Abstract
Diabetic kidney disease typically develops over decades, but only outcomes associated with late disease stages are currently approved for clinical trials. Participants at greatest risk for these outcomes are currently selected using various combinations of traditional risk factors, including blood pressure, urine albumin excretion, and estimated glomerular filtration rate. Nevertheless, most clinical trial participants still do not reach the endpoints of interest, requiring very large trials or prolonged follow-up to achieve adequate statistical power. In this issue, Yamanouchi et al. used a Classification and Regression Trees analysis to further enrich enrollment criterion for patients at high risk for approved clinical trial outcomes. Selection criteria included novel biomarkers of diabetic kidney disease, which greatly enhanced the prognostic value of criterion based solely on traditional risk factors. These findings suggest a greater role for newly identified biomarkers of diabetic kidney disease in the selection of participants for clinical trials.
Despite improvements in treatment, diabetic kidney disease (DKD) remains the leading cause of end-stage renal disease (ESRD) in much of the world, and new medicines are urgently needed to reduce the incidence of this outcome. For new medicines to receive regulatory approval for treatment of DKD, however, requires that they demonstrate efficacy beyond established treatments in reducing this and other late-stage events such as doubling of serum creatinine concentration, or death. Accordingly, if we continue to select participants for clinical trials using traditional risk factors for DKD progression, future renoprotective trials will need to be larger and/or longer than those conducted in the past, adversely affecting both trial cost and feasibility. Enriching clinical trials to selectively enroll those at highest risk of progression may offer a viable and lower cost alternative.
In this issue of Kidney International, Yamanouchi et al1 describe an approach to optimizing enrollment criteria for Phase III clinical trials by using a Classification and Regression Trees (CART) analysis, a machine-learning method designed for creating simple rules to identify patients who are at high risk for a particular outcome. The investigators used extensive data on the natural history of DKD from patients with either type 1 or type 2 diabetes who were receiving care at the Joslin Clinic to identify optimal prognostic criterion for rapid DKD progression. All participants had chronic kidney disease (CKD stages 3 or 4), a history of elevated albuminuria, and up to 15 years of follow-up data. A composite end-point was defined that included ESRD, a >40% decline in estimated glomerular filtration rate (eGFR), or death unrelated to ESRD. Using a time-frame of 3 years, which is typical for DKD clinical trials, they identified 222 cases where the composite endpoint was met – 134 in the type 1 diabetes cohort and 88 in the type 2 diabetes cohort.
The CART analysis was limited to eight measures: four clinical risk factors – age, sex, systolic blood pressure and HbA1c; two established biomarkers – eGFR and albumin:creatinine ratio (ACR); and two novel serum biomarkers – tumor necrosis factor receptor (TNFR) 1 and 2 concentrations. Using the type 1 diabetes cohort for development and the type 2 diabetes cohort for validation they found that only two measures – serum TNFR1 and ACR – were needed to optimize discrimination between cases and non-cases. Those at highest risk for progression were defined based either on a serum TNFR1 concentration >4.3 ng/ml or a combination of serum TNFR1 concentration of 2.9–4.3 ng/ml and an ACR >1.9 g/g. This model performed well in both type 1 and type 2 diabetes cohorts, despite their different clinical characteristics, and greatly increased the prognostic value of the enrollment criterion relative to traditional risk factors alone. It was also encouraging to see that the model performed better when deaths not due to ESRD were excluded from the composite end-point, indicating that the model’s strengths were in identifying the DKD progressors rather than frailty per se.
This is certainly not the first effort to enhance risk prediction for CKD progression,2 but it does have certain elements that are worth highlighting. The authors focus on a few prediction metrics, namely the positive predictive value and sensitivity, because these are the most useful metrics for identifying those who will progress to the endpoint within the abbreviated time-frame of a clinical trial. The more traditional c-statistic or area under the receiver operating characteristic curves, by contrast, are driven by performance in classifying both cases and non-cases. Thus, where non-cases make up the majority of individuals included, as is the case in past clinical trials for DKD, these metrics may be influenced more by the correct assignation of non-cases rather than by correctly identifying cases, which is the priority for clinical trial enrichment. Although CART analyses have been used for many years, the application of CART for this purpose is novel and appealing due to the simplicity of the resultant decision tree rules compared with the mathematical complexity found in risk equations based on regression models.
