Abstract
Objective:
Increasing age has been associated with higher risk of chemotherapy-related toxicities, often resulting in treatment disruptions or discontinuations. Age has also been evaluated as a potential risk factor for chemotherapy-induced peripheral neuropathy (CIPN), but current understanding of recovery from CIPN in older adults after treatment is limited. The goal of the current study was to: 1) evaluate longitudinal change in patient-reported CIPN symptoms from the start of chemotherapy to one year post-chemotherapy; and 2) examine treatment modifications in older (≥65 years) and younger patients (<65 years).
Methods:
As part of a larger ongoing study, gynecologic cancer patients (n= 90) treated with cytoxic chemotherapy reported their CIPN symptoms via the EORTC-CIPN20 three times during active treatment and at 6 and 12 months post-treatment. Medical record reviews were conducted to abstract clinical information during active treatment.
Results:
Piecewise mixed models revealed that older and younger patients reported similar increases in CIPN during the active treatment phase. However, older patients did not recover from CIPN after treatment completion, whereas younger patients exhibited significant declines in CIPN symptoms post-treatment. No age differences were observed in the presence of provider-recorded sensory neuropathy and pain; neuropathy-related treatment delays, changes in chemotherapy dose, regimen, or discontinuations; or falls (all p-values>0.05).
Conclusions:
Results from the current study indicate that older adults are at higher risk for chronic CIPN. Older survivors may require additional education and treatment for chronic CIPN symptoms. Additional studies are needed to explore novel interventions to manage chronic CIPN in older cancer survivors.
Keywords: chemotherapy-induced peripheral neuropathy, age-related differences
INTRODUCTION
More than half of new cancer cases are diagnosed in adults over the age of 65.[1] Moreover, cancer incidence among older adults is projected to increase by 67% by 2030.[2] Older cancer patients are at high risk for chemotherapy-related toxicities, with over 50% of patients over the age of 65 developing severe, disabling, or fatal treatment-related toxicities.[3–6] For example, older cancer patients treated with chemotherapy are significantly more likely to experience life-threatening toxicity (i.e., grade 4), to report treatment disruptions (e.g., treatment discontinuations due to such toxicity), and to die from treatment-related complications when compared to their younger counterparts.[7, 8] As a result, even low-grade chemotherapy toxicities (i.e., ≤ grade 2) are considered clinically significant in older adults and can be sufficient cause for modifying or discontinuing chemotherapy.[9]
One common chemotherapy-related toxicity in adult patients regardless of age is peripheral neuropathy. Chemotherapy-induced peripheral neuropathy (CIPN) is characterized by a variety of sensory and motor symptoms such as numbness, tingling, reduced sense of touch, reduced proprioception, pain, weakness, balance disturbances, and deficits in motor skills.[10] From a patient perspective, CIPN is one of the least expected and most distressing side effects of chemotherapy.[11] Symptoms typically increase in severity during treatment, but at least partially remit after treatment is completed.[12] However, a subset of patients experience chronic CIPN symptoms that continue past treatment and into survivorship.[13, 14] Chronic CIPN is particularly common following treatment with cytoxic chemotherapy agents.[15] At 6 months post-treatment, up to 30% of patients treated with chemotherapy report chronic CIPN symptoms.[16] Chronic CIPN has a negative impact on patients’ quality of life, even years after treatment completion.[17] CIPN may also result in gait instability, an area of particular concern for older adults who are at higher risk of falls and subsequent injury.[10, 18]
Identification of patients at high risk for chronic CIPN is important in order to educate patients, manage expectations, and enact interventions. Age has been evaluated as a potential risk factor for CIPN, but methodological limitations constrain current understanding of recovery from CIPN in older adults. No known studies have longitudinally examined the progression of patient-reported CIPN symptomatology in older adults from before chemotherapy to the survivorship period. Among studies examining CIPN during active treatment or shortly thereafter (i.e., within 3 months), older patients typically report similar CIPN severity compared to younger patients.[19–22] Literature evaluating CIPN symptomatology after treatment in older adults is sparse. In one study with ovarian cancer survivors, older age was associated with higher incidence of patient-reported CIPN [23], and a second study with testicular cancer survivors found that older age was associated with greater physician-assessed CIPN severity[24]. However, these studies were cross-sectional and it is unclear when these associations emerged. Thus, further research is needed to better understand the longitudinal course of CIPN in older versus younger patients.
