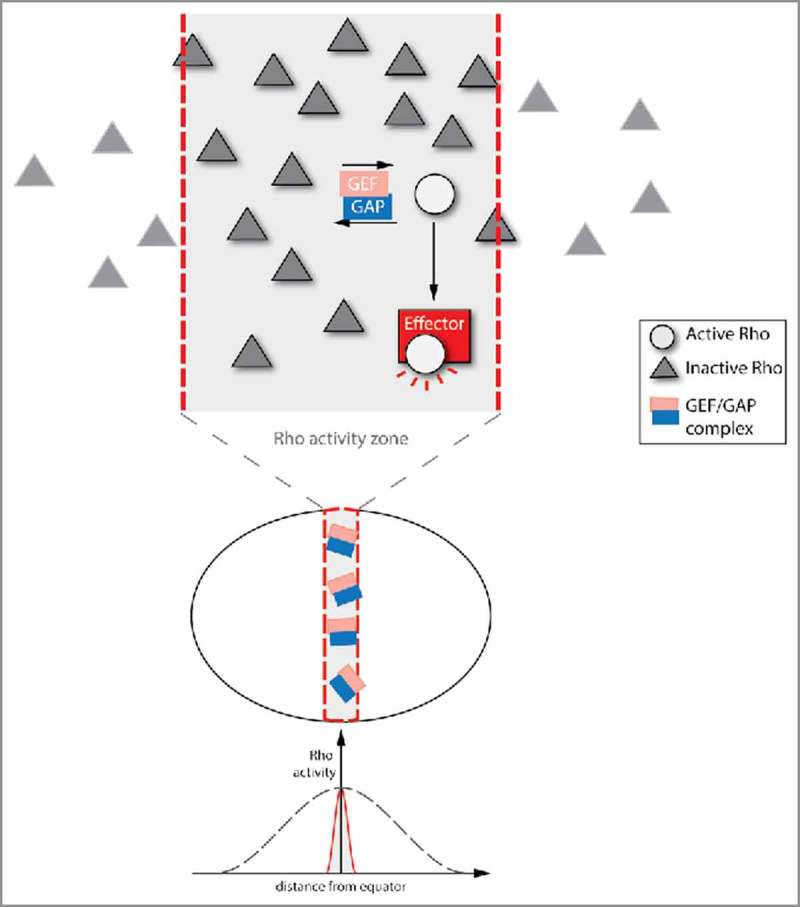
Cycling between Rho GTPase inactivation and activation creates a restricted Rho activity zone. The Rho activity cycling that emerges from spatially and temporally overlapping GAP and GEF activities can create spatially defined Rho activity zones. In this model, the Rho GTPase is only activated by a GEF within a specific part of the cell. The active form of the Rho GTPase is anchored in the Rho activity zone by the GAP, which is also area-specific (or even in a complex with the GEF). GAP-bound active Rho GTPase is immediately inactivated, leading to constant cycling between active and inactive states. However, effectors can bind to the active form of the Rho GTPase, and thereby enable downstream Rho signaling. This type of spatial restriction is necessary for successful cytokinesis in the Xenopus embryo.
