Abstract
Background
Kilohertz frequency alternating currents (KHFAC) produce rapid nerve conduction block of mammalian peripheral nerve and have potential clinical applications in reducing peripheral nerve hyperactivity. The experimental investigation of KHFAC nerve block requires a robust output measure and this has proven to be the block threshold (BT), the lowest current or voltage at which the axons of interest are completely blocked. All significant literature in KHFAC nerve block, both simulations and experimental, were reviewed to determine the block threshold method that was used. The two common methods used are the High-Low method experimentally and the Binary search method for simulations.
New Method
Four methods to measure the block threshold (High-Low, High-Low-High, Binary and Random) at three frequencies (10, 20 and 30 kHz) were compared through randomized repeated experiments in the in-vivo rodent sciatic nerve-gastrocnemius model.
Results
The literature review showed that more than 50 % of publications did not measure the block threshold. The experimental results showed no statistical difference in the BT value between the four methods.
Comparison with Existing Method(s)
However, there were differences in the number of significant onset responses, depending on the method. The run time for the BT determination was the shortest for the High-Low method.
Conclusions
It is recommended that all research in electrical nerve block, including KHFAC, should include measurement of the BT. The High-Low method is recommended for most experimental situations but the Binary method could also be a viable option, especially where onset responses are minimal.
Keywords: Electrical Nerve Block, Kilohertz Frequency Nerve Block, Block Threshold, Motor Block, Rodent, Sciatic Nerve
1. Introduction
Over the past two decades, there has been increasing interest in the use of kiloHertz frequency alternating currents (KHFAC) for the purpose of blocking action potentials in peripheral nerves [1]. KHFAC nerve block initiates a fast-acting axonal block which is rapidly and completely reversible. This type of block is a true localized conduction block of axons as opposed to a muscle fatigue or neuro-muscular junction depletion block [2]. KHFAC peripheral nerve block holds the potential for ameliorating disorders of peripheral motor, sensory and autonomic origin. It is being studied experimentally for spasticity reduction, pain control and autonomic applications e.g. cardiac disease and asthma. It is already clinically used in some recent medical applications. Enteromedics [St. Paul., MN] has developed an obesity control application, the “VBLOC” system, which uses a 5 kHz KHFAC waveform applied to the vagal nerve [3]. A 10 kHz KHFAC waveform is being applied for post-amputation neuroma pain relief [Neuros Medical, Inc., Willoughby, OH] with encouraging results [4]. Relief of back pain [Nevro Corp., Menlo Park, CA] has been obtained using a current-controlled 10 kHz waveform delivered to the thoracic spinal cord [5].
It is important to have a measure of the block input parameters (waveform shape, frequency, and amplitude) that determine the effectiveness of block. This is essential for comparing results of nerve block across experiments, electrodes, waveforms and nerves/axons. It is also equally important to have a robust output measure of successful nerve block. One measure that has been used has been termed the block threshold (BT) and is the minimum voltage or current amplitude of the KHFAC waveform at each selected frequency that results in complete block of the system under investigation [2]. Thus, this could be block of all the motor axons in a mixed nerve, a specific population of sensory axons in a nerve or even single axons where single fiber recording techniques are used.
The common method that has been used in many laboratories, including ours, to determine the BT is described below. We have named this method the “High-Low” method. The application of KHFAC typically induces an initial, brief onset response (less than one second in motor axons), a period of rapid neuronal firing, before conduction block is established [2]. The onset response is lower for higher amplitudes and higher frequencies of the KHFAC [2]. When the KHFAC is on for any amount of time, further increasing the amplitude produces new onset responses but decreasing the amplitude does not produce any more. This occurs because the onset is produced by a positive change in the charge delivered to the axons by the KHFAC waveform. The onset response is pronounced in motor axons. As yet, there is no dependable data on the severity of the onset response in sensory and autonomic nerves. This onset response influenced creation of the High-Low method used to measure block thresholds experimentally [2]. This method starts with test stimulation of the nerve/axon at one end and then initiation of the KHFAC (at the selected frequency/frequencies) at a high amplitude (to minimize the onset response) [2]. Once the onset response has abated the amplitude is decreased in discrete steps until the test stimulation response is no longer blocked. The lowest amplitude where the system is completely blocked is identified as the block threshold. The amplitude step size is the resolution of the block threshold measurement.
The block threshold is also widely used for computer simulation studies of KHFAC block. Here, the common technique to determine block thresholds has been to use a Binary Search method [6]. An initial high KHFAC amplitude is selected. The choice of this amplitude is dictated by the axonal model, electrode geometry, waveform shape, frequency and other input factors and has to be determined for each particular condition. The interval from zero to this amplitude is repeatedly tested at the mid-point values with the results from one run determining the interval to test in the next run. The search ends when the pre-determined resolution of the search amplitude has been reached.
