Abstract
Background:
Older hospitalized acute decompensated heart failure (ADHF) patients have persistently poor outcomes and delayed recovery regardless of ejection fraction. We hypothesized that impairments in physical function, frailty, cognition, mood and quality-of-life (QoL) potentially contributing to poor clinical outcomes would be similarly severe in ADHF patients ≥60 years of age with preserved versus reduced ejection fraction (HFpEF, HFrEF).
Methods and Results:
In 202 consecutive older (≥60 years)hospitalized ADHF patients in a multicenter trial, we prospectively performed at baseline: Short Physical Performance Battery (SPPB), six-minute walk distance (6MWD), frailty assessment, Geriatric Depression Scale (GDS), Montreal Cognitive Assessment, and QoL assessments. Older acute decompensated HFpEF (EF ≥45%, n=96) and HFrEF (EF<45%, n=106) patients had similar impairments in all physical function measures (SPPB [5.9±0.3 versus 6.2±0.2]; 6MWD [184±10 vs 186±9m]; and gait speed [0.60±0.02 versus 0.61±0.02m/sec]) and rates of frailty (55% versus 52%; p=0.70) and cognitive impairment (77% versus 81%; p=0.56) when adjusted for differences in gender, BMI, and comorbidities. However, depression and QoL were consistently worse in HFpEF versus HFrEF. Depression was usually unrecognized clinically with 38% having GDS ≥5 and no documented history of depression.
Conclusion:
Patients ≥60 years hospitalized with ADHF patients have broad, marked impairments in physical function and high rates of frailty and impaired cognition: these impairments are similar in HFpEF versus HFrEF. Further, depression was common and QOL was reduced, and both were worse in HFpEF than HFrEF. Depression was usually unrecognized clinically. These findings suggest opportunities for novel interventions to improve these important patient-centered outcomes.
Clinical Trial Registration:
https://clinicaltrials.gov/ct2/show/NCT02196038 Identifier: NCT02196038
Keywords: acute decompensated heart failure, heart failure with preserved ejection fraction, physical function, quality of life, depression, frailty, self-care, aging
Introduction
The burden of heart failure (HF) is increasing in the United States particularly among older adults, who comprise the majority of the HF population.1 Acute decompensated HF (ADHF) is a leading cause of hospitalization among older Americans, and hospitalization is associated with markedly adverse outcomes including increased mortality, morbidity, and health care expenditures.2–4 While these outcomes have received considerable attention, less is known regarding key patient-centered outcomes of physical function, frailty, cognition, depression, and quality-of-life (QoL) among older ADHF which are important to patients independent of mortality, and are also strong predictors of clinical events.5
In a small study of older hospitalized ADHF patients we previously found severe impairments in multiple domains of physical function including balance, strength, mobility and endurance, and these were accompanied by high rates of frailty, cognitive dysfunction, and depression, and were associated with poor QoL.6 These impairments were much more severe than those observed in age-matched patients with chronic stable HF, and were similar in severity to patients with advanced HF awaiting left ventricular assist device implantation.7 It has been shown by our group and others that these impairments, which are often unrecognized and not addressed by current care pathways, could help account for the persistently poor outcomes after hospitalization among older ADHF patients.8–10 However, the sample size in our pilot study was very small (n=27), limiting confidence in the point estimates and generalizability of our results, as well as precluding subgroup analyses.10
Among subgroups of older ADHF patients, HF with preserved ejection fraction (HFpEF) is the most important phenotype. HFpEF is the most common form of HF in the elderly, accounting for nearly 90% of incident HF among older women.11 The cardiovascular substrate of HFpEF differs significantly from HF with reduced EF (HFrEF) and the pathophysiology of HFpEF is not well understood. Furthermore, responses to interventions in chronic HFpEF frequently diverge markedly from those of HFrEF, where many therapies proven highly effective for HFrEF have shown little or no benefit for HFpEF.12 Following ADHF, all-cause outcomes for HFpEF are similarly adverse as for HFrEF, but with differences in HF disease-specific outcomes.3 The mechanisms contributing to these outcomes are not well understood.