The goal of the current study was to evaluate longitudinal change in patient-reported CIPN in older (≥65 years) and younger patients (<65 years) from the start of chemotherapy to one year post-chemotherapy. In this study, gynecologic cancer patients reported their CIPN symptoms via questionnaire three times during active treatment and at 6 and 12 months post-treatment. We anticipated that older and younger adults would report similar CIPN symptom severity during active treatment, but older patients would demonstrate poor recovery from CIPN symptoms after treatment compared to their younger counterparts. We also expected that older participants would 1) report more treatment disruptions (e.g., delays, regimen changes, and discontinuations) due to CIPN, and 2) sustain more falls during the active treatment phase than their younger counterparts.
METHODS
Participant Eligibility and Recruitment
Participants were recruited as part of an ongoing, larger IRB-approved study examining side effects of cytoxic chemotherapy in patients with gynecologic cancer. To be eligible, participants had to: (1) be 18–89 years of age; (2) be diagnosed with gynecologic cancer; (3) be scheduled to undergo intravenous (IV) or intraperitoneal (IP) cytoxic chemotherapy at Moffitt Cancer Center (MCC); (4) have not received chemotherapy or radiation in the month prior to enrollment; (5) have no documented or observable psychiatric or neurological disorders that would interfere with study participation (e.g., dementia, psychosis); (6) have no reported or documented diagnosis of immune-related disease (7) not be pregnant; (8) be able to speak and read English; and (9) be able to provide informed consent. Participants were recruited between August 2013 and January 2018.
Potential participants were identified via medical chart review in consultation with their treating physician at MCC and were contacted via phone by a trained research assistant in order to determine initial eligibility and interest in the study. Eligible women who wished to participate provided informed consent during an outpatient clinic visit prior to the start of chemotherapy. A total of 127 patients consented to participate in the study. Because the EORTC-CIPN20 was added later, 90 patients provided complete demographic data and at least one measure of CIPN, and were included in analyses. Participants completed CIPN and other questionnaires at five timepoints: (1) following chemotherapy cycle 1 (post-cycle 1), (2) following chemotherapy cycle 3 (post-cycle 3), (3) following chemotherapy cycle 6 (post-cycle 6), (4) 6 months post-chemotherapy (6mo post-chemo), and (5) 12 months post-chemotherapy (12mo post-chemo). A study flow diagram is presented in Figure 1.
Figure 1:
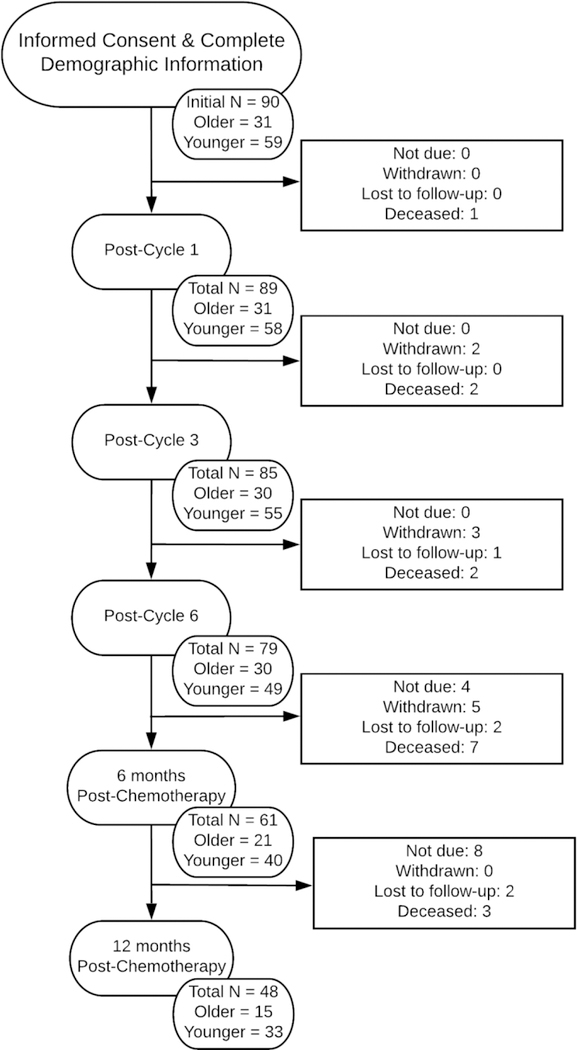
Study Flow Diagram (CONSORT)
Measures
Demographic and Clinical Data
Sociodemographic characteristics were assessed via patient self-report prior to beginning chemotherapy. These characteristics included age, race/ethnicity, marital status, education level, and income. Medical comorbidities, including presence of diabetes, were ascertained via patient report on the Charlson Comorbidity Index.