We reviewed most of the significant experimental and modeling publications on KHFAC nerve block, and tabulated the information on the block threshold (Table 1). The search criteria used to identify these publications used the terms; high frequency nerve block and kilohertz frequency nerve block but was also based on following up the citations of known electrical nerve block papers. Many papers did not mention measuring the block threshold. Usually, in these papers arbitrary amplitudes were used to show that nerve block could be obtained, but no attempts were made to map the amplitude versus frequency relationship. Some papers mentioned the block threshold but did not describe the method used to determine it. The remaining papers used one of four methods: High-Low (described above), Low-High (where the KHFAC amplitude is initiated at a low level and increased in steps or ramped up until complete block is obtained), Binary Method (described above), or Random Method (where random amplitudes are chosen over a given range).
Table 1:
A summary of block threshold information from simulation and experimental publications on KHFAC nerve block. The underlined numbers indicate the highest incidences.
| Block Threshold Information in the publication |
Simulation publications Total = 14 |
Experimental publications Total = 44 |
||
|---|---|---|---|---|
| # out of 14 | References | # out of 44 | References | |
| Not measured | 2 of 14 | [11, 12] | 23 of 44 | [3, 5, 11, 13-31] |
| Mentioned but method was not described | 7 of 14 | [32-38] | 6 of 44 | [12, 39-43] |
| Method : High-Low | 1 of 14 | [44] | 11 of 44 | [2, 9, 45-53] |
| Method : Binary Search | 4 of 14 | [6, 53-55] | 0 of 44 | |
| Method : Random Search | 0 of 14 | 1 of 44 | [56] | |
| Method : Low-High | 1 of 14 | [44] | 3 of 44 | [7-9] |
The KHFAC onset is less with higher amplitudes and there is no new onset firing when transitioning from high amplitudes to low amplitudes. This is the key reason the High-Low method was originally developed since most of the earlier work in KHFAC nerve block was conducted on peripheral motor nerves and the method minimized the problem of the onset response. There is one concern about using the High-Low method, which is that the amplitudes tested decrease sequentially and are monotonic and could have a potential summative effect. It was unknown if this affected the determination of the true block threshold. The best way to investigate this would be to compare versus randomized amplitudes (Random method). However, a random method implies separate trials of the KHFAC at different amplitudes with each trial producing an initial onset response. The Binary method was added since it is used so commonly in simulations. A binary search has the same problem with separate trials producing onsets. However, a binary search is very efficient in finding the BT with the minimum number of trials. The Low-High method was added since it was used in three experimental papers [7-9]. However, testing a pure Low-High method would guarantee large onsets at every increasing step change in a motor system due to the positive injection of charge. Therefore, we tested it as an extension of the High-Low method as described below and named it the High-Low-High method.
The purpose of this research was to evaluate the four methods using randomized and repeated acute in-vivo experiments. The aim was to measure the error in the evaluation of the block threshold using these four methods and identify which method, if any, was superior. The methods chosen were the following; High-Low, Low-High, Binary and Random. The High-Low method was used as the standard method for comparison. The Low-High method was tested by using a variation of the High-Low method, which consisted of finding the BT in the High-Low method, then reducing the amplitude further down and then increasing again to measure the BT again (the High-Low-High method). This gave the block threshold for the Low-High method and was done to reduce the number of trials and to acquire the Low-High block thresholds without significantly larger onsets.
2. Materials and Methods
2.1. Surgical Procedure
Acute experiments were conducted on adult rats (Sprague-Dawley ~ 400 grams) anesthetized with inhaled Isoflurane. For each experiment an incision was made 1cm lateral to the spine, the sciatic nerve was exposed, and the superior gluteal nerve was severed. A second incision was made on the hind leg extending from the plantar surface of the foot to 1cm distal of the first incision. The sciatic nerve was exposed at its terminal branching point into the tibial, peroneal, and sural nerves, and the peroneal and sural nerves were severed. The hamstring was cut to expose the gastrocnemius-soleus muscle, which was freed from the surrounding tissue. After the Achilles tendon was cut, the tendon and gastrocnemius-soleus muscle were attached to a force transducer (Entran, Fairfield, NJ) via a customized setup. The muscle was tightened to create 1-2 N of tension and the gastrocnemius muscle was protected from drying with mineral oil. Our institutional animal care and use committee approved all animal studies.