In older adults in general, severe physical impairments and accompanying frailty, cognitive impairment, depression, and diminished QoL are associated with adverse clinical outcomes8, 13, 14 but, to our knowledge, there is no previous report comparing these key patient-centered outcomes in older adults with acute decompensated HFpEF versus HFrEF. Accordingly, we performed prospective, comprehensive assessments at baseline in 202 consecutive patients in the NIH-funded, multi-center Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial (NCT02196038). We hypothesized that impairments in physical function, frailty, cognitive function, depression, and quality-of-life (QoL) would be similarly severe across all assessments in ADHF patients ≥60 years of age with preserved versus reduced ejection fraction (HFpEF, HFrEF).6
Methods
Inclusion & Exclusion Criteria
The present study is a cross-sectional analysis of the baseline assessment of the first n=202 consecutively enrolled participants in the REHAB-HF clinical trial. Full details regarding REHAB-HF trial design have been published.15,16 We recruited patients at 7 sites ≥60 years old and hospitalized for at least 24 hours with ADHF. HFrEF was defined as EF <45% and HFpEF was defined as EF ≥45% based on the REHAB-HF trial protocol. For the purposes of this analysis, we also conducted comparisons using more contemporary 3 EF categories, which also includes HF with borderline EF (HFbEF): EF ≤35%, EF >35% to <50% and EF ≥ 50%. EF was assessed during the course of clinical care via echocardiogram, MRI, cardiac catheterization, or nuclear medicine scan and was abstracted from the medical record; 68% of participants had EF evaluated during the index hospitalization, while the rest had an EF evaluation prior to hospitalization [median (interquartile range): 2.1 months (1.1–5.4 months)]. Criteria for ADHF included at least 2 signs of HF (pulmonary congestion by x-ray or examination, elevated central or jugular venous pressure, peripheral edema and elevated B-type natriuretic peptide >100 pg/ml or N-terminal pro-hormone of B-type natriuretic peptide >220 pg/ml); acute worsening of at least 1 HF symptom (exertional dyspnea or fatigue, swelling of legs or abdomen, paroxysmal nocturnal dyspnea, or orthopnea); and change in medical treatment consistent with ADHF (e.g. change in dose or initiation of diuretics, vasodilators, or inotropes). In all patients, the diagnosis of HF was confirmed by a REHAB-HF investigator, board-certified in cardiology with expertise in HF. Additional inclusion criteria included independence with basic activities of daily living prior to admission, achievement of clinical stability allowing participation in physical function assessments, ability to walk at least 4 meters with or without assistive device, and planned discharge to home. All patients provided written informed consent and the study was approved by the Institution Review Boards of all participating sites. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Variables
Assessments were collected during hospitalization, after successful initial treatment for ADHF and clinical stability had been achieved, and prior to hospital discharge. A trained assessor using a standardized protocol assessed all physical function measures, which included the Short Physical Performance Battery (SPPB), six-minute walk distance (6MWD), and handgrip strength. The SPPB is a well-established, standardized, reproducible measure of physical function in older patients that strongly predicts key clinical outcomes including hospitalization, death, and nursing home placement.13 The three components of the SPPB are static standing balance, 4 meter walk time, and time to complete 5 repeated chair stands. Each component is scored from 0–4, for a total score of 0–12; patients with a score of <10 are considered at high risk for mobility disability with lower score identifying progressively higher risk.17–20 The 6MWD was assessed in an unobstructed hallway. Participants were allowed to use an assist device if needed. A distance of ≤300 meters identifies patients with severe functional impairment.21 Frailty phenotype was assessed as previously described10, 22 based on domains originally described by Fried (slowness [gait speed], weakness [hand grip], weight loss, exhaustion and low physical activity).23 Gait speed, a component of both the SPPB and Fried frailty phenotype, is also of independent importance with <0.8 m/s identifying patients at increased risk for disability and mortality that increases incrementally with each 0.1 m/s additional reduction in gait speed.19, 20 Handgrip strength was assessed using a hand dynamometer. Weak handgrip has been associated with increased risk of poor clinical outcomes;8, 24 recommended cut-offs for weakness are <28.5 kg in men and <18.5 kg in women.25
Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) with a score of <26 indicating at least mild cognitive impairment.26 A score of ≥5 on the Geriatric Depression Scale 15-item survey (GDS-15) was considered consistent with depression.27 Clinical diagnosis of depression was based on documentation of the diagnosis in the medical record. QoL was assessed using the Kansas City Cardiomyopathy Questionnaire (Overall and Physical Limitation Scores [PLS]), the Short Form-12 (physical and mental composite scores [PCS and MCS]) and EuroQol-5D-5L (EQ-5D-5L).
Statistical Analysis
Categorical variables were analyzed as frequencies (percentages) and group differences were compared using the Chi-Square test. Continuous variables were compared by t-test and presented as mean ± standard deviation if normality assumptions appeared to be reasonable. BNP and NT-ProBNP were log-transformed before analysis. Highly skewed continuous variables were analyzed using non-parametric Wilcoxon rank sum tests in unadjusted analyses. Negative binomial regression was used for count data (e.g., hospitalizations). Due to significant differences between patient groups, adjusted analyses were conducted to control for potential confounders that may affect physical function and QOL; these included sex, BMI, non-white race, and comorbidities. We did not adjust GDS score for comorbidities given collinearity between GDS and clinical depression. For continuous variables, analysis of covariance (ANCOVA) was used and least square means based on observed sample margins were presented in adjusted analysis. Rank transformed ANCOVA was used for group comparisons for highly skewed continuous variables. Similarly, binary variables were adjusted using logistic regression for sex, BMI, non-white race, and comorbidities. Adjusted proportions and associated 95% confidence intervals (CI) were estimated using non-linear contrasts. Group differences and 95% CIs were reported in all adjusted analyses. Pearson’s correlation coefficients were used to assess the relation between QOL and depression measures. In the primary analysis, EF categories were derived from the parent trial: EF <45% and EF ≥45%. We also replicated the primary analysis using 3 EF categories: EF ≤35%; EF >35% and <50%; and EF ≥50%. Additional details regarding statistical methods for this analysis are provided in the Supplement. Two tailed p value <0.05 was considered significant. All analyses were performed using SAS Enterprise Guide 7.1 (Cary, NC).
Results
Participant Characteristics
A total of 202 consecutively enrolled patients from September 2014 through February 2017, and were included in this analysis. HFpEF patients represented 48% (n=96/202) of the population, and were more likely to be female, white, have not graduated high school, and have higher BMI than patients with HFrEF; there was no difference in age (Table 1). HFpEF patients were less likely to have been hospitalized for HF within 6 months prior to enrollment and were more likely to be hospitalized for non-HF causes than HFrEF patients. Comorbidities were frequent in both groups (mean >5), with HFpEF patients having a higher burden of overall comorbidities than HFrEF patients, including more arthritis/connective tissue disease, obstructive sleep apnea and depression. Patients with HFpEF were less likely to be on beta-blockers and angiotensin converting enzyme inhibitors, and had higher calcium channel blocker use.
