Abstract
Background
Type 2 diabetes (T2D) results from a complex interplay between genetics and the environment. Several epigenome-wide association studies (EWAS) have found DNA methylation loci associated with T2D in European populations. However, data from African populations are lacking. We undertook the first EWAS for T2D among sub-Saharan Africans, aiming at identifying ubiquitous and novel DNA methylation loci associated with T2D.
Methods
The Illumina 450k DNA-methylation array was used on whole blood samples of 713 Ghanaian participants (256 with T2D, 457 controls) from the cross-sectional Research on Obesity and Diabetes among African Migrants (RODAM) study. Differentially methylated positions (DMPs) for T2D and HbA1c were identified through linear regression analysis adjusted for age, sex, estimated cell counts, hybridization batch, array position and body mass index (BMI). We also did a candidate analysis of previously reported EWAS loci for T2D in non-African populations, identified through a systematic literature search.
Results
Four DMPs [cg19693031 (TXNIP), cg04816311 (C7orf50), cg00574958 (CPT1A), cg07988171 (TPM4)] were associated with T2D after correction for inflation by possible systematic biases. The most strongly associated DMP—cg19693031, TXNIP (P = 2.6E-19) —showed hypomethylation in T2D cases compared with controls. Two out of the four DMPs [cg19693031 (TXNIP), cg04816311 (C7orf50)] remained associated with T2D after adjustment for BMI, and one locus [cg07988171 (TPM4)] that has not been reported previously.
Conclusions
In this first EWAS for T2D in sub-Saharan Africans, we have identified four DMPs at epigenome-wide level, one of which is novel. These findings provide insight into the epigenetic loci that underlie the burden of T2D in sub-Saharan Africans.
Keywords: DNA-methylation, Africans, type 2 diabetes, epigenetic epidemiology, EWAS, RODAM study
Key Messages
This is the first epigenome-wide study on type 2 diabetes in sub-Saharan Africans.
A novel DNA methylation locus was associated with type 2 diabetes in a sample of Ghanaians.
The three DNA methylation loci most strongly associated with type 2 diabetes were also previously reported for other populations, indicating that these are ubiquitous.
The four epigenetic loci that were genome-wide associated with type 2 diabetes explained 25% of variance in type 2 diabetes.
Background
Type 2 diabetes (T2D) is estimated to affect 415 million adults worldwide, contributing greatly to mortality and health care costs.1 It is a complex multifactorial disease, meaning that many genetic loci as well as environmental factors play a role.2,3 The explained heritability of T2D is 5–10%,4 of an estimated overall heritability of about 10–20%,4 suggesting that additional genetic factors remain to be identified and that gene-environment interactions may contribute to the onset of T2D.5
Epigenetics is the study of mitotically heritable yet reversible molecular modifications to the DNA without altering the DNA sequence.6 Epigenetic processes play a role in organism development, cell differentiation and genome stability. Furthermore, epigenetics is considered a means of interaction between genes and environment, and one of its main read-outs, DNA methylation, has been previously associated with T2D in European populations.7,8 Additionally, a great variety of health-related behaviours, such as diet and physical activity, can affect DNA methylation even in a short time span.9,10 T2D is frequently accompanied by health-related behaviours such as reduced physical activity and unhealthy diet.11 These health-related behaviours may affect T2D risk through epigenetics.12
The prevalence of T2D in sub-Saharan Africa is increasing rapidly, and the International Diabetes Federation estimates that the number of people with T2D in the African region may rise from 14.2 million to 34.2 million in 2040.1 In addition, sub-Saharan African migrants in Europe are about three times more likely to have T2D compared with European populations.13 This increasing T2D burden in sub-Saharan Africa, as well as among migrants, likely reflects increasing obesity rates, diet changes and a reduction in physical activity.14 Environmental risk factors may be mediated (at least partially) through changes in DNA methylation. Previous epigenome-wide association studies (EWAS) found DNA methylation at multiple loci associated with T2D in blood,7,8 pancreatic,15 adipose,16 liver17 and muscle tissue18 from European populations. However, similar data on any tissue from sub-Saharan African populations are lacking. Sub-Saharan Africans are characterized by wide genetic variation, as well as marked differences in genetic variation and linkage disequilibrium, when compared with populations from other continents. The differences in the genetics of sub-Saharan African populations compared with Europeans, as well as differences in environmental exposures and health behaviours, could influence epigenetic loci associated with traits and disorders in sub-Saharan Africans.
In the present study, we conducted an EWAS for T2D in sub-Saharan Africans, aiming at identifying DNA methylation loci in blood associated with T2D using an epigenome-wide association study. Second, we aimed at identifying loci shared between populations of different ancestry (i.e. ubiquitous variants), as well as loci that have not been previously reported in other populations and are therefore potentially unique to sub-Saharan Africans.
Methods
Study population
This study is part of the Research on Obesity and Diabetes among African Migrants (RODAM) study. Details of the design and data collection of the RODAM study are described elsewhere.19,20 In brief, the RODAM study collected samples and data between 2012 and 2015 on 6385 Ghanaians resident in five geographical locations. An individual was considered Ghanaian when born in Ghana and at least one of the parents was born in Ghana, or when both parents were born in Ghana. Ethical approval was obtained from the ethical committees of the institutions involved in Ghana, The Netherlands, Germany and the UK. Written informed consent was obtained from all study participants. The EWAS was performed using a subset of 736 participants (265 T2D cases, 471 controls) of the RODAM cohort, of which 713 samples (256 T2D cases, 457 controls) passed quality control (see below). All T2D cases not on glucose-lowering medication were selected with a group of T2D controls on an approximate 1:2 ratio, respectively, which is generally accepted to favour statistical power. The final sample size had over 80% power to detect a 6% difference in methylation between T2D cases and controls with epigenome-wide significance, and over 99% power to detect a 10% difference in methylation between cases and controls.21
Phenotypic measurements
Information on demographics and self-reported T2D were collected by self- or interviewer-administered questionnaire. Self-reported T2D comprised an affirmative response to the question ‘Have you ever been diagnosed with diabetes by a doctor or health care worker?’ Participants were physically examined; height (SECA 217 stadiometer) and weight (SECA 877 scale) were assessed in light clothing, and body mass index (BMI) was calculated (kg/m2). Participants were instructed to fast from 10 pm on the evening before the physical examination. Fasting venous blood samples were collected, and fasting glucose in mmol/l was measured by the ABX PENTRA 400 and HbA1c in mmol/mol by high-performance liquid chromatography (TOSOH G8 HPLC analyser). T2D was defined according to self-reported diabetes and/or fasting glucose ≥7.0 µl/l. In a sensitivity analysis, we additionally applied an HbA1c cut-off of ≥6.5% (48 µl/mol) to the T2D definition (Supplementary Material 1, available as Supplementary data at IJE online).