[25] Clinical characteristics were obtained by a medical record review, which included cancer type, stage, previous chemotherapy, and provider-recorded CIPN symptoms. ASCO-recommended medications for CIPN,[26] including duloxetine, gabapentin, nortriptyline/SSRIs, and/or a topical gel comprised of baclofen, amitryptiline, and ketamine were also recorded if present at any time, for any reason, during treatment. Deviations from the planned regimen (e.g., treatment delays, dose and regimen changes, and discontinuations), patient-reported falls, and reasons for each were abstracted from clinical notes. Treatment modifications were considered to be possibly related to CIPN if they were 1) recorded as occurring due to CIPN symptoms, or 2) occurred in a patient with CIPN with no defined reason. Definitions of treatment modifications are as follows: Treatment delays were defined as delays in scheduled chemotherapy infusions due to CIPN symptoms. Dose changes were recorded when the dosage of chemotherapy drugs were lowered due to the presence of neuropathy. In both treatment delays and dose changes, the chemotherapy agent remained stable. In contrast, regimen changes were noted when the chemotherapy agent was changed to another drug in response to increasing neuropathy. Instances in which one agent in a combination was removed (e.g., removing taxanes from a platinum-taxane combination regimen) were also recorded as a regimen change. Finally, treatment discontinuations were recorded when chemotherapy was discontinued entirely due to CIPN symptoms, with no plans for subsequent treatment.
Chemotherapy-Induced Peripheral Neuropathy
The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Chemotherapy-Induced Peripheral Neuropathy-20 (EORTC QLQ-CIPN20) is a 20-item quality of life questionnaire developed to assess symptoms and functional limitations related to CIPN.[27] Items assess sensory, motor, and autonomic symptoms. Participants rate their symptoms over the past week on a 1 (not at all) to 4 (very much) scale. Summed scores are converted to a 0–100 scale for analysis, with higher scores indicating worse CIPN. Studies assessing the QLQ-CIPN20 indicate that the measure distinguishes between contrasting groups (e.g., those who have received neurotoxic chemotherapy vs. those who have not), has acceptable validity and reliability, and demonstrates sensitivity to change over time.[28]
Statistical Analysis
Patients were categorized into one of two age groups (<65 and ≥65 years old), consistent with previous epidemiological and clinical literature defining 65 as the cut-off age for older adults.[19] Patients’ demographic and clinical characteristics were summarized using descriptive statistics. T-tests and chi-square tests were used to examine age group differences in potential sociodemographic and clinical covariates. Sociodemographic and clinical characteristics that differed at a significance of p<0.10 were included as covariates in multivariate models. Mean CIPN scores by age group were plotted to visually inspect symptom trajectories during active treatment and post-treatment. SAS PROC MIXED was used to examine age-related longitudinal changes in CIPN from treatment initiation to one year post-chemotherapy.[29] Mixed models use all available data at every timepoint, making it an appropriate choice for longitudinal designs. Given that there are two distinct phases being assessed – on chemotherapy and post-chemotherapy – we used piecewise models[30] with post-cycle 6 as the cut-point. This analytic approach yielded information about associations between age and self-reported CIPN change during chemotherapy (at post-cycle 1, post-cycle 3, and post-cycle 6), and assessed separate relationships between age and CIPN change during the post-treatment phase (from post-cycle 6 to 6m and 12m post-chemo). Interactions were then decomposed using follow-up mixed models separately within age group. Chi-square analyses or Fisher’s exact tests were used to assess group differences in provider-reported sensory neuropathy and pain, treatment disruptions, and falls. All statistical analyses were performed using SAS 9.4 (Cary, NC).
RESULTS
Sample Characteristics
Sociodemographic and clinical characteristics of the 90 participants are shown in Table 1. The majority of the sample was non-Hispanic, white, and married. College degrees were held by 42% of the sample, and almost three-quarters of patients had a household income of at least $40,000 per year. Patients were most often diagnosed with ovarian cancer (56%), followed by endometrial cancer (22%). Approximately 70% of patients had advanced (stages III and IV) cancer. The majority of patients (96%) received intravenous (IV) chemotherapy. Of the 4 patients who received intraperitoneal (IP) treatment, one patient received three IV/IP infusions (of six total) at the conclusion of her treatment, one patient received four IV/IP infusions (of six total) prior to switching to IV-only infusions due to increasing neuropathy, one patient received two IV/IP infusions and was still on study at the time of analysis, and one patient received a single IV/IP infusion before being discharged to hospice. The majority of patients were chemotherapy-naïve (49/90, 54%) and were receiving a taxane-platinum combination regimen (59/90, 66%). Information about current chemotherapy regimen and cycle length is shown in Table 1.
Table 1:
Participant Characteristics
| Overall N = 90 | <65y old n = 59 | ≥ 65y old n = 31 | Comparison p-value | |
|---|---|---|---|---|
| Age: M(SD) | 60.1 (10.9) | 54.2 (8.0) | 71.5 (4.8) | |
| Race: N (% white)* | 84 (93) | 53 (90) | 31 (100) | 0.07 |
| Ethnicity: N (% non-Hispanic) | 87 (97) | 58 (98) | 29 (94) | 0.33 |
| Marital Status: N (% married) | 60 (67) | 41 (69) | 19 (63) | 0.83 |
| Education: N (% college grad) | 38 (42) | 27 (45) | 11 (35) | 0.35 |
| Household Income: N (% >$40k) | 52 (73) | 40 (78) | 12 (60) | 0.12 |
| Cancer Stage | 0.13 | |||
| I | 17 (20) | 14 (26) | 3 (10) | |
| II | 8 (9) | 7 (13) | 1 (3) | |
| III | 47 (55) | 27 (50) | 20 (67) | |
| IV | 13 (15) | 7 (13) | 6 (20) | |
| Chemotherapy Regimen* | <0.0001 | |||
| Platinum-Taxane | 59 (66) | 44 (75) | 15 (48) | |
| Platinum-containing | 19 (21) | 13 (22) | 6 (19) | |
| Taxane-containing | 4 (4) | 0 (0) | 4 (13) | |
| Other (non-platinum or taxane) | 8 (9) | 2 (6) | 6 (19) | |
| Chemotherapy Cycle | ||||
| 21 day | 66 | 45 (76) | 21 (68) | 0.38 |
| 28 day | 24 | 14 (24) | 10 (32) | |
| Diabetes: N (%) | 0.18 | |||
| Yes | 9 (10) | 4 (7) | 5 (16) | |
| No | 81 (90) | 55 (93) | 26 (84) | |
| Previous Chemotherapy* | 0.08 | |||
| Yes | 41 (46) | 23 (39) | 18 (58) | |
| No | 49 (54) | 36 (61) | 13 (42) |
Denotes group differences at p<0.10, which were considered for inclusion in subsequent models as covariates.
