Abstract
Tissue engineering holds great promise for the advancement of cardiovascular surgery as well as other medical fields. Tissue-engineered vascular grafts have the ability to grow and remodel and could therefore make great advances for pediatric cardiovascular surgery. In 2001, we began a human clinical trial evaluating these grafts in patients with a univentricular physiology. Herein, we report the long-term results of patients who underwent implantation of tissue-engineered vascular grafts as extracardiac total cavopulmonary conduits. Tissue-engineered vascular grafts seeded with autologous bone marrow mononuclear cells were implanted in 25 patients with univentricular physiology. The graft is composed of a woven fabric of poly-l-lactide acid or polyglycolic acid and a 50:50 poly (l-lactic-co-ε-caprolactone) copolymer. Patients were followed up with postoperatively in a multidisciplinary clinic. Median patient age at operation was 5.5 years and the mean follow-up period was 11.1 years. There was no graft-related mortality during the follow-up period. There was also no evidence of aneurysmal formation, graft rupture, graft infection, or calcification. Seven (28%) patients had asymptomatic graft stenosis and underwent successful balloon angioplasty. Stenosis is the primary complication of the tissue-engineered vascular graft. Avoidance of anticoagulation therapy would improve patients’ quality of life. Tissue-engineered vascular grafts have feasibility in pediatric cardiovascular surgery.
Keywords: congenital heart disease, pediatric cardiac surgery, univentricular physiology, tissue-engineering, Fontan surgery
Graphical Abstract
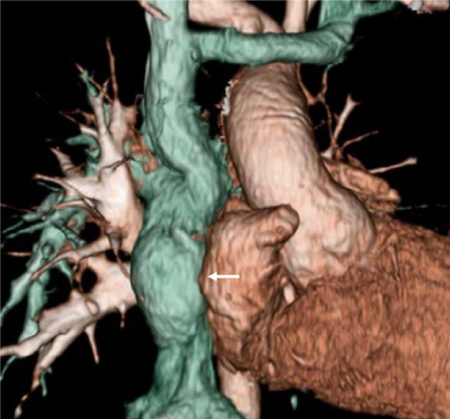
Three-dimensional computed tomography of the tissue-engineered vascular graft.
In pediatric cardiovascular surgery, many patients require surgical intervention with foreign materials to repair congenital anomalies. However, the materials commonly used lack growth potential, and cause several material-related failures, including stenosis, thromboembolism, calcification, or infection. To solve these problems, an implant with growth potential is needed. To address this challenge, we created tissue-engineered vascular grafts (TEVGs) seeded with bone-marrow mononuclear cells, which are entirely reconstituted by host-derived cells over the course of an inflammation-mediated degradation process.1 Previously, we reported the satisfactory late-term (mean follow-up 5.8 years) results of the TEVGs.2 In this study, we report the long-term clinical and radiologic examination results of patients with a mean-follow up period of 11 years.
MATERIALS AND METHODS
Biodegradable Scaffolds
Bioresorbable vascular scaffolds were constructed using a dual-cylinder chamber molding system from a woven fabric of poly-l-lactide acid (PLA) or poly (glycolic acid) (PGA) coated with a 50:50 copolymer sealant solution of poly(l-lactic-co-ε-caprolactone) (PLCL), as previously described.3 The scaffolds were 0.6–0.7 mm in thickness and 13 cm in length. On the basis of vessel size and hemodynamics, the appropriate diameter scaffold was selected. The length of the scaffold was modified by the surgical team, as necessary, to accommodate each patient’s anatomy. PLA and PGA fibers degrade in 2 years and 2–4 weeks, respectively. PLCL fibers degrade within several months.
Cell Collection and Scaffold Preparation
As described previously,3 with the patient under general anesthesia, and before median sternotomy for the definitive operation, bone marrow (5 mL/kg body weight) was aspirated from the anterosuperior iliac spine. The mononuclear cell component of the marrow (2.26 ± 1.02 × 108 cells) was collected with Histopaque-1077 (Sigma Chemical Co, St. Louis, MO) centrifugation, and these cells were seeded onto the scaffold by manual pipetting. The seeded scaffold was incubated in diluted autologous plasma for 2 hours before implantation.
