Abstract
Background
Few studies have examined the independent and combined relationships of body mass index (BMI) peak and rebound with adiposity, insulin resistance and metabolic risk later in life. We used data from Project Viva, a well-characterized birth cohort from Boston with repeated measures of BMI, to help fill this gap.
Methods
Among 1681 children with BMI data from birth to mid childhood, we fitted individual BMI trajectories using mixed-effects models with natural cubic splines and estimated age, and magnitude of BMI, at peak (in infancy) and rebound (in early childhood). We obtained cardiometabolic measures of the children in early adolescence (median 12.9 years) and analysed their associations with the BMI parameters.
Results
After adjusting for potential confounders, age and magnitude at infancy BMI peak were associated with greater adolescent adiposity, and earlier adiposity rebound was strongly associated with greater adiposity, insulin resistance and metabolic risk score independently of BMI peak. Children with a normal timing of BMI peak plus early rebound had an adverse cardiometabolic profile, characterized by higher fat mass index {β 2.2 kg/m2 [95% confidence interval (CI) 1.6, 2.9]}, trunk fat mass index [1.1 kg/m2 (0.8, 1.5)], insulin resistance [0.2 units (0.04, 0.4)] and metabolic risk score [0.4 units (0.2, 0.5)] compared with children with a normal BMI peak and a normal rebound pattern. Children without a BMI peak (no decline in BMI after the rise in infancy) also had adverse adolescent metabolic profiles.
Conclusions
Early age at BMI rebound is a strong risk factor for cardiometabolic risk, independent of BMI peak. Children with a normal peak-early rebound pattern, or without any BMI decline following infancy, are at greatest risk of adverse cardiometabolic profile in adolescence. Routine monitoring of BMI may help to identify children who are at greatest risk of developing an adverse cardiometabolic profile in later life and who may be targeted for preventive interventions.
Keywords: Lifecourse epidemiology, body mass index peak, body mass index rebound, cardiometabolic outcomes, growth trajectories
Key Messages
Few studies have examined the relationships of body mass index (BMI) peak in infancy and rebound later in childhood, with later cardiometabolic risk.
We examined these relationships among 1681 children participating in Project Viva, a Boston-area birth cohort study.
Age at BMI rebound was a strong risk factor for cardiometabolic risk in adolescence, independently of BMI peak. Children with a normal timing of BMI peak plus an early adiposity rebound, or without any BMI decline after infancy, were at greatest risk of having adverse cardiometabolic profiles in adolescence.
Routine monitoring of BMI trajectory patterns may help to identify children who are at greatest risk of developing an adverse cardiometabolic profile in later life and who may be targeted for preventive interventions.
Introduction
The obesity epidemic remains a global public health challenge.1 Studies have shown that child and adolescent obesity tracks strongly into adulthood,1 which likely increases the risk of developing later chronic disorders such as metabolic syndrome and type 2 diabetes.2,3 Understanding early life predictors of later obesity is therefore important for developing and testing preventive interventions.
In the past decade, researchers have studied two milestones of early life body mass index (BMI) trajectories: the BMI peak, which typically occurs during infancy, and the BMI rebound, which occurs during early childhood.4 Previous studies have reported that characteristics of the BMI peak (e.g. later age and higher magnitude at peak) predicted obesity and cardiometabolic risk later in childhood,5,6 and characteristics of the adiposity (BMI) rebound (e.g. age at rebound <4 years) predicted increased adiposity,7 risk of obesity8 and metabolic dysfunction9 in adolescence. Those studies were limited, however, by relatively short follow-up (e.g. from birth to early childhood5,6 or from early to late childhood7,9) and did not include the repeated BMI measures in infancy and childhood required to assess both BMI peak and BMI rebound. Furthermore, the associations of BMI peak and rebound with later cardiometabolic health outcomes have not been well characterized, which should help in developing potential interventions to prevent later cardiometabolic consequences in children with at-risk BMI peak-rebound patterns.
To address these gaps, we used data from a prospective birth cohort with repeated measures of BMI in infancy and early childhood and assessment of cardiometabolic outcomes in early adolescence. We hypothesized that: (i) later age and higher magnitude at BMI peak and earlier age at BMI rebound would be associated with greater adiposity, insulin resistance and metabolic risk; and (ii) at-risk patterns of BMI peak and rebound (late peak and early rebound) would confer greater risks of adverse cardiometabolic outcomes in early adolescence.
Methods
Study population
Children were participants in Project Viva, an ongoing prospective cohort study of pre- and perinatal influences on maternal, fetal and child health. Between 1999 and 2002, we recruited eligible pregnant women at clinical visits during the first trimester of pregnancy from eight obstetric offices of Atrius Harvard Vanguard Medical Associates, a multisite group practice in Eastern Massachusetts.10 During research examinations at birth, in infancy (median 6.3 months; range 4.9–10.6 months), early childhood (37.9; 33.6–72.5 months) and mid childhood (92.5; 78.8–131.2 months),10 trained research assistants measured weight and length/height, using standardized protocols detailed previously.11–13 We also obtained additional data on weight and length/height from medical records where paediatric clinics recorded length/height and weight data at routine well-child visits during infancy and childhood. As described previously, clinicians used the paper-and-pencil technique for measuring recumbent length for infants 0–2 years at paediatric clinics.14 We applied a correction algorithm to account for overestimation of length measured below 24 months, resulting from the paper-and-pencil technique.14 Using both research and clinical measurements, we calculated BMI (in kg/m2) as weight divided by length or height squared.
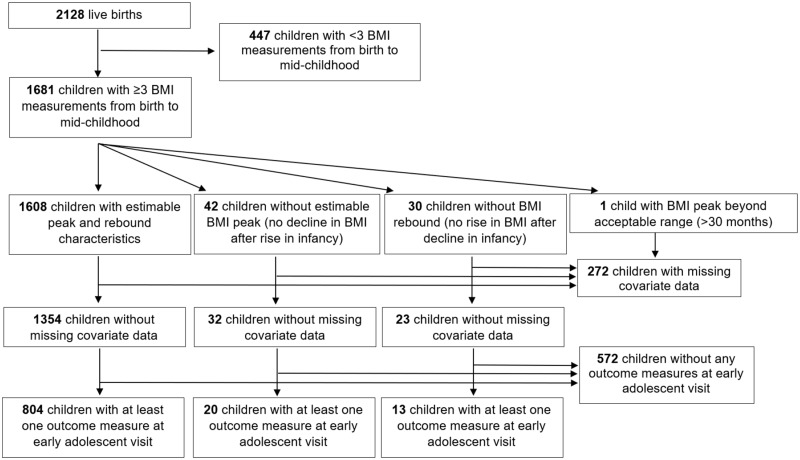
Of 2128 live singleton births, we modelled BMI trajectories in 1681 children who satisfied the inclusion criteria of having three or more BMI measurements from birth to mid childhood15 (Figure 1). Mothers provided written informed consent at enrolment and at each postnatal follow-up visit, and children provided verbal assent at the early adolescent visit. The Institutional Review Board of Harvard Pilgrim Health Care approved the project in line with ethical standards established by the Declaration of Helsinki.
Figure 1.
Flow chart of study sample.
Exposure: BMI peak and rebound
In an earlier publication, we provided details on deriving the BMI curves and BMI peak and rebound.15 We used actual BMI values, rather than z-scores, to assess each child’s absolute BMI peak and rebound. This approach has been used in other studies.5–7 Briefly, we fitted individual BMI curves using mixed-effects models with natural cubic spline functions for age, to capture the non-linear trend in BMI. We derived sex-specific trajectories by including interactions of child sex with spline terms as fixed parameters in the model. Random effects for the intercept, linear age slopes and spline functions were included in the model to account for repeated measures in the same child, as detailed previously.15 We estimated the age (months) at peak and rebound by differentiating each child-specific BMI curve; the peak and rebound are located at ages where the derivative of the curve equals to zero (i.e. at the maximum inflection point during infancy for peak, and the minimum inflection point during childhood for rebound). We estimated the magnitude (kg/m2) of BMI at peak and rebound as the highest and lowest points, respectively, of each child-specific BMI curve.
Among 1681 BMI trajectories modelled, 1608 had estimable BMI peak and rebound, 42 did not exhibit a BMI peak (i.e. no decline in BMI was observed after the rise in infancy) and 30 children did not exhibit a BMI rebound (i.e. no rise in BMI was observed after a decline in early childhood) (Figure 1).
Outcomes: Early adolescent cardiometabolic measures
Adiposity
Trained research assistants measured weight, standing height and waist circumference (WC) according to standardized protocols.16 We then calculated BMI and waist-to-height ratio (WHtR), and computed age- and sex-specific height and BMI z-scores using Centers for Disease Control and Prevention reference data.17 We performed whole-body dual X-ray absorptiometry scans with a Hologic Model Discovery (Hologic, Bedford, MA). A single trained investigator assessed fat, lean and trunk fat mass according to a standardized protocol.10,18 We calculated the following adiposity indices (all in kilograms of mass/height in metres squared): fat mass index (FMI), lean mass index (LMI) and trunk fat mass index (TFMI).
Metabolic risk score and its components
Trained research assistants measured systolic blood pressure (SBP) five times on the child’s upper arm at 1-min intervals, using calibrated automated oscillometric monitors (Omron HEM-907XL, IL). We used the mean of the five measurements for SBP to improve precision, given individual BP variability. We calculated age-, sex- and height-specific SBP z-scores according to the NHANES BP reference for children and adolescents.19 Trained technicians collected fasting blood specimens; all samples were centrifuged within 24 h, with plasma aliquots stored at -80°C. Fasting glucose, insulin, high-density lipoprotein (HDL) cholesterol and triglycerides were measured according to standard protocols.16 We calculated insulin resistance using the homeostasis model assessment (HOMA-IR), and log-transformed the values using natural logarithms to normalize the distribution. As described elsewhere,20 we calculated a metabolic risk z-score using the mean of sex- and cohort-specific z-scores for waist circumference, SBP, log-transformed triglycerides, HDL cholesterol (inverted) and log-transformed HOMA-IR.
Covariates
Mothers reported their pre-pregnancy weight, height, smoking history and highest education and their partner’s weight, height and highest education via questionnaires and interviews at recruitment. We calculated maternal pre-pregnancy and paternal BMI and categorized parental obesity as follows: both parents without obesity (pre-pregnancy and paternal BMI <30 kg/m2), only mother with obesity (pre-pregnancy BMI ≥30 kg/m2), only father with obesity or both parents with obesity. We categorized parental educational level as neither parent university-educated or mother, father or both university-educated. We used the last prenatal weight (within 4 weeks of delivery) recorded in prenatal care records and self-reported pre-pregnancy weight to calculate total gestational weight gain. We obtained results of two-stage clinical glycaemic screening to categorize women as having normal glucose tolerance, isolated hyperglycaemia, impaired glucose tolerance, or gestational diabetes (GDM).13 We extracted data on gestational hypertensive disorders, infant birthweight and delivery date from hospital medical records. We calculated length of gestation in days by subtracting the date of the last menstrual period (LMP) from the date of delivery. If gestational age according to the second-trimester ultrasound differed from that according to the LMP by >10 days, we used the ultrasound result to determine gestational duration. We calculated birthweight for gestational age z-scores using national reference data.21 Mothers reported their child’s race/ethnicity, which we categorized as White, Black, Hispanic, Asian or other. We obtained information on initiation of breastfeeding at post-delivery interviews.22 Trained research assistants also obtained parent-reported assessment of pubertal characteristics at early adolescence (for boys: voice deepening, facial and body hair growth, skin changes and growth spurt; for girls: breast development, menstrual period, facial and body hair growth, skin changes and growth spurt) using questionnaires, from which we derived a pubertal score.
Statistical analyses
We assessed associations between the BMI trajectory milestones and cardiometabolic outcomes using multivariable linear regression. We separately modelled each outcome, expressed per standard deviation (1 SD) increase in each trajectory parameter, to allow direct comparison of the magnitude of effect for different trajectory milestones. We adjusted for factors associated with both BMI trajectory parameter and outcome (selected a priori from previous publications4–9,15,23). Parental covariates included educational attainment, obesity status, total gestational weight gain, maternal smoking history (never, smoked before pregnancy or smoked during pregnancy), prenatal glucose tolerance status (normoglycaemia, isolated hyperglycaemia, impaired glucose tolerance or GDM) and gestational hypertensive disorders (normal blood pressure, chronic hypertension, gestational hypertension and pre-eclampsia). Child covariates included gestational age at delivery, race/ethnicity (White, Black, Hispanic, Asian or other), sex, birthweight for gestational age z-score, breastfeeding initiation (yes or no) and age at outcome. We included both age and magnitude (at peak or rebound) in the same regression model with each outcome. For models with age and magnitude at rebound as the primary predictors, we additionally adjusted for age and magnitude at peak (which occurs at an earlier age than rebound) but did not adjust models of BMI peak for characteristics of BMI rebound, which occurs later.
As normal ranges for age at peak or rebound have not been established, we categorized each as early (<25th percentile), normal (≥25th to ≤75th percentile) or late (>75th percentile) internally within the cohort, resulting in nine groups based on the combined timing of BMI peak and rebound (see illustration in Supplementary Figure 1, available as Supplementary data at IJE online). We assessed associations between peak-rebound pattern (with ‘normal’ timing of BMI peak and rebound as the reference category) and each cardiometabolic outcome, using multivariable linear regression models adjusted for the covariates listed above. We also examined outcomes of children without estimable trajectory characteristics, compared with those whose trajectories were estimable. We investigated effect modification by child sex by adding multiplicative interaction terms with each BMI milestone characteristic to each fully adjusted model.
In all analyses, we used chained equation multiple imputation to impute values for children with missing covariate or outcome data. We generated 50 imputed datasets for all 2128 Project Viva participants with live births. The imputation model included all exposures, outcomes and covariates under study. In final analytical models after imputation, we combined imputed datasets using MI ESTIMATE in Stata, after excluding 447 subjects who did not satisfy the inclusion criteria for this study, that is children without three or more BMI measurements from birth to mid childhood.15 Last, to assess robustness of our study findings, we repeated all analyses in subjects without missing covariate or outcome data (n = 837; Figure 1). We performed all analyses using Stata 15 software (StataCorp LP, TX).
Results
Cohort description
Table 1 describes the anthropometric characteristics of children with estimable BMI peak and rebound. The mean (SD) age at peak was 8.4 (2.7) months and at rebound was 60.9 (21.0) months, and the mean (SD) magnitude of BMI at peak was 18.0 (1.4) kg/m2 and at rebound was 15.9 (1.2). Asian children had an earlier age at peak (7.5 vs 8.6 months) and Black children had an earlier age at rebound (55.2 vs 62.3 months) compared with White children. Girls had later age at peak, earlier age at rebound and lower magnitude at peak and rebound compared with boys. In adolescence, girls had higher FMI, TFMI, HOMA-IR and pubertal score, but lower height, SBP z-score and LMI than boys.
Table 1.