Chi-square analyses indicated that older participants were marginally more likely to be white (X2 (1) = 3.38, p=0.07), to have had prior chemotherapy (X2 (1) = 2.98, p=0.08), and were less likely to have received a platinum/taxane combination regimen (p<0.0001). Thus, race and receipt of prior chemotherapy were included in subsequent models. Because regimen type was confounded with receipt of prior chemotherapy (e.g., platinum/taxane given almost exclusively fire-line), inclusion of prior chemotherapy as a covariate also largely controlled for regimen. No other demographic or clinical characteristics were significantly different between the age groups, including ethnicity, marital status, education, household income, and presence of diabetes (all p values>0.10).
Piecewise Mixed Models
Adjusted analyses indicated that during chemotherapy, there were no age by time interactions during the active phase of treatment above and beyond the influence of prior chemotherapy, suggesting that younger and older patients have similar increases in CIPN during this time (p>0.05). CIPN trajectories varied as a result of prior chemotherapy (p=0.01) but not race (p>0.05).
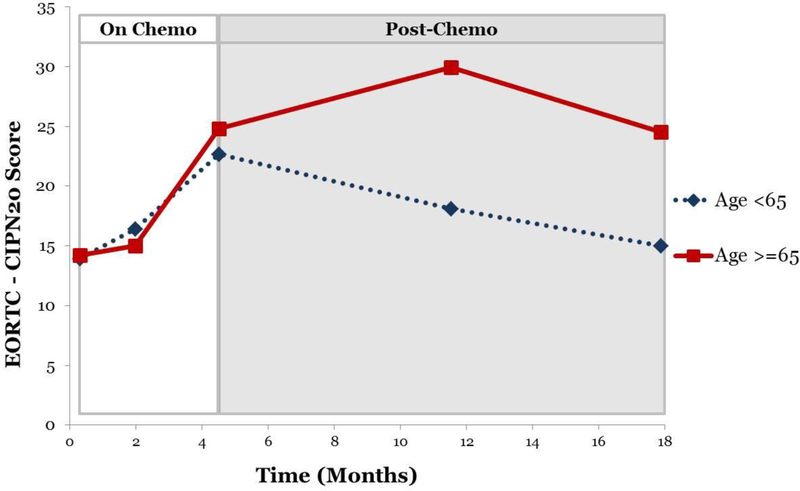
In the post-treatment assessment period, CIPN trajectories over time varied as a result of age group (p=0.01), while neither race nor prior chemotherapy significantly impacted the trajectory of CIPN symptoms after the conclusion of chemotherapy (both p values>0.05).. Post-hoc analyses indicate that older patients did not exhibit changes in their CIPN symptoms post-treatment (p>0.05), whereas younger patients exhibited significant declines in CIPN symptoms (p<0.01). Results of the piecewise mixed models analyses are shown in Table 2 and displayed in Figure 2.
Table 2:
Piecewise Mixed Model Unstandardized Parameter Estimates Demonstrating an Age Group*Time Interaction in the Post-Chemo Phase, Adjusting for Race and Prior Chemotherapy
| Effect | Estimate | p-value |
|---|---|---|
| Intercept | 21.29 | <0.01 |
| Race | −9.91 | 0.13 |
| Prior Chemotherapy | 2.73 | 0.39 |
| Age Group | 0.47 | 0.89 |
| OnChemo | 2.22 | 0.13 |
| OnChemo*Race | 0.54 | 0.73 |
| OnChemo*Prior Chemotherapy | −2.07 | 0.01 |
| OnChemo*Age Group | 0.58 | 0.49 |
| Post-Chemo | −0.46 | 0.30 |
| PostChemo*Race | 0.86 | 0.06 |
| PostChemo*Prior Chemotherapy | −0.35 | 0.10 |
| Post-Chemo*Age Group | 0.57 | 0.01 |
Figure 2:
Piecewise CIPN Scores, by Age Group
CIPN-related Treatment Modifications
Presence of provider-recorded sensory neuropathy and pain, treatment disruptions possibly due to CIPN (delays, dose changes, regimen changes, and discontinuations without subsequent chemotherapy), and falls are shown by age group in Table 3. Sensory neuropathy and pain were commonly documented in the medical records, with the majority of patients in both age groups reporting these symptoms at least once during treatment. Four patients were prescribed ASCO-recommended medication at any point in their treatment, including three patients using gabapentin (all in the younger group) and one patient using duloxetine (in the older group). Presence of medication management did not differ by age group. Treatment modifications (e.g., dose and regimen changes, delays, and discontinuations) due to CIPN were infrequent, with the most common modification being chemotherapy regimen changes. Falls were rare, with only one older patient reporting a fall during treatment. Chi-square analyses and Fisher’s exact tests revealed no statistically significant differences in the frequency of provider-reported sensory neuropathy and pain, treatment modification, or falls between the age groups (all p-values > 0.05).