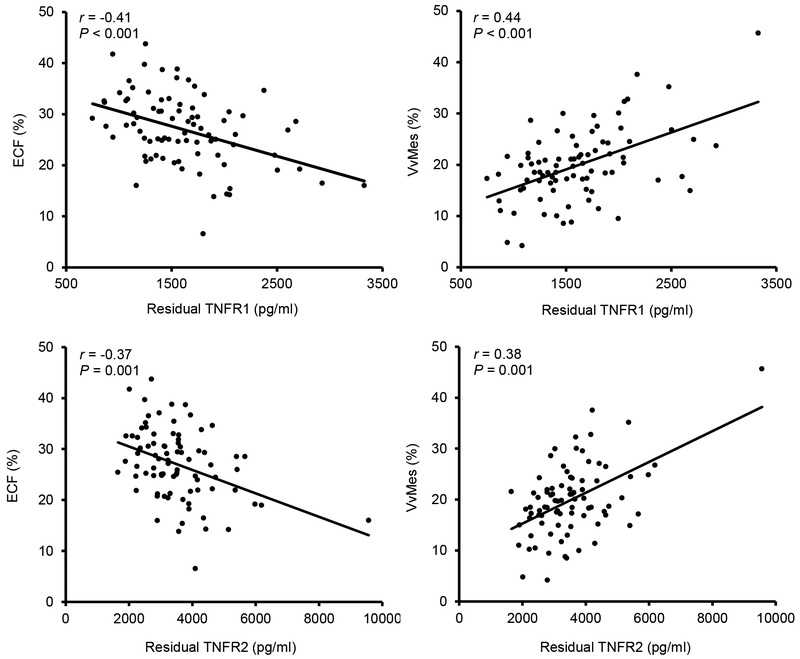
Both TNFR1 and TNFR2 are associated with increased DKD risk,3–5 and correlate with glomerular lesions that predict progressive DKD as illustrated in Figure 1.6 However, the authors note that while higher TNFR1 and TNFR2 concentrations are associated with increased risk, the absolute values associated with risk are far higher in American Indians than in other populations.5 Thus, while the combination of serum TNFR1 and ACR are useful for prediction, the cut points reported in this study may vary by racial or ethnic group. Other risk factors for progression may also vary in people not represented in the Joslin cohorts, such as those with youth onset type 2 diabetes or in the elderly. As such, it would be valuable to assess this simple set of rules in other populations to understand if the optimal criterion that divide patients by risk in this cohort are the same in other populations or if a change in cut-points or a whole new set of rules will be required. The authors only considered a limited number of risk measures in their models. While few would argue with the chosen traditional risk factors, other measures such as blood pressure treatment or serum KIM-17 may also be useful. Moreover, there was little advantage in measuring both TNFR1 and TNFR2, which in this study captured essentially the same information.
Figure 1 |. Partial regression residual plot of the associations between TNFRs, percent of normal endothelial cell fenestration (ECF), and mesangial fractional volume (VvMes).
The residuals were computed from regressing each of these variables on age, sex, diabetes duration, A1c, body mass index, and mean arterial pressure. Exclusion of the single outlier did not change the significance of the associations with the two morphometric variables. A1c, hemoglobin A1c; TNFR, tumor necrosis factor receptor. Reprinted with permission from Pavkov ME, Weil EJ, Fufaa GD, et al. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int. 2016;89:226–234. Copyright © by permission of Elsevier.6
That the optimal prognostic criterion identified by Yamanouchi et al yielded similar results in people with type 1 and type 2 diabetes is intriguing. At least in Europeans, CKD in type 2 diabetes tends to reflect a variety of pathologies with different rates of progression and not just DKD.8 Nondiabetic causes of CKD may have been excluded in the current study, at least in part, by restricting the study to individuals with a history of elevated albuminuria as well as low eGFR in the type 2 diabetes cohort.