This mode of signal restriction has been proposed to create a RhoA activity zone at the cell equator during cytokinesis in Xenopus laevis embryos.30 Restricting RhoA activity to this zone is essential for proper formation of the cytokinetic ring. This process was found to depend on the GEF Ect2 and the GAP MgcRacGAP. In a classic, sequential model of RhoA signaling during cytokinesis, RhoA would be first activated by the GEF, Ect2, and then inactivated by the GAP, MgcRacGAP, when cytokinesis finished. In contrast, the GAP activity of MgcRacGAP is already required during cytokinesis.30 MgcRacGAP depletion leads to an unfocused Rho activity zone, with instability and oscillations. Consistent with MgcRacGAP and Ect2 functioning together, they form a physical complex, which accumulates at the position of the RhoA activity zone.49,57 It is possible that MgcRacGAP anchors GTP-bound RhoA in the zone where it has been activated by the GEF Ect2. Secondly, the GEF/GAP complex leads to rapid turnover/flux of active RhoA. RhoA that has been activated by the GEF Ect2 is either bound by MgcRacGAP and inactivated immediately, or tethered away by binding to an effector, which initiates signaling. The combination of: 1) anchoring of active RhoA; and 2) sustained cycling between active and inactive RhoA within the Rho activity zone, mediated by the Ect2-McgRacGAP complex, appears to stop active RhoA from moving out of the Rho activity zone during its lifetime. These 2 effects could lead to the creation of a stable RhoA activity zone during cytokinesis. It has also been proposed that the GAP domain of MgcRacGAP could promote Rho activation by Ect2.25
In the model of GTPase cycling above, effectors are predicted to play a role in tethering the GTPase. Effector binding to RhoA could tether active RhoA to a certain domain and possibly prevent RhoA inactivation. However, there are many open questions about the role of effectors in creating a restricted RhoA activity zone. For example, how is effector-bound active RhoA restricted to that zone? Is the effector-RhoA complex still bound and anchored by the GAP? And in that case, how is RhoA kept from being inactivated by the GAP immediately? Experimentally investigating these questions will be essential to understand this module better.
A similar module may be at play during vertebrate development. In zebrafish and other vertebrates, neural crest cells undergo epithelial-to-mesenchymal transition (EMT) on-path to giving rise to craniofacial cartilage and other cell types.48 EMT requires drastic changes in cell morphology, adhesion, and cytoskeletal behavior. In a critical early step, cells detach from the apical side and retract their apex as they delaminate from the epithelium. Apical detachment of zebrafish neural crest cells depends on RhoA and myosin 2 activity.9 On the other hand, the RhoGAP, ArhGAP1, a negative regulator of both RhoA and Cdc42 signaling,23 is also required for efficient apical detachment. Similar to cytokinesis, ArhGAP1 depletion leads to a less focused zone of active RhoA, which might be the cause of reduced apical detachment. Exogenous ArhGAP1-GFP is evenly distributed throughout the cell, although an occasional reduction in apical domain was reported.9 The available data on this system allows for several different models. The authors of the study propose a model whereby global inhibition by a ArhGAP1 is combined with local activation by an unknown GEF. In this case, GTPase cycling might restrict RhoA signaling, similar to the GTPase flux model for cytokinesis.
Because endogenous ArhGAP1 localization is not known, it is not yet clear whether there could be compartmentalized GAP activity, as described previously (Fig. 1).47 If this were the case, one would expect ArhGAP1 to be excluded from the cell apex where RhoA is normally active. In addition, ArhGAP1 exhibits GAP activity toward both RhoA and Cdc42 and it is not clear which GTPase ArhGAP1 regulates to modulate the polarity of RhoA.23
Co-localized GEFs and GAPs lead to dynamic Rho activity
In contrast to establishing a setpoint of Rho signaling activity, another possible outcome of GEF and GAP activity being present in the same cellular domain is the possibility of signaling dynamics. This category includes the classic model of a ‘molecular switch’, where a GEF might appear first and a GAP then subsequently turns off the signal. In this case, the combined presence of GEF and GAP can result in unique functional outcomes in terms of Rho signaling activity, such as oscillations (Fig. 4).
Figure 4.
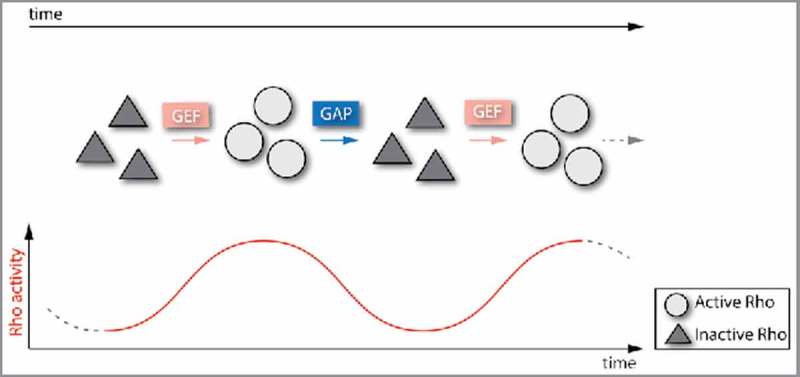
Dynamic GEF and GAP activities lead to oscillatory/pulsatile Rho activity. Co-localized GEFs and GAPs can also create temporal dynamics of Rho activity levels. For example, alternating GEF-mediated activation with GAP-mediated inactivation can result in oscillatory or pulsatile Rho activity, which is observed in the C. elegans single cell embryo and the Drosophila ventral furrow.
