Abstract
Higher-grade meningiomas (WHO grade II and III) represent a diagnostic and prognostic challenge. We assessed the pathological and molecular characteristics of 94 higher-grade meningiomas (85 grade II, 9 grade III) to identify novel prognostic parameters. Higher mitotic count (p = 0.018), diffuse (≥50%) prominent nucleoli (p < 0.001), and sheeting (p < 0.001) were associated with recurrence. Lower SSTR2a-positive cells median rate (p = 0.048) and TERT promoter mutations (p = 0.014) were associated with recurrence and patient death, respectively; further analyses did not identify other outcome associations. Presence of Ki67 hot spots was associated with a shorter progression-free survival (PFS), independently of WHO grade at multivariate analysis (HR = 3.35, p = 0.008). Necrosis was related to a poorer overall survival (OS) at univariate (focal: HR = 4.55, p = 0.041 and diffuse: HR = 7.38, p = 0.020) and Kaplan-Meier analyses. A prognostic score was designed based on previous results: Presence of diffuse (≥50%) prominent nucleoli (0/1 point), diffuse (≥50%) sheeting (0/1 point), focal (<50%) or diffuse (≥50%) necrosis (0/1/2 points), and Ki67 hot spots (0/1 point). A total score ≥4 predicted poorer PFS and OS by Kaplan-Meier (PFS: 1.7 vs 6.4 years, p < 0.001 and OS: 5.2 vs 10.8 years, p = 0.001) and multivariate (PFS: HR = 5.98, p < 0.001 and OS: HR = 2.99, p = 0.048) analyses. These results were confirmed in an independent series of 58 grade II meningiomas (PFS: HR = 7.22, p = 0.002 and OS: HR = 9.69, p = 0.003). These associations and the integrated score could complement WHO grading.
Keywords: Ki67, Meningioma, Prognosis, Prognostic factors, Score, SSTR2a, TERT
INTRODUCTION
Meningiomas are the most common primary tumors of the CNS with an incidence of 8.14/100 000 population according to the latest Central Brain Tumor Registry of the United States (CBTRUS) (1). According to the World Health Organization (WHO) classification criteria, a tumor grade between I and III is assigned based upon the assessment of specific morphological features (2). Diagnosis of atypical grade II meningioma is defined by an increased mitotic count (≥4/10 high-power fields [HPF]) or histological evidence of brain invasion or the presence of 3 out of 5 characteristics: Prominent nucleoli, sheeting, hypercellularity, small cells with a high nucleus/cytoplasm ratio, and foci of spontaneous necrosis. Grade III anaplastic meningiomas are defined by elevated mitotic activity (≥20/HPF) or overtly malignant cytology. Also, some histotypes harbor an independent grading implication. Overall, higher-grade meningiomas (II and III) represent a significant subgroup of patients, amounting to 25% and 5% of all meningiomas, respectively (3–5).
Surgical resection is considered the first-line option for patients needing treatment and gross total resection should be achieved whenever possible, limiting patient observation to selected cases (i.e., small asymptomatic meningiomas). Although most grade I meningiomas are cured by resection alone, grade II and grade III tumors show high recurrence rates and may thus require adjuvant treatments (6). Adjuvant radiotherapy is usually advised for incompletely resected grade II and for all grade III meningiomas (7); however, this approach is not universally agreed upon and grey zones do exist, thus making meaningful and reproducible prognostic factors especially warranted when tailoring patient treatment. For recurrent meningiomas, if additional surgery or re-irradiation is not feasible or indicated, medical therapies can be considered although efficacy is usually very limited (7–11); however, this could change in the near future thanks to targeted approaches (12).
WHO grading criteria suffer from subjective interpretation, which could explain the different frequencies of grade II and III tumors observed among institutions and, more importantly, the wide differences in patients’ outcome reported within the same grade group. Although phospho-histone H3 (PHH3)-based mitotic count can improve interobserver reproducibility and prognostic stratification by increasing mitotic count reliability (5, 13), the evaluation of other criteria heavily depends on pathologists’ subjective evaluation. Correlation between tumor grade and Ki67 labeling index is known, but at present it is not considered a grading variable (2). Moreover, in our experience, a significant number of meningiomas show Ki67 hot spots (i.e., focal areas of high Ki67 labeling index) despite a low overall median index, but no data have been reported about the possible prognostic significance of this feature. Regarding other immunohistochemical prognostic factors, expression of the progesterone receptor was found to be associated with a better outcome, but its prognostic role could overlap with tumor grade (14). Somatostatin receptors are commonly expressed by meningiomas and their prognostic role has been shown in other tumors (15). In meningiomas, no relationship with outcome was found, but most of the analyzed cases were grade I meningiomas, thus it could be of interest to specifically evaluate a larger series of higher-grade meningiomas (16). More recently, the absence of H3K27 trimethylation, detected by immunohistochemistry, was found to be prognostic of a poorer outcome (17).
Besides morphological and immunohistochemical features, molecular factors could play a prognostic role as well. In particular, both DNA methylation-based classification (18, 19) and telomerase reverse transcriptase (TERT) promoter mutations (a common alteration of many human cancers, although rare in meningiomas) (20–23) showed promising results. However, the cost of comprehensive molecular analyses like methylation-based classification is not negligible, thus its widespread implementation for routine diagnostic workup will require some time, especially for a pathology with a relatively high incidence like meningioma.
Lastly, most studies focused on discerning between benign (grade I) and aggressive (grade II–III) meningiomas, while only few data are available regarding possible prognosticators within the latter group, despite the potential clinical usefulness.