Do these findings indicate that we should make greater use of biomarkers to enhance the power of clinical trials? Perhaps so. Beyond the obvious cost savings, the PONTIAC (NT-proBNP Selected PreventiOn of cardiac eveNts in a populaTion of dIabetic patients without A history of Cardiac disease) study is just one example that illustrates the potential clinical value of this approach. PONTIAC was a clinical trial of intensive primary prevention for cardiovascular disease in which high concentrations of the biomarker N-Terminal Prohormone B-type Natriuretic Peptide (NT-ProBNP) were used as an entry criterion.9 This intervention had not provided clinical benefit in a wider population selected using only conventional risk-factors for cardiac disease, but among individuals who also had a raised NT-ProBNP there was a statistically significant reduction in cardiac events, indicating that the high NT-ProBNP subset received a benefit that was not apparent in people without the elevated biomarker. On the other hand, in DKD, many of those who do not progress rapidly to ESRD, still ultimately reach this endpoint, and studies that examine efficacy only in the subset of rapid progressors may not provide useful treatment information for many who ultimately develop progressive kidney disease, but at a somewhat slower rate.
As clinical trials have a limited time-frame, those seeking licensing for a new medicine often concentrate on late stages of disease where hard end-points are more likely to occur. While averting ESRD is a primary goal of DKD treatment, interventions at such late stages may be of limited value. Nevertheless, enriching these late-stage trials for patients at greatest risk may reduce costs and assist investigators in finding new efficacious late-stage treatments for this disease. Where the method proposed by Yamanouchi et al may have its greatest value, however, is in early DKD, but this will require identification of valid early disease markers that optimize selection criteria, and the approval of suitable surrogate endpoints or the granting of “subpart H” marketing approval for existing surrogates.10 Without these incentives, many trials will not focus on early intervention, where the greatest clinical benefit may be found, and we may fail to recognize interventions that are highly effective in early DKD, particularly if their efficacy diminishes at later stages.
ACKNOWLEDGMENTS
The authors are supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
DISCLOSURE
The authors declare no competing interests.
REFERENCES
- 1.Yamanouchi M, Skupien J, Niewczas MA et al. Improved clinical trial enrollment criterion to identify patients with diabetes at risk of ESRD. Kidney Int. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangri N, Kitsios GD, Inker LA et al. Risk prediction models for patients with chronic kidney disease: a systematic review. Ann Intern Med. 2013; 158:596–603 [DOI] [PubMed] [Google Scholar]
- 3.Gohda T, Niewczas MA, Ficoceiello LH et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsblom C, Moran J, Harjutsalo V et al. Added value of soluble tumor necrosis factor alpha receptor 1 as a biomarker of ESRD risk in patients with type 1 diabetes. Diabetes Care. 2014; 37:2334–42 [DOI] [PubMed] [Google Scholar]
- 5.Pavkov ME, Nelson RG, Knowler WC et al. Elevation of circulating TNR receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015;87:821–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavkov ME, Weil EJ, Fufaa GD et al. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int. 2016;89:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbisetti VS, Waikar SS, Antoine DJ et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell S, Fletcher EH, Brady I et al. End-stage renal disease and survival in people with diabetes: a national database linkage study. QJM. 2015. February;108(2):127–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huelsmann M, Neuhold S, Resl M et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013. 8;62:136572 [DOI] [PubMed] [Google Scholar]
- 10.Perkovic V, Agarwal R, Fioretto P et al. ; for Conference Participants. Management of patients with diabetes and CKD: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;90:1175–1183 [DOI] [PubMed] [Google Scholar]