One such example of Rho-mediated dynamics is that there are assembly and disassembly cycles of actin and myosin 2 (actomyosin pulses).33 These actomyosin pulses are found in single cells,33,46 but are also found associated with a variety of tissue-level phenomena.4,19,26,39 It was shown that actomyosin pulses coincide with increases and decreases of RhoA effectors, such as Rho-associated and coiled-coil kinase (ROCK).32,54 In the Drosophila early embryo, a RhoGEF, called RhoGEF2, is required for contraction of the cell's apical side (apical constriction) and tissue folding.1,17 A RhoA GAP, called Cumberland GAP (C-GAP), was identified as being critical for tissue folding, as well.29 Interestingly, both RhoGEF2 and C-GAP localize to the apical surface, suggesting that both RhoA activation and inhibition are required in the same cellular domain to fold the tissue.
The RhoGEF2 protein exhibits dynamic behavior, exhibiting pulses that are similar in timescale to RhoA/ROCK pulses.29 RhoGEF2 appearance precedes that of ROCK and actomyosin, suggesting that RhoA activation initiates an actomyosin pulse. Depletion or overexpression of C-GAP changes the amount of myosin 2 that is left remaining at the end of a pulse, suggesting that C-GAP's function is to inactivate RhoA at the end of the pulse. The dynamics of C-GAP activity and localization are still not known. However, analysis of pulsing in the C. elegans embryo has demonstrated that the Rho GAP Rga-3 exhibits delayed recruitment relative to RhoA activity.41 This suggests that there is delayed negative feedback in the system and that increases and decreases of GEF and GAP activities that are offset, but in the same general location can create dynamic changes in Rho activation. Importantly, we pointed out before, that C-GAP also appears to restrict actomyosin to the apical domain. Therefore, C-GAP functions in 2 modules during tissue folding: 1) localizing with RhoGEF2 to promote Rho1 dynamics, and 2) localizing to a distinct compartment to restrict Rho1 activity to the apical side of the cell. This example illustrates that different modules, i.e. GEF/GAP relationships of different categories, can be combined to fulfill complex tasks regarding proper Rho signaling activity.
Another example in which the timing of a Rho GAP is regulated to promote cyclical actomyosin contraction is during fertilization in the C.elegans spermatheca.52 In this process, oocytes enter the spermatheca, remain inside it for fertilization, and are subsequently expulsed by Rho1-mediated contraction. Depletion of the GAP, Spv-1, leads to premature contraction of the spermatheca, which causes embryo deformation and increased lethality of the offspring. Interestingly, the Spv-1 GAP also contains an F-BAR domain, which binds to curved phospholipid bilayers. When the spermatheca is empty, and plasma membranes are possibly corrugated, the Spv-1 GAP is present on the plasma membrane where it inhibits Rho-1 and actomyosin contractility. When an oocyte enters the spermatheca and stretches out the cell membrane folds, Spv-1 is released from the membrane and RhoA is then activated. This mechanism creates a timed cycle between Rho activation and inactivation, allowing cycles of spermatheca relaxation and then contraction that are connected with when an oocyte is present.
In many cases, it is unknown what regulates the localization of GAPs; for instance, C-GAP does not have any other protein interaction motifs. One possibility is that the GAP is localized through feedback by downstream effectors of the Rho signaling pathway. Feedback loops have been observed as crucial elements of the RhoA signaling pathway in several contexts.10,32,35 Several RhoGAPs seem to bind to actin filaments or cables,11,41 which suggests that actin filament assembly downstream of Rho signaling might have a role in recruiting RhoGAPs. This actin-mediated GAP recruitment could represent delayed negative feedback. However, it should be noted that active RhoA and ROCK can also be associated with actin structures and that RhoGAPs could be binding to the active form of the GTPase.20,51 In the case of the F-BAR-containing GAP, Spv-1, its membrane localization is linked to whether or not the spermatheca cells are stretched, suggesting mechanical feedback in localization.