DNA methylation profiling and processing
DNA extraction and methylation profiling were performed on whole-blood samples by Source BioScience, Nottingham, UK. Bisulphite DNA treatment was achieved using the Zymo EZ DNA MethylationTM kit, and the quality of the conversion was determined by high-resolution melting analyses.22 The converted DNA was amplified and hybridized on the Illumina Human Methylation 450 K array, which measures DNA methylation levels of approximately 485 000 CpG sites. The samples were randomly divided over nine bisulphite conversion and hybridization batches. The quality control procedures are described in detail in Supplementary Material 2, available as Supplementary data at IJE online. After quality control a sample size of 713 remained for the current analyses.
Statistical analysis
Differentially methylated positions (DMPs)
Linear association analyses were performed in ‘R’, using the lmFit function from the Limma package to identify DMPs. DNA methylation levels were the dependent variable in all analyses. Age, sex, estimated cell counts and technical effects (hybridization batch and array position) were included as covariates both for the epigenome-wide approach and for the candidate approach. Candidate loci were selected from the literature as described below. Correlation of the included covariates with DNA methylation is shown in Supplementary Material 2, Figures 2 and 3, available as Supplementary data at IJE online. Plate position was additionally added as covariate as identified in principal component analysis (Supplementary Material 2, Figure 4, available as Supplementary data at IJE online).
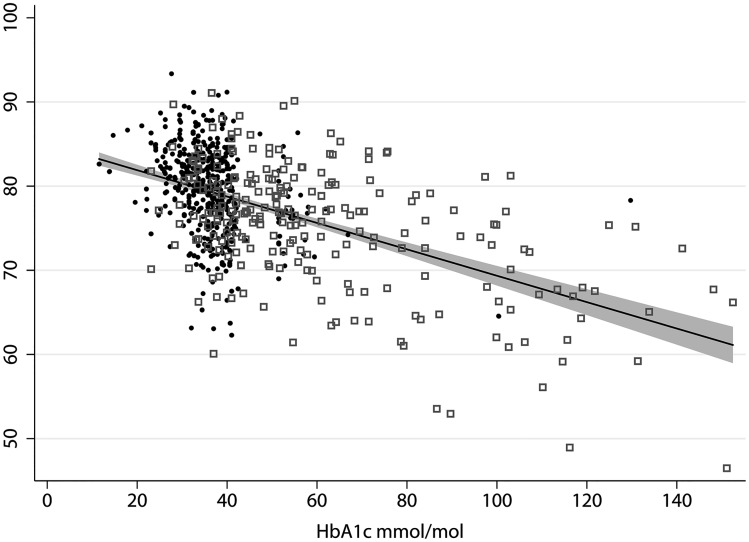
Figure 2.
Scatterplot of HbA1c and percentage methylation for differentially methylation position cg19693031 on gene TXNIP by sample. Black dot = T2D control, grey square = T2D case, line = fitted values, grey-shaded area = 95% CI.
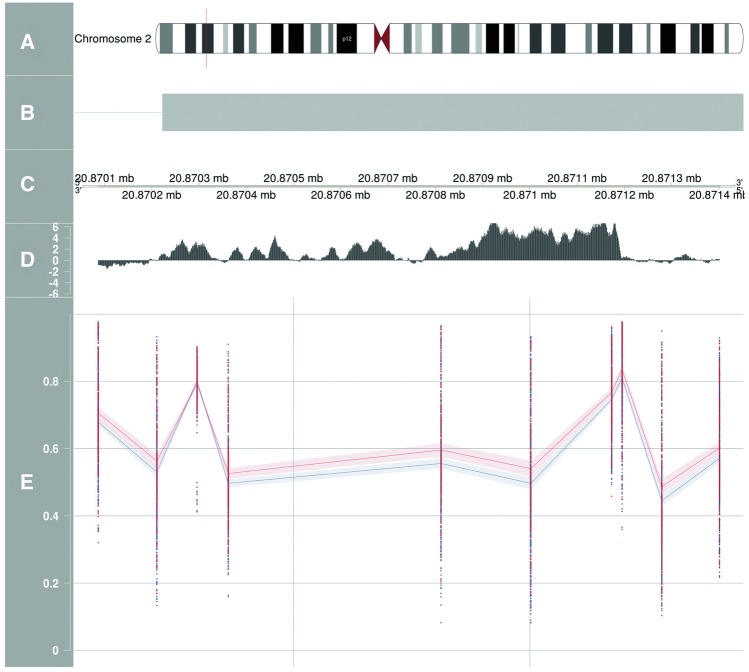
Figure 3.
Differentially methylated region located near gene GDF7 associated with type 2 diabetes. A = chromosome, B = reference gene, C = position, D = conservation species track, E = betas 450k DNA methylation + 95% confidence intervals. Blue = diabetes cases, red = diabetes controls.
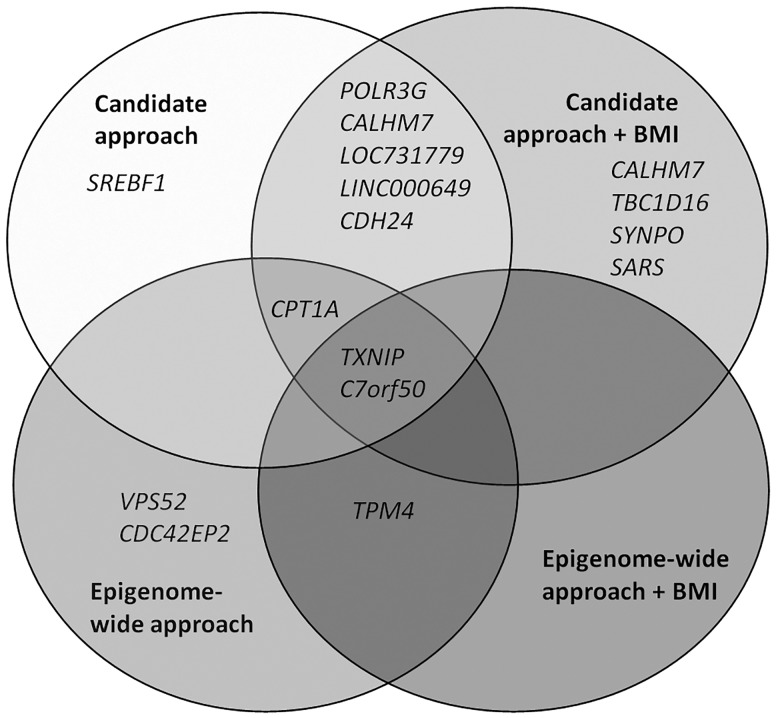
Figure 4.
Venn diagram of overlapping genes. Gene names are genes on which identified differentially methylated positions were located in epigenome-wide analyses and in analyses on differentially methylated positions previously reported in other populations (candidate approach).
