Abstract
Background
Socioeconomic inequalities in neonatal mortality are substantial in many developing countries. Little is known about how to address this problem. Trials in Asia and Africa have shown strong impacts on neonatal mortality of a participatory learning and action intervention with women’s groups. Whether this intervention also reduces mortality inequalities remains unknown. We describe the equity impact of this women’s groups intervention on the neonatal mortality rate (NMR) across socioeconomic strata.
Methods
We conducted a meta-analysis of all four participatory women’s group interventions that were shown to be highly effective in cluster randomized trials in India, Nepal, Bangladesh and Malawi. We estimated intervention effects on NMR and health behaviours for lower and higher socioeconomic strata using random effects logistic regression analysis. Differences in effect between strata were tested.
Results
Analysis of 69120 live births and 2505 neonatal deaths shows that the intervention strongly reduced the NMR in lower (50–63% reduction depending on the measure of socioeconomic position used) and higher (35–44%) socioeconomic strata. The intervention did not show evidence of ‘elite-capture’: among the most marginalized populations, the NMR in intervention areas was 63% lower [95% confidence interval (CI) 48–74%] than in control areas, compared with 35% (95% CI: 15–50%) lower among the less marginalized in the last trial year (P-value for difference between most/less marginalized: 0.009). The intervention strongly improved home care practices, with no systematic socioeconomic differences in effect.
Conclusions
Participatory women’s groups with high population coverage benefit the survival chances of newborns from all socioeconomic strata, and perhaps especially those born into the most deprived households.
Keywords: Developing countries, obstetric, infant mortality, intervention studies, inequalities
Key Messages
This is one of the first studies to report on the equity impact of an intervention on neonatal mortality across socioeconomic strata.
Based on a meta-analysis of four cluster randomized controlled trials, including 69120 live births and 2505 neonatal deaths, we find that community-based women’s groups reduced neonatal mortality equitably and substantially across socioeconomic strata. This contrasts with concerns about elite capture of community-based interventions and with evidence that health interventions generally benefit the better-off most.
We conclude that community-based women’s groups can substantially contribute to the survival of all newborns, perhaps especially of disadvantaged infants, and to progress towards the targets set in the Every Newborn Action Plan.
Introduction
A world without preventable newborn deaths, as envisaged in the Every Newborn Action Plan led by WHO and UNICEF, is only achievable when strategies are available to close the gap in newborn mortality between socioeconomic groups.1,2 The odds of surviving the first month of life are grossly unequal between infants born in poor and rich households and to higher and less educated mothers within the same country.3–5 Unfortunately, little is known about what works to close these mortality gaps.
The strongest evidence on intervention effects comes from randomized controlled trials (RCT). Four cluster randomized controlled trials, in Asia and Africa, have shown that community-based women’s groups can substantially reduce neonatal mortality, perhaps especially through improved home care practices, provided that coverage of the women’s groups in the population is sufficiently high (≥ 30% of pregnant women participating in groups).6 These trials provide an opportunity to assess the equity impact of community-based interventions on neonatal mortality using a gold standard study design. Whereas community-based interventions run the risk of elite capture, in which the best-off reap more benefits,7–11 there are indications that community-based women’s groups can reduce mortality inequalities by reaching all socioeconomic strata through their soft-targeting approach (i.e. tailoring the intervention design to the target group rather than applying strict in-/exclusion criteria).12,13 The equity impact of the groups has, however, not yet been evaluated systematically across the trials, and a meta-analysis will generate evidence on approaches that may reduce inequalities in neonatal mortality.
In this study, we analysed the effect of community-based women’s groups on neonatal mortality, health care use and home care practices in lower and higher socioeconomic strata, and tested for differences in effect between strata, using data from four RCTs.
Methods
We included all four RCTs of community-based women’s groups that had sufficient population coverage to be able to have an effect on the neonatal mortality rate (NMR). These studies were identified in two previously published systematic reviews and a meta-analysis.6,14 That meta-analysis, and a corresponding heterogeneity test, have shown that the women’s group intervention only has an effect on neonatal mortality when coverage is high. Three women’s group trials had had too low coverage to be able to have an effect on NMR. In those trials, 1%, 3% and 10% of pregnant women in intervention areas attended groups, respectively,15–17 compared with around 35–50% in the other trials.6 Given that we were interested in the distribution of the substantial mortality effects shown in the highly effective women’s groups interventions, we considered further sub-group analyses of the low-coverage trials a priori as futile.
Nonetheless, we conducted a secondary analysis of all six published trials that contained data on socioeconomic position, i.e. including the four ‘high coverage trials’18–21 and two ‘low coverage trials’.15,16 In a third ‘low coverage trial’,17 no information on socioeconomic position was collected, so the trial had to be excluded. We also had to exclude a trial in Dhanusha district in Nepal, of which the findings have not yet been published.22
The four ‘high coverage trials’ were located in rural areas of Nepal, India, Bangladesh and Malawi. One ‘low coverage trial’ was located in rural Bangladesh, and one in urban India (Mumbai). In the trial sites, geographical clusters were randomly allocated to the intervention or control arm (Table S1, available as Supplementary data at IJE online). In the intervention areas, participatory learning and action groups were set up. The groups identified and prioritized maternal and newborn health problems, and developed and evaluated strategies to address them. Strategies included, among others, home visits to pregnant women, emergency funds, arrangements for emergency transport to a health facility, and the preparation and distribution of safe delivery kits. The groups met monthly under the guidance of a facilitator for 2–3 years. The intervention and evaluation design were similar across the study sites.6 This intervention led to a reduction in neonatal mortality of around 30–40% when intervention coverage was high.6,18–21
The women’s groups are a community-based intervention, in which active engagement of the wider community played an important role. The women’s groups, for example, organized community meetings in which they explicitly asked support from the wider community for the implementation of their strategies. The strategies were meant to benefit all pregnant women, also those not attending groups. Women’s group members, for example, made home visits to women who were not members, to tell them about what they have learned in the group and/or to support them during delivery and the newborn period. Therefore, intervention effects are expected to be seen community-wide, not just among women’s group members.
The trials were conducted in large surveillance sites where the total population was prospectively followed up and all births and birth outcomes were registered. Identification was done by local key informants who typically covered 200–250 households. Interviewers verified the key informants’ information and interviewed all mothers around 6 weeks postpartum.23,24 We obtained the full individual-level trial datasets from the trial analyst or database manager and included all live births to residents of the study areas. For the Bangladesh trial, the migrant tea garden populations, which were socioculturally distinct from the other study areas, were excluded, following the original trial paper (we examined sensitivity of our main findings against the in/exclusion of the tea garden population and found that findings remained very similar).
We examined intervention effects on NMR (number of deaths in the first 28 days of life/1000 live births) and health care use and home care practices (i.e. the primary and secondary outcomes of the original trials) among lower and higher socioeconomic strata. The strongest effects were generally reported in the final trial year, probably due to a lag time during which the groups become established and active. Given our interest in the distribution of these strong effects, we conducted our main analysis for the final year, as a priori planned, and secondary analyses for the last two trial years (time window for evaluation in several trials).