2.2. Electrical Stimulation and Block
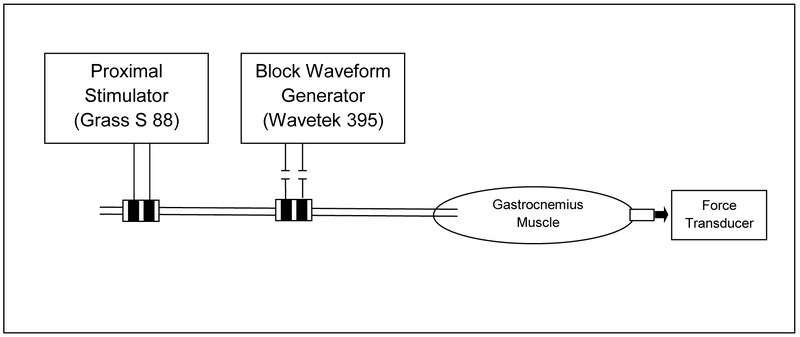
A bipolar electrode was placed on the proximal sciatic nerve and was used to stimulate the nerve and generate a response in the gastrocnemius muscle (Figure 1). A second bipolar electrode was placed on the distal sciatic nerve and was used to deliver the blocking kilohertz frequency alternating current (KHFAC) waveform. Both bipolar electrodes had platinum contacts with a width of 1mm and a height of 3 mm, 2 mm spacing between contacts, and 1mm of insulation on either side of the electrode. Including the insulation, the overall electrodes have a width of 6mm and a height of 9mm. Any tissue between the cuff and the nerve was cleared in order to optimize the position of the blocking electrode and to minimize the onset response, which was characterized in Bhadra & Kilgore 2005. A distal electrode was not used in these experiments since it has previously been proven that the KHFAC block is a localized nerve block and not a neuromuscular junction fatigue block [2].
Figure 1:
In-vivo setup for testing KHFAC nerve block thresholds.
A voltage-controlled stimulator (Grass Model S88) connected to a constant current stimulus isolation unit (Grass Model PSIU6) was used to deliver stimulating pulses through the bipolar electrode proximal to the spine. Stimulation was delivered as square pulses at 1Hz with 20 μs duration [2]. The minimum amount of current needed to elicit a muscle response (activation threshold) and the minimum amount of current needed to elicit the maximum amount of force from the muscle (saturation threshold) were mapped at the beginning of each experiment and after every set of 9 trials to monitor any change in these values. The current amplitude was set to a value 0.1-0.2 mA higher than the saturation threshold to ensure maximal activation of the gastrocnemius muscle.
A voltage-controlled waveform generator (Wavetek Model 395, Fluke, Everett, Washington) was used to deliver the KHFAC sinusoidal waveform to the distal bipolar block electrode. The three frequencies tested during the experiment were 10 kHz, 20 kHz, and 30 kHz, with amplitudes varying as described under Experimental Protocol. A 3-μF capacitor was placed in series with the output of the Wavetek generator in order to minimize direct current (DC) offsets. All noted amplitude values are the peak-to-peak voltage (V). Data were recorded using a data acquisition interface (CED Power 1401-3, CED, Cambridge, England) and Spike2 software (CED, Cambridge, England) with a sampling rate of 100 kHz. The KHFAC waveform, stimulation pulse triggers and force were recorded.
2.3. Experimental Protocol
Four different methods for determining block threshold were evaluated (Table 2). The methods are designated by names as well as numbers (for the statistical analysis). Method 1 was the High-Low method, which was similar to that used by Bhadra and Kilgore 2005. A step size of −1 V was used and the block threshold was determined to be the lowest value at which complete block was achieved and no muscle twitches were recorded. For most trials, the starting amplitude was 10V (34 of 36 trials). Rarely complete block was not achieved at that amplitude and a higher value of 16V was used as the starting amplitude (2 of 36 trials). Method 2 was the High-Low-High method, which is similar to Method 1 where the starting block was a suprathreshold value (also 10V or 16V). Block threshold was determined after decreasing KHFAC amplitude two V past block threshold, and then increasing the amplitude again in one V steps. Since a block threshold using Method 1 could be determined in the process of conducting Method 2, these two methods were combined and evaluated within the same trial. Method 3 was the Binary method used to explore a range of values within the range of 0-16V. Method 4 was the Random method. The amplitude ranges for the random search were selected for each frequency and were based on a preliminary experiment. Lower and upper amplitudes were determined for the three frequencies using non-randomized experiments to find the amplitude range which would definitely include the BT (BT amplitude range shown in Table 2, last three lines).
Table 2:
Summary of experimental procedure for the four methods
| Method Number |
Method Name | Description All voltages are peak to peak |
|---|---|---|
| 1 | High-Low | Start block suprathreshold at 10 V or 16 V. Decrease in 1 V decrements until twitches return. |
| 2 | High-Low-High | Start block suprathreshold at 10 V or 16 V. Decrease in 1V increments until twitches return. Continue to decrease until 2 V below first threshold. Increase in 1 V increments until twitches disappear |
| 3 | Binary | Search a range from 0-16V starting in the middle at 8V. Two possible next steps: 1) If complete block achieved then values above 8V eliminated and test middle of remaining range, from 4V. 2) If complete block was not achieved, then values below 8 V eliminated and test middle of remaining range, or from 12V. Repeat steps 1 and 2 until block threshold is determined within a 1V resolution |
| 4 | Random | Test block values in a randomly generated order within the below ranges (ranges based on preliminary data from animal # 1): 10 kHz: 2-9 V 20 kHz: 4-11 V 30 kHz: 5-12 V |
The KHFAC was applied five seconds after the initiation of proximal stimulation. Each KHFAC application was maintained until the onset response disappeared, and block could be correctly evaluated. This determination was an interactive process and therefore these times varied with each trial. Each step was maintained for approximately 4 to 10 seconds with 3 to 10 seconds before the next KHFAC amplitude was tested.
Three sets of randomized trials were conducted for each animal. Each set included the four different blocking threshold methods at frequencies of 10 kHz, 20 kHz, and 30 kHz. Since Methods 1 and 2 were evaluated within a single trial, this resulted in 9 trials per set and a total of 27 trials per animal. Each trial started and ended with proximal electrode stimulation alone. The conduction block was initiated approximately 5 seconds after proximal stimulation and was left on for varying times depending on the block threshold method being tested (average 30.7 seconds, standard deviation 17.85). For Methods 1 and 2 the Wavetek output was not turned off between each block value tested, whereas for Methods 3 and 4 the Wavetek output was turned off for 1-2 seconds in between each block value. A two-minute wait was used in between each trial.
Block threshold data was collected from a total of five animals. The first animal was a preliminary experiment, and the results were used to determine the range of amplitudes tested for the random trials. The preliminary experiment data was not included in the final data analysis since the protocol for all following experiments was modified to include Method 4. The bipolar electrodes were cleaned between experiments using an ultrasonic bath.
2.4. Data Analysis
Digitized data sets were processed using commercial software (MATLAB, Math Works, Natick, MA) to derive the required data variables. Extracted data were analyzed using a commercial statistical software package (JMP, SAS Institute, Cary, NC). A one-way fixed effects analysis of variance (ANOVA) model was applied to the data sets to test the null hypothesis of equality of means, to determine the influence of each of the independent parameters as well as interactions between the parameters on the response variables. The control variables were: animal number, trial number, set number, frequency, and method number. The output variables were: the determined block threshold from each trial, the sum of the onset responses in each trial (area under the curve in Newton.seconds), and the time taken to determine the block threshold from the start of the first KHFAC application. A one way analysis of variance (ANOVA) and post-hoc Tukey Kramer HSD (honestly significant difference) tests were used to analyze the collected block threshold data. Three separate Tukey Kramer HSD tests were used to evaluate the block threshold against method type, set number, and KHFAC frequencies. These tests compare all possible pairs and were utilized to determine which group or groups were significantly different.
3. Results
3.1. Complete Motor Block
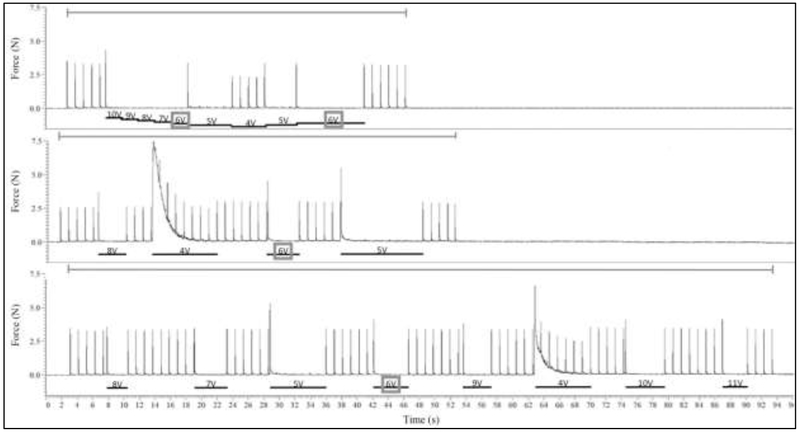
A complete and reversible motor block was achieved in all trials performed for all frequencies tested. In all animals tested, block thresholds were successfully determined using each method. The block threshold values ranged from 3 V to 13 V. Figure 2 displays an example of trials conducted for each block threshold method tested. For the binary and random method trials, the Wavetek output was turned off between the different amplitudes tested. Pulses from proximal stimulation can therefore be seen prior to block onset. In this example, the High-Low was able to determine the block threshold in the least amount of time, followed by Method 2, Method 3, and finally Method 4, which took the longest. Randomization was tested primarily to evaluate if there was a time or order bias within the other thresholding methods. These examples (Figure 2) were taken from the same experiment, and each method resulted in the same block threshold (6 V).
Figure 2:
Example of three trials evaluating four different block threshold methods. Force is shown in Newtons. All these three trials evaluated a 20 kHz KHFAC blocking waveform. The top graph displays a typical trial for Methods 1 (High-Low) and 3 (High-Low-High). The blocking waveform is initiated at 10 V to determine block threshold using the High-Low Method, and then once block threshold is passed the blocking amplitude is lowered by 2 V and then increased again and block threshold is re-determined through the High-Low-High Method. The middle graph displays a typical trial for Method 2, the Binary Method. Blocking amplitude begins at 8 V. The bottom graph displays a typical trial for Method 4, the Random Method. The gray bars above each graph indicate when proximal stimulation was being delivered. The black bars below each graph and accompanying text indicate the duration and amplitude of the block. A gray box shows the determined block threshold, 6 V for all cases in this example.
3.2. Block Threshold Method Comparison
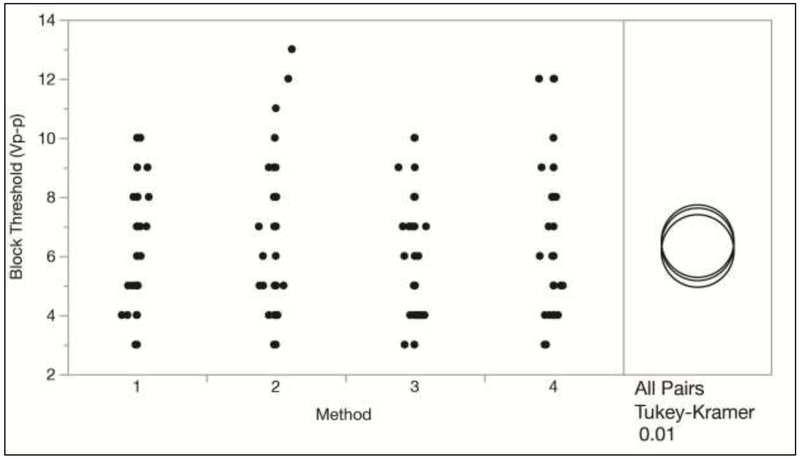
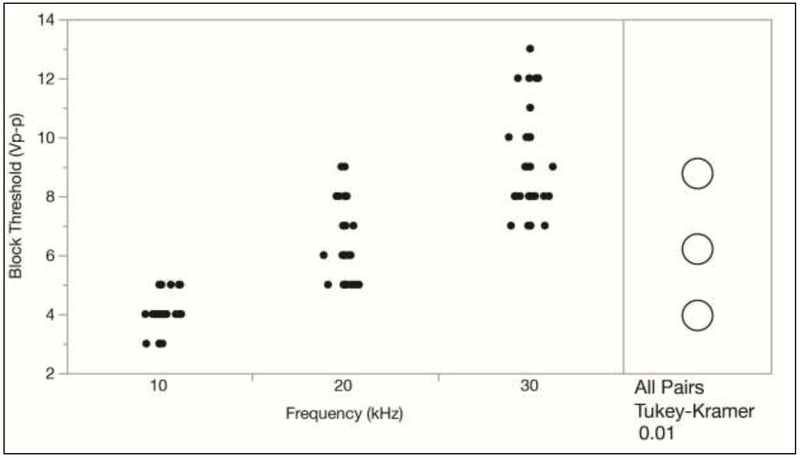
An ANOVA effects test resulted in a p-value of 0.4024 for the block threshold methods, indicating that there was no significant difference in the measured block thresholds between the four methods tested. This is also shown in Figure 3 with the Tukey-Kramer test. Also, no significant differences were found between the three sets of trials performed for each rat, which is shown in Figure 4. This indicates that there was no significant change in the block threshold over the duration of the experiments. However, the Tukey Kramer HSD test does show a significant difference between the block thresholds determined at all KHFAC frequencies (10, 20, and 30 kHz), as is expected. Figure 5 shows that higher KHFAC frequencies require higher block amplitudes in order to achieve a complete block.
Figure 3:
Block threshold versus method with the Tukey Kramer HSD Test. Block thresholds from all trials conducted on four animals (N = 108) are plotted. The overlap between the circles in the All Pairs test shows that there is no significant difference between the group means for all possible pairs of method types at α = 0.01.
Figure 4:
Block threshold versus set number with the Tukey Kramer HSD Test. The All Pairs test shows that there is no significant difference between the groups. Block thresholds are comparable over the time of the acute experiments at α = 0.01.
Figure 5:
Block threshold versus KHFAC frequency with the Tukey Kramer HSD Test indicates that there is a significant difference between the group means for all possible pairs of KHFAC frequencies at α = 0.01.
The onset area during each trial was analyzed for the four methods and is shown in Figure 6. The results are as follows. The High-Low method had a mean of 9.48 N.s with a standard deviation of 20.48 and a range of 0.13 to 93.1 N.s. The High-Low-High method had a mean of 11.48 N.s with a standard deviation of 21.44 and a range of 0.13 to 93.8 N.s. The Binary method had a mean of 48.41 N.s with a standard deviation of 50.6 and a range of 5.58 to 190.78 N.s. The Random method had a mean of 49.19 N.s with a standard deviation of 57.91 and a range of 2.89 to 227.42 N.s. An ANOVA effects test resulted in a p-value of <0.0001 for the BT method indicating significant differences in the total onset area between the four methods.
Figure 6:
Onset area and trial time needed to measure the BT versus the four methods.
The time needed to determine the BT was also analyzed for every trial (Figure 6). The results are as follows. The High-Low method had a mean of 30.72 s with a standard deviation of 17.84 and a range of 10 to 83 s. The High-Low-High method had a mean of 57.33 s with a standard deviation of 23.3 and a range of 35 to 120 s. The Binary method had a mean of 89.91 s with a standard deviation of 44.78 and a range of 40 to 200 s. The Random method had a mean of 168.44 s with a standard deviation of 85.76 and a range of 70 to 420 s. An ANOVA effects test resulted in a p-value of <0.0001 for the BT method indicating significant differences in the time to determine the BT between the four methods.