Table 1:
Baseline Characteristics, Comorbidities, and Medications Among Patients ≥60 years Hospitalized with ADHF by Heart Failure Phenotype
| Characteristics | HFrEF (n=106) | HFpEF (n=96) | P-value |
|---|---|---|---|
| Age (years) | 72.3 ± 7.7 | 71.7 ± 7.4 | 0.54 |
| Women | 50 (47%) | 59 (61%) | 0.042 |
| White | 42 (39%) | 55 (57%) | 0.012 |
| Black or African American | 62 (58%) | 38 (40%) | 0.007 |
| Hispanic or Latino ethnicity | 2 (2%) | 1 (1%) | 0.62 |
| Less than high school education | 24 (23%) | 13 (14%) | 0.002 |
| Body Mass Index (kg/m2) | 30.6 ± 7.5 | 36.1 ± 9.3 | <0.001 |
| New York Heart Association Class | |||
| − II | 16 (15%) | 16 (17%) | 0.62 |
| − III | 57 (53%) | 48 (50%) | |
| − IV | 22 (21%) | 23 (24%) | |
| Ejection Fraction (%), median (IQR) | 28 (20, 35) | 55 (55, 60) | <0.001 |
| Days from initial presentation to baseline assessment, median (IQR) | 3 (2, 4.5) | 2.5 (2, 4) | 0.41 |
| Patients with previous hospitalizations within 6 months | 46 (43%) | 43 (45%) | 0.84 |
| Patients with previous HF hospitalizations within 6 months | 36 (34%) | 19 (20%) | 0.024 |
| All-cause hospitalizations in last 6 months, total (mean ± SD) | 83 (0.8±1.1) | 86 (0.9±1.3) | 0.52 |
| HF hospitalizations in last 6 months, total (mean ± SD) | 55 (0.5±0.8) | 30 (0.3±0.8) | 0.07 |
| Non-HF hospitalizations in last 6 months, total (mean ± SD) | 28 (0.3±0.6) | 56 (0.6±0.9) | 0.004 |
| B-type natriuretic peptide (pg/mL), (n=117) | 1344 ± 1118 | 525 ± 535 | <0.001 |
| N-terminal brain natriuretic peptide (pg/mL), (n=77) | 8259 ± 12450 | 2750 ± 3185 | <0.001 |
| Live Alone | 36 (34%) | 30 (31%) | 0.68 |
| Current smoking | 12 (11%) | 11 (11%) | 0.98 |
| Alcohol Abuse | 6 (6%) | 4 (4%) | 0.63 |
| Comorbidities | |||
| Diabetes mellitus | 55 (52%) | 56 (58%) | 0.36 |
| Hypertension | 96 (91%) | 91 (95%) | 0.25 |
| Hyperlipidemia | 74 (70%) | 66 (69%) | 0.87 |
| Atrial Fibrillation | 45 (42%) | 48 (50%) | 0.28 |
| Coronary artery disease (previous MI, PCI, or CABG) | 43 (41%) | 30 (31%) | 0.17 |
| Chronic obstructive pulmonary disease | 29 (27%) | 36 (38%) | 0.12 |
| Chronic kidney disease | 35 (33%) | 32 (33%) | 0.96 |
| Stroke | 15 (14%) | 17 (18%) | 0.49 |
| Arthritis / Connective Tissue Disease | 41 (39%) | 53 (55%) | 0.019 |
| Cancer | 24 (23%) | 18 (19%) | 0.50 |
| Depression | 12 (11%) | 21 (22%) | 0.043 |
| Anemia (by Hgb) | 77 (73%) | 67 (70%) | 0.65 |
| Peripheral vascular disease | 10 (9%) | 16 (17%) | 0.13 |
| Peptic ulcer disease | 4 (4%) | 5 (5%) | 0.62 |
| Dementia | 1 (1%) | 3 (3%) | 0.27 |
| Obstructive sleep apnea | 20 (19%) | 41 (43%) | <0.001 |
| AIDS/HIV | 2 (2%) | 0 (0%) | 0.18 |
| Total number of comorbidities | 5.8 ± 1.8 | 6.5 ± 2.3 | 0.021 |
| Current Medications | |||
| Loop diuretics | 99 (93%) | 89 (94%) | 0.93 |
| Beta blockers | 98 (92%) | 70 (74%) | <0.001 |
| Angiotensin converting enzymes inhibitors | 52 (49%) | 32 (34%) | 0.03 |
| Angiotensin receptor blockers | 24 (23%) | 20 (21%) | 0.79 |
| Calcium channel blockers | 14 (13%) | 46 (48%) | <0.001 |
Values presented as frequency (%) or mean ± standard deviation unless otherwise indicated. Anemia was defined as hemoglobin <12g/dl for women or <13g/dl for men. HIV/AIDs was determined by medical record. Abbreviations: HIV/AIDS – human immunodeficiency virus/acquired immunodeficiency syndrome, IQR – interquartile range
Physical Function, Frailty, and Cognitive Function
Both older acute HFpEF and HFrEF had physical function impairments in physical in all domains (balance, mobility, strength, and endurance). Performance score on the SPPB, gait speed, 6MWD, and handgrip strength were well below recognized cut-offs for poor performance associated increased risk (Table 2). For context, in the overall study population, balance deficits rendered 36% unable to attempt tandem stance (one foot directly in front of the other, heel to toe) and lower extremity weakness rendered 25% unable to stand even once from a chair without assistance. For comparison, in a prior analysis of subjects of similar age range, such deficits were present in only 6–7% of chronic HF patients and in no healthy adults.6 Additionally, we found that 79% of older ADHF patients had impaired mobility based on slow gait (<0.8 m/s), 85% had severely reduced endurance (6MWD <300m), and 34% had generalized weakness based on low grip strength. Unadjusted SPPB score, 6MWD, and gait speed were significantly lower in HFpEF than HFrEF. However, these between-group differences were no longer observed after adjusting for inter-group differences in sex, race, BMI and comorbidities (Table 2, Figure 1). Overall, 53% of patients met formal criteria for physical frailty, and frailty rate was similar between HFpEF and HFrEF (Table 2). At least mild cognitive impairment, defined as MoCA score <26, was present in 78% of patients despite that patients with severe cognitive impairments or dementia were excluded from trial recruitment and only 2% of patients had any degree of dementia or cognitive impairment noted in the medical record. Cognitive impairment based on MoCA score was similar between HFpEF and HFrEF (Table 2).