Distributions of growth parameters and cardiometabolic outcomes in children
| Boys n = 417 | Girls n = 387 | Totaldn = 804 | |
|---|---|---|---|
| Gestational age at deliverya | 39.6 (1.6) | 39.6 (1.5) | 39.6 (1.6) |
| Race/ethnicityb | |||
| White | 279 (66.9) | 284 (73.4) | 563 (70.0) |
| Black | 56 (13.4) | 41 (10.6) | 91 (12.1) |
| Hispanic | 19 (4.6) | 12 (3.1) | 31 (3.9) |
| Asian | 14 (3.4) | 13 (3.4) | 27 (3.4) |
| Others | 49 (11.8) | 37 (9.6) | 86 (10.7) |
| Birthweight-for-gestational age z-scorea | 0.2 (0.9) | 0.2 (1.0) | 0.2 (0.9) |
| Age at peak (months)a | 7.9 (1.7) | 9.0 (3.4)c | 8.4 (2.7) |
| BMI at peak (kg/m2)a | 18.3 (1.4) | 17.6 (1.3)c | 18.0 (1.4) |
| Age at rebound (months)a | 63.7 (21.1) | 57.8 (20.5)c | 60.9 (21.0) |
| BMI at rebound (kg/m2)a | 16.0 (1.2) | 15.8 (1.2)c | 15.9 (1.2) |
| Lean mass | |||
| Height z-score (SD units) | 0.4 (1.0) | 0.2 (1.0)c | 0.3 (1.0) |
| Lean mass index (kg/m2) | 15.0 (1.8) | 14.5 (1.8)c | 14.7 (1.8) |
| Total adiposity | |||
| BMI z-score (SD units) | 0.4 (1.0) | 0.3 (1.0) | 0.3 (1.0) |
| Fat-mass index (kg/m2) | 5.8 (2.8) | 6.4 (2.5)c | 6.1 (2.7) |
| Central adiposity | |||
| Waist-to-height ratio | 0.4 (0.07) | 0.4 (0.06) | 0.4 (0.06) |
| Trunk fat-mass index (kg/m2) | 2.2 (1.3) | 2.4 (1.2)c | 2.3 (1.2) |
| Metabolic risk score components | |||
| Waist circumference (cm) | 72.6 (11.4) | 71.7 (9.6) | 72.1 (10.6) |
| Systolic BP z-score (SD units) | 0.09 (0.8) | 0.3 (0.8)c | 0.2 (0.8) |
| Log HOMA-IR | 0.9 (0.6) | 1.1 (0.6)c | 0.9 (0.6) |
| HDL cholesterol (mg/dl) | 55.4 (14.3) | 56.0 (12.6) | 55.7 (13.5) |
| Triglyceride (mg/dl) | 69.5 (32.7) | 71.8 (30.1) | 70.5 (31.5) |
| Global metabolic risk score (units) | −0.02 (0.62) | −0.08 (0.53) | −0.04 (0.58) |
| Pubertal score | 2.2 (0.8) | 2.6 (0.4)c | 2.4 (0.6) |
aMean (SD).
bn (%).
cP < 0.05 compared with boys, using two-sample t test.
dSample is restricted to children with no missing covariates, at least one outcome measure and estimable BMI peak and rebound.
Children without outcome measurements at adolescence were more likely to be Black, not to initiate breastfeeding, had parents without university education and mothers who smoked during pregnancy, compared with children with at least one outcome measurement (Supplementary Table 1, available as Supplementary data at IJE online). Children who did not exhibit a BMI peak (i.e. no decline in BMI was observed after the rise in infancy) were more likely to have obese parents (either mother, father or both), and less likely to have both parents with university education compared with children with estimable BMI peak and rebound.
Associations of BMI peak and rebound with cardiometabolic outcomes in early adolescence
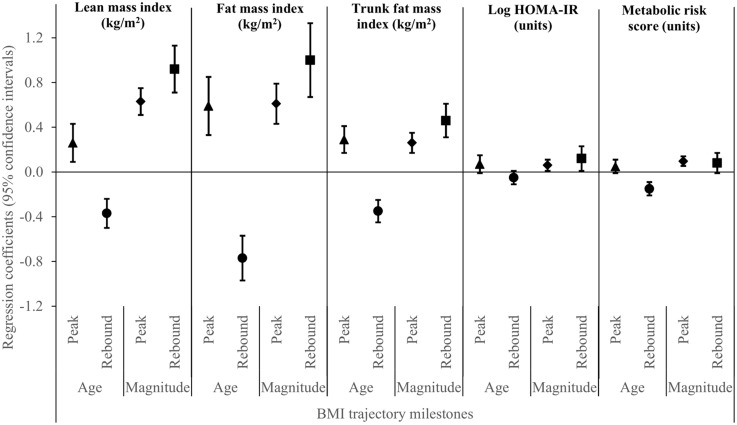
Age at BMI peak was positively associated, and age at rebound was strongly inversely associated, with lean mass and total and central adiposity in early adolescence. Age at rebound was inversely associated with metabolic risk score [−0.1units (−0.2,−0.09)] (Figure 2). Age at BMI rebound significantly interacted with child sex in its association with metabolic risk score; the relationship of age at rebound with metabolic risk score was pronounced in boys [−0.2 units (−0.3,−0.1)] but not in girls [0.007 units (−0.1, 0.1); pint < 0.001].
Figure 2.
Associations of ages at BMI peak and rebound, and magnitudes of BMI at peak and rebound with lean-, fat- and trunk fat-mass indices, insulin resistance and metabolic risk score. ▴ = age at BMI peak; • = age at BMI rebound; ♦ = magnitude of BMI at peak; ▪ = magnitude of BMI at rebound.
Magnitude of BMI at both peak and (especially) rebound was positively associated with lean mass and total and central adiposity (Figure 2). Magnitudes of BMI at both peak and rebound were associated with insulin resistance [peak: 0.05 units (0.004, 0.1); rebound: 0.1 units (0.0, 0.2)], and magnitude of BMI at peak was associated with metabolic risk score [0.1 units (0.05, 0.1)]. Neither magnitude significantly interacted with child sex for any of the study outcomes.