4. Discussion
These experiments successfully tested four methods of determining the KHFAC block threshold in complete block of the motor component of the rat sciatic nerve. Complete motor block was obtained for all trials at all the three tested frequencies. The statistics of the data from the repeated, randomized trials showed that there were no differences between the four methods tested in determining the block threshold at a resolution of 1 Vpp. There were also no differences in the set number thus confirming that the position of the trial chronologically in the experiment did not affect the block threshold determination. As expected, the block thresholds were statistically different for the three frequencies tested. The onset area and time to determine BT was similar for the High-Low and High-Low-High methods and significantly higher for the Binary and Random methods.
Recently it has been shown that KHFAC nerve block has a carryover effect where the nerve block can persist even after the KHFAC has been turned on [10]. However, this effect appears after a minimum KHFAC application time of 15 minutes. The average duration of KHFAC application for the block threshold testing in the present research was 30.72 seconds (High-Low method) to 168.44 seconds (Random method) and the range of durations for all methods was 10 seconds to 420 seconds. Therefore, it is very unlikely that the carryover block effect would have any influence on the block threshold measurements.
Our recommendations for choosing a method to measure block thresholds are based on four parameters. These are; any differences in the BT between methods, onset area, time to measure the BT, and the system being studied (motor, sensory, autonomic or mixed). This study shows no differences between methods. The onset is lowest for the High-Low method (Method 1) and for the High-Low-High method (Method 2). It should be noted that if a pure Low-High method was used as opposed to our choice of a High-Low-High method, the onsets would be as severe as for the random method, with onsets at every increasing step. Similarly, the time to measure the BT was shortest for the High-Low method and slightly longer for the High-Low-High method.
Based on these findings, we believe that the High-Low method (Method 1) is most appropriate for determining KHFAC block thresholds of motor and autonomic motor systems. The method minimizes the onset response yet is efficient in determining the block threshold rapidly (in less than ~ 90 seconds). However, if there is need for the resolution of the block threshold amplitude to be finer, then it does imply much longer trials. The Low-High method (Method 2) does not grant any extra advantages compared to Method 1 and is not recommended. The binary search method (Method 3) is very efficient for this requirement as the resolution can be increased with the addition of a small number of extra trials. However, the binary search method will induce an onset response if used to determine block thresholds for motor systems. There is some indication that suggests that the onset response may be smaller in pure sensory systems and the binary search method may be appropriate (unpublished data). The Random search method (Method 4) is not recommended, due to both larger onsets and longer time to detect the BT, unless the requirements for statistical power in the experiment dictate it.
The literature review shows how common it has been to not measure the block threshold. Experimentally more than 50% of publications did not measure the KHFAC block thresholds. We advocate that all research in electrical nerve block should include a paradigm to rigorously evaluate and measure block thresholds. This will allow relevant comparisons within and between experiments, interventions and across laboratories. We suggest the usage of the High-Low method for most determinations but the binary method could also be a viable option.
HIGHLIGHTS.
A literature review showed 50% of papers on electrical nerve block did not measure block thresholds.
Four block threshold measurement methods were evaluated using randomized in-vivo experiments.
There were no statistical differences between the four methods.
The High-Low method is recommended as the most efficient way to measure the block threshold.
5. Acknowledgements
This research was supported by NIH R01-EB-024860.