Table 2.
Adjusted and unadjusted differences in physical function, quality of life, depression, and cognition in patients ≥60 years hospitalized with ADHF by heart failure phenotype
| Unadjusted Analysis | Adjusted Analysis | ||||||
|---|---|---|---|---|---|---|---|
| HFrEF (n=106) |
HFpEF (n=96) |
P-value | HFrEF (n=106) |
HFpEF (n=96) |
Group Difference (95% CI) |
P-value | |
| SPPB Total Score | 6.4 ± 2.5 | 5.6 ± 2.4 | 0.044 | 6.2 ± 0.2 | 5.9 ± 0.3 | 0.3 (−0.5 to 1.0) | 0.46 |
| Balance Score | 2.8 ± 1.3 | 2.6 ± 1.3 | 0.29 | 2.7 ± 0.1 | 2.7 ± 0.1 | 0.0 (−0.4 to 0.4) | 0.84 |
| 4-meter Walk Score | 2.5 ± 0.9 | 2.1 ± 1.0 | 0.005 | 2.3 ± 0.1 | 2.2 ± 0.1 | 0.1 (−0.1 to 0.4) | 0.33 |
| Chair Stand Score | 1.1 ± 1.1 | 1.0 ± 1.1 | 0.33 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.1 (−0.2 to 0.4) | 0.42 |
| 6-Minute Walk Distance (m) | 200 ± 95 | 167 ± 103 | 0.021 | 186 ± 9 | 184 ± 10 | 2 (−25 to 30) | 0.88 |
| Gait speed (m/s) | 0.64 ± 0.23 | 0.57 ± 0.21 | 0.031 | 0.61 ± 0.02 | 0.60 ± 0.02 | 0.01 (−0.05 to 0.08) | 0.60 |
| Handgrip strength (kg) | |||||||
| Men | 28.5 ± 10.9 | 26.7 ± 7.1 | 0.36 | 27.7 ± 1.3 | 27.8 ± 1.6 | −0.1 (−4.5 to 4.3) | 0.96 |
| Women | 18.5 ± 5.6 | 19.0 ± 8.0 | 0.74 | 18.5 ± 1.0 | 19.0 ± 1.0 | −0.5 (−3.4 to 2.4) | 0.73 |
| Frailty Phenotype | |||||||
| Frail (≥ 3 of 5 domains met) | 51 (48%) | 56 (58%) | 0.15 | 52% (42 to 62%) | 55% (44 to 65%) | −3% (−19 to 13%) | 0.70 |
| Disease-Specific Quality of Life | |||||||
| KCCQ Overall Score | 46 ± 21 | 35 ± 19 | <0.001 | 44 ± 2 | 37 ± 2 | 7 (1 to 13) | 0.030 |
| KCCQ Physical Limitation Score | 53 ± 23 | 41 ± 23 | <0.001 | 52 ± 2 | 42 ± 2 | 10 (3 to 16) | 0.007 |
| Non-Specific Quality of Life | |||||||
| Short Form-12 PCS | 29 ± 9 | 26 ± 8 | 0.023 | 28 ± 1 | 27 ± 1 | 1 (−1 to 4) | 0.28 |
| Short Form-12 MCS | 47 ± 14.2 | 41 ± 14 | 0.003 | 47 ± 2 | 41 ± 2 | 6 (2 to 10) | 0.007 |
| EQ-5D-5L Components | |||||||
| Walking | 2.3 ± 1.0 | 2.8 ± 0.9 | <0.001 | 2.4 ± 0.1 | 2.6 ± 0.1 | −0.2 (−0.5 to 0.0) | 0.09 |
| Self-Care | 1.5 ± 0.8 | 2.0 ± 0.9 | <0.001 | 1.5 ± 0.1 | 1.9 ± 0.1 | −0.4 (−0.7 to −0.2) | 0.002 |
| Usual Activities | 2.5 ± 1.2 | 2.8 ± 1.2 | 0.030 | 2.6 ± 0.1 | 2.7 ± 0.1 | −0.2 (−0.5 to 0.2) | 0.32 |
| Pain/Discomfort | 2.3 ± 1.1 | 2.5 ± 1.1 | 0.31 | 2.4 ± 0.1 | 2.4 ± 0.1 | 0.0 (−0.3 to 0.3) | 0.94 |
| Depression/Anxiety | 1.7 ± 1.0 | 2.0 ± 1.1 | 0.025 | 1.7 ± 0.1 | 1.9 ± 0.1 | −0.2 (−0.5 to 0.1) | 0.10 |
| Visual Analog Scale (0–100) | 61 ± 22 | 53 ± 22 | 0.009 | 61 ± 2 | 54 ± 2 | 7 (0 to 14) | 0.05 |
| Depression | |||||||
| Geriatric Depression Scale Score* | 4.2 ± 3.3 | 5.5 ± 3.5 | 0.007 | 4.4 ± 0.3 | 5.4 ± 0.4 | −1.0 (−2.1 to 0.0) | 0.046 |
| Geriatric Depression Scale ≥5* | 43 (41%) | 50 (52%) | 0.10 | 42% (33 to 52%) | 50% (40 to 60%) | −8% (−23 to 7%) | 0.30 |
| Cognitive Function | |||||||
| MoCA Score | 21.3 ± 4.9 | 22.2 ± 4.0 | 0.19 | 21.5 ± 0.5 | 21.9 ± 0.5 | −0.4 (−1.8 to 0.9) | 0.53 |
| MoCA Score <26 | 86 (81%) | 72 (75%) | 0.29 | 81% (72 to 88%) | 77% (67 to 85%) | 4% (−9 to 16%) | 0.56 |
Unadjusted values shown as mean ± standard deviation or frequency (%). Adjusted values shown as LSmean ± standard error or percent (95% CI), adjusted for sex, race, and BMI and total number of comorbidities. For KCCQ, SF-12 and Visual Analog Scale, higher score indicates better health status; for EQ-5D components higher score indicates worse health status
Adjusted for sex, race, and BMI only
Abbreviations: KCCQ – Kansas City Cardiomyopathy Questionnaire, PCS – physical composite score, MCS – mental composite score, EQ-5D-5L – EuroQol, MoCA – Montreal Cognitive Assessment
Figure 1.

Differences in physical function and quality of life between patients ≥60 years of age hospitalized with ADHF. Values adjusted for sex, race, BMI, and total comorbidities, except GDS, which was adjusted for sex, race, and BMI only. Abbreviations: 6MWD – six-minute walk distance, GDS – geriatric depression scale score, KCCQ – Kansas City cardiomyopathy questionnaire, SPPB – short physical performance battery.
Depression and QOL
Compared with HFrEF patients, HFpEF patients had more depressive symptoms by GDS score (5.4±0.3 vs. 4.4±0.4, p=0.046). Consistent with this, self-reported depression and anxiety on the EQ-5D-5L were more common in HFpEF versus HFrEF (58% vs. 39%, p=0.005) as was depression by clinical diagnosis (22% vs. 11%, p=0.043). While 16% (n=33) of patients overall had a clinical diagnosis of depression documented in the medical record, an additional 38% (n=65) had symptomatic depression based on GDS ≥5 with no clinical recognition of depression in the medical record. Further, presence of depression strongly predicted poor QOL, based on significant correlations of the GDS score with QoL measures (KCCQ, r=−0.58; SF-12 PCS, r=−0.63, Table 3).
Table 3:
Correlation of Geriatric Depression Scale with measures of quality of life
| Measure | R | P-value |
|---|---|---|
| KCCQ Overall Score | −0.58 | <0.001 |
| KCCQ Physical Limitation Score | −0.38 | <0.001 |
| Short Form-12 PCS | −0.63 | <0.001 |
| Short Form-12 MCS | −0.26 | <0.001 |
| EQ-5D-5L Components | ||
| Walking | 0.31 | <0.001 |
| Self-Care | 0.41 | <0.001 |
| Usual Activities | 0.46 | <0.001 |
| Pain/Discomfort | 0.29 | <0.001 |
| Depression/Anxiety | 0.48 | <0.001 |
| Visual Analog Scale | −0.38 | <0.001 |
Abbreviations: KCCQ – Kansas City Cardiomyopathy Questionnaire, PCS – physical composite score, MCS – mental composite score, EQ-5D-5L – EuroQol
HF-specific (KCCQ) and general QoL (SF-12; EQ-5D-5L) were reduced in both groups; in unadjusted analyses this was worse in HFpEF patients, and remained so in adjusted analyses (Table 2, Figure 1). This included self-reported walking difficulty, which, consistent with objective assessments of impairments in physical function, was common (≥75%) in both groups, more frequent in HFpEF patients in the unadjusted analysis, and similar in the adjusted analysis (Table 2). However, difficulty with self-care was nearly twice as frequent in HFpEF patients (Figure 2) and self-care difficulties remained greater in HFpEF patients even after adjustments (Table 2).
Figure 2.
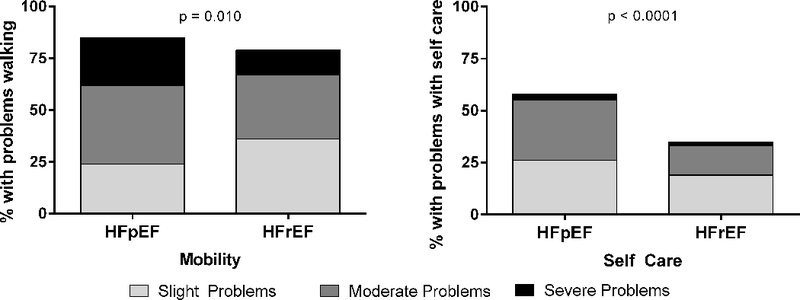
EQ-5D-5L dimensions of self-reported mobility and self-care in older hospitalized patients with ADHF. P-value represents difference in frequency of problem of any severity.
Analysis with 3 EF categories
In the supplemental analysis comprised of three EF categories (35% [HFrEF; n=86]; >35% and <50% [HFbEF; n=28]; ≥50% [HFpEF; n=88]), adjusted differences between the groups were largely consistent with the primary analysis except that significant between group differences in GDS were no longer noted (Supplementary Tables 1–3). HFbEF patients constituted a relatively small portion of the study sample (14% of participants) and had characteristics generally intermediate between patients with HFrEF and HFpEF (Supplementary Tables 1–3), consistent with prior reports.28
Discussion
Within an ongoing multi-center trial, we prospectively conducted a comprehensive assessment of multiple key patient-centered outcomes relating to physical function, frailty, cognition, depression, and QoL in 202 consecutive patients ≥60 years hospitalized with ADHF and analyzed outcomes by HF phenotype (HFpEF vs HFrEF). We found broad, marked impairments in all domains of physical function—balance, strength, mobility and endurance. Approximately 50% of patients met formal criterial for physical frailty, >75% had significant cognitive impairment, nearly 50% had significant depressive symptoms, and HF-specific and general QoL were severely reduced. Consistent with our hypothesis, physical dysfunction, frailty, and cognitive impairments were similar in HFpEF versus HFrEF when adjusted for differences in sex, race, BMI, and comorbidities. However, depression appeared to be more common or severe in HFpEF. Importantly, the majority of depression in both HFpEF and HFrEF appeared to be clinically unrecognized. Health-related QoL (HF-specific and general) was consistently worse in HFpEF patients and remained so after adjustment.
To our knowledge, these patient-centered outcomes have not previously been systematically and comprehensively assessed in the large and growing high risk population of older adults with ADHF and examined by EF subtypes, particularly HFpEF which is the most common among older patients. We also selected an age cut-off (≥60 years) that is inclusive of the vast majority of HF patients. These findings are valuable in that each of these impairments is clinically meaningful, has independent prognostic importance, and is usually not addressed in current care models or disease management pathways.21 Prior reports in older hospitalized patients in general indicated that these types of impairments are strong, independent predictors of rehospitalization, nursing home placement, and all-cause mortality.8, 29 A large recent study reported that, surprisingly, the majority of rehospitalizations in older ADHF patients are not for recurrent HF.3 Thus, these data provide potentially valuable insights and facilitate the development of novel interventions and care models targeting these impairments, as discussed below. Doing so has the potential to improve patient-centered outcomes as well as clinical event rates adjudicated by changes in these outcomes.
Frailty, present in >50% of both HFpEF and HFrEF patients, was much more common in ADHF than observed in chronic stable HF patients (≤ 20%) and is particularly notable given the inclusion of patients aged 60–69 years where lower frailty rates would be expected.6, 8, 22 Physical function impairments likely account for the high frailty rates we observed. Impairments were present across all domains (endurance, strength, balance and mobility) and were similarly severe in HFrEF and HFpEF despite very different underlying cardiovascular substrates. These included 6MWD that was approximately half that reported in chronic HF, weakness such that 25% were unable to stand unassisted from a chair even once, and difficulties in balance and mobility not typically seen in chronic HF patients.6, 7, 31 The severity and breadth of impairments is also notable given that assessments were conducted after successful treatment for the acute HF decompensation in patients who were deemed nearing readiness for discharge to home and excluding those requiring subacute rehabilitation.
The severity and breadth of impairments are likely due to the combined effects of pre-existing impairments from aging, chronic HF, comorbidities, and chronic skeletal muscle abnormalities common to HFpEF and HFrEF, including reduced muscle mass, adipose infiltration, shift in fiber type, reduced capillary density, and mitochondrial dysfunction.30, 31 These are likely additionally compounded by the systemic effects of ADHF mediated through activation of inflammatory and neurohumoral pathways, hospital-associated immobility (older hospitalized patients spend >80% of time lying in bed and <5% ambulating36) and skeletal myopathy that progresses rapidly after hospitalization (Figure 3).35
Figure 3.
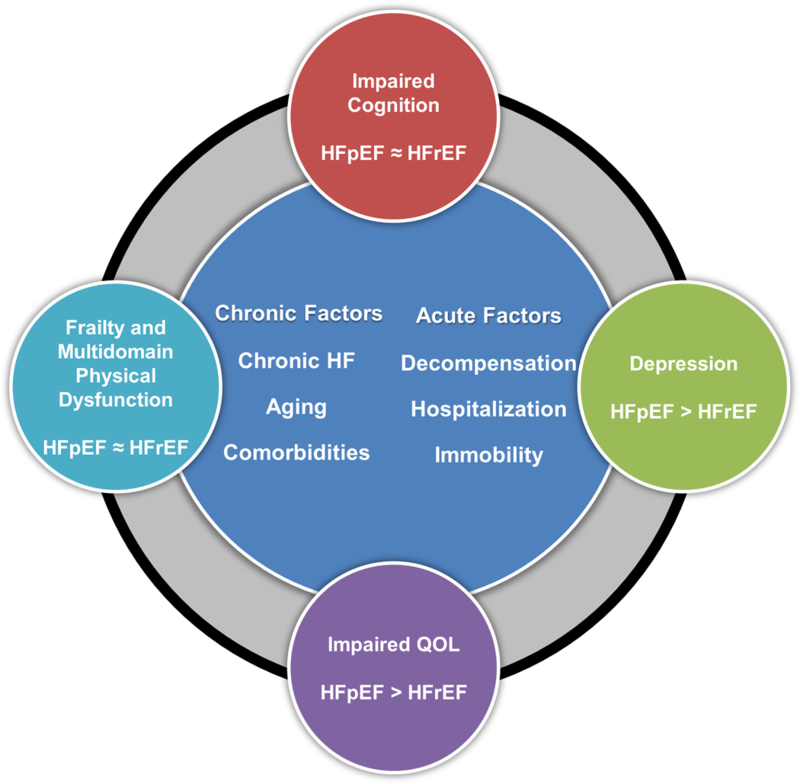
Profile of patient-centered outcomes in acute decompensated HF with preserved and reduced ejection fraction.
These findings have important implications for physical rehabilitation interventions for recently hospitalized, older ADHF patients. Standard, endurance-based cardiac rehabilitation (CR) improves physical function and QoL in chronic stable HFrEF and may provide similar benefit in chronic, stable HFpEF.33–35 However CR is not approved for Medicare reimbursement in patients with ADHF or a recent hospitalization due to a lack of evidence supporting its safety or efficacy,36, 37 and is not approved at all for HFpEF.37 Furthermore, conventional CR does not usually address the broad impairments in balance, mobility and functional strength we identified in these very frail patients ≥60 years of age. For example, 40% of HFpEF patients in our study were unable to even attempt tandem stance due to severe balance deficits, 89% reported difficulty with walking, and 60% had difficulty with self-care. Thus, even if eligible for Medicare coverage, these patients are likely ill-suited for standard CR. Indeed, it has been shown in other older frail populations that undertake endurance-based rehabilitation without first addressing these deficits may increase the risk of injuries.38 Thus, addressing these marked, wide-spread physical function deficits in ADHF patients may require a tailored physical rehabilitation intervention addressing deficits in balance, mobility, strength and endurance, as is being testing in the ongoing, NIH-funded, REHAB-HF trial (NCT02196038), of which a subset of cross-sectional data is presented here.15
Clinically important impairments were not limited to the physical function. Cognitive impairment was present in >75% of the cohort and may be linked to physical frailty through common underlying mechanisms, including complex interplay of neurohormonal, inflammatory, hemodynamic and nutritional pathways.39 This may limit patients’ ability to adhere to complex self-care and medication regimens41, 42 and is independently associated with adverse outcomes.14 However, cognitive impairment is often unrecognized and has not been accounted for or addressed in conventional HF care pathways and models. Recognition of difficulties in self-care and cognition could lead to increased use of specific strategies such as support systems within transitional care programs, which could potentially improve outcomes.58
Similarly, depression was common and generally under-recognized clinically by approximately 50%. The lack of focus on depression may in part be due to the lack of benefit from standard pharmacotherapy in depressed HF patients.45 However, these trials did not include HFpEF patients, in whom our findings suggest depression may be more frequent and more severe, at least in the context of ADHF. Additionally, non-pharmacologic therapies, such as exercise training and cognitive behavior therapy, have shown promise in alleviating depression in HF patients,46, 47 supporting that depression in HF patients may be amenable to alternative treatment modalities.
Depression is strongly linked to poor health status in older HF patients42–44 and QoL was worse in HFpEF versus HFrEF by all 3 QOL instruments utilized, despite similar impairments in physical function, cognition, and frailty. For perspective, global QoL reported by HFpEF patients is nearly identical to ambulatory end-stage HFrEF patients for whom LVAD therapy may be justified (53 and 52 out of 100, respectively).47 Difficulties in self-care and consequent loss of independence were more frequent in HFpEF versus HFrEF, and may also have contributed to the lower QoL since these are important determinants of QoL among older adults.
Overall, these findings support a more comprehensive and multidimensional approach to the care of older patients hospitalized with ADHF irrespective of EF, such as that recently described by Gorodeski and colleagues.8, 48 This model addresses impairments in physical function, cognition, mood, and social support needs in addition to medical management of HF and comorbidities. Ultimately, such an approach could help reduce the ‘post-hospital syndrome’, associated with delayed and incomplete recovery and frequent re-hospitalization and death experienced by >70% of older HFrEF and HFpEF patients within 1-year post-hospitalization, at least half of which are due to non-cardiac causes.3, 10, 49
Limitations
The study cohort was limited to patients eligible for participation in a clinical trial. However, the trial inclusion/exclusion criteria were specifically designed to be broadly inclusive of typical older, frail ADHF patients with multiple comorbidities.15 Additionally, HFpEF patients were more likely to be female, white, have higher BMI, and greater comorbidity burden than patients with HFrEF, which is in accord with reports from population-based studies,50 supporting the generalizability of these results. The severity and breadth of impairments are consistent with previous reports, including observational cohorts8, 29 further supporting generalizability. It is possible we underestimated the severity because the trial excluded patients who were expected to be discharged to a rehabilitation or skilled nursing facility. Given the cross-sectional study design, we cannot assess the relative importance of these impairments to subsequent outcomes, although prior research indicates these impairments strongly predict subsequent clinical events. Finally, we cannot exclude potential residual confounding causing observed differences in patients by HF phenotype.
Conclusion
Hospitalized ADHF patients ≥60 years of age had markedly impaired physical function and high rates of frailty and cognitive impairments, and these were similar in HFpEF vs HFrEF. QOL was severely reduced and depression was common, and both were worse in HFpEF than HFrEF. These findings may help explain the high frequency of ‘post-hospital syndrome’,49 delayed recovery, and rehospitalization and suggest opportunities for novel interventions to improve the persistently poor outcomes in hospitalized older ADHF patients.
Supplementary Material
What is new?
Hospitalized, older (≥60 years) ADHF patients with both preserved and reduced ejection fraction have severe impairments across multiple patient-centered outcomes including physical function, quality of life, cognition, and depression.
HFpEF and HFrEF patients show similar degrees of impairment in physical function, including endurance, strength, balance, and mobility, and high rates of cognitive impairment and frailty.
Despite these similarities, HFpEF patients report worse QoL and higher burden of depression.
What are the clinical implications?
These patient-centered outcomes are of independent importance and strongly associated with clinical outcomes.
However, these impairments are not addressed by current ADHF care models.
These findings suggest opportunities for novel interventions and multi-dimensional care models to improve clinical outcomes, functional status, and quality of life in older patients recovering from ADHF across HF phenotypes.
Acknowledgments
Funding
This study was supported in part by the following research grant awards from the National Institutes of Health: R01AG045551 and R01AG18915. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine (DWK); the Claude D. Pepper Older Americans Independence Center NIH Grants P30AG021332 (DWK) and P30AG028716 (AMP); and the Wake Forest Clinical and Translational Science Award, NIH Grant UL1TR001420.
Footnotes
Disclosures
Dr. Kitzman has been a consultant for Relypsa, Abbvie, GlaxoSmithKline, Merck, Corvia Medical, Bayer, CinRx, Boehringer-Ingleheim, and St. Luke’s Medical Center in Kansas City, Kansas; received grant support from Novartis, Bayer, and St. Luke’s Medical Center in Kansas City, Kansas; and owns stock in Gilead Sciences. Dr. Mentz receives research support from Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, Luitpold, Medtronic, Merck, Novartis, Otsuka, and ResMed; honoraria from Abbott, Bayer, Janssen, Luitpold Pharmaceuticals, Merck, Novartis, and ResMed; and has served on an advisory board for Amgen, Luitpold, Merck and Boehringer Ingelheim.
References:
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krumholz HM, Nuti SV, Downing NS, Normand SL and Wang Y. Mortality, Hospitalizations, and Expenditures for the Medicare Population Aged 65 Years or Older, 1999–2013. JAMA. 2015;314:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW and Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL and Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelho R, Ramos S, Prata J, Bettencourt P, Ferreira A and Cerqueira-Gomes M. Heart failure and health related quality of life. Clin Pract Epidemiol Ment Health. 2005;1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves GR, Whellan DJ, Patel MJ, O’Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva AM and Kitzman DW. Comparison of Frequency of Frailty and Severely Impaired Physical Function in Patients >/=60 Years Hospitalized With Acute Decompensated Heart Failure Versus Chronic Stable Heart Failure With Reduced and Preserved Left Ventricular Ejection Fraction. Am J Cardiol. 2016;12:1953–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, Edwards BS, Park S, John R, Conte JV, Farrar DJ, Slaughter MS and HeartMate III. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–34. [DOI] [PubMed] [Google Scholar]
- 8.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA and Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–75. [DOI] [PubMed] [Google Scholar]
- 9.Alahdab MT, Mansour IN, Napan S and Stamos TD. Six minute walk test predicts long-term all-cause mortality and heart failure rehospitalization in African-American patients hospitalized with acute decompensated heart failure. J Card Fail. 2009;15:130–5. [DOI] [PubMed] [Google Scholar]
- 10.Reeves GR, Whellan DJ, O’Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva A, Patel MJ and Kitzman DW. A Novel Rehabilitation Intervention for Older Patients With Acute Decompensated Heart Failure: The REHAB-HF Pilot Study. JACC Heart Fail. 2017;5:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J and Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–8. [DOI] [PubMed] [Google Scholar]
- 12.Pandey A, Kitzman DW, Brubaker P, Haykowsky MJ, Morgan T, Becton JT and Berry JD. Response to Endurance Exercise Training in Older Adults with Heart Failure with Preserved or Reduced Ejection Fraction. J Am Geriatr Soc. 2017;65:1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R and Guralnik JM. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodson JA, Truong TT, Towle VR, Kerins G and Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013;126:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves GR, Whellan DJ, Duncan P, O’Connor CM, Pastva AM, Eggebeen JD, Hewston LA, Morgan TM, Reed SD, Rejeski WJ, Mentz RJ, Rosenberg PB and Kitzman DW. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J. 2017;185:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastva AM, Duncan PW, Reeves GR, Nelson MB, Whellan DJ, O’Connor CM, Eggebeen JD, Hewston LA, Taylor KM, Mentz RJ, Rosenberg PB and Kitzman DW. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemp Clin Trials. 2018;64:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC, Kritchevsky SB, Manini TM, McDermott MM, Miller ME, Newman AB, Rejeski WJ, Sink KM and Williamson JD. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME and Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M and Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–9. [DOI] [PubMed] [Google Scholar]
- 20.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L and Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 22.McNallan SM, Singh M, Chamberlain AM, Kane RL, Dunlay SM, Redfield MM, Weston SA and Roger VL. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA and Cardiovascular Health Study Collaborative Research G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry SI, McAvay G, Chen S, Whitson H, Newman AB, Krumholz HM and Gill TM. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the Cardiovascular Health Study. J Am Coll Cardiol. 2013;61:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L and Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL and Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 27.Wancata J, Alexandrowicz R, Marquart B, Weiss M and Friedrich F. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scand. 2006;114:398–410. [DOI] [PubMed] [Google Scholar]
- 28.Hsu JJ, Ziaeian B and Fonarow GC. Heart Failure With Mid-Range (Borderline) Ejection Fraction. Clinical Implications and Future Directions. 2017;5:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez E, Vidan MT, Serra JA, Fernandez-Aviles F and Bueno H. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart. 2011;97:1602–6. [DOI] [PubMed] [Google Scholar]
- 30.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitzman DW, Haykowsky MJ and Tomczak CR. Making the Case for Skeletal Muscle Myopathy and Its Contribution to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM and Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. [DOI] [PubMed] [Google Scholar]
- 33.Haykowsky M, Brubaker P and Kitzman D. Role of physical training in heart failure with preserved ejection fraction. Current heart failure reports. 2012;9:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J and Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolte K, Herrmann-Lingen C, Wachter R, Gelbrich G, Dungen HD, Duvinage A, Hoischen N, von Oehsen K, Schwarz S, Hasenfuss G, Halle M, Pieske B and Edelmann F. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. European journal of preventive cardiology. 2015;22:582–93. [DOI] [PubMed] [Google Scholar]
- 36.Ades PA, Keteyian SJ, Balady GJ, Houston-Miller N, Kitzman DW, Mancini DM and Rich MW. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail. 2013;1:540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleg JL, Cooper LS, