Abstract
This study examined differences in neuropsychological test scores between individuals with primary age-related tauopathy (PART) and Alzheimer disease (AD) using cross-sectional data from the National Alzheimer’s Coordinating Center. Linear regression tested for differences in 4 cognitive domains stratified by cognitive status (global Clinical Dementia Rating [CDR]). The sample included 240 participants with no neuritic plaques (NP) (definite PART), 186 with sparse NP (possible PART), and 510 with moderate/frequent NP (AD). Four cognitive domain z-score outcome variables (memory, attention, executive function, and semantic memory/language) were calculated using 12 neuropsychological tests. Definite PART participants had a sparing of semantic memory/language compared to those with AD, with a mean adjusted z-score difference of 0.37 (95% confidence interval [CI]: 0.16–0.58) for those with CDR = 0.5 or 1 and of 0.92 (CI: 0.22–1.63) for those with CDR = 2 or 3. Compared to participants with AD, definite PART participants with CDR = 0.5 or 1 had sparing of memory (adjusted z-score difference: 0.61; CI: 0.39–0.84) and definite PART participants with CDR = 2 or 3 had sparing of attention (adjusted z-score difference: 0.76: CI: 0.09–1.43). Patterns of cognitive impairment differed between definite PART and AD, suggesting significant differences in clinical presentation between individuals from these 2 groups.
Keywords: AD, Alzheimer disease, Cognition, Cognitive, Neuropathology, PART, Primary age-related tauopathy
INTRODUCTION
A variety of neuropathologic changes underlie the cognitive patterns observed in mild cognitive impairment and dementia (1–4). Primary age-related tauopathy (PART), one such pattern of neuropathologic change associated with cognitive impairment, is characterized by the presence of neurofibrillary tangles (NFT) composed of tau protein in regions comparable to early- to moderate-stage Alzheimer disease (AD), independent of amyloid plaque deposition (5, 6). Higher NFT burden in individuals with PART has been associated with more rapid decline in episodic and semantic memory/language and processing speed/attention (7) and worse scores on the WAIS-R Block Design and Trail Making Tests (8). Otherwise, cognitive changes associated with PART have been minimally addressed. Furthermore, few studies have investigated differences in cognitive domain scores between those with PART and AD. One study suggested that Mini-Mental State Exam (MMSE) scores are relatively preserved at higher Braak stages in PART compared to AD (defined as moderate to frequent neuritic plaques [NP]), but the study did not control for potential confounding factors such as apolipoprotein E (APOE) genotype and did not examine differences in sensitive neuropsychological tests (6).
Hence, we sought to compare neuropsychological test scores for several cognitive domains (i.e. episodic memory, attention, executive function, semantic memory/language) among individuals who subsequently had either PART or AD at autopsy, and to examine their score differences by disease stage. By so doing, we aimed to increase understanding of the cognitive consequences of the biologic changes occurring in PART and to test the hypothesis that differences in cognition between PART and AD can be demonstrated during life.
MATERIALS AND METHODS
Sample
The study sample originated from the National Alzheimer’s Coordinating Center’s (NACC) Uniform Data Set (UDS) (9) and Neuropathology Data Set (10, 11). UDS clinical data, including demographics, health history, clinical symptoms, neuropsychological test scores, and diagnoses have been collected longitudinally on participants at approximately 30 Alzheimer Disease Centers (ADCs) since September 2005. A subset of UDS participants consent to autopsy and have neuropathological exam data collected on standardized forms. Data collected up through the end of August 2017 were included in this study.
Standard Protocol Approvals, Registration, and Patient Consents
Each ADC obtained written informed consent from all participants and use of NACC data for research has been approved by University of Washington’s Institutional Review Board.
Participants
Our sample was restricted to participants with: (i) a neuropathological exam; (ii) nonmissing data on NP; (iii) a UDS visit conducted ≤2 years of autopsy; (iv) Version 2 of the neuropsychological battery at the last visit before death; (v) ≥1 nonmissing calculated cognitive domain z-score from the last visit before death; and (vi) none of the following comorbidities: clinical and neuropathological Lewy body disease (including Parkinson disease), Huntington disease, clinical or neuropathological prion disease, clinical corticobasal syndrome or progressive supranuclear palsy, frontotemporal lobar degeneration (FTLD) with tau inclusions, FTLD with transactive response DNA-binding protein 43 (TDP-43), or FTLD not otherwise specified. Participants were stratified by cognitive status at their last visit before death (Clinical Dementia Rating [CDR] = 0, CDR = 0.5 or 1, or CDR = 2 or 3) and by neuropathologic lesion burden at autopsy as follows: definite PART (i.e. no NP), possible PART (i.e. sparse NP), and AD (moderate to frequent NP). These groups were based on a Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) score for neocortical NP density (plaques with argyrophilic, thioflavin S-positive or tau-positive dystrophic neurites with/without dense amyloid cores [12]), which ADCs ascertain using standardized definitions/techniques (13, 14). Unlike Thal phase for amyloid deposits, which is available for a smaller subset of autopsied participants, CERAD NP are available for the vast majority and have been suggested as reasonable alternatives in classifying PART (6).
We describe the sample according to demographic, clinical, and neuropathologic characteristics, including Braak stage for neurofibrillary degeneration (15), age at last visit (years), sex, education (years), nonwhite race (yes/no), ≥1 APOE ɛ4 allele, family history of cognitive impairment, global CDR at last visit, self-reported history of stroke, hypertension, depression, diabetes, and traumatic brain injury, and vascular brain injury (VBI) at autopsy (hemorrhage, microbleed, infarct/lacune, or microinfarct). Braak stage, expected to be associated with cognitive impairment (5), was described for the sample and controlled for in multivariable analyses. The definite PART, possible PART, and AD groups were compared on these characteristics using unadjusted logistic regression, with alpha = 0.05 determining statistical significance.
Neuropsychological Tests
The UDS neuropsychological test battery (Version 2) (16) was used to calculate z-scores for 4 cognitive domains and a z-score for global cognition. Unadjusted z-scores were calculated for each of 12 neuropsychological tests by comparing to the mean test scores of 10 988 individuals with a global CDR = 0 at their initial visit (i.e. norms sample). To obtain a z-score for each test, we subtracted the norms sample’s mean score from each individual’s raw neuropsychological test score and divided by the standard deviation of the norms sample’s score. Then for each individual, the cognitive domain scores were determined by calculating a simple average of Logical Memory Immediate and Delayed z-scores for the episodic memory domain; Digit Span Forward and Backward correct trials and span length z-scores for the attention domain; Trail Making Test Parts A and B and Digit Symbol z-scores for the executive function domain; and Animals, Vegetables, and Boston Naming test z-scores for the semantic memory/language domain. These 4 composites were previously determined for the UDS neuropsychological battery using a factor analysis (17). We diverge from that prior factor analysis by referring to “semantic memory/language” instead of language because word fluency tasks and naming tasks additionally reflect semantic memory/retrieval and are distinct from specific language impairments in aging populations (18, 19). A simple average of the z-scores for the 4 cognitive domains provided the z-score for the global composite. The mean unadjusted cognitive domain scores were stratified by cognitive status at the last visit before death and comparison group (Supplementary DataTable S1).
Statistical Analyses
Unadjusted and adjusted linear regression analyses with generalized estimating equations (accounting for clustering by Center), employing an exchangeable working correlation structure, were run to compare cognitive domain z-scores between those with definite or possible PART and AD (using 2 dummy variables with AD as reference group). As differences between definite PART and AD groups might vary significantly based on cognitive status, analyses were stratified by global CDR score. The multivariable models controlled for variables that are either common confounders in dementia research or were differentially distributed between the groups in our study (Tables 1 and 2): age at last visit; sex; education; ≥1 APOE ɛ4 allele; family history of cognitive impairment; history of hypertension, stroke, diabetes, or traumatic brain injury; and Braak stage (dichotomized as 0–II and III–VI due to small numbers with definite PART and high Braak stage and with AD and low Braak stage). In addition, we present the same adjusted analyses for those with CDR = 0 or CDR = 0.5–1 stratified by Braak stages (I–II, III–IV) instead of controlling for Braak stage (sample too sparse to examine CDR = 2–3 or Braak stages V–VI). Statistical significance was based on alpha = 0.05.
TABLE 1.
Sample Demographics in Primary Age-Related Tauopathy Versus Alzheimer Disease
| Characteristic* | Definite PART | Possible PART | AD | Definite PART Versus AD | Possible PART Versus AD |
|---|---|---|---|---|---|
| p Value† | p Value† | ||||
| Sample size, n | 240 | 186 | 510 | NA | NA |
| Age at last visit (years), n (%) | |||||
| <60 | 9 (3.8%) | 0 (0.0%) | 16 (3.1%) | 0.29 | <0.001 |
| 60–69 | 15 (6.3%) | 9 (4.8%) | 30 (5.9%) | ||
| 70–79 | 44 (18.3%) | 27 (14.5%) | 94 (18.4%) | ||
| 80–89 | 86 (35.8%) | 67 (36.0%) | 240 (47.1%) | ||
| 90+ | 86 (35.8%) | 83 (44.6%) | 130 (25.5%) | ||
| Male sex, n (%) | 115 (47.9%) | 87 (46.8%) | 281 (55.1%) | 0.07 | 0.05 |
| Education (years), mean (SD) | 15.3 (2.7) | 14.8 (3.4) | 15.3 (3.2) | 0.95 | 0.13 |
| Nonwhite race (yes versus no), n (%) | 15 (6.3%) | 13 (7.0%) | 33 (6.5%) | 0.92 | 0.82 |
Abbreviations: PART, primary age-related tauopathy; AD, Alzheimer disease; NA, not applicable.
Missing data (definite PART, possible PART, AD): education (n = 4; n = 0; n = 3); race (n = 2; n = 0; n = 3).
Unadjusted logistic regression.
Bold = statistically significant at p < 0.05.
TABLE 2.
Braak Stage and Clinical Characteristics in Primary Age-Related Tauopathy Versus Alzheimer Disease
| Characteristic* | Definite PART | Possible PART | AD | Definite PART Versus AD | Possible PART Versus AD |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | p Value† | p Value† | |
| Braak stage | |||||
| 0 | 35 (14.8%) | 5 (2.7%) | 3 (0.6%) | <0.001 | <0.001 |
| I–II | 126 (53.4%) | 53 (28.5%) | 63 (12.4%) | ||
| III–IV | 71 (30.1%) | 94 (50.5%) | 158 (31.0%) | ||
| V–VI | 4 (1.7%) | 34 (18.3%) | 285 (56.0%) | ||
| Presence of ≥1 APOE ε4 allele | 31 (13.6%) | 60 (34.3%) | 212 (45.3%) | <0.001 | 0.01 |
| Family history, cognitive impairment | 93 (42.5%) | 93 (52.3%) | 273 (56.8%) | 0.001 | 0.30 |
| Global CDR score, last visit | |||||
| 0 | 122 (50.8%) | 64 (34.4%) | 74 (14.5%) | Ref. | Ref. |
| 0.5 | 60 (25.0%) | 61 (32.8%) | 116 (22.8%) | <0.001 | 0.03 |
| 1 | 38 (15.8%) | 29 (15.6%) | 127 (24.9%) | <0.001 | <0.001 |
| 2 | 14 (5.8%) | 24 (12.9%) | 123 (24.1%) | <0.001 | <0.001 |
| 3 | 6 (2.5%) | 8 (4.3%) | 70 (13.7%) | <0.001 | <0.001 |
| History of stroke | 45 (18.8%) | 37 (20.0%) | 66 (13.1%) | 0.04 | 0.03 |
| History of hypertension | 173 (72.4%) | 140 (76.1%) | 329 (64.6%) | 0.04 | 0.005 |
| History of depression | 92 (38.7%) | 60 (32.4%) | 192 (37.9%) | 0.84 | 0.19 |
| History of diabetes | 49 (20.4%) | 28 (15.1%) | 61 (12.0%) | 0.003 | 0.27 |
| History of traumatic brain injury | 30 (12.5%) | 34 (18.7%) | 58 (11.5%) | 0.70 | 0.02 |
| Vascular brain injury at autopsy‡ | 106 (44.2%) | 95 (51.1%) | 205 (40.2%) | 0.30 | 0.01 |
Abbreviations: APOE, apolipoprotein E; CDR, Clinical Dementia Rating; PART, primary age-related tauopathy; AD, Alzheimer disease; NA, not applicable.
Missing data (definite PART, possible PART, AD): APOE genotype (n = 12; n = 11; n = 42); family history (n = 21; n = 8; n = 29); stroke (n = 1; n = 1; n = 6); hypertension (n = 1; n = 2; n = 1); depression (n = 2; n = 1; n = 3); diabetes (n = 0; n = 1; n = 1); TBI (n = 0; n = 4; n = 7); Braak stage (n = 4; n = 0; n = 1).
Unadjusted logistic regression.
Vascular brain injury included hemorrhage, microbleed, infarct/lacune, and microinfarct.
Bold = statistically significant at p < 0.05.
In post hoc analyses, we reran the multivariable linear regression analyses with each semantic memory test z-score (Animals, Vegetables, Boston Naming test) entered as separate outcome variables to investigate in more detail the finding that semantic memory/language was the most notably different domain between PART and AD. Each regression model included individuals who were not missing the examined cognitive domain z-score. Additional sensitivity analyses included rerunning the adjusted models after: (1) redefining definite PART based on the absence of diffuse plaques instead of NP, and (2) removing vascular risk factors (hypertension, diabetes, stroke) as covariates and adding a variable for VBI observed at autopsy. The former sensitivity analysis was conducted to determine if the results would change significantly after defining PART in a manner consistent with Thal phase 0. Data on Thal phase were only collected in the most recent version of the NP Form and thus were missing for 80% of the sample. Therefore, data on diffuse plaques, which were collected since the inception of the NP Form, were used in the place of Thal phase.
RESULTS
The sample included 240 participants with definite PART, 186 with possible PART, and 510 with AD (Fig. 1; Table 1). A larger percentage with possible PART were ≥90 years old (last visit before death), compared to AD (Table 1; Fig. 2). No significant differences were observed by sex (male: 48% definite PART; 47% possible PART; 55% AD). The majority with definite PART had Braak stages I–II, the majority with possible PART had Braak stages III–IV, and the majority with AD had Braak stages V–VI (Table 2). Compared to AD, those with definite or possible PART less often had ≥1 APOE ɛ4 allele. A family history of cognitive impairment was reported less often in definite PART versus AD. Higher global CDR scores indicating greater impairment were more likely in AD than in definite and possible PART. Compared to AD, those with definite PART were more likely to report a history of stroke, hypertension, and diabetes. Likewise, compared to AD, those with possible PART were more likely to report a history of stroke, hypertension, and traumatic brain injury, and were more likely to have VBI at autopsy. The unadjusted cognitive domain z-scores by group and cognitive status are reported in Supplementary DataFigure S1 and Table S1.
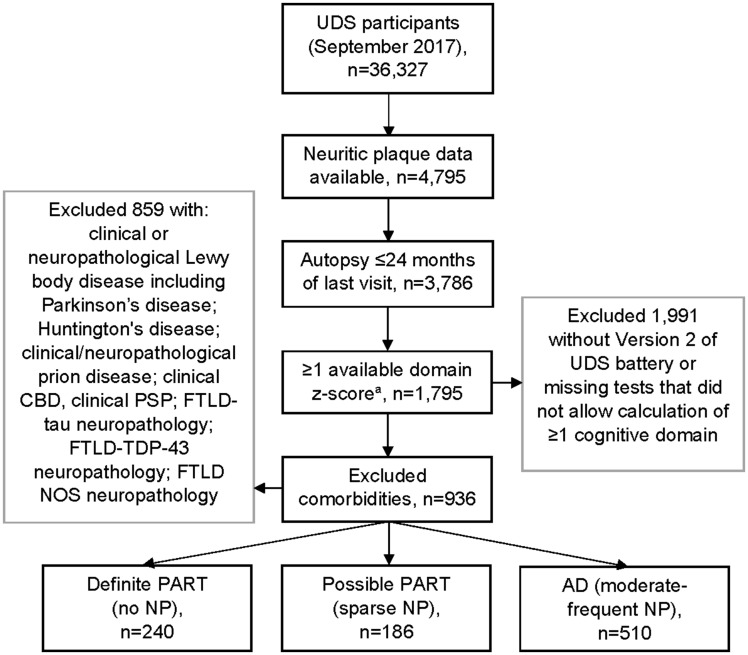
FIGURE 1.
Sample selection flow diagram. Over 60% of all 3 neuropathologic groups with CDR = 2 or 3 had missing executive function z-scores. Negative z-scores = scored worse than UDS norms sample mean score; positive z-scores = scored better than UDS norms sample mean score. aNonmissing domain based on Version 2 of the UDS neuropsychological test battery. UDS, Uniform Data Set; CBD, corticobasal degeneration; PSP, progressive supranuclear palsy; FTLD, frontotemporal lobar degeneration; TDP, TAR DNA-binding protein 43; NOS, not otherwise specified; CDR, Clinical Dementia Rating.
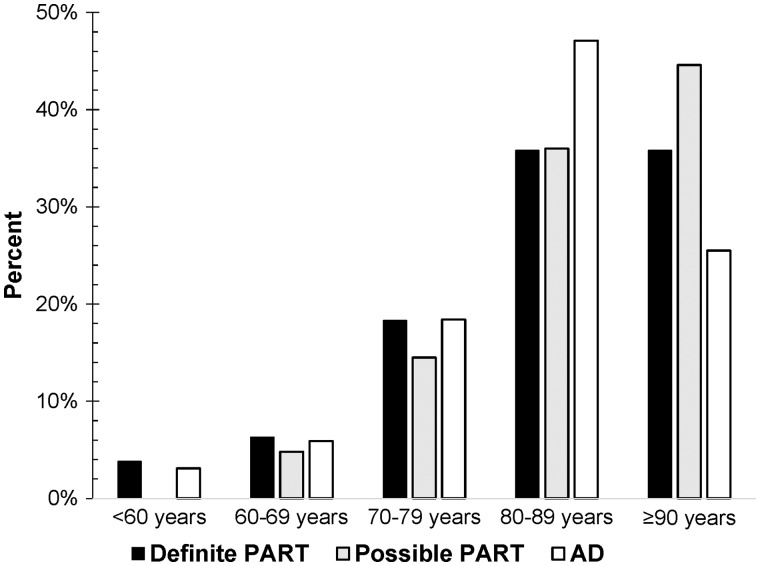
FIGURE 2.
Distribution of definite PART, possible PART, and AD sample by age group. For a given neuropathologic group, the sum of the 5 age group percentages equals 100 percent. The majority of the entire sample were 80 years or older. When comparing the 3 neuropathologic groups, a larger proportion of those with AD were 80–89 years old and a larger proportion of the Definite and Possible PART groups were ≥90 years old. PART, primary age-related tauopathy; AD, Alzheimer disease.
In the unadjusted linear regression analyses, individuals with definite PART scored significantly better than those with AD on episodic memory regardless of cognitive status (Supplementary DataTable S2). In those with CDR = 0.5–1 and CDR = 2–3, individuals with definite PART scored significantly better than those with AD on the global composite and semantic memory/language domain. In addition, in those with CDR = 2–3, individuals with definite PART scored better than those with AD on attention and executive function. Compared to AD, individuals with possible PART scored worse on attention among those with CDR = 0, better on episodic memory among those with CDR = 0.5–1, and better on semantic memory/language in those with CDR = 2–3.
In the adjusted linear regression analyses comparing individuals with definite PART and AD, there were no significant differences in cognitive domain scores among those with CDR = 0 (Table 3; Fig. 3). However, compared to AD, the definite PART group scored better on the global composite, episodic memory, and semantic memory/language domains among those with CDR = 0.5–1, and the definite PART group scored better on attention and semantic memory/language among those with CDR = 2–3. Individuals with possible PART scored worse on attention than those with AD among those with CDR = 0, and there were no other statistically significant differences when comparing individuals with possible PART and AD.
TABLE 3.
Adjusted Difference in Cognitive Domains at Last Visit in Primary Age-Related Tauopathy Versus Alzheimer Disease
| Adjusted Mean Difference in z-Scoresa,b (95% CI) |
||||||
|---|---|---|---|---|---|---|
| CDR = 0 |
CDR = 0.5 or 1 |
CDR = 2 or 3 |
||||
| Cognitive domainc | Definite PART Versus AD | Possible PART Versus AD | Definite PART Versus AD | Possible PART Versus AD | Definite PART Versus AD | Possible PART Versus AD |
| Global composite | 0.07 (−0.08, 0.21) | −0.03 (−0.19, 0.13) | 0.36*(0.08, 0.64) | 0.20 (−0.06, 0.46) | 0.49 (−0.09, 1.07) | 0.33 (−0.30, 0.95) |
| Episodic memory | 0.21 (−0.02, 0.44) | 0.15 (−0.07, 0.36) | 0.61**(0.39, 0.84) | 0.32 (−0.05, 0.68) | 0.40 (−0.13, 0.93) | 0.11 (−0.25, 0.47) |
| Attention | 0.11 (−0.15, 0.37) | −0.22*(−0.40, −0.04) | 0.27 (−0.06, 0.60) | 0.15 (−0.14, 0.43) | 0.76*(0.09, 1.43) | −0.08 (−0.53, 0.36) |
| Executive function | −0.09 (−0.36, 0.18) | −0.01 (−0.28, 0.27) | 0.28 (−0.13, 0.68) | 0.23 (−0.13, 0.59) | 0.29 (−0.80, 1.37) | 0.59 (−0.82, 2.01) |
| Semantic memory/language | 0.08 (−0.06, 0.21) | −0.18 (−0.43, 0.07) | 0.37**(0.16, 0.58) | 0.16 (−0.10, 0.42) | 0.92*(0.22, 1.63) | 0.23 (−0.24, 0.71) |
Abbreviations: CDR, Clinical Dementia Rating; PART, primary age-related tauopathy; AD, Alzheimer disease; CI, confidence interval; vs, versus.
Statistically significant at p < 0.05.
Statistically significant at p < 0.01.
Controlling for age at last visit, sex, education (years), presence of at least 1 APOE ε4 allele; family history of cognitive impairment; history of: hypertension, stroke, diabetes, traumatic brain injury, Braak stage.
Negative mean difference in z-scores = scored worse than those with moderate to frequent neuritic plaques; positive mean difference in z-scores = scored better than those with moderate to frequent neuritic plaques.
For all tests but Trail Making Parts A and B, lower score is worse score; therefore Trail Making Part A and B scores (within the executive function domain) were multiplied by −1 to make them consistent with the other tests.
Bold = statistically significant at p < 0.05.
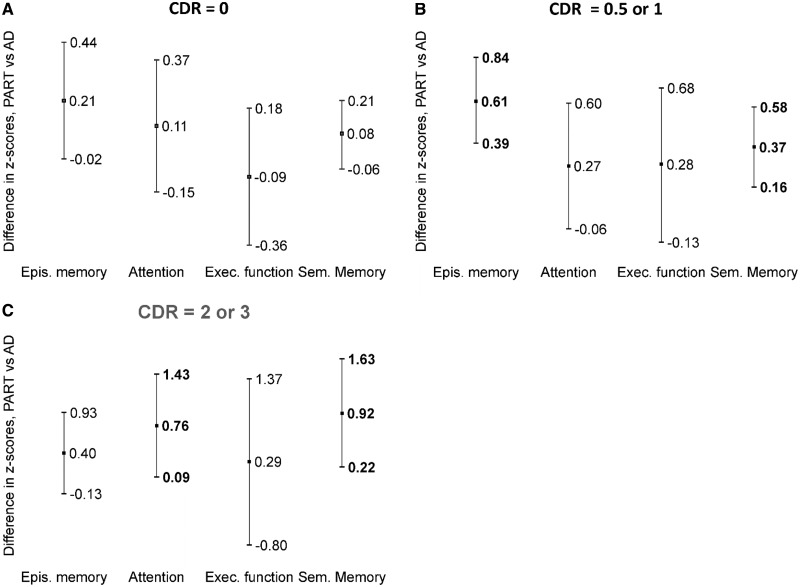
FIGURE 3.
Cognitive domain score differences between definite PART and AD by CDR. Estimates (center marker) and upper and lower 95% confidence intervals are presented. Statistically significant differences (p < 0.05) are bolded. (A) No significant differences between definite PART and AD among those with CDR = 0. (B) Among those with CDR = 0.5 or 1, individuals with definite PART scored better than those with AD on tests of episodic memory and semantic memory. (C) Among those with CDR = 2 or 3, individuals with definite PART scored better than those with AD on tests of attention and semantic memory. PART, primary age-related tauopathy; AD, Alzheimer disease; CDR, Clinical Dementia Rating; Epis., episodic; Exec, executive.
Multivariable analyses stratifying by Braak stages (I–II, III–IV) instead of controlling for it are presented in Tables 4 and 5. New findings emerged through stratification. Focusing on Braak stages III–IV, CDR = 0 individuals with definite PART demonstrated a sparing of semantic memory/language compared to CDR = 0 individuals with AD (Table 4), and CDR = 0.5–1 individuals with definite PART demonstrated a sparing of executive function compared to CDR 0.5–1 individuals with AD (Table 5). Stratification revealed that the findings for sparing of global cognition, episodic memory, and semantic memory/language among definite PART individuals with CDR = 0.5–1 in the main analyses (Table 3) were restricted to those with Braak stages III–IV (Table 5).
TABLE 4.
Adjusted Difference in Cognitive Domains at Last Visit Among Individuals With CDR = 0
| Cognitive domainc | Adjusted Mean Difference in z-Scoresa,b (95% CI) |
|||
|---|---|---|---|---|
| Braak Stages I–II |
Braak Stages III–IV |
|||
| Definite PART Versus AD | Possible PART Versus AD | Definite PART Versus AD | Possible PART Versus AD | |
| Global composite | −0.01 (−0.20, 0.18) | 0.01 (−0.22, 0.24) | 0.23 (−0.10, 0.56) | −0.12 (−0.39, 0.16) |
| Episodic memory | −0.06 (−0.56, 0.44) | −0.01 (−0.52, 0.50) | 0.56 (−0.02, 1.14) | 0.29*(0.00, 0.58) |
| Attention | 0.10 (−0.22, 0.41) | −0.17 (−0.52, 0.17) | 0.08 (−0.35, 0.51) | −0.35*(−0.69, −0.01) |
| Executive function | −0.12 (−0.36, 0.11) | 0.19 (−0.09, 0.46) | −0.18 (−0.68, 0.32) | −0.39 (−0.83, 0.06) |
| Semantic memory/language | −0.01 (−0.22, 0.19) | −0.22 (−0.56, 0.12) | 0.37*(0.08, 0.66) | −0.04 (−0.29, 0.21) |
Abbreviations: CDR, Clinical Dementia Rating; NP, neuritic plaques; CI, confidence interval; vs, versus; NA, too few with Braak.
Statistically significant at p < 0.05.
Controlling for age at last visit, sex, education (years), presence of at least 1 APOE ε4 allele; family history of cognitive impairment; history of: hypertension, stroke, diabetes, traumatic brain injury.
Negative mean difference in z-scores = scored worse than those with moderate to frequent neuritic plaques; positive mean difference in z-scores = scored better than those with moderate to frequent neuritic plaques.
For all tests but Trail Making Parts A and B, lower score is worse score; therefore Trail Making Part A and B scores (within the executive function domain) were multiplied by −1 to make them consistent with the other tests.
Bold = statistically significant at p < 0.05.
TABLE 5.
Adjusted Difference in Cognitive Domains at Last Visit Among Individuals With CDR = 0.5 or 1
| Cognitive domainc | Adjusted Mean Difference in z-Scoresa,b (95% CI) |
|||
|---|---|---|---|---|
| Braak Stages I–II |
Braak Stages III–IV |
|||
| Definite PART Versus AD | Possible PART Versus AD | Definite PART Versus AD | Possible PART Versus AD | |
| Global composite | 0.30 (−0.17, 0.76) | 0.44 (−0.30, 1.17) | 0.48**(0.15, 0.82) | 0.10 (−0.32, 0.51) |
| Episodic memory | 0.29 (−0.27, 0.84) | −0.19 (−0.92, 0.54) | 0.60**(0.15, 1.06) | 0.22 (−0.41, 0.85) |
| Attention | 0.30 (−0.21, 0.80) | 0.04 (−0.38, 0.46) | 0.23 (−0.16, 0.62) | 0.08 (−0.25, 0.42) |
| Executive function | 0.20 (−0.40, 0.80) | 1.17*(0.11, 2.22) | 0.57*(0.03, 1.11) | 0.04 (−0.36, 0.44) |
| Semantic memory/language | −0.02 (−0.59, 0.54) | −0.13 (−0.61, 0.34) | 0.41*(0.04, 0.78) | 0.14 (−0.22, 0.51) |
Abbreviations: CDR, Clinical Dementia Rating; NP, neuritic plaques; CI, confidence interval; vs, versus.
Statistically significant at p < 0.05.
Statistically significant at p < 0.01.
Controlling for age at last visit, sex, education (years), presence of at least 1 APOE ε4 allele; family history of cognitive impairment; history of: hypertension, stroke, diabetes, traumatic brain injury.
Negative mean difference in z-scores = scored worse than those with moderate to frequent neuritic plaques; positive mean difference in z-scores = scored better than those with moderate to frequent neuritic plaques.
For all tests but Trail Making Parts A and B, lower score is worse score; therefore Trail Making Part A and B scores (within the executive function domain) were multiplied by −1 to make them consistent with the other tests.
Bold = statistically significant at p < 0.05.
In post hoc analyses, we found that definite PART participants scored significantly better on all 3 semantic memory/language tests compared to AD participants (Table 6; Fig. 4). However, in the CDR = 0.5–1 and CDR = 2–3 groups, the biggest differences between these 2 groups were observed for the Boston Naming Test. In the sensitivity analysis redefining definite PART based on diffuse plaques instead of NP, the overall results remained similar except that individuals with definite PART (n = 148) scored significantly better than those with AD in the attention and executive function domains (Supplementary DataTable S3). Lastly, in the sensitivity analyses examining the independent contribution of VBI to cognitive domain scores, we found that among those with CDR = 0, those with VBI scored worse than those without VBI on the global composite, attention, and executive domains (data not shown). In addition, among those with CDR = 0.5 or 1, individuals with VBI scored worse in the attention and executive domains, and no differences were observed between those with and without VBI among individuals with CDR = 2 or 3.
TABLE 6.
Adjusted Difference in Semantic Memory/Language at Last Visit in Primary Age-Related Tauopathy Versus Alzheimer Disease
| Cognitive test | Adjusted Mean Difference in z-Scoresa,b (95% CI) |
|||||
|---|---|---|---|---|---|---|
| CDR = 0 |
CDR = 0.5 or 1 |
CDR = 2 or 3 |
||||
| Definite PART Versus AD | Possible PART Versus AD | Definite PART Versus AD | Possible PART Versus AD | Definite PART Versus AD | Possible PART Versus AD | |
| Animals | −0.06 (−0.25, 0.14) | −0.08 (−0.36, 0.19) | 0.27**(0.08, 0.47) | 0.14 (−0.03, 0.31) | 0.65**(0.24, 1.05) | 0.26*(0.00, 0.51) |
| Vegetables | 0.19*(0.02, 0.35) | 0.03 (−0.23, 0.30) | 0.39**(0.19, 0.59) | 0.28**(0.10, 0.45) | 0.66**(0.25, 1.07) | 0.13 (−0.24, 0.51) |
| Boston Naming Test | 0.11 (−0.11, 0.33) | −0.40**(−0.69, −0.10) | 0.53**(0.21, 0.85) | 0.19 (−0.35, 0.73) | 1.69*(0.14, 3.24) | 0.27 (−0.56, 1.10) |
Abbreviations: CDR, Clinical Dementia Rating; PART, primary age-related tauopathy; AD, Alzheimer disease; CI, confidence interval; vs, versus.
Statistically significant at p < 0.05.
Statistically significant at p < 0.01.
Controlling for age at last visit, sex, education (years), presence of at least 1 APOE ε4 allele; family history of cognitive impairment; history of: hypertension, stroke, diabetes, traumatic brain injury, Braak stage.
Negative mean difference in z-scores = scored worse than those with moderate to frequent neuritic plaques; positive mean difference in z-scores = scored better than those with moderate to frequent neuritic plaques.
Bold = statistically significant at p < 0.05.
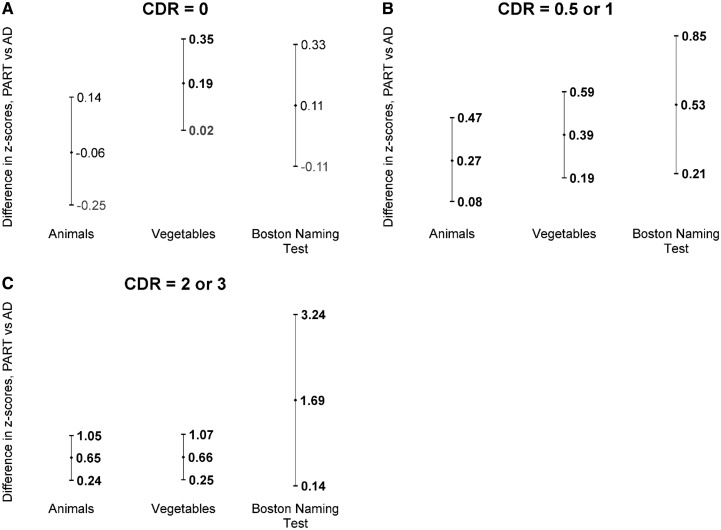
FIGURE 4.
Semantic language/memory test score differences between definite PART and AD by CDR. Estimates (center marker) and upper and lower 95% confidence intervals are presented. Statistically significant differences (p < 0.05) are bolded. (A) Among those with CDR = 0, individuals with definite PART scored better than those with AD on the Vegetables List Generation test. (B) Among those with CDR = 0.5 or 1, individuals with definite PART scored better than those with AD on the Animals and Vegetables List Generation tests and on the Boston Naming Test. (C) Among those with CDR = 2 or 3, individuals with definite PART scored better than those with AD on the Vegetables List Generation test. PART, primary age-related tauopathy; AD, Alzheimer disease; CDR, Clinical Dementia Rating.
DISCUSSION
We sought to compare antemortem neuropsychological profiles between individuals who at autopsy had PART or AD, and to examine how differences between these groups alter when stratified by disease stage. We found that even within the same stratum of CDR scores, individuals with PART had relative sparing of semantic memory/language in comparison to AD. In addition, when comparing individuals with PART to AD, episodic memory was relatively spared in the moderately severe stratum (CDR 0.5–1) and attention was relatively spared in the most severe stratum (CDR 2–3). Within the asymptomatic stratum there were no differences between PART and AD. Minimal differences in neuropsychological scores were observed when comparing possible PART and AD. It is important to note that individuals in this transitional category might represent either early AD or, alternatively, resilient individuals who are resistant to further degeneration.
Previous studies suggest that those with PART have less severe cognitive decline than those with AD neuropathologic change (6, 20). This is reinforced by the current study’s finding that more PART participants had CDR = 0 and fewer had CDR = 2–3 in comparison to AD, and the aforementioned findings of relative sparing of semantic memory/language, episodic memory, and attention in PART versus AD. Nonetheless, PART does have cognitive consequences, which are more notable with more advanced pathology. For example, when PART is present, it is more severe (more likely to be Braak stages III/IV) in individuals with mild disease than in asymptomatic individuals (1). In addition, increasing Braak stage has been associated with an increased odds of having a CDR >0 among individuals with PART (5).
The topic of neuropsychological changes in PART has received minimal attention thus far. Two studies have looked at associations between neuropsychological test scores and PART. One study using longitudinal NACC data found progressive cognitive decline in individuals with PART, in particular for tasks involving episodic memory, processing speed, and semantic memory/language (7). Individuals with higher NFT burden were shown to have more rapid decline in episodic and semantic memory/language, as well as processing speed/attention. There was also a difference between those with definite and possible PART. Unlike the former, with increasing Braak stage, the latter had a faster rate of decline for delayed recall and global impairment on the MMSE. Another study showed that among individuals with PART, there was an association between higher Braak NFT stage and poorer performance on WAIS R Block Design and Trail Making Tests (8).
This study adds to the literature by providing details on the specific neuropsychological domains that are spared in PART versus AD, which has been nominally investigated to date. Moreover, this study adds evidence to the ongoing debate about whether temporal lobe NFTs in the absence of amyloid deposits are necessarily on the AD spectrum (21, 22) or part of an alternative pathway (6, 23). The observed differential pattern of neuropsychological change in PART in comparison to AD provides further evidence that PART might have a distinct trajectory and pathogenesis. In contradistinction, the pattern of neuropsychological change was virtually identical for individuals with possible PART in comparison to AD, suggesting that unlike individuals with definite PART, those with possible PART may not be easily distinguished from AD based on cognitive testing. However, this inability to distinguish the 2 groups clinically does not allow any conclusions about the underlying biologic processes. An individual with sparse amyloid and tauopathy might be on either the AD or PART pathway, or some hybrid thereof. Likewise, individuals with sparse amyloid and tauopathy might represent early AD or alternatively resilience. Other factors, such as genetics or environmental exposure, may also differ among individuals with possible PART and AD. Of note, the differences in cognitive domain scores between the groups did not follow a specific additive pattern. This further reinforces the hypothesis that AD and PART are different pathologic entities with different trajectories rather than different speeds of progression along the same trajectory.
Another study examined MRIs for individuals with PART and found that the posterior but not anterior hippocampus was relatively spared from atrophy (8). The authors suggested that the pattern of atrophy observed in PART, affecting only the anterior hippocampus, may be similar to patterns previously observed in semantic dementia, which is characterized cognitively by deficits in naming and word comprehension with relative preservation of episodic memory and other cognitive domains (24). However, they did not investigate this hypothesis further, but instead proposed that future studies should examine differences in NFT distribution between individuals with PART and AD. In contrast, our study found that semantic memory/language was relatively preserved in PART compared to AD, which is inconsistent with their hypothesis. To help elucidate how differences in anatomic distribution and burden of NFT may affect expression of cognitive symptoms differently in PART and AD, future studies are needed to examine differences in atrophy on MRIs and NFT burden on neuropathological exam by brain region.
Our study has some limitations, including potential residual confounding by disease severity within the CDR strata. Among those in the CDR = 0.5–1 stratum, there were relatively fewer individuals with CDR = 1 in PART (39%) than AD (52%). However, the possible PART group had even fewer (32%) and within the CDR = 2–3 stratum, all 3 neuropathologic groups had similar percentages with CDR = 3 (25%–36%). Hence, this is unlikely to explain the differences in neuropsychologic test scores. Moreover, limitations of sample size did not allow narrower categories and similar concerns would apply if the groups were defined by clinical diagnosis. In addition, although we interpreted significant results at an alpha level of 0.05, this is a limitation due to multiple comparisons. To help address that concern, we also presented associations significant at p < 0.01, finding that individuals with definite PART performed statistically significantly better than those with AD on the episodic memory and semantic memory/language, in those with CDR = 0.5 or 1.
Study participants were more likely to be white and highly educated than the general population, which limits generalizability of the findings. Seventy-four percent were ≥80 years old. Thus, the results may not generalize to younger individuals, although PART is typically considered a neurodegenerative condition affecting older ages (i.e. ≥80 years), and the age distribution was similar between those with definite PART and AD. In addition, data were missing for some of the neuropsychological tests. Typically, ≤10% of the data were missing across domains and missingness was generally similar for the neuropathologic groups. A major exception was that although the neuropathologic groups had similar levels of missingness of executive function scores, the percent missing by neuropathologic group was much higher for this domain than other domains (range: 13%–75%). However, missing data is unlikely to have substantially affected our main findings regarding episodic memory, attention, and semantic memory/language. The relative sparing of attention in PART participants with CDR >0 may be explained by the limited tests measuring attention in UDS neuropsychological battery or residual confounding by unreported vascular disease (which affects attention) due to the self-reported nature of the stroke and hypertension control variables.
PART was defined based on CERAD NP, which is not synonymous with amyloid plaques, and future studies of PART will need to assess whether CERAD NP are an adequate substitute. In our sensitivity analysis of definite PART, substituting the absence of diffuse plaques (proxy for Thal phase 0) for the absence of CERAD NP, the overall findings remained similar to the main analyses, but suggested that those with no diffuse plaques also performed better than those with AD in 2 additional domains (executive function and attention). Future studies will be needed to determine if these results hold when PART is defined using Thal phase. In addition, individuals with PART more frequently had lower Braak stages and individuals with AD more frequently had higher Braak stages, leading to potential limitations in the comparisons. However, we accounted for Braak NFT stage to the extent possible in our analyses.
We applied a number of exclusions in this study to help assure that we were assessing PART, not other pathologies. VBI at autopsy was not excluded due to its high prevalence in the sample, and a sensitivity analysis indicated that PART and VBI independently contributed to differences in cognition. As PART becomes better characterized in the literature, future studies examining PART with and without additional copathologies will be of increasing interest.
Despite these limitations, this study has major strengths. It provides multi-institutional data on a large group of individuals who had PART or AD, identified by neuropathological exam at autopsy using standardized techniques. It is one of the first studies to compare scores from a battery of standardized neuropsychological tests between individuals with either autopsy-confirmed PART or autopsy-confirmed AD. In addition, this study allows us to draw reasonable conclusions about the pattern of neuropsychological changes at several stages of PART.
Our study confirms prior studies showing that PART is less likely to be associated with more severe dementia than is AD. Within the same stratum of CDR scores, PART was associated with a different pattern of neuropsychological change than AD, with relative sparing of semantic memory/language among those with any cognitive impairment, and in the most severe stratum, with additional sparing of attention. The relatively small observed differences in cognitive domain scores suggests that more sensitive neuropsychological tests or biomarkers/neuroimaging may be better suited to clinically distinguish PART from AD during life. Nonetheless, the study adds further evidence for the hypothesis that PART is a distinct pathologic entity, with different biologic consequences than AD.
Supplementary Material
ACKNOWLEDGMENTS
The authors thankfully acknowledge the patients and families enrolled at the ADCs who contributed data to the UDS and the faculty and staff of the ADCs who conducted the evaluations and collected the data used in these analyses. The authors would also like to thank the NIA, which provided support for the ADCs and NACC, as well as NACC staff (George Thomas, Janene Hubbard, Mary Jacka, Joylee Wu, Elizabeth Robichaud, Nicole Barlow, Kathryn Gauthreaux, Kristen Schwabe-Fry, Carolyn Velez, Ayushi Divecha, Zack Miller, Karyn Vo, Margaret Dean) who help in programing of the data submission systems, data management, research coordination, and administration, and without whom this research would not be possible.
Footnotes
The authors have no duality or conflicts of interest to declare.
The NACC database is funded by NIA/NIH Grant U01 AG016976. Dr John Crary is supported by National Institutes of Health Grants R01 NS095252 (PI), R01 AG054008 (PI), RF1 AG06096 (PI), R01 AG062348 (PI), Alzheimer’s Association NIRG-15-363188 (PI), and the Tau Consortium (PI). NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
REFERENCES
- 1. Abner EL, Kryscio RJ, Schmitt FA, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol 2017;81:549–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–204 [DOI] [PubMed] [Google Scholar]
- 3. Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol 2012;71:362–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montine KS, Montine TJ.. Anatomic and clinical pathology of cognitive impairment and dementia. J Alzheimers Dis 2013;33(Suppl 1):S181–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Besser LM, Crary JF, Mock C, et al. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology 2017;89:1707–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jefferson-George KS, Wolk DA, Lee EB, et al. Cognitive decline associated with pathological burden in primary age-related tauopathy. Alzheimers Dement 2017;13:1048–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Josephs KA, Murray ME, Tosakulwong N, et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: A clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol 2017;133:705–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–6 [DOI] [PubMed] [Google Scholar]
- 10.National Alzheimer’s Coordinating Center. NP Data Set Forms and Documentation Available at: http://www.alz.washington.edu/WEB/forms_np.html. Accessed September 29, 2018
- 11. Besser LM, Kukull WA, Teylan MA, et al. The revised National Alzheimer's Coordinating Center’s Neuropathology form—Available data and new analyses. J Neuropathol Exp Neurol 2018;77:717–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Alzheimer’s Coordinating Center. Neuropathology Data Set: Coding Guidebook Available at: http://www.alz.washington.edu/NONMEMBER/NP/npguide10.pdf. Accessed September 29, 2018
- 13. Montine TJ, Phelps CH, Beach TG, et al. National Institute on aging-Alzheimers Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mirra SS, Hart MN, Terry RD.. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch Pathol Lab Med 1993;117:132–44 [PubMed] [Google Scholar]
- 15. Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayden KM, Jones RN, Zimmer C, et al. Factor structure of the National Alzheimer’s Coordinating Centers uniform dataset neuropsychological battery: An evaluation of invariance between and within groups over time. Alzheimer Dis Assoc Disord 2011;25:128–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balthazar ML, Cendes F, Damasceno BP.. Semantic error patterns on the Boston Naming Test in normal aging, amnestic mild cognitive impairment, and mild Alzheimer’s disease: Is there semantic disruption? Neuropsychology 2008;22:703–9 [DOI] [PubMed] [Google Scholar]
- 19. Albert MS, Heller HS, Milberg W.. Changes in naming ability with age. Psychol Aging 1988;3:173–8 [DOI] [PubMed] [Google Scholar]
- 20. Kryscio RJ, Abner EL, Jicha GA, et al. Self-reported memory complaints: A comparison of demented and unimpaired outcomes. J Prev Alzheimers Dis 2016;3:13–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duyckaerts C, Braak H, Brion JP, et al. PART is part of Alzheimer disease. Acta Neuropathol 2015;129:749–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braak H, Del Tredici K.. Are cases with tau pathology occurring in the absence of Aβ deposits part of the AD-related pathological process? Acta Neuropathol 2014;128:767–72 [DOI] [PubMed] [Google Scholar]
- 23. Crary JF. Primary age-related tauopathy and the amyloid cascade hypothesis: The exception that proves the rule? J Neurol Neuromed 2016;1:53–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodges JR, Patterson K.. Semantic dementia: A unique clinicopathological syndrome. Lancet Neurol 2007;6:1004–14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.