Patients
In 2001, the ethics committee at Tokyo Women’s Medical University approved the implantation of TEVGs in human subjects. The following inclusion criteria were used for patient screening: elective surgery, age younger than 30 years, full understanding of the procedure by the patient or family, and minimal extracardiac disease burden. Before proceeding, informed consent was obtained from each patient or from the parent or guardian if the patient was a minor. Between September 2001 and December 2004, 25 patients underwent an extracardiac total cavopulmonary connection using a TEVG. After opening the chest, we measured the diameter of the inferior vena cava (IVC) just above the diaphragm. We determined the graft size as a little bit larger than the IVC diameter; for instance, if the IVC diameter is 14.5 mm, then we would select a graft size of 16 mm. The graft was anastomosed with running suture using 6–0 polypropylene suture. Anticoagulation therapy with warfarin sodium and aspirin was started 2 days postoperatively and continued for 3–6 months. We performed angiography before the patients entered the next stage of education. Patients were followed up postoperatively in a multidisciplinary clinic.
RESULTS
Characteristics of the Patients
Patient demographics, diagnoses, and graft type and size at the time of implantation are presented in Table 1. The median patient age was 5.5 years (range 1–24 years). The range of the graft size we used was 12–24 mm.
Table 1.
Characteristics of the Patients
| Patient | Main Diagnosis | Age | Graft Type | Graft Size |
|---|---|---|---|---|
| 1 | Asplenia, AVSD(A), small RV | 2 | PLA | 16 |
| 2 | Asplenia, SRV, TAPVC(Ib+III) | 1 | PLA | 20 |
| 3 | Concordant criss-cross heart, DORV, PAA, MS | 8 | PLA | 18 |
| 4 | TA(Ib) | 22 | PLA | 24 |
| 5 | PPA, ASD(II), sinusoidal communication | 13 | PLA | 22 |
| 6 | SRV, DORV, AVVA | 4 | PLA | 20 |
| 7 | Total sinus defect, ASD, TR(IV) | 14 | PLA | 24 |
| 8 | Asplenia, SLV, CAVVR | 17 | PLA | 24 |
| 9 | TA(Ib) | 22 | PLA | 22 |
| 10 | Polysplenia, SRV | 4 | PLA | 12 |
| 11 | HLHS, MA, IAA(A) | 2 | PLA | 16 |
| 12 | Asplenia, SRV, PAA, nonconfluent PA | 2 | PGA | 16 |
| 13 | SLV, lt AVVA | 2 | PGA | 16 |
| 14 | DORV, small LV, VSD, PS, ASD(II) | 2 | PGA | 18 |
| 15 | Polysplenia, cAVSD, DORV, PS | 2 | PGA | 12 |
| 16 | Asplenia, SRV, CA, TAPVC(Ib) | 2 | PGA | 16 |
| 17 | PPA, RA thrombosis, AFL, af | 24 | PGA | 18 |
| 18 | SRV, DIRV, PA, ASD(II) | 1 | PGA | 16 |
| 19 | Asplenia, SRV, PS, CA | 11 | PGA | 18 |
| 20 | Polysplenia, cAVSD, PS, CAVVR | 2 | PGA | 14 |
| 21 | DORV, VSD, small RV, PLSVC, TAPVC(IIb) | 3 | PGA | 16 |
| 22 | PPA, ASD(II), PS, Sinusoidal communication | 5 | PGA | 18 |
| 23 | SLV, DILV, PA, ASD, bilateral SVC | 4 | PGA | 18 |
| 24 | Asplenia, SRV | 13 | PGA | 16 |
| 25 | TA(IIc), SAS | 2 | PGA | 18 |
af, atrial fibrillation; AFL, atrial flutter; ASD, atrial septal defect; AVSD, atrioventricular septal defect; AVVA, atrioventricular valve atresia; CA, common atrium; cAVSD, complete atrioventricular septal defect; CAVVR, common atrioventricular valve regurgitation; CAVV, common atrioventricular valve; DILV, double-inlet left ventricle; DIRV, double-inlet right ventricle; DORV, double-outlet right ventricle; HLHS, hypoplastic left heart syndrome; IAA, interruption of aortic arch; LV, left ventricle; MA, mitral atresia; MS, mitral stenosis; PA, pulmonary artery; PAA, pulmonary artery atresia; PLA, polylactide acid; PLSVC, persistent left superior vena cava; PPA, pure pulmonary atresia; PS, pulmonary stenosis; RA, right atrium; RV, right ventricle; SAS, subaortic stenosis, SLV, single left ventricle; SRV, single right ventricle; SVC, superior vena cava; TA, tricuspid atresia; TAPVC, total anomalous pulmonary venous connection; TR, tricuspid regurgitation; VSD, ventricular septal defect.