The equity impact was examined for dimensions of socioeconomic position that were relevant across all sites: literacy (can read vs cannot read, based on respondent’s ability to read a brief passage) and economic status. Household ownership of assets, combined into an index using principal component analysis for each trial, was used as indicator of economic status. A high percentage of births (50–60% in the Nepal and India trials) took place in households with none or few of the asset items for which data were available. Based on their asset index score, live births were categorized into two roughly equally sized groups, poor and less poor, using Stata’s quantile function. For the Nepal trial, we were restricted by the pre-defined asset levels in the postpartum interview, which we combined into two wealth groups (poorest: owns none of the asset items; less poor: owns at least one asset item) (see Table 1). Given our interest in intervention effects on the bottom end of the socioeconomic ladder, we also constructed a multidimensional measure of socioeconomic position, including both literacy and economic status, with those who were illiterate and poor categorized as ‘most marginalized’ and the rest of the population as ‘less marginalized’. The definitions of these dimensions of socioeconomic position were specified in advance of the analysis.
Table 1.
Number of live births and neonatal deaths in intervention and control areas, baseline and trial periods combined
| Nepala |
India |
Bangladesh |
Malawi |
Total |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Intervention |
Control |
Intervention |
Control |
Intervention |
Control |
Intervention |
|||||||||||||||||||
| Live births | % | Neonatal deaths | Live births | % | Neonatal deaths | Live births | % | Neonatal deaths | Live births | % | Neonatal deaths | Live births | % | Neonatal deaths | Live births | % | Neonatal deaths | Live births | % | Neonatal deaths | Live births | % | Neonatal deaths | Live births | Neonatal deaths | |
| Total | 3264 | 100 | 121 | 2923 | 100 | 78 | 10981 | 100 | 634 | 11735 | 100 | 542 | 13373 | 100 | 411 | 13647 | 100 | 359 | 6776 | 100 | 202 | 6421 | 100 | 158 | 69120 | 2505 |
| Marginalization | ||||||||||||||||||||||||||
| Less marginalized | 1727 | 55 | 56 | 1846 | 65 | 45 | 5675 | 52 | 297 | 4668 | 40 | 205 | 11084 | 83 | 330 | 10986 | 81 | 269 | 5162 | 77 | 152 | 4964 | 78 | 127 | 46112 | 1481 |
| Most marginalized | 1433 | 45 | 60 | 996 | 35 | 32 | 5296 | 48 | 337 | 7063 | 60 | 337 | 2283 | 17 | 81 | 2655 | 19 | 89 | 1562 | 23 | 50 | 1400 | 22 | 31 | 22688 | 1017 |
| Literacy | ||||||||||||||||||||||||||
| Literate | 717 | 23 | 25 | 1111 | 40 | 19 | 3363 | 31 | 170 | 2795 | 24 | 112 | 10237 | 77 | 289 | 10199 | 75 | 249 | 4123 | 61 | 116 | 4101 | 65 | 112 | 36646 | 1092 |
| Illiterate | 2364 | 77 | 91 | 1668 | 60 | 56 | 7598 | 69 | 462 | 8932 | 76 | 429 | 3133 | 23 | 122 | 3437 | 25 | 109 | 2590 | 39 | 86 | 2257 | 35 | 46 | 31979 | 1401 |
| Economic status | ||||||||||||||||||||||||||
| Less poor | 1437 | 44 | 46 | 1427 | 49 | 36 | 4570 | 42 | 232 | 3391 | 29 | 142 | 6386 | 48 | 171 | 5959 | 44 | 128 | 3021 | 45 | 86 | 2654 | 42 | 51 | 28845 | 892 |
| Poorest | 1818 | 56 | 75 | 1484 | 51 | 42 | 6411 | 58 | 402 | 8343 | 71 | 400 | 6978 | 52 | 240 | 7684 | 56 | 230 | 3692 | 55 | 115 | 3700 | 58 | 106 | 40110 | 1610 |
Summing of the sub-groups does not always add up to the totals because of missing values.
Note on measurement of economic status: Nepal: predefined asset levels in the surveillance questionnaire, based on household ownership of one or more of the items in the list, were as follows: richest (bus, truck, motorcycle, TV, motor tractor, fridge, hand tractor), next-rich (sewing machine, cassette player, fan, radio, camera, bicycle), next-poor (wall clock, iron), poorest (none of the above). As over 50% of live births were in households that owned none of the asset items, we combined the richest, next-rich and next-poor level into the category ‘less poor’. India, Bangladesh, Malawi: principal component analysis-based asset index. List of asset items for which data were available varied between trials: India (electricity, generator, battery, fan, TV, radio, tape, fridge, bicycle, motor), Bangladesh (electricity, generator, fan, TV, radio, fridge, bicycle, telephone), Malawi (electricity, radio, bicycle, motor, car, lamp, oxcart).
aFor the original Nepal trial paper, data entry was frozen on 1 December 2003. However, after this date, the monitoring and evaluation team continued to receive a few additional forms for births that took place within the trial period (38 live births, of which 2 NND in control areas, and 24 live births of which 2 NND in intervention areas). We included the data for these records in our analysis. Although the inclusion of these births that took place within the trial period is certainly more accurate, the very small difference in numbers of records will have had no effect on the findings.
Statistical analyses
In sum, we obtained our findings using logistic regression analysis in which we adjusted for the trials’ sampling schemes. The clustered design of the trials was adjusted for using random effects modelling; for trials with a stratified design (India, Bangladesh), we included stratum as covariate. We checked the quadrature approximation used in the random effects estimators using the quadchk command in Stata.
For the Nepal trial, with pair-matched clusters, we included a fixed effect for matching pair in the models. In the Malawi trial, which used a factorial design, the mortality effect of the intervention was based on a ‘treatment group analysis’, comparing the ‘women’s group only’ arm with the ‘no intervention’ arm. We replicated this in our analysis.
The original trial papers usually estimated the difference in log odds of neonatal death between intervention and control arm at endline only, adjusted for sampling design, essentially using the following model:
| (1) |
Interv.Arm is the dummy for intervention vs control arm. β1 represents the difference in log odds of neonatal death (NND) between intervention and control arm at endline, β2 the adjustment for the stratified sampling design and u the random effect term for cluster.
We expanded this by adding information about baseline neonatal mortality, thereby adjusting for the difference in odds of neonatal death between intervention and control arm at baseline (equation 2):
| (2) |
‘Endline’ is the dummy for endline vs baseline. β1 now stands for the difference in log odds of NND between intervention and control arm at baseline, β2 for the difference in log odds of NND between endline and baseline in the control arm, and β3 for the effect of the intervention, i.e. difference in log odds of NND between intervention and control arm at endline, adjusted for baseline differences in neonatal mortality. The exponent of β3 provides the odds ratio (OR) for the intervention effect in the total population adjusted for baseline differences.
As most studies had more than one intervention year, we expanded equation 2 by distinguishing the intervention effect between follow-up years (equation 3):
| (3) |
Year is the categorical variable for the separate intervention years, where β2y splits up β2 into two or three (depending on the number of intervention years) separate effects of year. β3y represents the difference in log odds of NND between intervention and control arm in year y, adjusted for baseline differences. For example, β33 gives the difference in log odds of NND between intervention and control arm in year 3 (adjusted for baseline differences). The exponents of β3y are given in the first row of Table 3 and provide the intervention effects for the total population, adjusted for baseline mortality differences between intervention and control.
Table 3.
Intervention effects on the neonatal mortality rate for lower and higher socioeconomic groups, per trial and pooled estimates, for the last study year
| Pooled estimates |
Nepal |
India |
Bangladesh |
Malawi |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORa | 95% CI | P- valueb | ORa | 95% CI | P- valueb | ORa | 95% CI | P- valueb | ORa | 95% CI | P- valueb | ORa | 95% CI | P- valueb | |
| Total | 0.51 | (0.42–0.63) | 0.000 | 0.64 | (0.40–1.03) | 0.064 | 0.46 | (0.32–0.65) | 0.000 | 0.45 | (0.31–0.64) | 0.000 | 0.80 | (0.43–1.51) | 0.498 |
| Marginalization | |||||||||||||||
| Less marginalized | 0.65 | (0.50–0.85) | 0.009 | 0.82 | (0.45–1.50) | 0.240 | 0.82 | (0.48–1.41) | 0.010 | 0.49 | (0.32–0.73) | 0.413 | 0.85 | (0.43–1.70) | 0.652 |
| Most marginalized | 0.37 | (0.26–0.52) | 0.45 | (0.20–1.01) | 0.32 | (0.20–0.51) | 0.33 | (0.14–0.76) | 0.67 | (0.25–1.79) | |||||
| Literacy | |||||||||||||||
| Literate | 0.56 | (0.41–0.76) | 0.587 | 0.50 | (0.20–1.27) | 0.551 | 0.71 | (0.33–1.50) | 0.240 | 0.46 | (0.30–0.70) | 0.798 | 0.91 | (0.43–1.94) | 0.547 |
| Illiterate | 0.50 | (0.37–0.66) | 0.70 | (0.40–1.23) | 0.42 | (0.28–0.63) | 0.41 | (0.20–0.84) | 0.66 | (0.27–1.59) | |||||
| Economic status | |||||||||||||||
| Less poor | 0.58 | (0.42–0.80) | 0.360 | 0.91 | (0.47–1.75) | 0.143 | 0.76 | (0.41–1.42) | 0.055 | 0.31 | (0.17–0.57) | 0.120 | 0.75 | (0.30–1.88) | 0.864 |
| Poorest | 0.48 | (0.36–0.62) | 0.44 | (0.22–0.90) | 0.36 | (0.24–0.56) | 0.57 | (0.36–0.90) | 0.83 | (0.39–1.76) | |||||
aThe ratio of the odds of neonatal mortality in the intervention compared with the control areas adjusted for baseline differences. For the Nepal trial, it was not possible to adjust for baseline mortality differences because of the absence of prospectively collected baseline data.
bP-value for the test on difference in OR between lowest and highest socioeconomic groups. For the total population, it gives the P-value for the difference between intervention and control. P-values for heterogeneity test in pooled analysis: marginalization (P = 0.3212), literacy (P = 0.3548), economic status (P = 0.04148). Absolute intervention effects for last study year (per 1000 live births): India, less marginalized −9 (95% CI: −32;15), most marginalized: −55 (95% CI: −78;−30), P-value for difference: 0.012. Nepal, less marginalized −5 (95% CI: −21;11), most marginalized −21 (95% CI: −42;0), P-value for difference: 0.212. Bangladesh, less marginalized −17 (95% CI: −27;−6), most marginalized −31 (95% CI: −53;−7), P-value for difference: 0.272. Malawi, less marginalized −3 (95% CI: −11;6), most marginalized −10 (95% CI: −29;11), P-value for difference: 0.542.
Then, we estimated the intervention effects for each socioeconomic stratum, while adjusting for baseline differences between intervention and control arm in socioeconomic inequality in neonatal mortality (equation 4):
| (4) |
SEP_L is the dummy for low vs high socioeconomic position. Now, β1 represents the difference in log odds of NND between intervention and control arm at baseline for the high socioeconomic group (the reference group); β2y the effect of the different years in the control arm for the high socioeconomic group; β3y the difference in odds of NND between intervention and control arm in year y for the high socioeconomic group, adjusted for baseline differences in log odds of NND (i.e. the intervention effect in year y for the high socioeconomic group adjusted for mortality baseline differences); β4 the difference in log odds of NND between low and high socioeconomic position at baseline; β5 the difference in effect of year between low and high socioeconomic position in the control arm; β6 the difference between low and high socioeconomic position in log odds of NND between intervention and control arm at baseline; and β7 the difference in intervention effect in year y between low and high socioeconomic position (adjusted for baseline mortality differences). The P-values for β7 are those for the difference in intervention effect between low and high socioeconomic position as given in Table 3. The exponent of β3 represents the intervention effect in the high socioeconomic group and the exponent of (β3 + β7) represents the intervention effect in the low socioeconomic group as given in Table 3. We transformed the estimated log odds into NMR by socioeconomic group by year and trial arm, as given in Table 2 and Figure 1.
Table 2.
Neonatal mortality rate (per 1000 live births) for baseline and intervention years, for intervention and control areas, four women’s group trials
| Nepal |
India |
Bangladesh |
Malawi |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Intervention |
Control |
Intervention |
Control |
Intervention |
Control |
Intervention |
|||||||||||||||||||
| Y1 | Y2 | Y1 | Y2 | B | Y1 | Y2 | Y3 | B | Y1 | Y2 | Y3 | B | Y1 | Y2 | B | Y1 | Y2 | B | Y1 | Y2 | Y3 | B | Y1 | Y2 | Y3 | |
| Total | 40 | 33 | 31 | 21 | 52 | 51 | 58 | 63 | 60 | 53 | 36 | 35 | 32 | 29 | 31 | 36 | 26 | 16 | 30 | 29 | 34 | 22 | 28 | 33 | 16 | 18 |
| Marginalization | ||||||||||||||||||||||||||
| Less marginalized | 34 | 30 | 25 | 24 | 49 | 43 | 58 | 56 | 44 | 56 | 35 | 41 | 32 | 27 | 30 | 34 | 23 | 16 | 33 | 26 | 35 | 20 | 31 | 33 | 17 | 17 |
| Most marginalized | 44 | 39 | 43 | 18 | 56 | 60 | 59 | 70 | 71 | 52 | 37 | 30 | 32 | 38 | 37 | 43 | 35 | 17 | 23 | 39 | 29 | 33 | 16 | 31 | 15 | 23 |
| Literacy | ||||||||||||||||||||||||||
| Literate | 38 | 32 | 18 | 16 | 44 | 42 | 54 | 59 | 36 | 51 | 37 | 34 | 29 | 26 | 29 | 34 | 23 | 16 | 31 | 25 | 34 | 18 | 35 | 36 | 17 | 17 |
| Illiterate | 40 | 36 | 40 | 26 | 55 | 55 | 60 | 64 | 67 | 54 | 36 | 35 | 39 | 41 | 36 | 42 | 33 | 16 | 30 | 36 | 33 | 29 | 16 | 27 | 15 | 19 |
| Economic status | ||||||||||||||||||||||||||
| Less poor | 33 | 30 | 24 | 27 | 49 | 44 | 56 | 51 | 49 | 57 | 26 | 40 | 27 | 25 | 28 | 33 | 21 | 11 | 35 | 27 | 28 | 20 | 18 | 25 | 17 | 15 |
| Poorest | 46 | 36 | 38 | 16 | 55 | 56 | 60 | 72 | 65 | 52 | 40 | 33 | 36 | 33 | 33 | 38 | 29 | 20 | 27 | 31 | 38 | 24 | 35 | 39 | 16 | 20 |
B, baseline; Y1, intervention year 1; Y2, intervention year 2; Y3, intervention year 3.
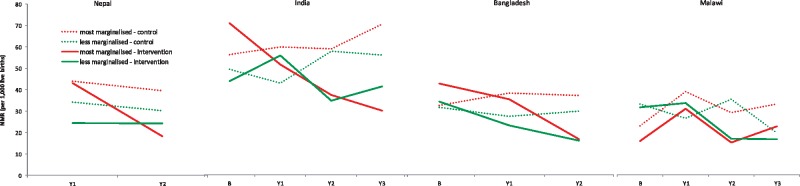
Figure 1.
Trends in the neonatal mortality rate (NMR) in intervention and control areas for the most and less marginalised, four women’s group trials. Note: women who were illiterate and poor were categorised as ‘most marginalised’; the rest of the population was categorised as ‘less marginalised’. B: baseline. Y1: intervention year 1, Y2: intervention year 2, Y3: intervention year 3.
For the Nepal trial, formal correction for baseline differences in NMR was impossible because there were no prospective baseline data. The retrospective baseline assessment (i.e. based on birth history data) in this trial showed no NMR differences between arms but lower rates than subsequently measured using prospective surveillance. The ORs for Nepal in Table 3 therefore give the ratio of the odds of neonatal death in intervention areas compared with control areas in the last study year, only adjusting for clustering and the pair-matched design. For Malawi (and urban India, Table S2, available as Supplementary data at IJE online) we adjusted for cluster-level average mortality rates at baseline, analogous to the original trial paper.
Using the same models as above, we also examined the intervention effects on health care use and home care practices in lower and higher socioeconomic groups. We used Stata 12 for the above analyses (Stata, College Station, TX, USA).
We also estimated the absolute intervention effects (per 1000 live births) for low and high SEP. We used a generalized linear mixed model to estimate mortality probabilities in a hypothetical new random cluster, for each of the strata or matched pairs. We combined the mortality predictions into average mortality probabilities weighted by the number at risk in each of the strata or matched pairs. Subtracting these averaged probabilities([intervention minus control at endline] minus [intervention minus control at baseline]) then provides the absolute intervention effect. A non-parametric bootstrap was done to get confidence intervals around the absolute effects (1000 replicas).
After we conducted the analyses for each trial separately, we used the beta and standard error of the effect estimate per trial to estimate pooled effects for all trials combined. We used random effects models for the meta-analysis, with trial as random effect, because we assumed that the (differential) effects of each trial were taken from an underlying distribution. The pooled analyses were replicated for early and late NMR. These analyses were conducted in R 3.0.1 (library lme4).
Approval for the trials was received from the Research Ethics Committee at the UCL Institute of Child Health and from appropriate national ethics committees.
Results
There were 69120 live births and 2505 neonatal deaths in the trial areas during the trial periods (Table 1). Births in Nepal and India were largely to illiterate women (60–77%), in contrast to Bangladesh and Malawi (23–39%). Of the study populations, 50–70% was categorized as poor. The percentage categorized as most marginalized varied from around 20% (Bangladesh, Malawi) to around 40% in Nepal and 55% in India. Despite the random allocation of clusters to the intervention and control arm, the population in the intervention arm in India was somewhat worse off compared with the control arm; in Nepal this was the other way around.
Baseline NMR varied from 30–40/1000 (Malawi, Bangladesh, Nepal) to 50–60/1000 (India) (Table 2, Figure 1). In India and Nepal, NMR declined more among lower socioeconomic strata than among higher strata in intervention areas. In control areas, NMR declined more slowly, especially among lower strata (Nepal), or even increased (India). In Bangladesh, NMR declined strongly in all strata in intervention areas, and remained roughly stable in control areas. In Malawi, NMR declined in intervention areas in years 2–3 in lower and higher socioeconomic groups and in control areas in year 3 in higher strata.
The meta-analysis shows that the intervention reduced NMR substantially and equitably across lower and higher socioeconomic groups (Table 3). Intervention effects ranged from a 35–44% mortality reduction in higher strata to a 50–63% reduction in lower strata in the last study year, depending on the measure of socioeconomic position used [last 2 years: 32–39% and 40–48%, respectively (Table S3, available as Supplementary data at IJE online)]. The effects in lower strata were as strong as or stronger than those in higher strata. In the last year, the effect was much stronger among the most marginalized (63% mortality reduction, OR 0.37; 95% CI: 0.26–0.52) than among the less marginalized (35% mortality reduction, OR 0.65; 95% CI: 0.50–0.85) (P-value for difference: 0.009), whereas the effects were similar by literacy and economic status. In the last 2 study years, effects were similar in lower and higher strata irrespective of the measure of socioeconomic position (SEP) used. In the individual trials, there was a tendency for stronger effects among lower socioeconomic groups, especially in the India trial, whereas the effects in the other trials were comparable across strata and/or confidence intervals were too large to detect differential effects. There was no heterogeneity between trials in the differential effect of the intervention on lower and higher socioeconomic groups, except when stratifying by economic status. The meta-analysis for all six trials, including the two trials with very low women’s group coverage and no intervention effect, was comparable to the results for the four trials presented above, albeit with point estimate closer to one [i.e. less marginalization OR (95% CI): 0.84 (0.64–1.10); more marginalized OR (95% CI): 0.48 (0.30–0.79), P = 0.056, see Table S2).
When examining absolute intervention effects (per 1000 live births), again there was a tendency for stronger effects among the most marginalized compared with the better-off in all trials; but only for the India trial, the P-value for the difference in effect between strata was small (P = 0.012, Table 3 footnote). The intervention in India was associated with an NMR change of −55/1000 (95% CI: −78 to −30) among the most marginalized and −9/1000 (95% CI: −32 to 15) in the less marginalized.
For early NMR, substantial effects were observed among lower and higher socioeconomic strata in the pooled analysis, with mortality declines of around 50% to 61% among lower strata and 44% to 53% among higher strata depending on the measure of socioeconomic position used (Table 4). No differential effects between lower and higher strata were detected. For late NMR, strong effects were only detected for lower strata (poorest: 56% mortality decline (95% CI: 29 73%), illiterate: 57% (95% CI: 29–74%), most marginalized: 72% (95% CI: 48–85%)), whereas the ORs for late NMR among higher strata were close to one. The P-values for the differences in effect on late NMR between lower and higher strata were mostly small (0.003 for marginalization, 0.051 for economic status, 0.107 for literacy). In the last 2 study years, the effects on early NMR were comparable across socioeconomic strata, but there was a tendency for stronger effects on late NMR among lower strata (results not shown).
Table 4.
Pooled effect estimates for the early and late neonatal mortality rate (NMR) for lower and higher socioeconomic groups, for the last study year
| Pooled estimates, early NMR |
Pooled estimates, late NMR |
|||||
|---|---|---|---|---|---|---|
| ORa | 95% CI | P-valueb | ORa | 95% CI | P-valueb | |
| Total | 0.47 | (0.37–0.61) | 0.000 | 0.58 | (0.40–0.84) | 0.004 |
| Marginalization | ||||||
| Less marginalized | 0.56 | (0.41–0.77) | 0.171 | 0.93 | (0.58–1.49) | 0.003 |
| Most marginalized | 0.39 | (0.26–0.59) | 0.28 | (0.15–0.52) | ||
| Literacy | ||||||
| Literate | 0.47 | (0.32–0.68) | 0.783 | 0.80 | (0.45–1.41) | 0.107 |
| Illiterate | 0.50 | (0.36–0.71) | 0.43 | (0.26–0.71) | ||
| Economic status | ||||||
| Less poor | 0.47 | (0.31–0.70) | 0.929 | 0.94 | (0.52–1.69) | 0.051 |
| Poorest | 0.48 | (0.35–0.66) | 0.44 | (0.27–0.71) | ||
aThe ratio of the odds of neonatal mortality in the intervention compared with the control areas adjusted for baseline differences.
bP-value for the test on difference in OR between lowest and highest socioeconomic groups. For the total population, it gives the P-value for the difference between intervention and control.
Among infants of women in the intervention arm who had not attended the groups, the (differential) effects on neonatal mortality were similar to, or only slightly smaller than, those in the full trial populations (Table S4, available as Supplementary data at IJE online).
Only the Nepal trial showed strong effects on antenatal and delivery care, in all strata (Table 5). The Bangladesh trial showed some improvements for the whole population and higher strata in health care use during the antenatal period [antenatal care (ANC) 3+, medical treatment for ill pregnant women], but not during delivery. No effects on health care use were observed in the trials in India and Malawi. The patterns for the last 2 trial years were similar to those in the last year (results not shown).
Table 5.
Intervention effects on behaviour for lower and higher socioeconomic groups per trial, for the last study year
| Nepal |
India |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
High SEP |
Low SEP |
Difference |
Total |
High SEP |
Low SEP |
Difference |
|||||||
| OR* | 95% CI | OR* | 95% CI | OR* | 95% CI | P-value* | OR* | 95% CI | OR* | 95% CI | OR* | 95% CI | P-value* | |
| Health care use | ||||||||||||||
| 3 + antenatal care visits to medical provider | 4.10 | (2.07–8.14) | 4.18 | (2.09–8.36) | 3.19 | (1.50–6.79) | 0.2510 | 1.12 | (0.92–1.35) | 1.02 | (0.78–1.32) | 1.10 | (0.81–1.48) | 0.715 |
| Medical treatment sought for illness during pregnancy | 4.79 | (2.85–8.05) | 6.90 | (3.55–13.38) | 2.35 | (1.13–4.90) | 0.0160 | 0.79 | (0.64–0.98) | 0.76 | (0.57–1.02) | 0.73 | (0.53–1.02) | 0.893 |
| Institutional delivery | 5.88 | (2.57–13.46) | 6.24 | (2.60–14.99) | 4.94 | (1.43–17.06) | 0.7300 | 0.87 | (0.70–1.10) | 0.79 | (0.59–1.06) | 1.03 | (0.69–1.52) | 0.299 |
| Institutional delivery for prolonged labour | 8.69 | (2.25–33.61) | 10.50 | (2.54–43.29) | 6.02 | (1.14–31.78) | 0.4930 | 1.02 | (0.53–1.97) | 1.51 | (0.60–3.79) | 0.61 | (0.23–1.62) | 0.176 |
| Home care: antenatal practices | ||||||||||||||
| Iron intake | 0.46 | (0.23–0.91) | 0.43 | (0.21–0.91) | 0.55 | (0.22–1.33) | 0.6150 | 1.47 | (1.23–1.76) | 1.24 | (0.94–1.64) | 1.63 | (1.29–2.06) | 0.141 |
| Home care: delivery practices | ||||||||||||||
| Birth attendant washed hands | 6.95 | (3.16–15.29) | 6.71 | (3.02–14.95) | 7.22 | (3.14–16.57) | 0.7790 | 4.19 | (3.38–5.21) | 4.73 | (3.43–6.53) | 3.85 | (2.86–5.18) | 0.358 |
| Clean delivery kit used | 5.90 | (3.72–9.36) | 5.01 | (3.10–8.10) | 7.74 | (3.58–16.73) | 0.2750 | 3.81 | (2.89–5.02) | 5.53 | (3.68–8.31) | 2.18 | (1.44–3.30) | 0.002 |
| Plastic sheet used | – | 3.40 | (2.38–4.85) | 5.65 | (3.37–9.45) | 1.92 | (1.12–3.28) | 0.004 | ||||||
| Cord cut with new or boiled blade | 3.06 | (1.78–5.26) | 3.36 | (1.93–5.85) | 2.55 | (1.38–4.72) | 0.2740 | 2.15 | (1.72–2.69) | 2.59 | (1.74–3.85) | 1.96 | (1.48–2.58) | 0.258 |
| Cord tied with boiled thread | – | 7.90 | (5.92–10.53) | 9.46 | (6.29–14.23) | 5.84 | (3.80–8.97) | 0.110 | ||||||
| Home care: postnatal practices | ||||||||||||||
| Appropriate cord care (nothing applied on stump) | 1.28 | (0.53–3.08) | 1.07 | (0.44–2.64) | 1.53 | (0.61–3.86) | 0.1290 | 1.86 | (1.41–2.47) | 1.34 | (0.88–2.02) | 2.42 | (1.66–3.53) | 0.037 |
| Baby wrapped or put on the skin within 10 min | 7.58 | (5.20–11.05) | 4.10 | (2.37–7.11) | 11.74 | (7.03–19.60) | 0.006 | |||||||
| Baby placed on mother's skin within 30 min | 0.47 | (0.17–1.32) | 0.44 | (0.16–1.23) | 0.37 | (0.11–1.20) | 0.6880 | 0.96 | (0.71–1.30) | 0.63 | (0.39–1.02) | 1.26 | (0.85–1.87) | 0.030 |
| Baby not bathed in first 6 h after birth | 3.95 | (2.42–6.46) | 3.93 | (2.38–6.51) | 3.78 | (2.01–7.09) | 0.8910 | 2.28 | (1.89–2.75) | 1.92 | (1.44–2.57) | 2.52 | (1.97–3.22) | 0.162 |
| Breastfeeding initiated within 1 h | 1.76 | (0.79–3.93) | 1.80 | (0.79–4.10) | 1.71 | (0.73–3.96) | 0.7870 | 3.29 | (2.60–4.18) | 2.02 | (1.41–2.91) | 5.15 | (3.74–7.10) | 0.000 |
| Only breastmilk given in first day | 1.52 | (1.22–1.91) | 1.89 | (1.35–2.65) | 1.31 | (0.97–1.78) | 0.112 | |||||||
| Exclusive breastfeeding in first 6 weeks after birth | 0.80 | (0.44–1.45) | 0.70 | (0.38–1.32) | 1.32 | (0.51–3.41) | 0.1820 | 1.39 | (1.13–1.72) | 1.73 | (1.25–2.37) | 1.22 | (0.92–1.62) | 0.110 |
| Bangladesh |
Malawi |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
High SEP |
Low SEP |
Difference |
Total |
High SEP |
Low SEP |
Difference |
|||||||
| OR* | 95% CI | OR* | 95% CI | OR* | 95% CI | P-value* | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | P-value* | |
| Health care use | ||||||||||||||
| 3 + antenatal care visits to medical provider | 1.55 | (1.37–1.75) | 1.60 | (1.39–1.83) | 1.39 | (0.98–1.98) | 0.476 | 0.94 | (0.56–1.55) | 0.92 | (0.53–1.61) | 0.87 | (0.43–1.74) | 0.843 |
| Medical treatment sought for illness during pregnancy | 1.18 | (1.04–1.34) | 1.22 | (1.06–1.40) | 0.98 | (0.71–1.36) | 0.235 | – | ||||||
| Institutional delivery | 1.12 | (0.97–1.28) | 1.13 | (0.97–1.31) | 0.97 | (0.62–1.53) | 0.545 | 1.23 | (0.69–2.19) | 1.28 | (0.67–2.42) | 1.17 | (0.55–2.52) | 0.771 |
| Institutional delivery for prolonged labour | – | – | ||||||||||||
| Home care: antenatal practices | ||||||||||||||
| Iron intake | 2.63 | (2.32–2.98) | 2.39 | (2.08–2.75) | 3.97 | (2.96–5.32) | 0.002 | 1.38 | (0.47–4.08) | 0.97 | (0.32–2.98) | 4.06 | (0.94–17.54) | 0.012 |
| Home care: delivery practices | ||||||||||||||
| Birth attendant washed hands | 3.05 | (2.39–3.87) | 3.58 | (2.68–4.79) | 2.15 | (1.38–3.37) | 0.061 | 0.19 | (0.06–0.53) | 0.18 | (0.07–0.47) | 0.25 | (0.08–0.73) | 0.434 |
| Clean delivery kit used | 5.46 | (4.51–6.62) | 5.67 | (4.59–7.01) | 4.98 | (3.13–7.90) | 0.611 | – | ||||||
| Plastic sheet used | 2.43 | (2.09–2.83) | 2.72 | (2.29–3.23) | 1.75 | (1.28–2.41) | 0.017 | – | ||||||
| Cord cut with new or boiled blade | 2.46 | (1.21–5.00) | 2.95 | (1.31–6.65) | 1.40 | (0.32–6.18) | 0.390 | – | ||||||
| Cord tied with boiled thread | 1.32 | (1.13–1.53) | 1.25 | (1.05–1.49) | 1.62 | (1.16–2.24) | 0.176 | – | ||||||
| Home care: postnatal practices | ||||||||||||||
| Appropriate cord care (nothing applied on stump) | 1.76 | (1.48–2.09) | 1.66 | (1.37–2.01) | 2.27 | (1.57–3.28) | 0.138 | – | ||||||
| Baby wrapped or put on the skin within 10 min | 1.49 | (1.28–1.73) | 1.40 | (1.18–1.66) | 1.88 | (1.36–2.62) | 0.113 | 1.61 | (0.39–6.71) | 2.40 | (0.49–11.80) | 0.67 | (0.11–4.31) | 0.180 |
| Baby placed on mother's skin within 30 min | 0.93 | (0.80–1.08) | 0.85 | (0.71–1.01) | 1.26 | (0.90–1.76) | 0.038 | – | ||||||
| Baby not bathed in first 6 h after birth | 1.74 | (1.43–2.12) | 1.76 | (1.40–2.20) | 1.61 | (1.06–2.45) | 0.711 | 1.51 | (0.48–4.69) | 1.76 | (0.53–5.82) | 0.72 | (0.20–2.63) | 0.013 |
| Breastfeeding initiated within 1 h | 2.55 | (2.08–3.11) | 2.59 | (2.06–3.26) | 2.45 | (1.63–3.67) | 0.812 | 1.79 | (0.44–7.37) | 2.32 | (0.54–10.06) | 1.49 | (0.30–7.49) | 0.340 |
| Only breastmilk given in first day | 2.28 | (1.75–2.97) | 2.42 | (1.79–3.27) | 1.79 | (1.00–3.21) | 0.370 | – | ||||||
| Exclusive breastfeeding in first 6 weeks after birth | 1.29 | (1.09–1.52) | 1.30 | (1.08–1.57) | 1.26 | (0.89–1.79) | 0.888 | – | ||||||
*P-value is for the difference in OR between lower and higher socioeconomic groups. Marginalization was used as indicator of socioeconomic position in the analyses for this table.
–, not available.
Conversely, effects on home care practices were substantial in all three Asian trials, especially for hygienic practices around delivery and for delayed bathing. The India and Bangladesh trials also showed strong effects on thermal care and breastfeeding. These effects were observed in lower and higher strata, with no systematic differences in effect. No effects on home care practices were observed in Malawi.
Discussion
Our analysis shows that community-based women’s groups with high population coverage reduced neonatal mortality equitably and substantially across socioeconomic strata. The reductions were at least as strong among poor, illiterate and most marginalized groups as in better-off strata, with particularly pronounced effects on lower strata in the India trial. The intervention also improved home care practices among lower and higher strata, with no systematic differences between strata.
Problems measuring NMR are unlikely to explain our findings. Measuring NMR reliably in poor settings is challenging with surveys using retrospective birth histories, but in the four trials a combined population of over 1 million was prospectively followed up and outcomes were registered for every birth.
We used random effects logistic regression to account for clustering, following the main publication of two of the trials. Although this was less robust for trials with relatively few clusters and pair-matched trials compared with cluster-summary methods of analysis, we found it important to use the same analytical approach for all trials. Also, our models assume that the period effects are constant across clusters, which may be a strong assumption.
For the Malawi trial, we conducted a treatment group analysis comparing the ‘women’s group only’ arm with the ‘no intervention’ arm, resembling the other trials. Our findings should be seen in the light of discussions on analysis strategy.25 A factorial analysis of the Malawi trial showed no NMR effect, but this was possibly caused by baseline imbalances.21 We therefore decided to explore the ‘treatment group’ findings further.
The intervention effects observed among women who had not attended women’s groups could be due to selection effects. As primigravid women (whose infants are at a higher risk of neonatal death) were less likely to participate in the intervention,13 our estimates among non-attenders are perhaps an underestimation of the real effect. Nevertheless, potential selection effects make the effect estimates among non-attenders uncertain.
The trial areas were located in rural areas. Rural communities in developing countries are not, however, homogeneously poor; there is still important socioeconomic differentiation by literacy, wealth and caste. These differences influence uptake of interventions, health care and healthy behaviours; in poor countries, inequalities of uptake are often very large.26
Our findings are important as, based on the inverse equity hypothesis27 and Tudor Hart’s inverse care law,28 we expect interventions – even ‘simple’ behavioural interventions – to reach higher socioeconomic groups better and to only benefit lower strata once higher strata reach a plateau beyond which further improvements are difficult to achieve. The reasons for this include inequitable intervention availability, elite capture at the community level when services and interventions are made available and a slower diffusion of behaviour change among lower socioeconomic strata.7,29,30 This is also reflected in, for example, substantial inequalities in uptake of low-cost interventions such as oral rehydration therapy for children with diarrhoea, with lower uptake among lower socioeconomic groups in many low- and middle-income countries.26 There are indications that community-based interventions can reinforce existing social hierarchies.8–11,31 Conversely, our findings show that lower and higher socioeconomic strata can experience survival and behavioural improvements in tandem.
The equitable distribution of the women’s group effects is important because little is known about how to reduce health inequalities.32,33 Health inequalities are a persistent problem in poor and rich countries, and few interventions are effective in reducing them. There is some evidence that home visits by community health workers can improve equity in antenatal and postnatal practices and coverage of childhood vaccinations,34,35 but studies examining differential impacts on neonatal mortality across socioeconomic groups remain extremely rare.32,33,36
An important contributor to the equitable intervention effects is probably the equitable reach of the intervention across strata, on which we reported in a separate paper.13 In that paper, we describe how equitable attendance at the women’s groups is related to intervention design, which included a combination of universal coverage (the groups were open to everyone who shared a concern about maternal and newborn health) and ‘soft targeting’ — meeting in places and at times that are convenient to lower socioeconomic strata, using methods and language that engage women with low levels of literacy and that involve the entire community and addressing issues around pregnancy and delivery that are important to everyone.2,13 And importantly, the women’s groups’ strategies focused on simple behavioural changes in the home, which were accessible and affordable to poor and illiterate families, and which could lead to survival benefits especially in contexts where home deliveries are common. In our current paper, we show that survival improvements were also observed among newborn infants of women who had not attended groups, in lower and higher strata, suggesting a diffusion of the group messages and actions across socioeconomic layers, not just the better-off.
The intervention effect across social strata was also observed for early NMR, which is important as early NMR is resistant to decline.37 The effect on early NMR is notable given that improvements in the institutional delivery rate were limited or absent. This suggests that behavioural improvements around delivery in the home, including prevention of infection and hypothermia, and handling of premature and perhaps even asphyxiated infants, played a role. Strong effects on late NMR were only observed for lower strata in the last trial year, suggesting that infection prevention and improvement in feeding practices were perhaps especially important among lower strata. So although the intervention design contributed to equitable uptake, its particularly strong mortality impacts among lower strata were possibly facilitated by socioeconomic differences in cause-of-death distribution, with arguably a greater importance of infections as cause of death among the poor.38
Policy implications
Women’s groups reduced newborn mortality among all socioeconomic layers within rural populations, especially among the most marginalized. The intervention can be scaled up through community health workers as group facilitators. Where a country-wide network of such health workers exits, such as the Accredited Social Health Activists (ASHAs) in India, the potential for scaling up is large. Currently, a trial to test the impact of women’s groups facilitated by ASHAs is ongoing.39 The potential contribution of women’s groups to a world without preventable newborn deaths, as envisaged in the Every Newborn Action Plan, can be large if our conclusions also hold in a programmatic (rather than trial) setting.1
We recommend that the women’s group intervention is implemented together with health system interventions to improve equitable uptake of professional maternity care, such as conditional cash transfers, voucher schemes and other measures to improve equitable uptake and quality of maternity care.40–42
The women’s group intervention is only effective when implemented well, i.e. with sufficient coverage, duration and intensity, as demonstrated in three other trials.15,16 Achieving high coverage is partly related to resources and knowledge about the importance of coverage, and partly related to context. Achieving high coverage in urban slum areas, with high migration levels and little space to meet, is difficult, as became evident in the Mumbai trial.16 In rural areas with high mortality levels, women’s groups, when implemented well, seem a safe bet to reduce newborn mortality among the poor and the less poor.
The principles of the women’s group intervention are arguably amenable to wider implementation. Principles for ensuring equitable intervention effects include universal coverage – making the intervention open to everyone – combined with soft-targeting to ensure uptake among lower strata, involving the wider community and thus ensuring diffusion, while focusing on those practices or problems that are important contributors to mortality and while also addressing the wider determinants of health.2
Conclusion
Community-based women’s groups with high population coverage benefit the survival chances of all newborn infants, and perhaps especially those born into the most deprived households.
Funding
This work was primarily supported by the Economic and Social Research Council and the Department for International Development (grant number ES/I033572/1). Additional support was provided by a Wellcome Trust Strategic Award (award number: 085417MA/Z/08/Z). T.A.J.H. was also supported by an EUR Research Excellence Initiative grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
Acknowledgement
We thank the reviewers of this manuscript for their helpful comments.
Author Contributions
T.A.J.H. initiated the study and study design. T.A.J.H. acquired the funding, together with D.O., K.A., D.S.M., S.L., P.T. and A.C. C.W.N.L. and T.A.J.H. designed the quantitative analysis strategy. The quantitative analyses were conducted by T.A.J.H., C.W.N.L., A.S., S.L. and C.K. All authors contributed to the interpretation of the data. T.A.J.H. wrote the first draft of the manuscript. All authors contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
Conflict of interest: All authors, except C.W.N.L., were involved in the trials under study. No other conflicts of interests declared.
References
- 1. Every newborn, every mother, every adolescent girl. Lancet 2014;383:755. [DOI] [PubMed] [Google Scholar]
- 2. Houweling TA, Morrison J, Azad K et al. . How to reach every newborn: three key messages. Lancet Glob Health 2014;2:e436–7. [DOI] [PubMed] [Google Scholar]
- 3. Houweling TA, Kunst AE. Socio-economic inequalities in childhood mortality in low and middle income countries: a review of the international evidence. Br Med Bull 2010;93:7–26. [DOI] [PubMed] [Google Scholar]
- 4. Houweling TA, Ronsmans C, Campbell OM, Kunst AE. Huge poor-rich inequalities in maternity care: an international comparative study of maternity and child care in developing countries. Bull World Health Organ 2007;85:745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKinnon B, Harper S, Kaufman JS, Bergevin Y. Socioeconomic inequality in neonatal mortality in countries of low and middle income: a multicountry analysis. Lancet Glob Health 2014;2:e165–73. [DOI] [PubMed] [Google Scholar]
- 6. Prost A, Colbourn T, Seward N et al. . Women's groups practising participatory learning and action to improve maternal and newborn health in low-resource settings: a systematic review and meta-analysis. Lancet 2013;381:1736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansuri G, Rao V. Community-based and driven development: a critical review. World Bank Research Observer 2004;19:1–39. [Google Scholar]
- 8. Bell D, Go R, Miguel C, Parks W, Bryan J. Unequal treatment access and malaria risk in a community-based intervention program in the Philippines. Southeast Asian J Trop Med Public Health 2005;36:578–86. [PubMed] [Google Scholar]
- 9. Kumar V, Kumar A, Darmstadt GL. Behavior change for newborn survival in resource-poor community settings: bridging the gap between evidence and impact. Semin Perinatol 2010;34:446–61. [DOI] [PubMed] [Google Scholar]
- 10. Thorp R, Steward F, Heyer A. When and How far is group formation a route out of chronic poverty? World Dev 2005;33:907–20. [Google Scholar]
- 11. Evans T, Adams A, Mohammed R. Demystifying nonparticipation in microcredit: a population-based analysis. World Dev 1999;27:419–30. [Google Scholar]
- 12. Houweling TA, Tripathy P, Nair N et al. . The equity impact of participatory women's groups to reduce neonatal mortality in India: secondary analysis of a cluster-randomized trial. Int J Epidemiol 2013;42:520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houweling TA, Morrison J, Alcock G et al. . Reaching the poor with health interventions: programme-incidence analysis of seven randomized trials of women's groups to reduce newborn mortality in Asia and Africa. J Epidemiol Community Health 2016;70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tripathy P, Nair N, Sinha R et al. . Effect of participatory women's groups facilitated by Accredited Social Health Activists on birth outcomes in rural eastern India: a cluster-randomized controlled trial. Lancet Glob Health 2016;4:e119–28. [DOI] [PubMed] [Google Scholar]
- 15. Azad K, Barnett S, Banerjee B et al. . Effect of scaling up women's groups on birth outcomes in three rural districts in Bangladesh: a cluster-randomized controlled trial. Lancet 2010;375:1193–202. [DOI] [PubMed] [Google Scholar]
- 16. More NS, Bapat U, Das S et al. . Community mobilization in Mumbai slums to improve perinatal care and outcomes: a cluster randomized controlled trial. PLoS Med 2012;9:e1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colbourn T, Nambiar B, Bondo A et al. . Effects of quality improvement in health facilities and community mobilization through women's groups on maternal, neonatal and perinatal mortality in three districts of Malawi: MaiKhanda, a cluster randomized controlled effectiveness trial. Int Health 2013;5:180–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manandhar DS, Osrin D, Shrestha BP et al. . Effect of a participatory intervention with women's groups on birth outcomes in Nepal: cluster-randomized controlled trial. Lancet 2004;364:970–79. [DOI] [PubMed] [Google Scholar]
- 19. Tripathy P, Nair N, Barnett S et al. . Effect of a participatory intervention with women's groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomized controlled trial. Lancet 2010;375:1182–92. [DOI] [PubMed] [Google Scholar]
- 20. Fottrell E, Azad K, Kuddus A et al. . The in Bangladesh: A cluster randomized trial. JAMA Pediatr 2013;167:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewycka S, Mwansambo C, Rosato M et al. . Effect of women's groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): a factorial, cluster-randomized controlled trial. Lancet 2013;381:1721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shrestha BP, Bhandari B, Manandhar DS, Osrin D, Costello A, Saville N. Community interventions to reduce child mortality in Dhanusha, Nepal: study protocol for a cluster randomized controlled trial. Trials 2011;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barnett S, Nair N, Tripathy P, Borghi J, Rath S, Costello A. A prospective key informant surveillance system to measure maternal mortality – findings from indigenous populations in Jharkhand and Orissa, India. BMC Pregnancy Childbirth 2008;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewycka S, Mwansambo C, Kazembe P et al. . A cluster randomized controlled trial of the community effectiveness of two interventions in rural Malawi to improve health care and to reduce maternal, newborn and infant mortality. Trials 2010;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirkwood B, Bahl R. Can women's groups reduce maternal and newborn deaths? Lancet 2013;381:e12–14. [DOI] [PubMed] [Google Scholar]
- 26. Gwatkin DR, Rutstein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Socio-economic Differences in Health, Nutrition, and Population Within Developing Countries: An Overview. Washington, DC: World Bank, 2007. [PubMed] [Google Scholar]
- 27. Victora CG, Vaughan JP, Barros FC, Silva AC, Tomasi E. Explaining trends in inequities: evidence from Brazilian child health studies. Lancet 2000;356:1093–98. [DOI] [PubMed] [Google Scholar]
- 28. Hart JT. The inverse care law. Lancet 1971;1:405–12. [DOI] [PubMed] [Google Scholar]
- 29. Rogers EM. Diffusion of Innovations. London: Free Press, 2003[1962]. [Google Scholar]
- 30. Callaghan-Koru JA, Nonyane BA, Guenther T et al. . Contribution of community-based newborn health promotion to reducing inequities in healthy newborn care practices and knowledge: evidence of improvement from a three-district pilot program in Malawi. BMC Public Health 2013;13:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UNICEF. What Works for Children in South Asia. Community Health Workers. Kathmandu: UNICEF Regional Office for South Asia, 2004. [Google Scholar]
- 32. Malqvist M, Yuan B, Trygg N, Selling K, Thomsen S. Targeted interventions for improved equity in maternal and child health in low- and middle-income settings: a systematic review and meta-analysis. PLoS One 2013;8:e66453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yuan B, Malqvist M, Trygg N, Qian X, Ng N, Thomsen S. What interventions are effective on reducing inequalities in maternal and child health in low- and middle-income settings? A systematic review. BMC Public Health 2014;14:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baqui AH, Rosecrans AM, Williams EK et al. . NGO facilitation of a government community-based maternal and neonatal health programme in rural India: improvements in equity. Health Policy Plan 2008;23:234–43. [DOI] [PubMed] [Google Scholar]
- 35. Bishai D, Suzuki E, McQuestion M, Chakraborty J, Koenig M. The role of public health programmes in reducing socioeconomic inequities in childhood immunization coverage. Health Policy Plan 2002;17:412–19. [DOI] [PubMed] [Google Scholar]
- 36. Kruk ME, Porignon D, Rockers PC, Van Lerberghe W. The contribution of primary care to health and health systems in low- and middle-income countries: a critical review of major primary care initiatives. Soc Sci Med 2010;70:904–11. [DOI] [PubMed] [Google Scholar]
- 37. Hill K, Choi Y. Neonatal mortality in the developing world. Demogr Res 2006;14:429–52. [Google Scholar]
- 38. Seward N, Osrin D, Li L et al. . Association between clean delivery kit use, clean delivery practices, and neonatal survival: pooled analysis of data from three sites in South Asia. PLoS Med 2012;9:e1001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tripathy P, Nair N, Mahapatra R et al. . Community mobilisation with women's groups facilitated by Accredited Social Health Activists (ASHAs) to improve maternal and newborn health in underserved areas of Jharkhand and Orissa: study protocol for a cluster-randomized controlled trial. Trials 2011;12:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meyer C, Bellows N, Campbell M, Potts M. The Impact of Vouchers on the Use and Quality of Health Goods and Services in Developing Countries: a Systematic Review. London: Institute of Education, University of London, 2011. [Google Scholar]
- 41. Bellows BW, Conlon CM, Higgs ES et al. . A taxonomy and results from a comprehensive review of 28 maternal health voucher programmes. J Health Popul Nutr 2013;31:106–28. [PubMed] [Google Scholar]
- 42. Giedion U, Alfonso EA, Díaz Y. The Impact of Universal Coverage Schemes in the Developing World: A Review of the Existing Evidence. Washington, DC: World Bank, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.