Concluding remarks
This review discusses a variety of modules for GEF-GAP interactions. These modules can precisely regulate Rho GTPase signals and in some cases operate together at the same time. Thus, these modules are not in conflict or mutually exclusive. All modules likely reflect some part of the reality of Rho regulation. Importantly, various GEF/GAP interactions can occur in the same organism and are likely even combined in the same processes. Even though we only consider interactions between 2 regulators here, we already describe a variety of elaborate Rho signal patterns; and combination of these modules renders an even larger toolbox for specialized, fine-tuned regulation. The amount of possible regulation is even larger, given the numbers of regulating RhoGEFs and RhoGAPs in various genomes. In addition, Rho GTPases are regulated on the levels of transcription and translation, by post-translational modifications, and by guanine dissociation inhibitors (GDIs), suggesting even more elaborate mechanisms of regulation.5,7
Another powerful role for GEF/GAP interactions, which we have not discussed in depth, is the crosstalk between different Rho GTPases.16 For example, if GAPs and GEFs which are specific to different Rho GTPases, respectively, are co-localized, in a complex, or even in the same protein, they can promote signaling activity of one Rho GTPase in that region, while inhibiting the other Rho GTPase in the same area.21,55 This signaling system can lead to mutually exclusive Rho activity zones and is also critical for Rho GTPase function.
Understanding how Rho GTPases are regulated and how they can be manipulated is important because of the ubiquitous medical relevance of Rho signaling pathways. Altered Rho GTPase signaling has been strongly implicated in diseases ranging from mental retardation and neurodegeneration to cancer and developmental defects.34 A telling example of the importance of Rho GTPase regulation in particular is cancer. Although over-activation of Rho pathway signaling has been implicated in many cancer types, the Rho GTPases themselves are not usually found to be mutated.13 This result implies that changes in the upstream GTPase regulation could be the cause of over-active Rho signaling. For X-linked mental retardation, genes implicated in the disease include the RhoGAP oligophrenin-1 and the RhoGEF αPIX.38 These findings emphasize the importance of understanding the general rules that define Rho GTPase regulation.
Here, we have categorized regulatory modules that have been found to operate on Rho GTPases in metazoan and predominantly developmental systems. As the mode of regulation used in a system depends strongly on the spatiotemporal relationship between regulators, better spatial and especially temporal resolution in experiments will be imperative. Furthermore, what exactly elicits the various specific activity patterns observed in GAPs and GEFs is unknown in most cases and will be an exciting area of future research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 1997; 91:905-915; PMID:9428514; http://dx.doi.org/ 10.1016/S0092-8674(00)80482-1 [DOI] [PubMed] [Google Scholar]
- [2].Bement WM, Miller AL, von Dassow G. Rho GTPase activity zones and transient contractile arrays. Bioessays 2006; 28:983-993; PMID:16998826; http://dx.doi.org/ 10.1002/bies.20477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bernards A. GAPs galore! A survey of putative ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta 2003; 1603:47-82; PMID:12618308 [DOI] [PubMed] [Google Scholar]
- [4].Blanchard GB, Murugesu S, Adams RJ, Martinez-Arias A, Gorfinkiel N. Cytoskeletal dynamics and supracellular organisation of cell shape fluctuations during dorsal closure. Development 2010; 137:2743-52; PMID:20663818; http://dx.doi.org/ 10.1242/dev.045872 [DOI] [PubMed] [Google Scholar]
- [5].Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 2007; 29:356-370; PMID:17373658; http://dx.doi.org/ 10.1002/bies.20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chau AH, Walter JM, Gerardin J, Tang C, Lim WA. Designing synthetic regulatory networks capable of self-organizing cell polarization. Cell 2012; 151:320-332; PMID:23039994; http://dx.doi.org/ 10.1016/j.cell.2012.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93:269-309; PMID:23303910; http://dx.doi.org/ 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- [8].Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell 2001; 104:593-604; PMID:11239415; http://dx.doi.org/ 10.1016/S0092-8674(01)00245-8 [DOI] [PubMed] [Google Scholar]
- [9].Clay MR, Halloran MC. Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition. Development 2013; 140:3198-3209; PMID:23804498; http://dx.doi.org/ 10.1242/dev.095448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Coravos JS, Martin AC. Apical sarcomere-like actomyosin contracts nonmuscle drosophila epithelial cells. Dev Cell 2016; 39:346-358; PMID:27773487; http://dx.doi.org/ 10.1016/j.devcel.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Diogon M, Wissler F, Quintin S, Nagamatsu Y, Sookhareea S, Landmann F, Hutter H, Vitale N, Labouesse M. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. Elegans epidermis to control morphogenesis. Development 2007; 134:2469-79; PMID:17537791; http://dx.doi.org/ 10.1242/dev.005074 [DOI] [PubMed] [Google Scholar]
- [12].Duman JG, Mulherkar S, Tu YK, X Cheng J, Tolias KF. Mechanisms for spatiotemporal regulation of Rho-GTPase signaling at synapses. Neurosci Lett 2015; 601:4-10; PMID:26003445; http://dx.doi.org/ 10.1016/j.neulet.2015.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ellenbroek SI, Collard JG. Rho GTPases: Functions and association with cancer. Clin Exp Metastasis 2007; 24:657-672; PMID:18000759; http://dx.doi.org/ 10.1007/s10585-007-9119-1 [DOI] [PubMed] [Google Scholar]
- [14].Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629-635; PMID:12478284; http://dx.doi.org/ 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- [15].Goryachev AB, Pokhilko AV. Computational model explains high activity and rapid cycling of Rho GTPases within protein complexes. PLoS Comput Biol 2006; 2:e172; PMID:17140284; http://dx.doi.org/ 10.1371/journal.pcbi.0020172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: Another social network?. Trends Cell Biol 2011; 21:718-726; PMID:21924908; http://dx.doi.org/ 10.1016/j.tcb.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hacker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev 1998; 12:274-284; PMID:9436986; http://dx.doi.org/ 10.1101/gad.12.2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature 1999; 402:C47-52; PMID:10591225; http://dx.doi.org/ 10.1038/35011540 [DOI] [PubMed] [Google Scholar]
- [19].He L, Wang X, Tang HL, Montell DJ. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol 2010; 12:1133-42; PMID:21102441; http://dx.doi.org/ 10.1038/ncb2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase–mediated contraction of isolated stress fibers. J Cell Biol 2001; 153:569-584; PMID:11331307; http://dx.doi.org/ 10.1083/jcb.153.3.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kutys ML, Yamada KM. An extracellular-matrix-specific GEF-GAP interaction regulates Rho GTPase crosstalk for 3D collagen migration. Nat Cell Biol 2014; 16:909-917; PMID:25150978; http://dx.doi.org/ 10.1038/ncb3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kutys ML, Yamada KM. Rho GEFs and GAPs: emerging integrators of extracellular matrix signaling. Small GTPases 2015; 6:16-19; PMID:25862162; http://dx.doi.org/ 10.4161/21541248.2014.989792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lancaster CA, Taylor-Harris PM, Self AJ, Brill S, van Erp HE, Hall A. Characterization of rhoGAP. A GTPase-activating protein for rho-related small GTPases. J Biol Chem 1994; 269:1137-42; PMID:8288572 [PubMed] [Google Scholar]
- [24].Lin R, Cerione RA, Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J Biol Chem 1999; 274:23633-41; PMID:10438546; http://dx.doi.org/ 10.1074/jbc.274.33.23633 [DOI] [PubMed] [Google Scholar]
- [25].Loria A, Longhini KM, Glotzer M. The RhoGAP domain of CYK-4 has an essential role in RhoA activation. Curr Biol 2012; 22:213-9; PMID:22226748; http://dx.doi.org/ 10.1016/j.cub.2011.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 2009; 457:495-9; PMID:19029882; http://dx.doi.org/ 10.1038/nature07522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Martin SG. Spontaneous cell polarization: Feedback control of Cdc42 GTPase breaks cellular symmetry. Bioessays 2015; 37:1193-1201; PMID:26338468; http://dx.doi.org/ 10.1002/bies.201500077 [DOI] [PubMed] [Google Scholar]
- [28].Mason FM, Martin AC. Tuning cell shape change with contractile ratchets. Curr Opin Genet Dev 2011; 21:671-9; PMID:21893409; http://dx.doi.org/ 10.1016/j.gde.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mason FM, Xie S, Vasquez CG, Tworoger M, Martin AC. RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J Cell Biol 2016; 214:603-617; PMID:27551058; http://dx.doi.org/ 10.1083/jcb.201603077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol 2009; 11:71-77; PMID:19060892; http://dx.doi.org/ 10.1038/ncb1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Motegi F, Sugimoto A. Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nat Cell Biol 2006; 8:978-985; PMID:16921365; http://dx.doi.org/ 10.1038/ncb1459 [DOI] [PubMed] [Google Scholar]
- [32].Munjal A, Philippe JM, Munro E, Lecuit T. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 2015; 524:351-5; PMID:26214737; http://dx.doi.org/ 10.1038/nature14603 [DOI] [PubMed] [Google Scholar]
- [33].Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell 2004; 7:413-424; PMID:15363415; http://dx.doi.org/ 10.1016/j.devcel.2004.08.001 [DOI] [PubMed] [Google Scholar]
- [34].Newell-Litwa KA, Horwitz R, Lamers ML. Non-muscle myosin II in disease: Mechanisms and therapeutic opportunities. Dis Model Mech 2015; 8:1495-1515; PMID:26542704; http://dx.doi.org/ 10.1242/dmm.022103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Priya R, Gomez GA, Budnar S, Verma S, Cox HL, Hamilton NA, Yap AS. Feedback regulation through myosin II confers robustness on RhoA signalling at E-cadherin junctions. Nat Cell Biol 2015; 17:1282-93; PMID:26368311; http://dx.doi.org/ 10.1038/ncb3239 [DOI] [PubMed] [Google Scholar]
- [36].Qiu RG, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol 1997; 17:3449-58; PMID:9154844; http://dx.doi.org/ 10.1128/MCB.17.6.3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Quintin S, Gally C, Labouesse M. Epithelial morphogenesis in embryos: Asymmetries, motors and brakes. Trends Genet 2008; 24:221-230; PMID:18375008; http://dx.doi.org/ 10.1016/j.tig.2008.02.005 [DOI] [PubMed] [Google Scholar]
- [38].Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci 2002; 25:191-9; PMID:11998687; http://dx.doi.org/ 10.1016/S0166-2236(00)02118-4 [DOI] [PubMed] [Google Scholar]
- [39].Rauzi M, Lenne PF, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 2010; 468:1110-4; PMID:21068726; http://dx.doi.org/ 10.1038/nature09566 [DOI] [PubMed] [Google Scholar]
- [40].Ridley AJ. Rho family proteins: Coordinating cell responses. Trends Cell Biol 2001; 11:471-7; PMID:11719051; http://dx.doi.org/ 10.1016/S0962-8924(01)02153-5 [DOI] [PubMed] [Google Scholar]