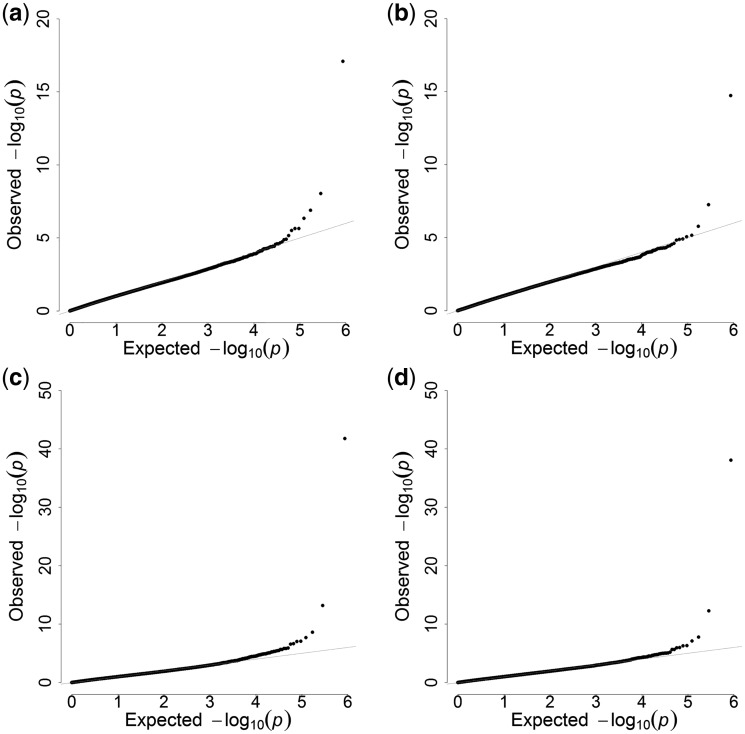
Since obesity is a major risk factor for T2D, methylation differences between T2D cases could be confounded by BMI. We therefore added BMI in a secondary model, to identify DMPs associated with T2D independent of BMI. In order to address possible inflation of our test statistics by systematic biases, we applied the recently reported EWAS method by van Iterson et al.,23 using the BACON package (version 1.4.0) in ‘R’ software. This package includes a Bayesian method that controls biases based on estimation of the empirical null distribution. Model fitting was evaluated using Q-Q plots (Figure 1a–d) . The lambdas for the T2D and HbA1c DMP analyses without and with adjustment for BMI reduced from 1.19, 1.31, 1.40 and 1.51 before implementation of the BACON package, to 1.04, 1.10, 1.10 and 1.14 after, respectively. Betas of DMPs identified were subsequently included as independent variables in logistic regression models to assess the association of 1% increase in DNA methylation of respective DMPs with T2D. The Nagelkerke’s R squared statistic from the logistic regression models with and without covariates was used to calculate trait variance explained by a locus. The results of the logistic regression analysis were presented as odds ratios with corresponding 95% confidence intervals (CI) and P-values.
Figure 1.
(a) Q-Q plot of EWAS P-values for DMP analyses on type 2 diabetes unadjusted for BMI after BACON correction. Lambda = 1.04. (b) Q-Q plot of EWAS P-values for DMP analyses on type 2 diabetes adjusted for BMI after BACON correction. Lambda = 1.10. (c) Q-Q plot of EWAS P-values for DMP analyses on HbA1c unadjusted for BMI after BACON correction. Lambda = 1.10. (d) Q-Q plot of EWAS P-values for DMP analyses on HbA1c adjusted for BMI after BACON correction. Lambda = 1.14.
To confirm our results from the binary T2D analyses, we additionally performed association analyses with the continuous measure of HbA1c in µl/mol as the dependent variable on a subset of 675 samples for which HbA1c measurements were available. HbA1c levels reflect a mean blood glucose over a period of 8–12 weeks24 and are therefore often used to get an impression of long-term glucose control in both T2D cases and healthy individuals. The same covariates as in the T2D analysis were selected.
For all DMP analyses, M-values were calculated as the log2 ratio of the intensities of methylated probes versus unmethylated probes. Identification of DMPs was based on M-values instead of beta-values, as M-values are more statistically valid.25 Corresponding beta-values were reported to facilitate biological interpretation.25 False discovery rate (FDR) adjusted P-values corresponding to M-values were used to adjust for multiple testing. FDR was calculated according to the Benjamini–Hochberg procedure.
Differentially methylated regions
To identify differentially methylated regions (DMRs), we fitted models similar to DMP analysis using the bumphunter function in the Minfi package.26 We calculated the DMRs with the methylation cut-off of 0.015 (corresponding to 1.5% difference in beta -alues) for T2D and 0.00025 for the continuous measure of HbA1c (corresponding to a 0.025 increase in beta-values per 1-unit increase in HbA1c) using bootstrapping with 500 permutations. In these analyses, beta-values were used instead of M-values as the bumphunter function currently does not yet support use of M-values. The DMR methylation cut-off was optimized based on observed effect sizes and significance levels in a volcano plot. We defined a DMR as three or more CpG sites in one cluster. Multiple testing adjustment was performed using family-wise error rate (FWER). We filtered CpG sites with FWER <0.2. Additional DMRs identified with less stringent thresholds are reported in Supplementary Material 3, available as Supplementary data at IJE online. Regulation and expression tracks (ENCODE RNA-seq track, ENCODE integrated regulation track and 100 Vertebrate Conservation track) were evaluated for the identified DMRs, using the University of California Santa Cruz (UCSC) human genome browser.27,28 In order to assess the odds for T2D per 1% increase in DNA methylation of respective DMRs, the average betas for each of the CpGs in a DMR were included in logistic regression models.
Candidate approach of loci reported in non-African populations
Previously reported DMPs and DMRs for T2D were identified through a systematic literature search in PUBMED (Supplementary Material 4, available as Supplementary data at IJE online) to use as candidate loci. Multiple testing thresholds using the FDR method were re-calculated for these candidate CpGs (n = 2876) due to the lower number of CpGs included. Only one previous EWAS found through the literature search-reported DMRs. Overlap with DMRs identified through bumphunter was studied.
Additional post-hoc analyses
We performed additional posthoc analyses on identified DMPs of both T2D and HbA1c analyses, including pathway enrichment analyses, evaluation of regulatory regions, evaluation of genetic interaction and gene expression using public databases, and evaluation of methylation levels across recruitment sites. A detailed description of methods for these post hoc analyses and their results can be found in Supplementary Material 3, available as Supplementary data at IJE online.
Results
Participant characteristics
Out of the 713 samples included in the analysis, 256 were T2D cases and 457 werecontrols (Table 1). Cases and controls were similar in age, gender distribution and sites of data collection. As expected, fasting glucose, HbA1c, BMI and positive family history of T2D were higher in cases than in controls (Table 1). Half of the T2D cases were unaware of their T2D status at the time of examination; the majority of unaware cases resided in Ghana. Among those aware, the average time since diagnosis was 2.4 years. Smoking and alcohol consumption were both low in our study population, with in total only 16 smokers. Both these lifestyle factors were similar between T2D cases and controls. The estimated distribution of cell types inferred from the methylation profile was similar between T2D cases and controls, except for CD4T cell count which was slightly lower in T2D cases (mean 17.3, 95% CI 16.6, 17.9) compared with controls (mean 18.6, 95% CI 18.0, 19.1).
Table 1.
Characteristics of type 2 diabetes (T2D) cases and controlsa
| T2D cases (n = 256) | T2D controls (n = 457) | Rural Ghana (n = 104) | Urban Ghana (n = 243) | Europe (n = 366) | |
|---|---|---|---|---|---|
| Age (years) | 51.9 (50.6, 53.2) | 50.7 (49.8, 51.5) | 56.2 (54.4, 57.9) | 50.7 (49.5, 51.9) | 49.9 (48.8, 50.9) |
| Sex (% male) | 46.5 | 40.4 | 30.8 | 29.6 | 54.6 |
| Geographical location (%) | |||||
| Rural Ghana | 16.4 | 13.6 | NA | NA | NA |
| Urban Ghana | 34.4 | 33.9 | NA | NA | NA |
| Europe | 49.2 | 52.5 | NA | NA | NA |
| BMI (kg/m2) | 27.8 (27.1, 28.4) | 26.6 (26.0, 27.1) | 22.9 (22.0, 23.7) | 26.2 (25.5, 27.0) | 28.7 (28.2, 29.2) |
| Fasting glucose (mmol/L)b | 9.1 (8.5, 9.6) | 5.0 (4.9, 5.0) | 6.7 (5.9, 7.5) | 7.3 (6.7, 7.8) | 5.8 (5.6, 6.0) |
| HbA1c (%)b | 7.7 (9.0, 10.1) | 5.5 (5.4, 5.5) | 5.7 (5.2, 6.1) | 6.7 (6.3, 7.0) | 6.1 (6.0, 6.2) |
| HbA1c (mmol/mol)b | 60.3 (56.8, 63.7) | 36.3 (35.4, 37.1) | 38.3 (33.6, 42.9) | 49.3 (45.9, 52.7) | 43.2 (41.6, 44.8) |
| HOMA-IRb | 2.74 (3.5) | 1.18 (1.04) | 1.17 (1.52) | 1.69 (2.30) | 1.40 (1.69) |
| HOMA-betab | 38.9 (56.8) | 74.3 (72.2) | 50.1 (59.4) | 54.6 (77.7) | 67.4 (65.0) |
| Immediate family with diabetes (%) | 37.7 (31.8, 43.9) | 17.1 (13.9, 20.9) | 17.8 (11.5, 26.6) | 29.0 (23.6, 35.1) | 23.2 (19.1, 27.9) |
| Newly detected T2D (%) | 47.8 (41.5, 54.1) | NA | 22.1 (15.1, 31.2) | 19.8 (15.2, 25.3) | 12.6 (9.5, 16.4) |
| Years since diagnosis (years) | 2.4 (1.8, 3.0) | NA | 2.6 (0.5, 4.6)c | 2.0 (1.3, 2.8)c | 2.6 (1.7, 3.5)c |
| Current smokers (%) | 2.0 (0.8, 4.8) | 2.4 (1.4, 4.4) | 0 (NA) | 0.4 (0.0, 2.9) | 4.2 (2.5, 6.8) |
| Average alcohol intake (units/week)d | 1.2 (0.8, 1.6) | 1.5 (0.9, 2.1) | 1.2 (0.1, 2.4) | 0.4 (0.2, 0.6) | 2.1 (1.3, 2.9) |
| Estimated cell counts (%)e | |||||
| CD8+ T cells | 10.7 (10.1, 11.3) | 11.0 (10.6, 11.5) | 12.0 (11.1, 13.0) | 11.9 (11.3, 12.4) | 9.9 (9.5, 10.4) |
| CD4+ T cells | 17.3 (16.6, 17.9) | 18.6 (18.0, 19.1) | 17.5 (16.5, 18.7) | 18.0 (17.2, 18.7) | 18.4 (17.8, 18.9) |
| Natural killer cells | 10.7 (10.0, 11.3) | 10.8 (10.3, 11.3) | 13.1 (11.9, 14.3) | 11.3 (10.6, 12.0) | 9.7 (9.2, 10.2) |
| B cells | 10.6 (10.2, 11.1) | 10.6 (10.3, 10.9) | 11.5 (10.7, 12.2) | 11.0 (10.6, 11.4) | 10.1 (9.8, 10.4) |
| Monocytes | 8.0 (7.8, 8.3) | 8.2 (7.9, 8.4) | 8.2 (7.7, 8.6) | 8.2 (7.9, 8.5) | 8.0 (7.7, 8.2) |
| Granulocytes | 46.5 (45.4, 47.6) | 44.6 (43.8, 45.5) | 42.0 (40.0, 44.0) | 43.5 (42.4, 44.6) | 47.4 (46.6, 48.4) |
Numbers are in means or percentages with corresponding 95% confidence intervals; NA, not applicable.
Number of missing results: fasting glucose = 7, HbA1c = 38, HOMA-IR = 6, HOMA-beta = 6. Glycaemic measures were higher in Ghana than in Europe due to exclusion of T2D cases on medication, the proportion of which was higher in Europe.
Years since diagnosed among T2D cases.
Alcohol consumption in units/week, with 500 ml of beer, 250 ml of wine or 80 ml of spirits counted as 1 unit of alcohol.
Cell counts were estimated used the method from Houseman et al.29
Differentially methylated positions
Six CpG sites (cg19693031, cg04816311, cg00574958, cg03078690, cg07988171, cg07477137) were associated with T2D (FDR <0.05) (Table 2). Implementation of the method to correct for systematic biases resulted in two of these differentially methylated positions (DMPs) (cg07988171, cg07477137) no longer passing the threshold for genome-wide significance (FDR = 0.16), but they remained in the top six DMPs. The difference in methylation between cases and controls was most pronounced for cg19693031 (4.1% less methylated in cases); it is annotated to the TXNIP gene (FDR = 3.15E-12) on chromosome 1 in a 3’UTR open sea region. Three out of six DMPs, annotated to genes TXNIP, C7orf50 and TPM4, remained associated with T2D after adjustment for BMI (Table 2). The DMPs annotated to TXNIP and TPM4 were hypomethylated in T2D cases, whereas the DMP annotated to C7orf50 was hypermethylated. The odds for T2D were highest for the DMP annotated to CDC42EP2 [odds ratio (OR) 1.48, 95% CI 1.27, 1.74] (Table 3). The explained variance in T2D was largest for the DMP annotated to TXNIP (14.6%). The combined six DMPs explained 24.6% of variance in T2D.
Table 2.