ABBREVIATIONS:
- KHFAC
Kilohertz Frequency Alternating Current
- BT
Block Threshold
- V
Vpp = Peak to Peak voltage
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- 1.Kilgore KL and Bhadra N, Reversible Nerve Conduction Block Using Kilohertz Frequency Alternating Current. Neuromodulation, 2014. 17(3): p. 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhadra N and Kilgore KL, High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve, 2005. 32(6): p. 782–90. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, et al. , Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery, 2008. 143(6): p. 723–31. [DOI] [PubMed] [Google Scholar]
- 4.Soin A Long-Term Human Testing of High-Frequency Nerve Block for Amputation Pain. in 16th Annual Meeting North American Neuromodulation Society 2012. Las Vegas, Nevada, USA. [Google Scholar]
- 5.Van Buyten JP, et al. , High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation, 2013. 16(1): p. 59–66. [DOI] [PubMed] [Google Scholar]
- 6.Bhadra N, et al. , Simulation of high-frequency sinusoidal electrical block of mammalian myelinated axons. J Comput Neurosci, 2007. 22(3): p. 313–26. [DOI] [PubMed] [Google Scholar]
- 7.Joseph L and Butera RJ, High-frequency stimulation selectively blocks different types of fibers in frog sciatic nerve. IEEE Trans Neural Syst Rehabil Eng, 2011. 19(5): p. 550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph L and Butera RJ, Unmyelinated Aplysia nerves exhibit a nonmonotonic blocking response to high-frequency stimulation. IEEE Trans Neural Syst Rehabil Eng, 2009. 17(6): p. 537–44. [DOI] [PubMed] [Google Scholar]
- 9.Reboul J and Rosenblueth A, The action of alternating currents upon the electrical excitability of nerve. Am. J. Physiology, 1939: p. 205–215. [Google Scholar]
- 10.Bhadra N, Foldes E, Vrabec T, Kilgore K, Bhadra N, Temporary persistence of conduction block after prolonged kilohertz frequency alternating current on rat sciatic nerve. Journal of Neural Engineering, 2018. 15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilgore KL and Bhadra N, Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput, 2004. 42(3): p. 394–406. [DOI] [PubMed] [Google Scholar]
- 12.Tandri H, et al. , Reversible cardiac conduction block and defibrillation with high-frequency electric field. Sci Transl Med, 2011. 3(102): p. 102ra96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Gawad M, et al. , Reduction of bladder outlet resistance by selective stimulation of the ventral sacral root using high frequency blockade: a chronic study in spinal cord transected dogs. J Urol, 2001. 166(2): p. 728–33. [PubMed] [Google Scholar]
- 14.Baratta R, et al. , Orderly stimulation of skeletal muscle motor units with tripolar nerve cuff electrode. IEEE Trans Biomed Eng, 1989. 36(8): p. 836–43. [DOI] [PubMed] [Google Scholar]
- 15.Bowman BR and McNeal DR, Response of single alpha motoneurons to high-frequency pulse trains. Firing behavior and conduction block phenomenon. Appl Neurophysiol, 1986. 49(3): p. 121–38. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, et al. , Selection of electrical algorithms to treat obesity with intermittent vagal block using an implantable medical device. Surg Obes Relat Dis, 2009. 5(2): p. 224–9; discussion 229-30. [DOI] [PubMed] [Google Scholar]
- 17.Cattel M and Gerard RW, The inhibitory effect of high-frequency stimulation and the excitation state of nerve. J. Physiology, 1935. 83: p. 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassouna M, et al. , Effect of early bladder stimulation on spinal shock: experimental approach. Urology, 1992. 40(6): p. 563–73. [DOI] [PubMed] [Google Scholar]
- 19.Li JS, et al. , Long-term effect of sphincteric fatigue during bladder neurostimulation. J Urol, 1995. 153(1): p. 238–42. [DOI] [PubMed] [Google Scholar]
- 20.Peng CW, et al. , High frequency block of selected axons using an implantable microstimulator. J Neurosci Methods, 2004. 134(1): p. 81–90. [DOI] [PubMed] [Google Scholar]
- 21.Perruchoud C, et al. , Analgesic Efficacy of High-Frequency Spinal Cord Stimulation: A Randomized Double-Blind Placebo-Controlled Study. Neuromodulation, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Sarr MG, et al. , The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg, 2012. 22(11): p. 1771–82. [DOI] [PubMed] [Google Scholar]
- 23.Sawan M, et al. , Stimulator design and subsequent stimulation parameter optimization for controlling micturition and reducing urethral resistance. IEEE Trans Rehabil Eng, 1996. 4(1): p. 39–46. [DOI] [PubMed] [Google Scholar]
- 24.Shaker HS, et al. , Reduction of bladder outlet resistance by selective sacral root stimulation using high-frequency blockade in dogs: an acute study. J Urol, 1998. 160(3 Pt 1): p. 901–7. [DOI] [PubMed] [Google Scholar]
- 25.Solomonow M, et al. , Fatigue considerations of muscle contractile force during high-frequency stimulation. Am J Phys Med, 1983. 62(3): p. 117–22. [PubMed] [Google Scholar]
- 26.Tai C, Roppolo JR, and de Groat WC, Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J Urol, 2004. 172(5 Pt 1): p. 2069–72. [DOI] [PubMed] [Google Scholar]
- 27.Tanner JA, Reversible Blocking of Nerve Conduction by Alternating Current Excitation. Nature, 1962. 195: p. 712–713. [DOI] [PubMed] [Google Scholar]
- 28.Tiede J, et al. , Novel Spinal Cord Stimulation Parameters in Patients with Predominant Back Pain. Neuromodulation, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Tweden KS, et al. , 46: Vagal Blocking for Obesity Control (VBLOC): Studies of pancreatic and gastric function and safety in a porcine model. Surgery for Obesity and Related Diseases, 2006. 2(3): p. 301–302. [Google Scholar]