Table 3:
Provider-Reported Symptomatology, Treatment Changes, and Falls
| Younger (<65y old) n = 59 | Older (≥ 65y old) n = 31 | p-value | |
|---|---|---|---|
| Sensory Neuropathy & Pain: N (%) | 44 (74.6) | 25 (80.1) | 0.52 |
| CIPN Medication Management: N (%) | 3 (5) | 1 (3) | 1.00 |
| Treatment Modifications Possibly Related to CIPN: | |||
| Delay: N (%) | 2 (3.4) | 2 (6.5) | 0.61 |
| Dose Change: N (%) | 0 (0) | 2 (6.5) | 0.12 |
| Regimen Change: N (%) | 9 (15.3) | 3 (9.7) | 0.53 |
| Treatment Discontinuation: N (%) | 2 (3.4) | 2 (6.5) | 0.61 |
| Falls: N (%) | 0 (0) | 1 (3.2) | 0.34 |
DISCUSSION
The goals of this study were to evaluate longitudinal change in patient-reported CIPN in older (≥65 years) and younger patients (<65 years) from the start of chemotherapy to one year post-chemotherapy. Results indicated that during active treatment, both older and younger patients reported similar CIPN trajectories. However, age differences were apparent in the post-treatment phase above and beyond the influence of prior chemotherapy and race, such that older adults continued to experience CIPN symptoms in the year following the completion of chemotherapy while younger patients exhibited a significant decline in CIPN symptoms. Though we anticipated that older patients may have a more difficult treatment course, resulting in more changes to planned treatment, there were no differences in the occurrence of treatment delays, dose or regimen changes, or treatment discontinuations during the active treatment phase between the age groups. Sensory neuropathy and pain were commonly reported symptoms across both age groups, while treatment changes and falls were relatively infrequent. Taken together, these data suggest that older patients are at higher risk of chronic CIPN than their younger counterparts, though their symptoms may be similar during active treatment. To our knowledge, this is the first longitudinal study to assess acute and chronic CIPN symptoms in the same cohort, allowing for a comparison of the CIPN symptom trajectory across age groups. That these age differences emerge shortly after the completion of treatment (e.g., 6 months post-chemotherapy) and continue until a year after treatment is a new finding.
The timing of age-related differences is notable, as older patients may not be aware that CIPN can continue or even worsen after the completion of treatment. Prior studies in this area were conducted in only the during- or post-treatment phase and were cross-sectional, which limited the ability to identify when age-related associations emerge. [19–23] This study adds to the literature by examining CIPN during and after treatment, identifying the post-treatment phase as an important period for age-related differences in CIPN. Thus, focused education outlining CIPN trajectories for older adults should be considered. Unfortunately, interventions to manage CIPN are limited. In this study, treatment modification decisions were made by each physician, based on the individualized needs of the patient and dependent on clinical variables such as the severity of CIPN, disease status, and preferences and goals of each patient. In terms of national guidelines for CIPN management, duloxetine is the best supported medication for use in patients with CIPN.[26] In light of the results of the current study, CIPN symptoms in patients over 65 years of age may warrant increased consideration of duloxetine in order to manage their CIPN. ASCO guidelines also tentatively support a few other medications (such as tricyclic antidepressants, gabapentin, and a compounded topical gel) based on their utility in other neuropathic pain conditions, but warn that it is unclear whether these agents are efficacious in treating CIPN specifically. Notably, medications were rarely used in this sample to manage CIPN; per medical record reviews, only four patients (4%) were using ASCO guideline-consistent medications at any time during treatment to manage their CIPN. Rather, the more common approach to CIPN management was to change treatment regimens (12/90, 13%). Older patients may also consider engaging in strength and balance training to help manage symptoms of CIPN that impact gait stability.[10] More research is needed to establish the efficacy of these interventions in older cancer survivors with CIPN.