- [41].Robin FB, Michaux JB, McFadden WM, Munro E. Excitable RhoA dynamics drive pulsed contractions in the early C. elegans embryo. BioRxiv 2016; 076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rolo A, Savery D, Escuin S, de Castro SC, Armer HE, Munro PM, Mole MA, Greene ND, Copp AJ. Regulation of cell protrusions by small GTPases during fusion of the neural folds. Elife 2016; 5:e13273; PMID:27114066; http://dx.doi.org/ 10.7554/eLife.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schmutz C, Stevens J, Spang A. Functions of the novel RhoGAP proteins RGA-3 and RGA-4 in the germ line and in the early embryo of C. Elegans. Development 2007; 134:3495-3505; PMID:17728351; http://dx.doi.org/ 10.1242/dev.000802 [DOI] [PubMed] [Google Scholar]
- [44].Schonegg S, Constantinescu AT, Hoege C, Hyman AA. The Rho GTPase-activating proteins RGA-3 and RGA-4 are required to set the initial size of PAR domains in caenorhabditis elegans one-cell embryos. Proc Natl Acad Sci U S A 2007; 104:14976-81; PMID:17848508; http://dx.doi.org/ 10.1073/pnas.0706941104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schonegg S, Hyman AA. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. Elegans embryos. Development 2006; 133:3507-16; PMID:16899536; http://dx.doi.org/ 10.1242/dev.02527 [DOI] [PubMed] [Google Scholar]
- [46].Sedzinski J, Biro M, Oswald A, Tinevez JY, Salbreux G, Paluch E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 2011; 476:462-6; PMID:21822289; http://dx.doi.org/ 10.1038/nature10286 [DOI] [PubMed] [Google Scholar]
- [47].Simoes S, Denholm B, Azevedo D, Sotillos S, Martin P, Skaer H, Hombria JC, Jacinto A. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development 2006; 133:4257-67; PMID:17021037; http://dx.doi.org/ 10.1242/dev.02588 [DOI] [PubMed] [Google Scholar]
- [48].Simoes-Costa M, Bronner ME. Establishing neural crest identity: A gene regulatory recipe. Development 2015; 142:242-257; PMID:25564621; http://dx.doi.org/ 10.1242/dev.105445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell 2003; 4:29-39; PMID:12530961; http://dx.doi.org/ 10.1016/S1534-5807(02)00402-1 [DOI] [PubMed] [Google Scholar]
- [50].Sweeton D, Parks S, Costa M, Wieschaus E. Gastrulation in Drosophila: The formation of the ventral furrow and posterior midgut invaginations. Development 1991; 112:775-789; PMID:1935689 [DOI] [PubMed] [Google Scholar]
- [51].Szulcek R, Beckers CM, Hodzic J, de Wit J, Chen Z, Grob T, Musters RJ, Minshall RD, van Hinsbergh VW, van Nieuw Amerongen GP. Localized RhoA GTPase activity regulates dynamics of endothelial monolayer integrity. Cardiovasc Res 2013; 99:471-482; PMID:23536606; http://dx.doi.org/ 10.1093/cvr/cvt075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tan PY, Zaidel-Bar R. Transient membrane localization of SPV-1 drives cyclical actomyosin contractions in the C. Elegans spermatheca. Curr Biol 2015; 25:141-151; PMID:25532891; http://dx.doi.org/ 10.1016/j.cub.2014.11.033 [DOI] [PubMed] [Google Scholar]
- [53].Um K, Niu S, Duman JG, Cheng JX, Tu YK, Schwechter B, Liu F, Hiles L, Narayanan AS, Ash RT, et al.. Dynamic control of excitatory synapse development by a Rac1 GEF/GAP regulatory complex. Dev Cell 2014; 29:701-715; PMID:24960694; http://dx.doi.org/ 10.1016/j.devcel.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vasquez CG, Tworoger M, Martin AC. Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J Cell Biol 2014; 206:435-450; PMID:25092658; http://dx.doi.org/ 10.1083/jcb.201402004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vaughan EM, Miller AL, Yu HY, Bement WM. Control of local Rho GTPase crosstalk by Abr. Curr Biol 2011; 21:270-7; PMID:21295482; http://dx.doi.org/ 10.1016/j.cub.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vincent S, Settleman J. Inhibition of RhoGAP activity is sufficient for the induction of Rho-mediated actin reorganization. Eur J Cell Biol 1999; 78:539-548; PMID:10494860; http://dx.doi.org/ 10.1016/S0171-9335(99)80019-3 [DOI] [PubMed] [Google Scholar]
- [57].Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol 2005; 170:571-582; PMID:16103226; http://dx.doi.org/ 10.1083/jcb.200501097 [DOI] [PMC free article] [PubMed] [Google Scholar]