- 30.Waataja JJ, Tweden KS, and Honda CN, Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng, 2011. 8(5): p. 056013. [DOI] [PubMed] [Google Scholar]
- 31.Zhou BH, Baratta R, and Solomonow M, Manipulation of muscle force with various firing rate and recruitment control strategies. IEEE Trans Biomed Eng, 1987. 34(2): p. 128–39. [DOI] [PubMed] [Google Scholar]
- 32.Elbasiouny SM and Mushahwar VK, Modulation of motoneuronal firing behavior after spinal cord injury using intraspinal microstimulation current pulses: a modeling study. J Appl Physiol, 2007. 103(1): p. 276–86. [DOI] [PubMed] [Google Scholar]
- 33.Tai C, Roppolo JR, and de Groat WC, Response of external urethral sphincter to high frequency biphasic electrical stimulation of pudendal nerve. J Urol, 2005. 174(2): p. 782–6. [DOI] [PubMed] [Google Scholar]
- 34.Tai C, de Groat WC, and Roppolo JR, Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE Trans Neural Syst Rehabil Eng, 2005. 13(3): p. 415–22. [DOI] [PubMed] [Google Scholar]
- 35.Tai C, et al. , Mechanism of conduction block in amphibian myelinated axon induced by biphasic electrical current at ultra-high frequency. J Comput Neurosci, 2011. 31 (3): p. 615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, et al. , Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE Trans Biomed Eng, 2006. 53(12 Pt 1): p. 2445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, et al. , Simulation analysis of conduction block in myelinated axons induced by high-frequency biphasic rectangular pulses. IEEE Trans Biomed Eng, 2006. 53(7): p. 1433–6. [DOI] [PubMed] [Google Scholar]
- 38.Lempka SF, McIntyre CC, Kilgore KL, & Machado AG, Computational Analysis of Kilohertz Frequency Spinal Cord Stimulation for Chronic Pain Management. . Anesthesiology, 2015. 122(6): p. 1362–1376. . [DOI] [PubMed] [Google Scholar]
- 39.Cuellar JM, et al. , Effect of High-Frequency Alternating Current on Spinal Afferent Nociceptive Transmission. Neuromodulation, 2012. [DOI] [PubMed] [Google Scholar]
- 40.De Ridder D, et al. , Burst Spinal Cord Stimulation for Limb and Back Pain. World Neurosurg, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Dowden BR, Wark HA, and Normann RA, Muscle-selective block using intrafascicular high-frequency alternating current. Muscle Nerve, 2010. 42(3): p. 339–47. [DOI] [PubMed] [Google Scholar]
- 42.Franke M, et al. , Combined KHFAC+DC nerve block without onset or reduced nerve conductivity post block. Journal of Neural Engineering, 2014. 11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaunt RA and Prochazka A, Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabil Neural Repair, 2009. 23(6): p. 615–26. [DOI] [PubMed] [Google Scholar]
- 44.Williamson RP and Andrews BJ, Localized electrical nerve blocking. IEEE Trans Biomed Eng, 2005. 52(3): p. 362–70. [DOI] [PubMed] [Google Scholar]
- 45.Ackermann D, et al. , Electrode design for high frequency block: effect of bipolar separation on block thresholds and the onset response. Conf Proc IEEE Eng Med Biol Soc, 2009. 2009: p. 654–7. [DOI] [PubMed] [Google Scholar]
- 46.Ackermann DM Jr., et al. , Conduction block of whole nerve without onset firing using combined high frequency and direct current. Med Biol Eng Comput, 2011. 49(2): p. 241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ackermann DM Jr., et al. , Effect of nerve cuff electrode geometry on onset response firing in high-frequency nerve conduction block. IEEE Trans Neural Syst Rehabil Eng, 2010. 18(6): p. 658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackermann DM Jr., et al. , Electrical conduction block in large nerves: high-frequency current delivery in the nonhuman primate. Muscle Nerve, 2011. 43(6): p. 897–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackermann DM, et al. , Nerve conduction block using combined thermoelectric cooling and high frequency electrical stimulation. J Neurosci Methods, 2010. 193(1): p. 72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhadra N, et al. , High frequency electrical conduction block of the pudendal nerve. J Neural Eng, 2006. 3(2): p. 180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boger A, Bhadra N, and Gustafson KJ, Bladder voiding by combined high frequency electrical pudendal nerve block and sacral root stimulation. Neurourol Urodyn, 2008. 27(5): p. 435–9. [DOI] [PubMed] [Google Scholar]
- 52.Gerges M, et al. , Frequency- and amplitude-transitioned waveforms mitigate the onset response in high-frequency nerve block. J Neural Eng, 2010. 7(6): p. 066003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miles JD, et al. , Effects of ramped amplitude waveforms on the onset response of high-frequency mammalian nerve block. J Neural Eng, 2007. 4(4): p. 390–8. [DOI] [PubMed] [Google Scholar]
- 54.Ackermann DM Jr., et al. , Effect of bipolar cuff electrode design on block thresholds in high-frequency electrical neural conduction block. IEEE Trans Neural Syst Rehabil Eng, 2009. 17(5): p. 469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ackermann DM, et al. , Dynamics and sensitivity analysis of high-frequency conduction block. J Neural Eng, 2011. 8(6): p. 065007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crosby ND, Janik JJ, & Grill WM, Modulation of activity and conduction in single dorsal column axons by kilohertz-frequency spinal cord stimulation. J Neurophysiol, 2017. 117(1): p. 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]