Mortality
Eight patients died during the follow-up period (Table 2, Fig. 1). We reviewed the charts of these eight patients and there was no graft-related mortality.
Table 2.
Cause of Death of Eight Patients
| Patient | Cause of Death | Year After Implantation |
|---|---|---|
| 3 | PLE, DIC, MOF | 13.7 |
| 5 | Sudden death | 5.5 |
| 7 | SLE, Aspergillus pneumonia, MOF, DIC | 3.8 |
| 9 | Sudden death (arrhythmia) | 3.6 |
| 10 | Pancytopenia, DIC, MOF | 11.8 |
| 11 | CHF, LOS | 0.4 |
| 16 | Severe AVVR, LOS | 10.1 |
| 25 | Sudden death | 1.8 |
AVVR, aorto-ventricular valve regurgitation: CHF, congestive heart failure; DIC, disseminating intravascular coagulopathy; LOS, low output syndrome; MOF, multiple organ failure; PLE, protein loosing enteropathy; SLE, systemic lupus erythematosus.
Figure 1.

Autopsy findings of TEVG 13 years after implantation in patient 10 show similar structure to the native vein or pulmonary artery. (This figure is reprinted from “What is the best material for extracardiac Fontan operation?” Shinoka et al. J Thorac Cardiovasc Surg. 2017;153:1551–1552.) LPA, left pulmonary artery; RPA, right pulmonary artery; SVC, superior vena cava; TEV, tissue-engineered vessel. (Color version of figure is available online.)
Adverse Events and Reintervention
Mean follow-up was 11.1 years (range 0.5–14.9 years, median 12.8 years). During the follow-up, there were no graft-specific lethal complications, such as rupture, aneurysmal formation, or ectopic calcification with any follow-up imaging modality (Fig. 2). Seven (28%) patients had asymptomatic graft stenosis noted on routine surveillance imaging and underwent successful balloon angioplasty (Table 3, Fig. 3), including 1 patient (patient 12) who required repeat balloon angioplasty and stent placement in the stenosed segment of the TEVG (Fig. 3C, D). There was no tendency in regard to where the graft stenosis occurred. There was stenosis at either the anastomosis site or mid graft. We used high pressure balloons for percutaneous transluminal angioplasty. The graft was covered by the adhesion tissues and the right pericardium. Graft rupture never happened during the percutaneous transluminal angioplasty. One patient had thrombus formation in the TEVG 1 year after surgery, which was successfully resolved by anticoagulation therapy.
Figure 2.