Differentially methylated positions associated with type 2 diabetes (T2D) with and without adjustment for body mass index (BMI)
| Base model |
Adjusted for BMI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CpG | CHR | Position | Genea | Featureb | Delta β-valuec | P-valued | FDRd | BACON FDRe | P-valued | FDRd | BACON FDRe |
| cg19693031 | 1 | 145 441 552 | TXNIP | 3’UTR | −0.0408 | 7.35E-18 | 3.15E-12 | 3.62E-12 | 2.45E-17 | 1.05E-11 | 8.28E-10 |
| cg04816311 | 7 | 1 066 650 | C7orf50 | Body | 0.0198 | 1.96E-09 | 4.21E-04 | 2.03E-03 | 1.99E-09 | 4.28E-04 | 1.22E-02 |
| cg00574958 | 11 | 68 607 622 | CPT1A | 5’UTR | −0.0159 | 9.26E-08 | 1.32E-02 | 1.84E-02 | 3.09E-06 | 1.66E-01 | 8.00E-01 |
| cg07988171 | 19 | 16 199 419 | TPM4 | Body | −0.0176 | 3.44E-07 | 3.69E-02 | 4.98E-02 | 2.73E-06 | 3.68E-02 | 2.47E-01 |
| cg07477137 | 11 | 65 083 332 | CDC42EP2 | 5’UTR | 0.0044 | 6.36E-07 | 4.64E-02 | 1.66E-01 | 5.86E-07 | 6.29E-02 | 7.62E-01 |
| cg03078690 | 6 | 33 235 504 | VPS52 | Body | 0.0089 | 6.48E-07 | 4.64E-02 | 1.66E-01 | 6.05E-06 | 1.96E-01 | 9.56E-01 |
CpGs are located in the gene if no distance is indicated (genome build Hg19).
Based on manifest feature annotation Illumina.
Beta coefficients were computed from methylation beta-values. Negative beta-values indicate lower DNA methylation (hypomethylation) in cases compared with controls.
P-values and FDRs corresponding to M-values.
FDRs corresponding to M-values for the models corrected for inflation by possible systematic biases using the BACON method. Results are sorted on P-values for the BACON-adjusted base model.
CHR, Chromosome.
Table 3.
Odds ratios for type 2 diabetes differentially methylated positions (DMPs) and differentially methylation regions (DMRs)
| Base model |
Adjusted for BMI |
Trait variance explained | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CpG | CHR | Position | Geneb | Featurec | ORd | 95% CI | P-value | ORd | 95% CI | P-value | % |
| cg19693031 | 1 | 145 441 552 | TXNIP | 3’UTR | 0.87 | 0.84, 0.90 | <0.001 | 0.87 | 0.84, 0.90 | <0.001 | 14.6 |
| cg04816311 | 7 | 1 066 650 | C7orf50 | Body | 1.09 | 1.06, 1.12 | <0.001 | 1.09 | 1.06, 1.12 | <0.001 | 6.6 |
| cg00574958 | 11 | 68 607 622 | CPT1A | 5’UTR | 0.86 | 0.81, 0.91 | <0.001 | 0.87 | 0.82, 0.92 | <0.001 | 4.7 |
| cg07988171 | 19 | 16 199 419 | TPM4 | Body | 0.89 | 0.85, 0.93 | <0.001 | 0.89 | 0.85, 0.93 | <0.001 | 4.6 |
| cg07477137 | 11 | 65 083 332 | CDC42EP2 | 5’UTR | 1.48 | 1.27, 1.74 | <0.001 | 1.51 | 1.28, 1.77 | <0.001 | 4.9 |
| cg03078690 | 6 | 33 235 504 | VPS52 | Body | 1.17 | 1.10, 1.25 | <0.001 | 1.16 | 1.09, 1.23 | <0.001 | 3.8 |
| DMRa | 2 | 20 870 087-20 871 401 | GDF7 | 4.37 | 1.43, 13.38 | 0.009 | 4.48 | 1.45, 13.84 | 0.009 | 1.2 | |
The average methylation level for the nine CpGs in this DMR was taken. There CpGs are: cg09074113, cg05481257, cg07755735, cg14780466, cg10687131, cg12334488, cg10553204, cg07021052, cg04082016.
CpGs are located in the gene if no distance is indicated (genome build Hg19).
Based on manifest feature annotation Illumina.
Odds ratios are per 1% increase in DNA methylation.
CHR, Chromosome.
In the inflation-adjusted models, we found eight DMPs associated with the continuous measure HbA1c unadjusted for BMI and six after adjustment for BMI (FDR <0.05) (Table 4). The DMP with the lowest FDR in association with HbA1c levels (BACON FDR = 3.71 x 10–33) was the same as in T2D analysis (cg19693031—TXNIP). A 0.1µl/mol (2.2%) increase in HbA1c was associated with 0.15% less methylation of cg19693031 (Figure 2).
Table 4.
Differentially methylated positions associated with HbA1c with and without adjustment for body mass index
| Base model |
Adjusted for BMI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CpG | CHR | Position | Nearest genea | Featureb | Delta β-valuec | P-valued | FDRd | BACON FDRe | P-valued | FDRd | BACON FDRe |
| cg19693031 | 1 | 145 441 552 | TXNIP | 3’UTR | −0.0016 | 8.26E-45 | 3.55E-39 | 7.74E-37 | 3.18E-44 | 1.36E-38 | 3.71E-33 |
| cg04816311 | 7 | 10 66 650 | C7orf50 | Body | 0.0008 | 1.87E-15 | 4.00E-10 | 1.37E-08 | 2.33E-15 | 5.00E-10 | 1.23E-07 |
| cg14020176 | 17 | 72 764 985 | SLC9A3R1 | 3’UTR | 0.0003 | 2.16E-10 | 3.09E-05 | 3.61E-04 | 4.45E-10 | 6.20E-05 | 2.32E-03 |
| cg00574958 | 11 | 68 607 622 | CPT1A | 5’UTR | −0.0004 | 5.16E-10 | 5.54E-05 | 2.16E-03 | 5.37E-09 | 4.61E-04 | 3.98E-02 |
| cg05201300 | 5 | 172 443 740 | ATP6V0E1 | Body | −0.0005 | 2.79E-09 | 2.16E-04 | 6.54E-03 | 5.77E-10 | 6.20E-05 | 8.48E-03 |
| cg08309687 | 21 | 35 320 596 | LINC00649 | IGR | −0.0006 | 3.01E-09 | 2.16E-04 | 6.54E-03 | 6.82E-09 | 4.88E-04 | 4.01E-02 |
| cg03078690 | 6 | 33 235 504 | VPS52 | Body | 0.0003 | 3.46E-08 | 1.86E-03 | 1.41E-02 | 1.77E-07 | 7.59E-03 | 9.76E-02 |
| cg12655112 | 15 | 42 261 154 | EHD4 | Body | −0.0005 | 1.11E-08 | 6.81E-04 | 1.49E-02 | 1.44E-08 | 8.78E-04 | 6.00E-02 |
CpGs are located in the gene if no distance is indicated (genome build Hg19).
Based on manifest feature annotation Illumina; IGR = intergenic region.
Beta coefficients were computed from methylation beta-values. Negative beta-values indicate lower DNA methylation (hypomethylation) for 0.1 µl/mol (2.2%) higher HbA1c.
Numbers are P-values and FDR corresponding to M-values.
FDR corresponding to M-values for the models corrected for inflation by possible systematic biases using the BACON method. Results are sorted on P-values for the BACON-adjusted base model.
CHR, Chromosome.