Strengths of this study include a longitudinal design, allowing for CIPN symptom assessment through the entire active treatment phase and into a year post-treatment, and the availability of treatment records, allowing for the examination of treatment-related events such as delays, dose changes, and regimen changes. Additionally, the innovative use of piecewise analyses allowed us to examine CIPN changes in separate assessment periods (e.g., during active treatment and post-treatment), while the mixed models approach allow for the use of all available data. Limitations of the study include a limited sample size and a relatively homogenous patient group comprised of primarily white, married, well-educated patients. Additionally, there were no differences in comorbidities between the age groups, suggesting that this sample of older adult patients may have been more healthy than average. CIPN trajectories and subsequent impact on treatment changes may have been more pronounced in a more representative sample. These factors may limit the generalizability of findings.
Despite limitations, this study advances our understanding of CIPN trajectories in older cancer patients. Given the rapidly rising age of the population, combined with the increase in effective treatments and higher survival rates, CIPN is likely to become an ever-increasing burden to older cancer survivors. Therefore, additional studies are needed to explore novel interventions to manage chronic CIPN in older cancer survivors.
Highlights:
Chemotherapy-induced peripheral neuropathy is commonly reported.
On average, neuropathy significantly decreases for younger patients after treatment.
Older adults report chronic neuropathy, extending well past the end of treatment.
No differences in treatment modifications by age group were observed.
Acknowledgments
Funding sources: This study was supported by National Cancer Institute grants R01 CA164109 (PI: Jim), R25 CA090314 (PI: Brandon), and P30 CA076292 (PI: Sellers).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
HWB: No conflicts of interest to disclose; AIH: No conflicts of interest to disclose; BK: No conflicts of interest to disclose; BWJ: No conflicts of interest to disclose; BA: No conflicts of interest to disclose; SA: No conflicts of interest to disclose; HSC: No conflicts of interest to disclose; BJS: No conflicts of interest to disclose; BDG: No conflicts of interest to disclose; HSLJ: No conflicts of interest to disclose.
REFERENCES
- [1].Age and cancer risk: National Cancer Institute; 2015. Available from: https://www.cancer.gov/about-cancer/causes-prevention/risk/age. [Google Scholar]
- [2].Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the united states: Burdens upon an aging, changing nation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009. June 10;27(17):2758–65. [DOI] [PubMed] [Google Scholar]
- [3].Aparicio T, Jouve JL, Teillet L, Gargot D, Subtil F, Le Brun-Ly V, et al. Geriatric factors predict chemotherapy feasibility: Ancillary results of ffcd 2001–02 phase iii study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013. April 10;31(11):1464–70. [DOI] [PubMed] [Google Scholar]
- [4].Hurria A, Mohile S, Gajra A, Klepin H, Muss H, Chapman A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. Journal of Clinical Oncology 2016. 2016/07/10;34(20):2366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011. September 1;29(25):3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kalsi T, Babic-Illman G, Ross PJ, Maisey NR, Hughes S, Fields P, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. British Journal Of Cancer 2015. 04/14/online;112:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The cancer and leukemia group b experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2007. August 20;25(24):3699–704. [DOI] [PubMed] [Google Scholar]
- [8].Lichtman SM, Hurria A, Cirrincione CT, Seidman AD, Winer E, Hudis C, et al. Paclitaxel efficacy and toxicity in older women with metastatic breast cancer: Combined analysis of calgb 9342 and 9840. Ann Oncol 2012. March;23(3):632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kalsi T, Babic-Illman G, Fields P, Hughes S, Maisey N, Ross P, et al. The impact of low-grade toxicity in older people with cancer undergoing chemotherapy. British Journal Of Cancer 2014. 09/30/online;111:2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marshall TF, Zipp GP, Battaglia F, Moss R, Bryan S. Chemotherapy-induced-peripheral neuropathy, gait and fall risk in older adults following cancer treatment. Journal of Cancer Research and Practice 2017. 2017/12/01/;4(4):134–8. [Google Scholar]