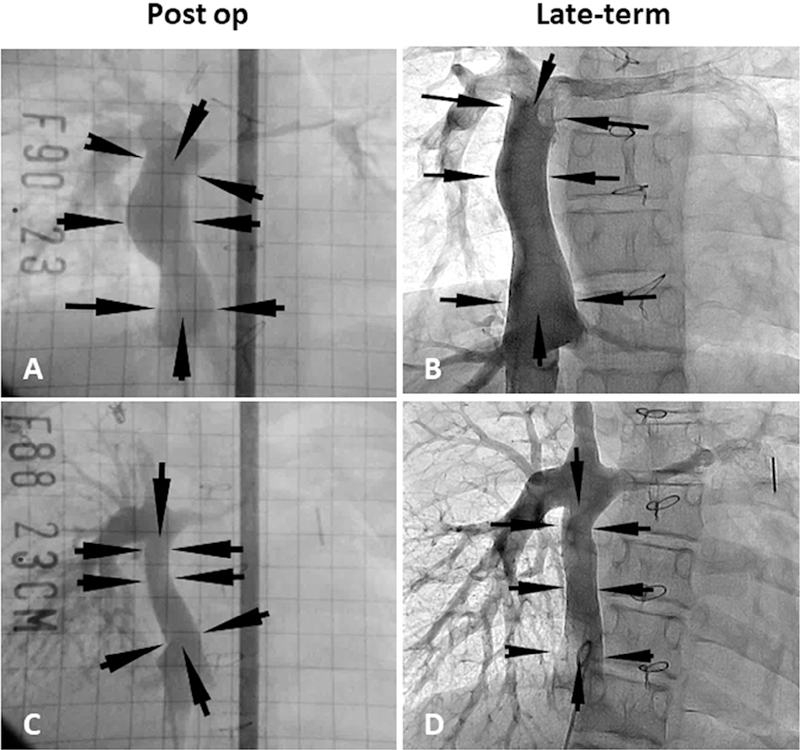
Postoperative and late-term TEVG angiography. (A) Postoperative angiography in patient 22. (B) Angiography 11 years after implantation in patient 22. (C) Postoperative angiography in patient 23. (D) Angiography 8 years after implantation in patient 23. Arrows indicate the TEVG location. Angiography shows macroscopic growth of the TEVGs.
Table 3.
Long-term Status After TEVG Implantation
| Patient | F/U Years | Status | Stenosis Requiring PTA | Postoperative Year of the PTA | Thrombus |
|---|---|---|---|---|---|
| 1 | 14.9 | Alive | No | No | |
| 2 | 14.8 | Alive | No | No | |
| 3 | 13.7 | Dead | Yes (×1) | 5.5 | No |
| 4 | 14.3 | Alive | No | No | |
| 5 | 5.5 | Dead | No | No | |
| 6 | 14.2 | Alive | Yes (×1) | 7.6 | No |
| 7 | 3.8 | Dead | No | No | |
| 8 | 14.1 | Alive | No | No | |
| 9 | 3.6 | Dead | No | No | |
| 10 | 11.8 | Dead | Yes (×3) | 5.1, 6.1, 9.9 | No |
| 11 | 0.5 | Dead | No | No | |
| 12 | 13.5 | Alive | Yes (×4, stent) | 1.5, 2.3, 4.3, 9.4 | No |
| 13 | 13.3 | Alive | Yes (×1) | 11.9 | Yes |
| 14 | 13.2 | Alive | No | No | |
| 15 | 13.2 | Alive | Yes (×1) | 13.1 | No |
| 16 | 10.1 | Dead | No | No | |
| 17 | 12.9 | Alive | No | No | |
| 18 | 12.9 | Alive | Yes (×2) | 3.8, 4.8 | No |
| 19 | 12.8 | Alive | No | No | |
| 20 | 12.8 | Alive | No | No | |
| 21 | 12.5 | Alive | No | No | |
| 22 | 12.5 | Alive | No | No | |
| 23 | 12.4 | Alive | No | No | |
| 24 | 11.9 | Alive | No | No | |
| 25 | 1.8 | Dead | No | No |
F/U, follow-up; PTA, percutaneous transluminal angioplasty.
Figure 3.