We performed in silico replication (nominal P-value of <0.05) in previously published EWAS for T2D for the DMPs annotated to VPS52 (cg03078690), TPM4 (cg07988171) and CDC42EP2 (cg07477137). None of the 11 EWAS included in the systematic literature search (Supplementary Material 4, available as Supplementary data at IJE online) reported VPS52 (cg03078690), TPM4 (cg07988171) or CDC42EP2 (cg07477137).
Differentially methylated regions
We identified one differentially methylated region (DMR) associated with T2D (OR 4.37, 95% CI 1.43, 13.38) annotated to an exon on gene GDF7 (Figure 3; Table 3). This region was hypomethylated in cases compared with controls and is strongly conserved between species, as is visualized in Figure 3 using data of the Vertebrate Multi Alignment and Conservation track of the UCSC browser.27 No signals were observed in histone modification track H3K27Ac or ENCODE RNA-seq expression track in muscle tissue-derived cells. One other DMR was associated with HbA1c. This DMR is annotated to chromosome 6 and is closest to gene ZFP57.27,28
Candidate analysis of DMPs reported in non-African populations
We performed a candidate analysis by fitting the T2D linear regression models to a subset of CpG sites extracted from the literature identified through a systematic search in PUBMED. We could confirm the association with T2D in our dataset for five of the 2876 CpG sites previously reported loci (FDR <0.05) (Table 5). We found a DMP (cg07988171) annotated to TPM4 associated with T2D in the present study, but this was a different DMP from previously reported DMPs in that gene (cg27377863,15cg07810884,15cg0659017315). None of these three CpG sites was associated with T2D in our population. In replication analysis adjusted for BMI, four DMPs were associated with T2D in our study population (Table 5). Only one DMP (cg11024682) did not remain associated with T2D after BMI adjustment (Figure 4). The DMPs annotated to TXNIP and C7orf50 were consistently found most strongly associated and with largest effect, that is 4.1% hypomethylation for the DMP annotated to TXNIP and 2.0% hypermethylation for the DMP annotated to C7orf50.
Table 5.
Type 2 diabetes differentially methylated positions in candidate analyses with and without adjustment for BMI
| Base model |
Adjusted for BMI |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CpG | Refa | CHR | Position | Gene (distance)b | Featurec | Delta β-valued | P-valuee | FDRe | BACON FDRf | P-valuee | FDRe | BACON FDRf |
| cg19693031 | 7,30–32 | 1 | 145 441 552 | TXNIP | 3’UTR | −0.0408 | 7.39E-18 | 1.97E-14 | 1.50E-12 | 2.47E-17 | 6.57E-14 | 2.21E-10 |
| cg04816311 | 30 | 7 | 1 066 650 | C7orf50 | Body | 0.0198 | 1.97E-09 | 2.62E-06 | 3.31E-05 | 2.00E-09 | 2.66E-06 | 1.70E-04 |
| cg00574958 | 30 | 11 | 68 607 622 | CPT1A | 5’UTR | −0.0159 | 9.28E-08 | 8.24E-05 | 7.01E-04 | 3.10E-06 | 2.06E-03 | 3.89E-02 |
| cg19266329 | 30 | 1 | 145 456 128 | POLR3GL(-108) | IGR | −0.0150 | 1.07E-05 | 7.15E-03 | 3.33E-02 | 1.70E-06 | 1.51E-03 | 3.27E-02 |
| cg26955383 | 30 | 10 | 105 218 660 | CALHM1 | TSS200 | 0.0104 | 2.00E-05 | 1.07E-02 | 4.39E-02 | 9.64E-06 | 5.13E-03 | 5.47E-02 |
Reference number for article in which candidate CpG was identified.
CpG’s are located in the gene if no distance is indicated (genome build Hg19). Distance is expressed in kb.
Based on manifest feature annotation Illumina; IGR = intergenic region.
Beta coefficients were computed from methylation beta-values. Negative beta-values indicate lower DNA methylation (hypomethylation) in cases compared with controls.
Numbers are P-values and FDR corresponding to M-values.
FDR corresponding to M-values for the models corrected for inflation by possible systematic biases using the BACON method. Results are sorted on P-values for the BACON-adjusted base model.
CHR, Chromosome.
Replicated DMRs established in non-African populations
Yuan et al.33 reported 20 DMRs identified in relation to T2D in European twins, using MeDIP sequencing and validation on the Illumina 450k array. We did not replicate any of these DMRs. Neither did we identify DMPs associated with T2D within the DMRs reported by Yuan et al.
Discussion
Key findings
We conducted an EWAS for T2D among people from Ghana, which to our knowledge, is the first such study in Africa. We identified several CpG sites that were differentially methylated between T2D cases and controls on an epigenome-wide level. Three ubiquitous methylation loci were consistently and strongly associated with T2D in Ghanaians (cg19693031—TXNIP, cg04816311—C7orf50 and cg00574958—CPT1A). Three loci (cg07988171, cg03078690 and cg07477137) were associated with T2D on an epigenome-wide level in this study which have not previously been reported in non-African populations. However, two of these no longer passed the epigenome-wide threshold (FDR = 0.16) after correction for possible systematic biases.
Discussion of the key findings
The three DMPs most strongly associated with T2D in our study population had previously been observed in non-African descent populations,7,30,34 indicating that these loci are associated with T2D across populations (ubiquitous). Out of these three, cg19693031 (TXNIP) has been reported to be associated with T2D in several other populations in blood7,30–32 and in liver.7 This DMP was 4.1% less methylated in our T2D cases compared with controls. This is similar to reported effect sizes ranging from -3% to -5%.7,30–32 Consistent with this finding, DMP cg19693031 (TXNIP) showed a substantial decrease in DNA methylation for higher levels of HbA1c. TXNIP is involved in insulin resistance; increased expression negatively affects insulin-mediated glucose uptake and correlates with elevated glucose concentrations.35 This is consistent with the hypomethylation we found in T2D cases and with the link between hypomethylation and increased HbA1c levels. On the other hand, GWAS in European populations suggest that impaired insulin secretion rather than insulin resistance is considered the main pathogenic cause of T2D.15 In post hoc analyses, we found insulin resistance as assessed by homeostatic modelling to attenuate odds for two of the six T2D DMPs, whereas adjustment for beta-cell dysfunction modelling resulted in hardly any changes (Supplementary Table 2, available as Supplementary data at IJE online). Intervention studies are needed to assess whether DNA methylation changes mediate their impact on T2D via insulin resistance or beta-cell dysfunction, in sub-Saharan African populations.