- [11].Sasane M, Tencer T, French A, Maro T, Beusterien K. Patient-reported outcomes in chemotherapy-induced peripheral neuropathy: A review. J Support Oncol 2010;8(6):e15–e21. [Google Scholar]
- [12].Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Frontiers in Pharmacology 2017;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cavaletti G, Alberti P, Frigeni B, Piatti M, Susani E. Chemotherapy-induced neuropathy. Current treatment options in neurology 2011. April;13(2):180–90. [DOI] [PubMed] [Google Scholar]
- [14].Argyriou AAB,J; Marmiroli P; Cavaletti G Chemotherapy-induced peripheral neurotoxicity (cipn): An update. Critical reviews in oncology/hematology 2012. April;82(1):51–77. [DOI] [PubMed] [Google Scholar]
- [15].Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro-Oncology 2012;14(Suppl 4):iv45–iv54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast cancer research and treatment 2016. 08/10;159(2):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ezendam NPP,B; Bhugwandass C; Pruijt JF; Mols F; Vos MC; Pijnenborg JM; van de Poll-Franse LV Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: Results from the population-based profiles registry. Gynecologic oncology 2014. December;135(3):510–7. [DOI] [PubMed] [Google Scholar]
- [18].Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. Journal of Clinical Oncology 2017;35(23):2604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Argyriou AAP, Panagiotis; Koutras Angelos; Iconomou Gregoris; Gourzis Philippos; Assimakopoulos Konstantinos; Kalofonos Haralabos P.; Chroni Elisabeth. Is advanced age associated with increased incidence and severity of chemotherapy-induced peripheral neuropathy? Supportive Care in Cancer 2006. March 01;14(3):223–9. [DOI] [PubMed] [Google Scholar]
- [20].Kiser DW, Greer TB, Wilmoth MC, Dmochowski J, Naumann RW. Peripheral neuropathy in patients with gynecologic cancer receiving chemotherapy: Patient reports and provider assessments. Oncology nursing forum 2010. November;37(6):758–64. [DOI] [PubMed] [Google Scholar]
- [21].Griffith KA, Couture DJ, Zhu S, Pandya N, Johantgen ME, Cavaletti G, et al. Evaluation of chemotherapy-induced peripheral neuropathy using current perception threshold and clinical evaluations. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2014. December/21;22(5):1161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schneider BP, Zhao F, Wang M, Stearns V, Martino S, Jones V, et al. Neuropathy is not associated with clinical outcomes in patients receiving adjuvant taxane-containing therapy for operable breast cancer. Journal of Clinical Oncology 2012;30(25):3051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ezendam NP, Pijlman B, Bhugwandass C, Pruijt JF, Mols F, Vos MC, et al. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: Results from the population-based profiles registry. Gynecol Oncol 2014. December;135(3):510–7. [DOI] [PubMed] [Google Scholar]
- [24].Glendenning JLB Y; Norman AR; Dearnaley DP; Horwich A; Huddart RA Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer 2010. May 15;116(10):2322–31. [DOI] [PubMed] [Google Scholar]
- [25].Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology 1994;47(11):1245–51. [DOI] [PubMed] [Google Scholar]
- [26].Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol 2014. June 20;32(18):1941–67. [DOI] [PubMed] [Google Scholar]
- [27].Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an eortc quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The qlq-cipn20. European journal of cancer (Oxford, England : 1990) 2005. May;41(8):1135–9. [DOI] [PubMed] [Google Scholar]
- [28].Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: The reliability and validity of the european organization for research and treatment of cancer qlq-cipn20 questionnaire. Quality of Life Research 2013. December 01;22(10):2787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. Sas for mixed models 2nd ed. Cary, NC: SAS Press.; 2006. [Google Scholar]
- [30].McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol 2009;60:577–605. [DOI] [PubMed] [Google Scholar]