Thus, we analyzed a retrospective series of grade II and III meningiomas with the aim of improving their prognostic characterization. Specifically, we (1) assessed the prognostic significance of the morphological features currently used for meningioma grading adopting specific predetermined definitions and cut-off values; (2) evaluated the role of Ki67 labeling index (including the specific assessment of Ki67 hot spots), somatostatin receptor subtype 2a (SSTR2a) expression and TERT promoter status; and (3) built and evaluated an integrated score based upon the association of the previous variables and aimed at predicting patients’ outcome in addition to WHO grade.
MATERIALS AND METHODS
Case Series and Tissue Samples
Grade II and grade III meningiomas, diagnosed at the Pathology Unit of AOU Città della Salute e della Scienza University Hospital of Turin between January 1994 and December 2015, were retrospectively collected. All patients underwent surgical resection at the Neurosurgery Unit of the same institution. Histological diagnosis was reviewed and confirmed by a senior neuropathologist (P.C.) according to the 2016 WHO classification. Follow up data were retrieved from patients’ charts and the Piedmont Tumor Registry. The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and within the guidelines and regulations defined by the Research Ethics Committee of the University of Turin. This study was approved by the Research Ethics Committee of the University of Turin; considered the retrospective nature of the research protocol and that it had no impact on patient care, no specific written informed consent was required.
Integrated Prognostic Score External Validation
A series of grade II meningiomas (according to WHO 2016 criteria) with follow up data was collected at the Neuropathology Unit of Fondazione IRCCS Istituto Neurlogico “C. Besta” (Milan, Italy) and morphological features required for score assessment were evaluated.
Morphological Features and Immunohistochemistry: Qualitative and Quantitative Evaluation
Tumor histotype, mitotic count, presence of hypercellularity (evaluated as absent vs present or as involving <50% vs ≥50% of tumor), small cells (absent/present), prominent nucleoli (absent/present or <50%/≥50%), sheeting (patternless growth) (absent/present or absent/<50%/≥50%), and spontaneous necrosis (absent/present or absent/<50%/≥50%) were assessed. Small cell presence was defined as presence of meningioma cells with a size up to 3 times that of a lymphocyte, involving ≥5% tumor sample. Immunohistochemistry for Ki67 (clone 30–9, Ventana Medical Systems, Tucson, AZ) and SSTR2a (clone UMB1, Abcam, Cambridge, UK) were performed on the BenchMark ULTRA platform (Ventana Medical Systems). Ki67 labeling index was assessed by manually counting 1000 cells, while Ki67 hot-spots were arbitrarily defined as intermediate power fields (×200) displaying at least a triple labeling index compared with the mean tumor labeling index. SSTR2a expression was evaluated both as the overall rate of positive cells and scored as previously described (24): Score 0: absence of immunoreactivity; score 1: pure cytoplasmic immunoreactivity, either focal or diffuse; score 2: membranous reactivity in <50% of tumor cells, irrespective of the presence of cytoplasmic staining; and score 3: circumferential membranous reactivity in >50% of tumor cells, irrespective of the presence of cytoplasmic staining.
TERT Promoter Sequencing
DNA extraction from formalin-fixed and paraffin-embedded (FFPE) tumor samples was performed as previously described (25), and concentrations/purity were measured by a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
Mutational status of the TERT promoter region from position –27 to –286 from ATG start site, including the polymorphic site represented by rs2853669, were determined by PCR and Sanger sequencing using the following primer pair: Promoter forward 5′-CAGCGCTGCCTGAAACTC-3′ and reverse 5′-GTCCTGCCCCTTCACCTT-3′, as described by Horn et al (26). PCR products were purified and used as template for the sequencing reactions that were performed with a BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). After purification, the sequences were analyzed by Sanger direct sequencing using the ABIPRISM 3130 Genetic Analyzer (Applied Biosystems).
Statistical Analyses
Statistical analyses were performed using Stata/MP 15.0 Statistical Software (StataCorp, College Station, TX). Categorical variables were compared using Pearson’s χ2 test. Kruskal-Wallis has been used to compare not normally distributed variables. The variables tested were as follows: Age at diagnosis, gender, tumor histotype/grade, tumor site, brain invasion and other morphological features, Ki67 labeling index, Ki67 hot-spots, SSTR2a expression, TERT promoter status, tumor recurrence, progression-free survival (PFS) and overall survival (OS).
Differences were considered significant when p < 0.05 was reported for two-sided p values. PFS was calculated from the initial diagnosis until there was radiologically confirmed tumor progression, with data censored at the last available date of follow-up. OS was defined as the interval from the initial diagnosis until death, with data censored at the last available date of follow-up. All patient data were updated as of December 31, 2016. Survival distribution curves were plotted using the Kaplan-Meier method and the statistical comparisons were performed using the log-rank test. Cox regression analyses were carried out on PFS and OS to calculate crude and adjusted hazard ratios (HRs) and 95% confidence intervals for the different study groups. A model was created for evaluating the prognostic role of different variables. The proportional hazard assumption was assessed with the Schoenfeld residuals which gave no reason to suspect violation of this assumption.
RESULTS
Clinical Characteristics
Ninety-four grade II and III meningiomas were retrospectively collected: 50/94 (53.2%) were female, median age at diagnosis was 61 (range: 25–83). Median follow up time was 3.9 years. Tumor site was supratentorial in 77/94 (81.9%), posterior fossa in 8/94 (8.5%), and cranial base in 7/94 (7.5%). Data were unavailable for 2/94 (2.1%) patients. Forty-seven (50%) patients received radiotherapy after surgical resection. Gender, age and tumor site were not significantly correlated with tumor grade (Table 1). Twenty-nine patients (30.9%) developed tumor recurrence and 70/94 (74.5%) patients were alive at the end of follow up. No association was found between recurrence and gender (p = 0.523), age (p = 0.294), tumor site (p = 0.272), and previous radiotherapy (p = 0.925).
TABLE 1.
Clinicopathological Characteristics of the Higher Grade Meningiomas and Tumor Grade/Tumor Recurrence
| Variable | All Cases | WHO Grade II | WHO Grade III | p | No Recurrence | Recurrent Cases | p | |
|---|---|---|---|---|---|---|---|---|
| Gender | Female | 50 | 44 | 6 | 0.394 | 36 | 14 | 0.523 |
| Male | 44 | 41 | 3 | 29 | 15 | |||
| Median age at diagnosis (range) | 61 (25–83) | 61 (25–83) | 53 (25–80) | 0.946 | 62(25–83) | 58 (25–80) | 0.294 | |
| Tumor site | Supratentorial | 77 | 70 | 7 | 0.202 | 51 | 26 | 0.272 |
| Posterior fossa | 8 | 7 | 1 | 6 | 2 | |||
| Cranial base | 7 | 7 | 0 | 7 | 0 | |||
| Data unavailable | 2 | 1 | 1 | 1 | 1 | |||
| Death | No | 70 | 65 | 5 | 0.226 | 56 | 14 | <0.001 |
| Yes | 24 | 20 | 4 | 9 | 15 | |||
| Histotype | Atypical | 79 | 79 | 0 | Not applicable | 59 | 20 | 0.001 |
| Clear cell | 3 | 3 | 0 | 2 | 1 | |||
| Chordoid | 3 | 3 | 0 | 3 | 0 | |||
| Anaplastic | 9 | 0 | 9 | 1 | 8 | |||
| WHO grade | II | 85 | – | – | – | 64 | 21 | <0.001 |
| III | 9 | – | – | – | 1 | 8 | ||
| Brain invasion | Absent | 76 | 69 | 7 | 0.805 | 51 | 25 | 0.361 |
| Present | 18 | 16 | 2 | 14 | 4 | |||
| Hypercellularity | Absent | 2 | 2 | 0 | 0.642 | 2 | 0 | 0.340 |
| Present | 92 | 83 | 9 | 63 | 29 | |||
| Hypercellularity | <50% | 60 | 59 | 1 | 0.001 | 43 | 17 | 0.483 |
| ≥50% | 34 | 26 | 8 | 22 | 12 | |||
| Prominent nucleoli | Absent | 20 | 19 | 1 | 0.433 | 15 | 5 | 0.523 |
| Present | 74 | 66 | 8 | 50 | 24 | |||
| Prominent nucleoli | <50% | 79 | 75 | 4 | 0.001 | 61 | 18 | <0.001 |
| ≥50% | 15 | 10 | 5 | 4 | 11 | |||
| Small cells | Absent | 72 | 65 | 7 | 0.930 | 49 | 23 | 0.678 |
| Present | 22 | 20 | 2 | 16 | 6 | |||
| Sheeting | Absent | 66 | 66 | 0 | <0.001 | 53 | 13 | <0.001 |
| Present | 28 | 19 | 9 | 12 | 16 | |||
| Sheeting | Absent | 66 | 66 | 0 | <0.001 | 53 | 13 | <0.001 |
| <50% | 8 | 6 | 2 | 6 | 2 | |||
| ≥50% | 20 | 13 | 7 | 6 | 14 | |||
| Necrosis | Absent | 31 | 31 | 0 | 0.027 | 25 | 6 | 0.090 |
| Present | 63 | 54 | 9 | 40 | 23 | |||
| Necrosis | Absent | 31 | 31 | 0 | <0.001 | 25 | 6 | 0.055 |
| <50% | 46 | 43 | 3 | 32 | 14 | |||
| ≥50% | 18 | 11 | 6 | 8 | 9 | |||
| Median mitotic count (range) | 4 (0–25) | 4 (0–14) | 20 (6–25) | 0.001 | 4 (0–23) | 6 (2–25) | 0.018 | |
| Median Ki67% (range) | 10 (2–30) | 10 (2–25) | 15 (10–30) | 0.014 | 10 (2–25) | 12 (2–30) | 0.155 | |
| Ki67 hot spots | Absent | 44 | 41 | 3 | 0.394 | 32 | 12 | 0.481 |
| Present | 50 | 44 | 6 | 33 | 17 | |||
| Median SSTR2a positive cells (range) | 55 (0–95) | 60 (0–95) | 30 (0–95) | 0.293 | 70 (0–95) | 40 (0–95) | 0.048 | |
| SSTR2a score | 0 | 7 | 6 | 1 | 0.451 | 6 | 1 | 0.582 |
| 1+ | 15 | 12 | 3 | 10 | 5 | |||
| 2+ | 14 | 13 | 1 | 8 | 6 | |||
| 3+ | 58 | 54 | 4 | 41 | 17 | |||
Significant p values (p < 0.05) are listed in bold.
Tumor Grade and Histological Features
Eighty-five cases (90.4%) were grade II meningiomas and 9/94 (9.6%) were grade III. Among grade II tumors, 3/85 (3.5%) were clear cell and 3/85 (3.5%) were chordoid meningiomas. Histological features according to tumor grade are listed in Table 1. In particular, mitotic count ranged from 0 to 25/10 HPF (median: 4) when considering all cases, whereas it was 0–14 (median: 4) and 6–25 (median: 20) when evaluating grade II and III meningiomas, respectively. Focal hypercellularity was present in almost all cases (92/94, 97.9%).
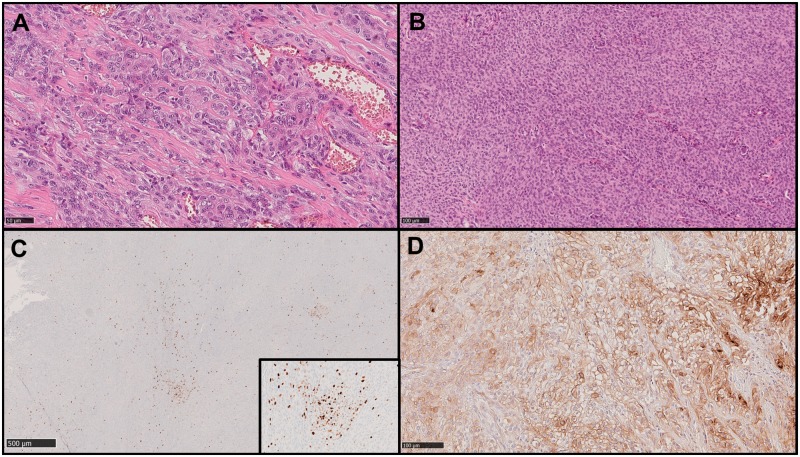
In our series, WHO tumor grade was associated with recurrence (p < 0.001), while brain invasion was not (p = 0.361; Table 1). Among the histological features, higher mitotic count (p = 0.018), diffuse (≥50%) prominent nucleoli (p < 0.001), and presence of sheeting (p < 0.001) were associated with recurrence. Necrosis was neither associated with recurrence when considered as a dichotomic variable (absent vs present) (p = 0.090) nor as a stratified variable (absent vs focal vs diffuse) (p = 0.055). Example images of the assessed morphological features are presented in Figure 1A, B.
FIGURE 1.
Example images showing some of the morphological and immunohistochemical features assessed. Hematoxylin and eosin (H&E) images showing diffuse prominent nucleoli (A) and sheeting (B). Original magnification: ×200. Immunohistochemical images showing Ki67 hot spots (C) and only partial staining of tumor cells for SSTR2a (D) in a meningioma which recurred during follow up.
Ki67 Labelling Index
Median Ki67 labeling index was significantly different between grade II and grade III meningiomas (10% vs 15%, p = 0.014), but not between nonrecurrent and recurrent cases (10% vs 12%, p = 0.155; Table 1). Presence of Ki67 hot spots (Fig. 1C) was not significantly different based on tumor grade (p = 0.396) or recurrence (p = 0.419).
SSTR2a Expression
Considering the ratio of SSTR2a-positive cells only (irrespective of the staining pattern), a significant lower median rate was observed in recurrent meningiomas (40% vs 70%, p = 0.048; Table 1), whereas no difference was observed between grade II and III meningiomas (p = 0.293; Table 1; Fig. 1D). Conversely, no specific association was found between the SSTR2a score and tumor grade (p = 0.451) or recurrence (p = 0.582).
TERT Promoter Sequencing
TERT promoter was successfully analyzed in 64/94 cases (68%). In the remaining cases (30/94, 32%), Sanger sequencing results were not conclusive or the available material was insufficient for analysis. Canonical TERT promoter mutations were detected in 5/64 (8%) cases (4 grade II and 1 grade III): The 1 295 228 C > T (C228T) and the 1 295 250 C > T (C250T) mutations, positioned respectively at 124 and 146 base pairs upstream of the ATG translational start site of TERT, were observed in 4/5 and 1/5 cases, respectively. In 23/64 (36%) noncanonical mutations were found, while the remaining 36/64 (56%) cases were wild type (Table 2). Approximately half of the cases (31/64 [48%]) cases harbored the rs2853669 single nucleotide polymorphism (SNP), while other SNPs were present in 9/64 (14%) cases (Table 2). Different SNPs were mutually exclusives. In our series, no association was identified between TERT promoter mutations and SNP or between them and clinical variables, histopathological features or outcome, except for TERT promoter mutations and patients’ death (p = 0.014 when considering canonical mutations [C228T and C250T]; p = 0.045 when considering all the observed mutations). Regarding this association, 4/5 patients with canonical TERT promoter mutations were no longer alive at the end of follow up: 2 died because of meningioma progression, while the cause of death of the remaining 2 patients was not available.
TABLE 2.
TERT Promoter Mutations and SNPs According to WHO Tumor Grade
| TERT Promoter Status | n | Grade II | Grade III | ||
|---|---|---|---|---|---|
| Mutations | Canonical mutations | –124 C>T | 4/64 | 3 | 1 |
| –146 C>T | 1/64 | 1 | None | ||
| Other mutations | –67 C>T | 1/64 | 1 | None | |
| –111 C>T | 1/64 | 1 | None | ||
| –125 C>T | 2/64 | 2 | None | ||
| –125_126 CC>TT | 1/64 | 1 | None | ||
| –126 C>T | 1/64 | 1 | None | ||
| –128 C>T | 2/64 | 2 | None | ||
| –144 C>T | 1/64 | 1 | None | ||
| –149 C>T | 1/64 | 1 | None | ||
| –150 C>T | 2/64 | 2 | None | ||
| –150 C>T –111 C>T | 1/64 | 1 | None | ||
| –150 C>T –128 C>T | 1/64 | 1 | None | ||
| –156 C>T | 2/64 | 2 | None | ||
| –159 C>T | 4/64 | 3 | 1 | ||
| –159 C>T –101 C>T | 1/64 | 1 | None | ||
| –166 C>T | 2/64 | 1 | 1 | ||
| SNP | SNP rs2853669 | 31/64 | 26 | 5 | |
| Other SNPs | 9/64 | 8 | 1 | ||
PFS Analysis
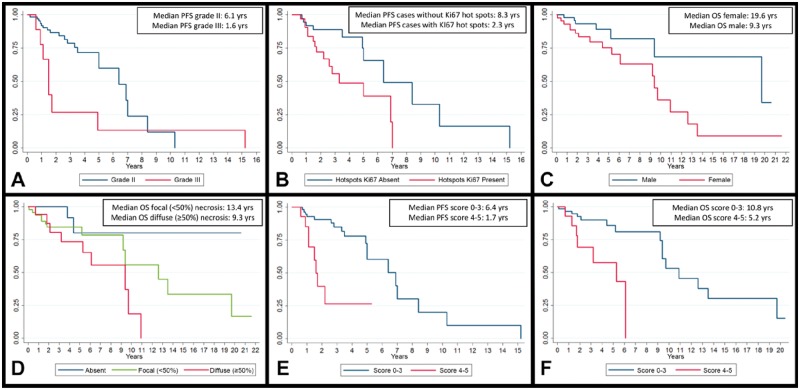
Overall, median PFS was 6.1 years (1-year PFS: 90.2%, 2-year PFS: 79.3%, and 5-year PFS: 52.6%). By univariate analysis, grade III (HR = 2.52, p = 0.044) and presence of Ki67 hot spots (HR = 2.93, p = 0.015) were associated with a poorer PFS, whereas gender, age at diagnosis and previous radiotherapy were not (Table 3). Kaplan-Meier analysis also confirmed these results: Tumor grade (1.6 vs 6.1 years, log-rank test p = 0.038; Fig. 2A) and presence of Ki67 hot spots (2.7 vs 8.3 years, log-rank test p = 0.0103; Fig. 2B) correlated with a shorter PFS. By multivariate analysis these parameters were confirmed to be independent (HR = 3.71, p = 0.009 for grade and HR = 3.35, p = 0.008 for Ki67 hot spots) when analyzed together with age at diagnosis (HR = 1.01, p = 0.488) and gender (HR = 0.94, p = 0.888).
TABLE 3.
Univariate Analysis of the Effect of the Clinicopathological Variables on PFS and OS
| Variable | PFS |
OS |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Gender (M vs F) | 1.02 | 0.48–2.19 | 0.952 | 2.78 | 1.14–6.72 | 0.024 | |
| Age at diagnosis (linear) | 0.99 | 0.98–1.03 | 0.984 | 1.08 | 1.03–1.13 | 0.001 | |
| Brain invasion (present vs absent) | 0.29 | 0.09–0.99 | 0.049 | 0.68 | 0.23–2.00 | 0.487 | |
| WHO grade (grade III vs II) | 2.52 | 1.02–6.22 | 0.044 | 1.25 | 0.42–3.74 | 0.679 | |
| Hypercellularity (≥50% vs <50%) | 1.32 | 0.60–2.90 | 0.493 | 1.08 | 0.47–2.48 | 0.851 | |
| Prominent nucleoli (present vs absent) | 0.79 | 0.29–2.13 | 0.644 | 2.38 | 0.55–10.21 | 0.243 | |
| Prominent nucleoli (≥50% vs <50%) | 1.44 | 0.63–3.31 | 0.387 | 0.76 | 0.28–2.04 | 0.587 | |
| Small cells (present vs absent) | 0.66 | 0.26–1.67 | 0.386 | 1.23 | 0.50–3.01 | 0.645 | |
| Sheeting (present vs absent) | 1.95 | 0.89–4.25 | 0.092 | 0.83 | 0.35–1.95 | 0.671 | |
| Sheeting | Absent | 1 | 1 | ||||
| <50% | 1.61 | 0.36–7.26 | 0.543 | 0.67 | 0.09–5.21 | 0.709 | |
| ≥50% | 2.03 | 0.89–4.61 | 0.088 | 0.86 | 0.35–2.12 | 0.745 | |
| Necrosis (present vs absent) | 1.74 | 0.69–4.35 | 0.233 | 4.55 | 1.06–19.40 | 0.041 | |
| Necrosis | Absent | 1 | 1 | ||||
| <50% | 1.40 | 0.52–3.76 | 0.499 | 3.63 | 0.82–16.17 | 0.090 | |
| ≥50% | 2.59 | 0.91–7.41 | 0.074 | 7.38 | 1.55–34.98 | 0.020 | |
| Mitotic count (linear) | 1.06 | 0.99–1.12 | 0.061 | 1.02 | 0.95–1.10 | 0.612 | |
| Ki67 (linear) | 1.05 | 0.98–1.13 | 0.122 | 1.05 | 0.98–1.12 | 0.142 | |
| Ki67 hot spots (present vs absent) | 2.93 | 1.23–6.93 | 0.015 | 1.46 | 0.62–3.42 | 0.376 | |
| SSTR2a positive cells (linear) | 0.99 | 0.65–1.52 | 0.971 | 1.00 | 0.67–1.51 | 0.968 | |
| SSTR2a score | 0 | 1 | 1 | ||||
| 1+ | 1.02 | 0.11–9.48 | 0.984 | 0.34 | 0.05–2.51 | 0.389 | |
| 2+ | 1.31 | 0.15–11.07 | 0.807 | 1.92 | 0.39–9.42 | 0.733 | |
| 3+ | 1.05 | 0.14–8.05 | 0.960 | 0.71 | 0.16–3.21 | 0.657 | |
| TERT promoter status (mutated vs wildtype) | 1.10 | 0.45–2.67 | 0.831 | 1.87 | 0.74–4.73 | 0.184 | |
| TERT promoter status | Wildtype | 1 | 1 | ||||
| Canonical mutations | 1.29 | 0.35–4.76 | 0.704 | 1.69 | 0.50–5.67 | 0.394 | |
| Other mutations | 1.03 | 0.38–2.77 | 0.951 | 2.02 | 0.69–5.95 | 0.201 | |
| TERT promoter SNP rs2853669 (present vs absent) | 1.71 | 0.68–4.26 | 0.251 | 0.81 | 0.32–1.99 | 0.647 | |
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression free survival.
Significant p values (p < 0.05) are listed in bold.
FIGURE 2.
Kaplan-Meier analyses curves of outcome-associated variables. (A) WHO grade and PFS (log-rank test p = 0.038). (B) Ki67 hot spots and PFS (log-rank test p = 0.0103). (C) Gender and OS (log-rank test p = 0.018). (D) Necrosis and OS (log-rank test p = 0.016). (E) Integrated score and PFS (log-rank test p < 0.001). (F) Integrated score and OS (log-rank test p = 0.001).
OS Analysis
Overall median OS was 9.7 years (1-year OS: 95.7%; 2-year OS: 88.7%; and 5-year OS: 82.5%). By univariate analysis, gender (male) (HR = 2.78, p = 0.024) and age at diagnosis (HR = 1.08, p = 0.001) correlated with a shorter OS, as expected (Table 3). Kaplan-Meier analysis confirmed this finding: Male gender showed a poorer outcome (9.3 vs 19.6 years, log-rank test p = 0.018; Fig. 2C). Among the histopathological features, necrosis was a negative prognostic variable (HR = 4.55, p = 0.041), particularly if diffuse (HR = 7.38, p = 0.020). Kaplan-Meier analysis showed similar findings: Median OS was lower in presence of necrosis (9.3 vs 13.4 years vs median not reached for diffuse, focal and absent necrosis, respectively; log-rank test p = 0.016; Fig. 2D). At multivariate analysis, only the association between age and OS was confirmed (HR = 1.07, p = 0.001), whereas patients’ gender (HR = 2.14, p = 0.111) and focal (HR = 3.09, p = 0.149) and diffuse necrosis (HR = 4.13, p = 0.091) were not significantly correlated with OS.
Integrated Prognostic Score
On the basis of the results previously observed, we designed a possible prognostic score aimed at identifying patients with shorter PFS and OS. This score is based on the following: (1) presence of diffuse (≥50%) prominent nucleoli (0 vs 1 point), (2) diffuse (≥50%) sheeting (0 vs 1 point), (3) focal (<50%) or diffuse (≥50%) necrosis (0 vs 1 vs 2 points), and (4) presence of Ki67 hot spots (0 vs 1 point); total score range: 0–5. Employing a cut-off value of ≥4 points, Kaplan-Meier analysis showed significant differences in median PFS (1.7 vs 6.4 years, log-rank test p < 0.001; Fig. 2E) and median OS (5.2 vs 10.8 years, log-rank test p = 0.001; Fig. 2F). By univariate analysis, this score was also significantly associated with both shorter PFS (HR = 5.44, p < 0.001) and OS (HR = 4.03, p = 0.009). Finally, multivariate analysis including age at diagnosis, gender and our score showed a significant independent association with PFS (HR = 5.98, p < 0.001) and OS (HR = 2.99, p = 0.048; Table 4).
TABLE 4.
Multivariate Analysis of the Prognostic Role of the Proposed Integrated Score in Terms of PFS and OS
| Variables | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Gender (M vs F) | 0.83 | 0.37–1.87 | 0.662 | 2.83 | 1.05–7.62 | 0.039 |
| Age at diagnosis | 0.99 | 0.96–1.02 | 0.541 | 1.08 | 1.03–1.13 | 0.002 |
| Integrated score (total score ≥4) | 5.98 | 2.24–15.97 | <0.001 | 2.99 | 1.01–8.86 | 0.048 |
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Significant p values (p < 0.05) are listed in bold.
To validate the integrated prognostic score, a series of 58 grade II meningiomas was collected at the Neuropathology Unit of the Fondazione IRCCS Istituto Neurologico “C. Besta” (Milan, Italy). It was decided to collect only grade II meningiomas since they represent the real grey zone in which a tool to stratify patients according to their outcome could be most useful for tailoring adjuvant treatments. Series characteristics are reported in Supplementary Data Table S1. Median follow up was 7.2 years. Multivariate analysis confirmed the prognostic capability of the integrated prognostic score both in terms of PFS (HR = 7.22, p = 0.002) and OS (HR = 9.69, p = 0.003; Table 5).
TABLE 5.
Multivariate Analyses of the Prognostic Role of the Proposed Integrated Prognostic Score in the External Validation Series
| PFS |
OS |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Gender (M vs F) | 1.05 | 0.41–2.70 | 0.917 | 0.93 | 0.25–3.37 | 0.913 |
| Age at diagnosis | 0.99 | 0.95–1.03 | 0.775 | 1.05 | 0.98–1.12 | 0.167 |
| Integrated score (total score ≥4) | 7.22 | 2.12–24.6 | 0.002 | 9.69 | 2.21–42.5 | 0.003 |
CI, confidence interval; HR, hazard ratio; OS, overall survival.
Significant p values (p < 0.05) are listed in bold.
DISCUSSION
The present study identifies a set of variables significantly associated with tumor recurrence and patients’ outcome in a series of higher-grade (II and III) meningiomas. Moreover, based on the results of this analysis, a possible prognostic score predictive of both PFS and OS is proposed and validated in an independent series. These results could prove useful for the management of these tumors in addition to WHO grade.
According to the latest WHO classification, grade II and III meningiomas represent 20%–25% and 1%–6% of all meningiomas (2), respectively, with a significant increment compared with the previous classifications (4.7%–7.2% for grade II and 1%–2.8% for grade III meningiomas) (27). It is difficult to ascertain the exact reasons behind this rise, but changes in the diagnostic criteria probably played a significant role (28). Whatever the reason, these data mean that the diagnosis of a higher-grade meningioma is a frequent occurrence in the clinical practice.
Higher-grade meningiomas, however, represent a peculiar grey zone both in terms of diagnosis and therapeutic management: The diagnostic criteria provided by the WHO classification are based, at least in part, upon a subjective evaluation and the clinical outcome can vary significantly among patients (2). In fact, reliability and reproducibility of many of the diagnostic criteria have been extensively questioned over time (29, 30) and cut-off values or clear definitions are still lacking, possibly favoring diagnostic inconsistencies between different institutions. In our study, trying to overcome this limitation, morphological traits were evaluated according to predetermined qualitative/quantitative criteria.
In terms of patient follow up and treatment, current guidelines suggest adjuvant radiotherapy for partially resected grade II and all grade III meningiomas (7), but the decision is not always easy, such as in completely resected grade II meningiomas with higher Ki67 labeling index. A better understanding of prognostic factors in this group of tumors is therefore warranted.
Our results show that only some of the routinely assessed morphological features are associated with tumor recurrence in higher-grade meningiomas: Higher mitotic count, diffuse prominent nucleoli and sheeting. In our series, no association was found between brain invasion and tumor recurrence. This parameter was added as a sufficient criterion for the diagnosis of atypical meningioma in the latest WHO classification based on its relationship with tumor recurrence (31), but conflicting evidence about its significance exists (32); differences in meningioma resection technique and sampling could explain, at least in part, our results as well as the discrepancies reported in literature (33–36). Accordingly, extensive sampling could be recommended in future prospective studies to reduce this possible source of variability.
Reduced expression of somatostatin receptors in higher-grade meningiomas has been reported in literature (16). A lower median rate of SSTR2a-positive cells was associated with tumor recurrence (40% vs 70%, p = 0.048) in our series, but we did not find significant associations between SSTR2a expression and other outcome measures.
TERT promoter status is reported to be mutated in a minority of meningiomas. As initially showed by Koelsche et al, this alteration is usually present in higher-grade meningiomas (20). Subsequent studies have demonstrated a relationship between this alteration and both malignant histological progression at recurrence (21) and a shorter time to progression (22). Our analysis found an association with patient death (p = 0.014 when considering canonical mutations only); recent data, specifically concerning nonbenign meningiomas, also support these negative prognostic correlations (23). Considering the overall limited number of mutated cases even in atypical and anaplastic meningiomas, evaluation of TERT promoter status does not seem to be warranted in routine diagnostics, but it can be taken into consideration in selected cases. Moreover, we also wanted to check for any possible effect of the TERT rs2853669 SNP considering its high frequency in the general population (∼50%), but no relationship with outcome was observed.
In terms of survival analysis, presence of Ki67 hot spots and necrosis resulted associated with PFS and OS, respectively. In our experience, presence of brisk Ki67 hot spots is a relatively common finding, even in meningiomas with low average Ki67 indices. The prognostic role of Ki67 labeling index in hot spots has been taken into consideration for many other tumors (37), but it is difficult to decide whether to integrate the hot spot index in the overall quantification or not (38). To overcome this problem, we decided to assess the prognostic significance of Ki67 hot spots presence irrespective of the specific labeling index values. In our series, this feature resulted significantly associated with a shorter PFS at univariate (HR = 2.93) and Kaplan-Meier analyses (2.7 vs 8.3 years, log-rank test p = 0.0103); even more interestingly, this association was confirmed at multivariate analysis (HR = 3.35) and resulted independent of WHO grade. In the future, the role of Ki67 hot spots could also be explored in benign (grade I) meningiomas to verify if this variable could help distinguish a subgroup with worse outcome. Regarding necrosis, its extent is a well-known prognosticator in many tumors (39, 40): Thus, the observed association with a poorer OS is consistent with previous data.
In our case series, the variables associated with tumor recurrence differ from those associated with PFS/OS, likely due to sample size and to the overall limited strength of the associations. To overcome this limit, we reconsidered the factors associated with tumor recurrence (prominent nucleoli and sheeting), PFS (Ki67 hot spots) and OS (necrosis) in an integrated score. On the basis of previous results and to reduce subjectivity in evaluation, prominent nucleoli and sheeting were only considered if diffuse (≥50% vs absent/<50%). A score value ≥4 was associated with both poorer PFS and OS at univariate (PFS: HR = 5.44 and OS: HR = 4.03) and Kaplan-Meier (PFS: 1.7 vs 6.4 years and OS: 5.2 vs 10.8 years) analyses. These associations were also confirmed by multivariate analysis (PFS: HR = 5.98, p < 0.001 and OS: HR = 2.99, p = 0.048) and by validation in an external series (PFS: HR = 7.22, p = 0.002 and OS: HR = 9.69, p = 0.003), thus this tool could provide valuable information, in addition to WHO grade. In particular, it could be useful for the management of grade II meningiomas or to “screen” cases in which additional testing, like DNA methylation-based classification, could be most useful. Further studies could specifically explore the reproducibility of this approach and possibly optimize the score parameters.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Francesca Veneziano and Chiara Musuraca for their excellent technical assistance.
Footnotes
The authors have no duality or conflicts of interest to declare.
Funding was received by Italian Ministry for Education, University and Research (Ministero dell’Istruzione, dell’Università e della Ricerca—MIUR): “Fondi di Ricerca Locale ex-60% 2016–2017” to C.B. and funding specifically dedicated to the Department of Medical Sciences, University of Turin under the programme “Dipartimenti di Eccellenza 2018 – 2022”, Project n° D15D18000410001 and by the Dipartimento Rete Oncologica Piemonte e Valle d’Aosta to P.C.
REFERENCES
- 1. Ostrom QT, Gittleman H, Liao P et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 2017;19:v1–v88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Ohgaki H, Wiestler OD et al. WHO Classification of Tumours of the Central Nervous System. Revised 4th edition Lyon: International Agency for Research on Cancer; 2016 [Google Scholar]
- 3. Perry A, Scheithauer BW, Stafford SL et al. “Malignancy” in meningiomas: A clinicopathologic study of 116 patients, with grading implications. Cancer 1999;85:2046–56 [DOI] [PubMed] [Google Scholar]
- 4. Sughrue ME, Sanai N, Shangari G et al. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J Neurosurg 2010;113:202–9 [DOI] [PubMed] [Google Scholar]
- 5. Olar A, Wani KM, Sulman EP et al. Mitotic index is an independent predictor of recurrence-free survival in meningioma. Brain Pathol 2015;25:266–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang YC, Chuang CC, Wei KC et al. Long term surgical outcome and prognostic factors of atypical and malignant meningiomas. Sci Rep 2016;6:35743 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN Clinical Practice Guidelines in Oncology – Central Nervous Systems Cancers (Version I.2017). 18/08/2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed June 28, 2018
- 8. Chamberlain MC, Glantz MJ, Fadul CE. Recurrent meningioma: Salvage therapy with long-acting somatostatin analogue. Neurology 2007;69:969–73 [DOI] [PubMed] [Google Scholar]
- 9. Johnson DR, Kimmel DW, Burch PA et al. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol 2011;13:530–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simo M, Argyriou AA, Macia M et al. Recurrent high-grade meningioma: A phase II trial with somatostatin analogue therapy. Cancer Chemother Pharmacol 2014;73:919–23 [DOI] [PubMed] [Google Scholar]
- 11. Mazza E, Brandes A, Zanon S et al. Hydroxyurea with or without imatinib in the treatment of recurrent or progressive meningiomas: A randomized phase II trial by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother Pharmacol 2016;77:115–20 [DOI] [PubMed] [Google Scholar]
- 12. Gupta S, Bi WL, Dunn IF. Medical management of meningioma in the era of precision medicine. Neurosurg Focus 2018;44:E3 . [DOI] [PubMed] [Google Scholar]
- 13. Duregon E, Cassenti A, Pittaro A et al. Better see to better agree: Phosphohistone H3 increases interobserver agreement in mitotic count for meningioma grading and imposes new specific thresholds. Neuro Oncol 2015;17:663–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roser F, Nakamura M, Bellinzona M et al. The prognostic value of progesterone receptor status in meningiomas. J Clin Pathol 2004;57:1033–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qian ZR, Li T, Ter-Minassian M et al. Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas 2016;45:1386–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva CB, Ongaratti BR, Trott G et al. Expression of somatostatin receptors (SSTR1-SSTR5) in meningiomas and its clinicopathological significance. Int J Clin Exp Pathol 2015;8:13185–92 [PMC free article] [PubMed] [Google Scholar]
- 17. Katz LM, Hielscher T, Liechty B et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol 2018;135:955–63 [DOI] [PubMed] [Google Scholar]
- 18. Sahm F, Schrimpf D, Stichel D et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol 2017;18:682–94 [DOI] [PubMed] [Google Scholar]
- 19. Olar A, Wani KM, Wilson CD et al. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol 2017;133:431–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koelsche C, Sahm F, Capper D et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol 2013;126:907–15 [DOI] [PubMed] [Google Scholar]
- 21. Goutagny S, Nault JC, Mallet M et al. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol 2014;24:184–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sahm F, Schrimpf D, Olar A et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst 2016;108:djv377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biczok A, Kraus T, Suchorska B et al. TERT promoter mutation is associated with worse prognosis in WHO grade II and III meningiomas. J Neurooncol 2018;139:671–8 [DOI] [PubMed] [Google Scholar]
- 24. Volante M, Brizzi MP, Faggiano A et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: A proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 2007;20:1172–82 [DOI] [PubMed] [Google Scholar]
- 25. Mariani S, Di Bello C, Bonello L et al. Flexible lab-tailored cut-offs for suitability of formalin-fixed tumor samples for diagnostic mutational analyses. PLOS ONE 2015;10:e0121815 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horn S, Figl A, Rachakonda PS et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013;339:959–61 [DOI] [PubMed] [Google Scholar]
- 27. Louis DN, Ohgaki H, Wiestler OD et al. WHO Classification of Tumours of the Central Nervous System. 4th edition Lyon: International Agency for Research on Cancer; 2007 [Google Scholar]
- 28. Kshettry VR, Ostrom QT, Kruchko C et al. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol 2015;17:1166–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nowosielski M, Galldiks N, Iglseder S et al. Diagnostic challenges in meningioma. Neuro Oncol 2017;19:1588–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willis J, Smith C, Ironside JW et al. The accuracy of meningioma grading: A 10-year retrospective audit. Neuropathol Appl Neurobiol 2005;31:141–9 [DOI] [PubMed] [Google Scholar]
- 31. Perry A, Stafford SL, Scheithauer BW et al. Meningioma grading: An analysis of histologic parameters. Am J Surg Pathol 1997;21:1455–65 [DOI] [PubMed] [Google Scholar]
- 32. Baumgarten P, Gessler F, Schittenhelm J et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol 2016;132:479–81 [DOI] [PubMed] [Google Scholar]
- 33. Pizem J, Velnar T, Prestor B et al. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin Neuropathol 2014;33:354–63 [DOI] [PubMed] [Google Scholar]
- 34. Spille DC, Heß K, Sauerland C et al. Brain invasion in meningiomas: Incidence and correlations with clinical variables and prognosis. World Neurosurg 2016;93:346–54 [DOI] [PubMed] [Google Scholar]
- 35. Brokinkel B, Stummer W. Brain invasion in meningiomas: The rising importance of a uniform neuropathologic assessment after the release of the 2016 World Health Organization Classification of Central Nervous System Tumors. World Neurosurg 2016;95:614–5 [DOI] [PubMed] [Google Scholar]
- 36. Brokinkel B, Hess K, Mawrin C. Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: A systematic review. Neuro Oncol 2017;19:1298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jang MH, Kim HJ, Chung YR et al. A comparison of Ki-67 counting methods in luminal Breast Cancer: The Average Method vs. the Hot Spot Method. PLOS ONE 2017;12:e0172031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dowsett M, Nielsen TO, A’Hern R et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011;103:1656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trojani M, Contesso G, Coindre JM et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984;33:37–42 [DOI] [PubMed] [Google Scholar]
- 40. Costa J, Wesley RA, Glatstein E et al. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer 1984;53:530–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.