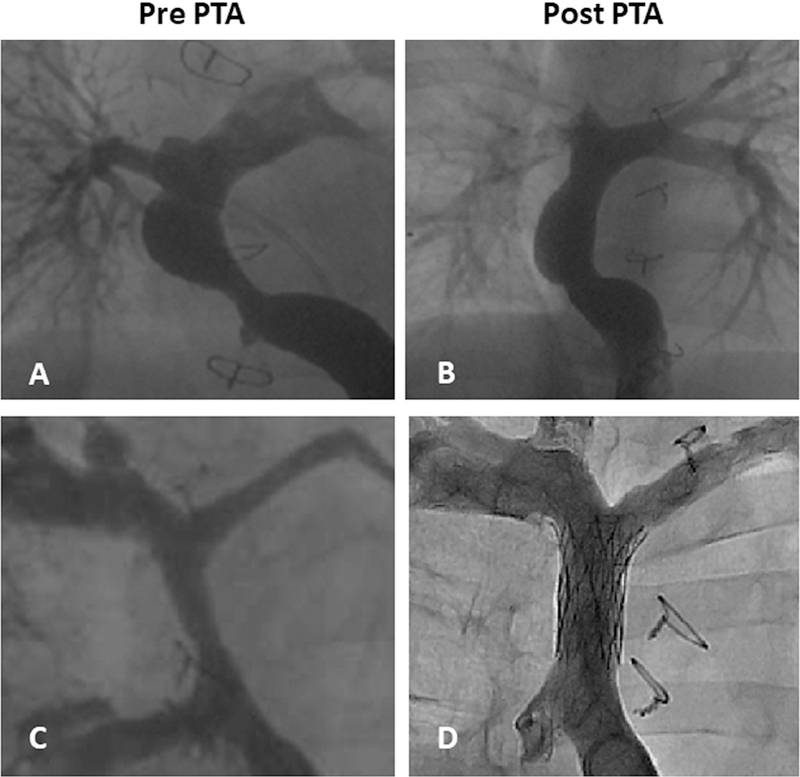
TEVG percutaneous transluminal angioplasty (PTA). (A) Pre-PTA in patient 3, (B) post-PTA in patient 3, (C) pre-PTA in patient 12, and (D) post-stent placement in patient 12.
DISCUSSION
Currently used prosthetic or bioprosthetic materials in surgery for congenital heart disease lack growth potential and often require surgical reintervention.4,5 Additionally, synthetic grafts are at risk for thrombosis or infection. To address this challenge, we applied tissue-engineered vascular grafts to the field of pediatric cardiovascular surgery.
We have previously reported the mid-term (median follow-up 16.7 months) and late-term (mean follow-up 5.8 years) results of this study.2,3 In this study, the mean follow-up is now 11.1 years, and to this point, still no graft-related deaths have occurred.
One of the merits of tissue-engineered vascular grafts is the potential for the graft to be entirely reconstituted by the host-derived cells and create autologous tissue, which eliminates the need for antiplatelet or anticoagulant therapy. In this study, antiplatelet and anticoagulation agents were discontinued 6 months postoperatively in all patients. Only 1 patient (4%) had graft thrombosis, which was successfully resolved by anticoagulation therapy. In contrast, in studies investigating artificial graft materials such as Dacron or polytetrafluoroethylene, most subjects received long-term oral anticoagulation with warfarin or aspirin.6,7
In this study, 7 (28%) patients had graft stenosis requiring balloon angioplasty. The indications for the angioplasty are (1) more than 2 mm Hg of the pressure gradient at the graft, or (2) more than 1 mm Hg of the pressure gradient at the graft in case there are collateral vessels. This stenosis rate is greater than in those studies investigating artificial graft materials.6,8 However, in this study, all patients who had graft stenosis were asymptomatic. Graft stenosis is a major adverse event for tissue-engineered vascular graft, but it can be successfully treated by balloon angioplasty.
In our group, we have previously conducted research on the prevention of graft stenosis. TEVG patency was significantly increased in a bone marrow mononuclear cell dose-dependent manner in a mouse IVC interposition graft model.9 In the human graft, more bone marrow used for seeding will result in prevention of graft stenosis. In addition, using mouse models, we have previously shown that stenosis is driven by TEVG infiltration by host monocytes,10,11 and that a transforming growth factor-β receptor 1 inhibitor could prevent stenosis.12 These methods will provide a viable strategy for preventing TEVG stenosis.
Recently, Bockeria et al from Russia reported human data in their clinical experience of new bioabsorbable vascular grafts in the Fontan circulation. They demonstrated anatomical and functional stability of the bioabsorbable grafts in all 5 patients and no graft-related adverse events during the 12-month follow-up period.13
There are several limitations in this study. First, although this trial represents the first use of tissue-engineered vascular grafts in humans, the number of study subjects was relatively small, and only a single institution was involved. Second, this study was not randomized with matched controls.