DMP cg04816311 annotated to C7orf50 was also associated with T2D in sub-Saharan Africans. This DMP has been previously linked with T2D in Mexican-Americans, Europeans and Arabs, in blood.30,34 The function of this locus is unknown, although it has been shown to be associated with lipid levels36 and human longevity.37
We also identified three loci—cg07988171 [TPM4], cg03078690 [VPS52] and cg07477137 [CDC42EP2] —not previously reported in non-African populations. Although two (cg03078690 and cg07477137) no longer met the epigenome-wide threshold of FDR <0.05 after correction for statistical inflation by possible systematic biases (FDR = 0.16), they remained in the top six loci and one of them (cg03078690) was associated with HbA1c. The DMP cg07988171 annotated to TPM4 remained associated with T2D also after correction for possible systematic biases. In our post hoc analyses (Supplementary Material 3, available as Supplementary data at IJE online), we found that within a 50-bp region around cg07988171, there are indications of H3K27Ac histone modifications (i.e. acetylation at the 27th lysine of the histone H3 protein), conservation between species and expression in muscle cells.27,28 In post hoc analyses for genetic interaction (Supplementary Material 3, available as Supplementary data at IJE online) around our DMPs, we found that cg07988171 methylation may interact with gene RAB8A which plays a role in glucose homeostasis and is therefore an important target to explore in future research. The DMPs annotated to VPS52 and CDC42EP2 showed slight (0.9% and 0.4%) hypermethylation but, whereas VPS52 is found to be expressed in blood, muscle and adipose tissue, CDC42EP2 is not.27,28 Replication studies are needed to confirm the association of these novel loci, in particular cg07988171, with T2D in sub-Saharan African populations.
Three DNA methylation loci were associated with T2D at an epigenome-wide level (FDR <0.05) in the model without BMI adjustment, but not after adjustment for BMI, suggesting that they may be mediating their effect on T2D via obesity or that increasing body mass may lead to greater methylation at these sites. It is possible that BMI increases may induce changes in DNA methylation at specific loci, which may in turn influence the risk of T2D. On the other hand, three other DMPs were not confounded by BMI, suggesting that they mediate T2D risk via a mechanism independent of obesity. Enrichment analysis indicated that the identified T2D DMPs might be involved both in muscular function pathways and metabolism pathways (Supplementary Material, available as Supplementary data at IJE online).
Our finding that methylation levels of identified DMPs differed by recruitment site should be interpreted with caution, given the limited sample size upon stratification, but it seems to suggest an environmental influence on the identified DMPs. Three of the six identified DMPs (cg19693031, cg04816311, cg00574958) had differential methylation levels between rural Ghana, urban Ghana and/or Europe (Supplementary Table 8, available as Supplementary data at IJE online). In general there was a trend of more hypomethylation in urban Ghana than in rural Ghana and in Europe than in urban Ghana. Possibly, health-related behaviour and environmental changes due to urbanization induce hypomethylation that contributes to a higher T2D prevalence among Ghanaians in Europe and urban Ghana compared with Ghanaians in rural Ghana.20 The geographical impact on DNA methylation and subsequent T2D requires further exploration.
In the candidate approach, we found five loci associated with T2D out of the 2876 CpGs candidates from previous studies, in either BMI-adjusted or unadjusted analyses. The low yield can be due to population differences, but could also indicate false-positives, particularly when considering the small sample sizes in previous studies (median sample size = 192). We did not find any of the 20 DMRs identified through the systematic literature search to be associated with T2D in our study. It is possible that DMRs associated with T2D differ between Africans and Europeans. However, the lack of overlap could also be attributed to the difference in methods used. Yuan et al.33 validated the DMRs on the Illumina 450k array, but initial detection of DMRs was performed using MeDip-sequencing. Also, differential statistical methods applied could explain the lack of overlap between the studies.
Strengths and limitations
This is the first EWAS for T2D in sub-Saharan Africans. Furthermore, it is one of the largest EWAS on T2D to date. Our sub-Saharan African study population is relatively homogeneous, that is all stem from one region in Ghana. Homogeneity of ethnic groups within Ghana is shown by annotated principal component analysis in Supplementary Figure 4, available as Supplementary data at IJE online. Our findings are unlikely to be driven by confounders such as smoking and alcohol intake, as both are low in this study population. Furthermore, smoking DMPs reported in a study among African Americans were not in the vicinity of our T2D DMPs.38
A potential limitation is the use of whole-blood samples. As DNA methylation is tissue specific, we ideally would have analysed target tissue in T2D including adipose tissue, skeletal muscle and pancreatic beta cells. However, sampling of these tissues in epidemiological studies is basically impossible, and the actual use of these tissues is very limited. Nevertheless, peripheral blood is thought to give a good reflection and is fairly concordant with other tissue types.39 More importantly, epigenetic biomarkers derived from a routine blood sample are more feasible for clinical use, for example for the development of biomarkers. A second possible limitation of the present study is the possibility that some of the hits reported are due to genetic [e.g. single nucleotide polymorphisms (SNPs)] rather than epigenetic variation. Unfortunately, we do not yet have GWAS SNP data on the RODAM cohort that would allow us directly test this hypothesis. Existing GWAS data based on non-African populations would not be appropriate for this purpose, due to differences in genetic variation, genetic linkage disequilibrium structure and other factors. Furthermore, we cannot disentangle whether the loci identified were a cause or a consequence of diabetes and its major risk factors, due to the cross-sectional nature of our study. Longitudinal studies assessing new-onset diabetes as an outcome with repeated measurements of phenotype and DNA methylation would be needed to assess whether the epigenetic changes identified occurred before T2D. Intervention studies could be used to study whether modifying DNA methylation affects T2D risk. These future studies should ideally have larger sample sizes and/or combine data in meta-analyses in order to overcome the potential limitation of the present study to detect additional epigenetic loci with small effect sizes (i.e. <3% methylation difference) due to its sample size.
Conclusions
In summary, this first EWAS on T2D in sub-Saharan Africans has identified methylation loci associated with T2D. We identified both ubiquitous loci and loci not previously reported in non-African populations. Further studies are needed to confirm and extend these findings, including evaluation of the functional relevance of the novel loci and replication in other sub-Saharan African populations. Our findings add to the slowly growing literature on the genetic architecture of T2D in African populations. The datasets created and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by the European Commission under the Framework Programme (Grant Number: 278901). C.G. is supported by NutriAct – Competence Cluster Nutrition Research Berlin-Potsdam funded by the German Federal Ministry of Education and Research (FKZ: 01EA1408A-G). C.N.R. and A.A. are supported by the Intramural Research Program of the National Institutes of Health in the Center for Research on Genomics and Global Health (CRGGH). The CRGGH is supported by the National Human Genome Research Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the Center for Information Technology, and the Office of the Director at the National Institutes of Health (1ZIAHG200362). The study sponsor was not involved in the design of the study; the collection, analysis and interpretation of data; writing the report; nor the decision to submit the report for publication.
Supplementary Material
Acknowledgements
The authors are very grateful to Dr Erik Beune (Department of Public Health, Academic Medical Center, The Netherlands) who, with great dedication and eye for detail, coordinated the RODAM study. Special thanks go to Prof. Karien Stronks (Department of Public Health, Academic Medical Center, The Netherlands) for her role in the conceptualization of the RODAM study, and to Prof. Ama de-Graft Aikins (Regional Institute for Populations Studies, University of Ghana, Ghana) for her valuable contribution to the conceptualization of the RODAM study and data collection in Ghana. We thank Nadine Huckauf and Candy Kalischke (Department of Endocrinology and Metabolism, Charité University Medicine, Berlin, Germany) for their excellent support in biochemical characterization of the participants and data handling. We gratefully acknowledge Jan van Straalen from the Academic Medical Center for his valuable support with standardization of the laboratory procedures, and the AMC Biobank for support in biobank management and storage of collected samples. Last but not least, the authors are very grateful to the advisory board members for their valuable support in shaping the methods, to the research assistants, interviewers and other staff of the five research locations who have taken part in gathering the data and, most of all, to the Ghanaian volunteers participating in this project.
Author Contributions
C.A., F.P.M., E.O.D., L.S., M.B.S., S.B., J.S., I.D., J.A., A.A., P.H., M.M.A.M., K.M., C.N.R. and M.H.Z. conceived and designed the study. C.A., E.O.D., F.P.M., J.A., I.D., C.G., J.S. and K.M. carried out the recruitment and data collection. T.B. and P.H. generated the epigenetics data. A.V. and K.M. performed the statistical analysis. All authors contributed to interpretation of the data. K.M. wrote the manuscript, with the cooperation of all co-authors. All authors read, contributed to and approved the final manuscript. C.A. is responsible for the integrity of the work as a whole.
Conflict of interest: The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.International Diabetes Federation (IDF). IDF Diabetes Atlas, 7th edn Brussels: International Diabetes Federation, 2015. [Google Scholar]
- 2. Patel CJ, Bhattacharya J, Butte AJ.. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One 2010;5:e10746.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeggini E, Scott LJ, Saxena R. et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 2008;40:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drong AW, Lindgren CM, McCarthy MI.. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther 2012;92:707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maher B. The case of the missing heritability. Nature 2008;456:18–21. [DOI] [PubMed] [Google Scholar]
- 6. Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 2002;3:662–73. [DOI] [PubMed] [Google Scholar]
- 7. Chambers JC, Loh M, Lehne B. et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol 2015;3:526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toperoff G, Aran D, Kark JD. et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet 2012;21:371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rönn T, Volkov P, Davegårdh C. et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet 2013;9:e1003572.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson OS, Sant KE, Dolinoy DC.. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem 2012;23:853–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nolan CJ, Damm P, Prentki M.. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011;378:169–81. [DOI] [PubMed] [Google Scholar]
- 12. Ling C, Groop L.. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes 2009;58:2718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meeks KAC, Freitas-Da-Silva D, Adeyemo A. et al. Disparities in type 2 diabetes prevalence among ethnic minority groups resident in Europe: a systematic review and meta-analysis. Intern Emerg Med 2016;11:327–40. [DOI] [PubMed] [Google Scholar]
- 14. Steyn NP, Mchiza ZJ.. Obesity and the nutrition transition in Sub‐Saharan Africa. Ann N Y Acad Sci 2014;1311:88–101. [DOI] [PubMed] [Google Scholar]
- 15. Dayeh T, Volkov P, Salö S. et al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet 2014;10:e1004160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ronn T, Volkov P, Gillberg L. et al. Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood. Hum Mol Genet 2015;24:3792–813. [DOI] [PubMed] [Google Scholar]
- 17. Nilsson E, Matte A, Perfilyev A. et al. Epigenetic alterations in human liver from subjects with type 2 diabetes in parallel with reduced folate levels. J Clin Endocrinol Metab 2015;100:E1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling C, Poulsen P, Simonsson S. et al. Genetic and epigenetic factors are associated with expression of respiratory chain component NDUFB6 in human skeletal muscle. J Clin Invest 2007;117:3427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agyemang C, Beune E, Meeks K. et al. Rationale and cross-sectional study design of the Research on Obesity and type 2 Diabetes among African Migrants: the RODAM study. BMJ Open 2015;4:e004877.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agyemang C, Meeks K, Beune E. et al. Obesity and type 2 diabetes in sub-Saharan Africans—is the burden in today’s Africa similar to African migrants in Europe? The RODAM study. BMC Med 2016;14:166.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai P-C, Bell JT.. Power and sample size estimation for epigenome-wide association scans to detect differential DNA methylation. Int J Epidemiol 2015;44:1429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alders M, Bliek J, Vd Lip K, Vd Bogaard R, Mannens M.. Determination of KCNQ1OT1 and H19 methylation levels in BWS and SRS patients using methylation-sensitive high-resolution melting analysis. Eur J Hum Genet 2009;17:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Iterson M, van Zwet EW, Heijmans BT.. Controlling bias and inflation in epigenome-and transcriptome-wide association studies using the empirical null distribution. Genome Biol 2017;18:19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bunn HF, Gabbay KH, Gallop PM.. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science 1978;200:21–27. [DOI] [PubMed] [Google Scholar]
- 25. Du P, Zhang X, Huang C-C. et al. Comparison of beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics 2010;11:587.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaffe AE, Murakami P, Lee H. et al. Bump hunting to identify differentially methylated regions in epigenetic epidemiology studies. Int J Epidemiol 2012;41:200–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kent WJ, Sugnet CW, Furey TS. et al. The human genome browser at UCSC (Human Assembly GRCh37/hg19). Genome Res 2002;12:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenbloom KR, Sloan CA, Malladi VS. et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res 2012;41:D56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houseman EA, Accomando WP, Koestler DC. et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 2012;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kulkarni H, Kos MZ, Neary J. et al. Novel epigenetic determinants of type 2 diabetes in Mexican-American families. Hum Mol Genet 2015;24:5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Florath I, Butterbach K, Heiss J. et al. Type 2 diabetes and leucocyte DNA methylation: an epigenome-wide association study in over 1,500 older adults. Diabetologia 2016;59:130–38. [DOI] [PubMed] [Google Scholar]
- 32. Soriano-Tárraga C, Jiménez-Conde J, Giralt-Steinhauer E. et al. Epigenome-wide association study identifies TXNIP gene associated with type 2 diabetes mellitus and sustained hyperglycemia. Hum Mol Genet 2016;25:609–19. [DOI] [PubMed] [Google Scholar]
- 33. Yuan W, Xia Y, Bell CG. et al. An integrated epigenomic analysis for type 2 diabetes susceptibility loci in monozygotic twins. Nat Commun 2014;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al Muftah WA, Al-Shafai M, Zaghlool SB. et al. Epigenetic associations of type 2 diabetes and BMI in an Arab population. Clin Epigenet 2016;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parikh H, Carlsson E, Chutkow WA. et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 2007;4:e158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ligthart S, Vaez A, Hsu Y-H. et al. Bivariate genome-wide association study identifies novel pleiotropic loci for lipids and inflammation. BMC Genomics 2016;17:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yashin AI, Wu D, Arbeev KG, Ukraintseva SV.. Joint influence of small-effect genetic variants on human longevity. Aging 2010;2:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun YV, Smith AK, Conneely KN. et al. Epigenomic association analysis identifies smoking-related DNA methylation sites in African Americans. Hum Genet 2013;132:1027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kato N, Loh M, Takeuchi F. et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat Genet 2015;47:1282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.