Associations of BMI peak-rebound combinations with cardiometabolic outcomes in early adolescence
Children with normal timing of BMI peak but early BMI rebound had a more adverse cardiometabolic profile than children with normal timing of both peak and rebound, characterized by higher total and central adiposity, HOMA-IR and metabolic risk score. In contrast, children with early BMI peak but late BMI rebound pattern were protected from an adverse cardiometabolic profile, as characterized by lower total and central adiposity, HOMA-IR and metabolic risk score (Table 2). Associations of normal peak but early rebound showed significant interactions with child sex for WC, WHtR, HOMA-IR, triglycerides and metabolic risk score. For example, the association between the normal peak-early rebound pattern and metabolic risk score was more pronounced in boys [0.6 units (0.3, 0.9) vs 0.3 units (0.03, 0.6); pint = 0.04], whereas the associations with HOMA-IR was pronounced only in girls [0.3 (0.01, 0.6) vs 0.1(−0.2, 0.4); pint = 0.02].
Table 2.
Associations between timing of BMI peak-rebound patterns and cardiometabolic outcomes at early adolescence
| β (95% CI)a |
|||||
|---|---|---|---|---|---|
| Fat-mass index (kg/m2)b | Trunk fat-mass index (kg/m2)b | Log HOMA-IR (units)b | Metabolic risk score (units)c | ||
| Early peak | Early rebound (n = 194) | 1.2 (0.5, 1.8) | 0.5 (0.2, 0.9) | −0.1 (−0.3, 0.1) | 0.2 (−0.02, 0.4) |
| Normal rebound (n = 118) | −0.4 (−1.1, 0.2) | −0.2 (−0.5, 0.1) | 0.0 (−0.2, 0.2) | 0.0 (−0.2, 0.2) | |
| Late rebound (n = 154) | −1.1 (−1.7, −0.5) | −0.4 (−0.7, −0.1) | −0.3 (−0.4, −0.1) | −0.2 (−0.4, 0.0) | |
| Normal peak | Early rebound (n = 118) | 2.2 (1.6, 2.9) | 1.1 (0.8, 1.5) | 0.2 (0.04, 0.4) | 0.4 (0.2, 0.5) |
| Normal rebound (n = 470) | Ref | Ref | Ref | Ref | |
| Late rebound (n = 177) | −0.8 (−1.4, −0.2) | −0.3 (−0.6, 0.0) | −0.2 (−0.3, 0.01) | −0.1 (−0.3, 0.0) | |
| Late peak | Early rebound (n = 81) | 2.0 (1.2, 2.8) | 1.0 (0.6, 1.3) | −0.1 (−0.3, 0.1) | 0.2 (−0.1, 0.4) |
| Normal rebound (n = 221) | 0.5 (−0.2, 1.1) | 0.3 (−0.1, 0.6) | 0.1 (−0.1, 0.3) | 0.2 (0.0, 0.4) | |
| Late rebound (n = 68) | −0.5 (−1.3, 0.3) | −0.2 (−0.6, 0.2) | −0.1 (−0.3, 0.2) | −0.1 (−0.3, 0.1) | |
aEffect estimates and 95% confidence intervals are relative to children with normal timing of both peak and rebound.
bAdjusted for parental university education, parental obesity status, maternal total gestational weight gain, smoking history, glucose tolerance status, hypertensive disorders of pregnancy, child sex, race/ethnicity, gestational age at delivery, birthweight for gestational age z-scores, breastfeeding initiation, magnitude at BMI peak and child age at outcome measurement.
cAdjusted for parental university education, parental obesity status, maternal total gestational weight gain, smoking history, glucose tolerance status, hypertensive disorders of pregnancy, child race/ethnicity, gestational age at delivery, birthweight for gestational age z-scores, breastfeeding initiation, magnitude at BMI peak and child age at outcome measurement.
No association with systolic BP z-score, HDL cholesterol or triglycerides was observed for any BMI milestone or peak-rebound combination. Children without an estimable BMI peak had outcomes that mirrored children with a normal peak-early rebound pattern (higher total and central adiposity, higher HOMA-IR and metabolic risk score), whereas those without an estimable BMI rebound had lower lean mass and total and central adiposity than children whose peak and rebound were both estimable (Supplementary Table 2, available as Supplementary data at IJE online). The associations of non-estimable v. estimable BMI peak with FMI (7.6 vs 3.7 kg/m2; pint = 0.007), trunk FMI (4.0 vs 1.9 kg/m2; pint = 0.002) and metabolic risk score (1.2 vs. 0.4 units; pint = 0.02) were more pronounced in boys than girls, respectively. For all analyses, we observed no appreciable changes in effect estimates after additional adjustment for pubertal score. We also observed similar patterns of associations of age and magnitude at BMI peak and rebound, and of peak-rebound combined timing categories, with the cardiometabolic outcomes in subjects without missing covariate or outcome data (Supplementary Table 3, available as Supplementary data at IJE online; n = 837).
Discussion
We found age at BMI rebound to be a strong risk factor for adiposity and markers of cardiometabolic risk almost a decade later, independently of age and magnitude at BMI peak. We also observed that children with a normal peak-early rebound pattern had a poorer cardiometabolic profile, exemplified by associations with higher total and central adiposity, insulin resistance and global metabolic risk score, than children with the normal peak-normal rebound. Furthermore, children without a BMI peak exhibited poorer cardiometabolic outcomes, than children with estimable BMI peak or rebound.
In line with recent studies,5,7,23 we observed adiposity outcomes to be positively associated with magnitude of BMI at peak and rebound. These associations may be explained by BMI tracking from infancy to middle childhood.2,24 Furthermore, we found age and magnitude at rebound to be more strongly associated with adiposity and markers of cardiometabolic risk compared with age and magnitude at peak, suggesting that BMI rebound, which occurs later in childhood than BMI peak and thus is more proximal to outcomes, is a stronger risk factor for later cardiometabolic health. Our findings corroborate those of Sovio et al.,23 which similarly reported stronger associations for age and magnitude at BMI rebound (compared with BMI peak) with adult cardiometabolic outcomes, and also those of other studies which have reported larger associations of later cardiometabolic health with growth during childhood than with growth during infancy.11,12,25–27 Although the exact mechanisms are unclear, we speculate that accumulation of fat during mid childhood is more likely to remain and less likely to be lost by early adolescence than accumulation of fat during infancy.
Viva children with a normal peak-early rebound timing pattern had a more adverse cardiometabolic profile, characterized by higher total and central adiposity, insulin resistance and metabolic risk score, compared with those with a normal peak-normal rebound pattern. Our results are consistent with recent studies reporting associations of early age at rebound with higher FMI,7 HOMA-IR28 and metabolic risk score.28 Other studies have also reported associations of earlier rebound with increased risk of glucose intolerance29 and type 2 diabetes mellitus30 in adults. Viva children whose BMI did not decline after its initial rise in infancy exhibited outcomes that mirrored those in children with normal peak-early rebound pattern, consistent with findings by Arisaka et al.31 Our finding that children with an early peak-late rebound pattern are protected from an adverse cardiometabolic profile also corroborates observations by Sovio et al.23 who reported that later age at rebound was associated with an improved cardiometabolic profile (lower WC, triglycerides, insulin and odds of metabolic syndrome). The link between BMI rebound and cardiometabolic risk could be due to the increased fat deposition often associated with early age at rebound.32 Taylor et al.33 reported a higher rate of fat gain in children with an early rebound than in those with a late rebound. Williams et al.34 also reported disproportionately high increases in fat mass in children with early BMI rebound. Excess fat can track into adulthood,35 leading to cardiometabolic consequences later in life. Gonzalez et al.28 had reported that total body fat is a mediator in the associations between age at rebound and metabolic markers such as insulin, triglycerides, HDL and metabolic risk score.
We observed sex differences in associations with cardiometabolic outcomes. The normal peak-early rebound pattern was more strongly associated with central adiposity and metabolic risk in boys, but with insulin resistance in girls. Our findings are consistent with studies that have reported sex differences in fat distribution and insulin resistance. Adolescent boys are known to accumulate more central adiposity,36 a known risk factor for increased metabolic risk,37 and adolescent girls are known to be more insulin-resistant.38 The underlying mechanism of these sex differences may involve sex hormone levels, which are known to have important effects on adiposity and insulin resistance among adolescents.38,39 These sex differences should be interpreted with caution, however. Children are typically more insulin-resistant during adolescence (during pubertal development), and girls are often further along in puberty compared with boys.40 Further studies should aim to understand these sex differences.
Our findings contribute important evidence concerning BMI peak and rebound. We have identified the independent and combined patterns of infant and early childhood BMI milestones related to risk of adverse cardiometabolic profiles in adolescence. Previous studies lacked the repeated BMI measures in infancy and childhood required to assess both BMI peak and rebound concurrently,5–7,9 nor have the relationships of BMI peak and rebound with later cardiometabolic health outcomes been well characterized. Assessing patterns of BMI peak and rebound that predict later cardiometabolic health outcomes may identify individuals to target for preventive interventions. Children with at-risk patterns cannot be identified until the peak and rebound have occurred, however. Therefore, prevention strategies can be implemented only during school age. The US Preventive Services Task Force (USPSTF) has recently provided evidence-based recommendations on comprehensive and multicomponent behavioural interventions to treat established obesity during school age.41–43 Recent interventions involving nutrition counselling, physical activity, parental support and behavioural knowledge implemented during school age have shown promise in effectively reducing BMI44 and other cardiometabolic risk markers45 in obese children. Further studies are needed to identify whether such interventions would be effective in preventing later obesity and its cardiometabolic consequences in children with at-risk BMI peak-rebound patterns.
Strengths of our study include its relatively large sample size, prospective study design, multiple measures of early life growth, long-term follow-up and wide range of cardiometabolic outcomes measured in early adolescence by highly trained staff using standardized protocols. This study is not without limitations, however. First, we estimated BMI peak and rebound from statistical models, rather than direct observations using the ‘gold standard’ of visual inspection of individual BMI-for-age curves.46 Our models, however, showed mean residual errors that were close to zero between observed and predicted BMI across all ages, suggesting that modelling the entire curve from birth provides more precise estimates of peak and rebound than visual inspection of raw BMI values, which is subject to large inter-observer variation.4,47 Second, a considerable number of children did not have outcome measures at early adolescence. Differences between children with and without outcome measures might conceivably have led to selection bias, but our multiple imputation analyses showed very similar findings compared with our complete-case analyses. Third, we derived the patterns of BMI peak-rebound combinations internally within our cohort, which therefore may not be generalizable to other populations. Fourth, we investigated multiple cardiometabolic outcomes, therefore increasing the risk of false-positive results. We chose not to adjust for multiple comparisons. Instead, the ‘significance’ of our findings is based on the strength and consistency of the associations observed across related outcomes.48 Fifth, residual confounding due to unmeasured risk factors of early adolescent outcomes (e.g. low physical activity or over-nutrition in mid childhood) could explain our observations. Sixth, early age at rebound may play a role in initiating puberty,49,50 which could explain the metabolic results among early adolescent subjects who had a more advanced pubertal stage than others.51 However, we observed no appreciable changes to our results after additional adjustment for pubertal score. Finally, our study findings may not be generalizable to other ethnic groups and populations from different settings, since many of our participants were White and university educated.10
In conclusion, we observed patterns of BMI peak and rebound in infancy and early childhood which are associated with risk of an adverse cardiometabolic profile later in life. Routine monitoring of BMI in young children and tracking its trajectory, as recommended by the US Preventive Services Task Force,41 may help to identify children at greatest risk of developing an adverse cardiometabolic profile in later life, and who may benefit from preventive interventions.
Funding
This work is supported by the National Institutes of Health (UG3OD023286, P30 DK092924, R01AI102960, R01 HD034568). I.M.A. is supported by the National University of Singapore Overseas Postdoctoral Fellowship (NUS OPF/2017). L-J.L. is supported by the Singapore National Medical Research Council (NMRC TA/0027/2014, NMRC/CG/C008A/2017 KKH).
Conflict of interest
All authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
Supplementary Material
References
- 1.Non-communicable Disease Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;10:32129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008;9:474–88. [DOI] [PubMed] [Google Scholar]
- 3. Naukkarinen J, Rissanen A, Kaprio J, Pietilainen KH. Causes and consequences of obesity: the contribution of recent twin studies. Int J Obes (Lond) 2012;36:1017–24. [DOI] [PubMed] [Google Scholar]
- 4. Wen X, Kleinman K, Gillman MW, Rifas-Shiman SL, Taveras EM. Childhood body mass index trajectories: modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC Med Res Methodol 2012;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aris IM, Bernard JY, Chen LW et al. . Infant body mass index peak and early childhood cardiometabolic risk markers in a multi-ethnic Asian birth cohort. Int J Epidemiol 2017;46:513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marinkovic T, Toemen L, Kruithof CJ et al. . Early infant growth velocity patterns and cardiovascular and metabolic outcomes in childhood. J Pediatr 2017;186:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes AR, Sherriff A, Ness AR, Reilly JJ. Timing of adiposity rebound and adiposity in adolescence. Pediatrics 2014;134:e1354–61. [DOI] [PubMed] [Google Scholar]
- 8. Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes 2006;30:S11–17. [DOI] [PubMed] [Google Scholar]
- 9. Koyama S, Ichikawa G, Kojima M, Shimura N, Sairenchi T, Arisaka O. Adiposity rebound and the development of metabolic syndrome. Pediatrics 2014;133:e114–19. [DOI] [PubMed] [Google Scholar]
- 10. Oken E, Baccarelli AA, Gold DR et al. . Cohort Profile: Project Viva. Int J Epidemiol 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perng W, Hajj H, Belfort MB et al. . Birth size, early life weight gain, and midchildhood cardiometabolic health. J Pediatr 2016;173:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perng W, Rifas-Shiman SL, Kramer MS et al. . Early weight gain, linear growth, and mid-childhood blood pressure: a prospective study in Project Viva. Hypertension 2016;67:301–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care 2013;36:3045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, Kleinman KP, Gillman MW. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed 2005;7:56. [PMC free article] [PubMed] [Google Scholar]
- 15. Aris IM, Rifas-Shiman S, Li LJ et al. . Pre-, perinatal and parental predictors of body mass index trajectory milestones. J Pediatr 2018;201:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li LJ, Rifas-Shiman SL, Aris IM et al. . Associations of maternal and cord blood adipokines with offspring adiposity in Project Viva: is there an interaction with child age? Int J Obes (Lond) 2017;13:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuczmarski RJ, Ogden CL, Guo SS et al. . 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190. [PubMed] [Google Scholar]
- 18. Boeke CE, Mantzoros CS, Hughes MD et al. . Differential associations of leptin with adiposity across early childhood. Obesity (Silver Spring) 2013;21:1430–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–76. [PubMed] [Google Scholar]
- 20. Haugaard LK, Baker JL, Perng W et al. . Growth in total height and its components and cardiometabolic health in childhood. PLoS One 2016;11:e0163564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States National Reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fairlie TG, Gillman MW, Rich-Edwards J. High pregnancy-related anxiety and prenatal depressive symptoms as predictors of intention to breastfeed and breastfeeding initiation. J Womens Health (Larchmt) 2009;18:945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sovio U, Kaakinen M, Tzoulaki I et al. . How do changes in body mass index in infancy and childhood associate with cardiometabolic profile in adulthood? Findings from the Northern Finland Birth Cohort 1966 Study. Int J Obes 2014;38:53–59. [DOI] [PubMed] [Google Scholar]
- 24. Kristiansen AL, Bjelland M, Brantsæter AL et al. . Tracking of body size from birth to 7 years of age and factors associated with maintenance of a high body size from birth to 7 years of age - the Norwegian Mother and Child Cohort study (MoBa). Public Health Nutr 2015;18:1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aris IM, Bernard JY, Chen LW et al. . Postnatal height and adiposity gain, childhood blood pressure and prehypertension risk in an Asian birth cohort. Int J Obes (Lond) 2017;41:1011–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tilling K, Davies N, Windmeijer F et al. . Is infant weight associated with childhood blood pressure? Analysis of the Promotion of Breastfeeding Intervention Trial (PROBIT) cohort. Int J Epidemiol 2011;40:1227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang X, Tilling K, Martin RM et al. . Analysis of ‘sensitive’ periods of fetal and child growth. Int J Epidemiol 2019;48:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonzalez L, Corvalan C, Pereira A, Kain J, Garmendia ML, Uauy R. Early adiposity rebound is associated with metabolic risk in 7-year-old children. Int J Obes (Lond) 2014;38:1299–304. [DOI] [PubMed] [Google Scholar]
- 29. Bhargava SK, Sachdev HS, Fall CH et al. . Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia 2003;46:190–94. [DOI] [PubMed] [Google Scholar]
- 31. Arisaka O, Sairenchi T, Ichikawa G, Koyama S. Increase of body mass index (BMI) from 1.5 to 3 years of age augments the degree of insulin resistance corresponding to BMI at 12 years of age. J Pediatr Endocrinol Metab 2017;30:455–57. [DOI] [PubMed] [Google Scholar]
- 32. Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts 2017;10:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor RW, Williams SM, Carter PJ, Goulding A, Gerrard DF, Taylor BJ. Changes in fat mass and fat-free mass during the adiposity rebound: FLAME study. Int J Pediatr Obes 2011;6:e243–51. [DOI] [PubMed] [Google Scholar]
- 34. Williams SM, Goulding A. Patterns of growth associated with the timing of adiposity rebound. Obesity (Silver Spring) 2009;17:335–41. [DOI] [PubMed] [Google Scholar]
- 35. Taylor RW, Grant AM, Goulding A, Williams SM. Early adiposity rebound: review of papers linking this to subsequent obesity in children and adults. Curr Opin Clin Nutr Metab Care 2005;8:607–12. [DOI] [PubMed] [Google Scholar]
- 36. Ohlsson C, Lorentzon M, Norjavaara E, Kindblom JM. Age at adiposity rebound is associated with fat mass in young adult males - the GOOD study. PLoS One 2012;7:e49404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ali O, Cerjak D, Kent JW, James R, Blangero J, Zhang Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatr Obes 2014;9:e58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeffery SC, Hosking J, Jeffery AN et al. . Insulin resistance is higher in prepubertal girls but switches to become higher in boys at age 16: a Cohort Study (EarlyBird 57). Pediatr Diabetes 2017;29:223–30. [DOI] [PubMed] [Google Scholar]
- 39. Agirbasli M, Agaoglu NB, Orak N et al. . Sex hormones, insulin resistance and high-density lipoprotein cholesterol levels in children. Horm Res Paediatr 2010;73:166–74. [DOI] [PubMed] [Google Scholar]
- 40. Levy-Marchal C, Arslanian S, Cutfield W et al. . Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab 2010;95:5189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Block JP, Oken E. Practical considerations for the US Preventive Services task force recommendations on obesity in children and adolescents. JAMA Intern Med 2017;1; 177:1077–79. [DOI] [PubMed] [Google Scholar]
- 42. Grossman DC, Bibbins-Domingo K, Curry SJ et al. . Screening for obesity in children and adolescents: US preventive services task force recommendation statement. JAMA 2017;317:2417–26. [DOI] [PubMed] [Google Scholar]
- 43. O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017;317:2427–44. [DOI] [PubMed] [Google Scholar]
- 44. Sobol-Goldberg S, Rabinowitz J, Gross R. School-based obesity prevention programs: a meta-analysis of randomized controlled trials. Obesity (Silver Spring) 2013;21:2422–28. [DOI] [PubMed] [Google Scholar]
- 45. Foster GD, Linder B, Baranowski T et al. . A school-based intervention for diabetes risk reduction. N Engl J Med 2010;363:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kroke A, Hahn S, Buyken AE, Liese AD. A comparative evaluation of two different approaches to estimating age at adiposity rebound. Int J Obes (Lond) 2006;30:261–66. [DOI] [PubMed] [Google Scholar]
- 47. Campbell MW, Williams J, Carlin JB, Wake M. Is the adiposity rebound a rebound in adiposity? Int J Pediatr Obes 2011;6:e207–15. [DOI] [PubMed] [Google Scholar]
- 48. Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity - whether and how to correct for many statistical tests. Am J Clin Nutr 2015;102:721–28. [DOI] [PubMed] [Google Scholar]
- 49. Marakaki C, Karapanou O, Gryparis A, Hochberg Z, Chrousos G, Papadimitriou A. Early adiposity rebound and premature adrenarche. J Pediatr 2017;186:72–77. [DOI] [PubMed] [Google Scholar]
- 50. German A, Shmoish M, Hochberg Z. Predicting pubertal development by infantile and childhood height, BMI, and adiposity rebound. Pediatr Res 2015;78:445–50. [DOI] [PubMed] [Google Scholar]
- 51. Berentzen NE, Wijga AH, van Rossem L, Postma DS, Gehring U, Smit HA. Pubertal timing and cardiometabolic markers at age 16 years. J Pediatr 2017;187:158–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.