CONCLUSIONS
In conclusion, the TEVG shows safe and feasible long-term results in pediatric cardiovascular surgery. Avoidance of anticoagulation therapy would improve patient’s quality of life. Therefore, a tissue-engineering approach may play an important role as an alternative to polytetrafluoroethylene grafts, especially in pediatric cardiovascular surgery.
Central Message
Tissue-engineered vascular graft shows safe and feasible long-term results in pediatric cardiovascular surgery. A tissue-engineering approach may play an important role as an alternative to polytetrafluoroethylene grafts.
Perspective Statement
Tissue-engineered vascular grafts have the ability to grow and remodel and could therefore make great advances for pediatric cardiovascular surgery. Our experience with 25 patients shows safe and feasible long-term results of tissue-engineered vascular grafts. There was no evidence of aneurysmal formation, graft rupture, graft infection, or calcification.
Acknowledgments
The authors acknowledge the editorial assistance of Avione Y. Lee, PhD, and video editing of Toshihiro Shoji.
Funding Source: This study was supported in part by grants from the National Institutes of Health (R01-HL098228 to Dr. Breuer). Dr. Sugiura was the recipient of a funding award from Kanae Foundation for the Promotion of Medical Science (Tokyo, Japan) and from Astellas Foundation for Research on Metabolic Disorders (Tokyo, Japan) in 2013.
Footnotes
Conflict of Interest Statement: Dr. Shinoka and Dr. Breuer receive grant support from Gunze Ltd. (Kyoto, Japan). The other authors report no conflicts of interest.
Meeting Presentation: This paper is to be presented at the AATS Centennial, Boston, Massachusetts, April 29 to May 3, 2017.
SUPPLEMENTARY MATERIAL
Supplementary materials associated with this article can be found in the online version at https://doi.org/10.1053/j.semtcvs.2018.02.002.
REFERENCES
- 1.Roh JD, Sawh-Martinez R, Brennan MP, et al. : Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation mediated process of vascular remodeling. Proc Natl Acad Sci USA 107:4669–4674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibino N, McGillicuddy E, Matsumura G, et al. : Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139:431–436, 436 e431–432, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Shin’oka T, Matsumura G, Hibino N, et al. : Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 129:1330–1338, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Albert JD, Bishop DA, Fullerton DA, et al. : Conduit reconstruction of the right ventricular outflow tract. Lessons learned in a twelve-year experience. J Thorac Cardiovasc Surg 106:228–235, discussion 235–226, 1993 [PubMed] [Google Scholar]
- 5.Breymann T, Thies WR, Boethig D, et al. : Bovine valved venous xenografts for RVOT reconstruction: Results after 71 implantations. Eur J Cardiothorac Surg 21:703–710, discussion 710, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Kim WH, Lim HG, et al. : Outcome of 200 patients after an extracardiac Fontan procedure. J Thorac Cardiovasc Surg 136:108–116, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Giannico S, Hammad F, Amodeo A, et al. : Clinical outcome of 193 extracardiac Fontan patients: The first 15 years. J Am Coll Cardiol 47:2065–2073, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Nakano T, Kado H, Tachibana T, et al. : Excellent midterm outcome of extracardiac conduit total cavopulmonary connection: Results of 126 cases. Ann Thorac Surg 84:1619–1625, discussion 1625–1616, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Lee YU, Mahler N, Best CA, et al. : Rational design of an improved tissue-engineered vascular graft: Determining the optimal cell dose and incubation time. Regen Med 11:159–167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibino N, Yi T, Duncan DR, et al. : A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 25:4253–4263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan DR, Chen PY, Patterson JT, et al. : TGFbetaR1 inhibition blocks the formation of stenosis in tissue-engineered vascular grafts. J Am Coll Cardiol 65:512–514, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YU, de Dios Ruiz-Rosado J, Mahler N, et al. : TGF-beta receptor 1 inhibition prevents stenosis of tissue-engineered vascular grafts by reducing host mononuclear phagocyte activation. FASEB J 30:2627–2636, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bockeria LA, Svanidze O, Kim A, et al. : Total cavopulmonary connection with a new bioabsorbable vascular graft: First clinical experience. J Thorac Cardiovasc Surg 153:1542–1550, 2017 [DOI] [PubMed] [Google Scholar]


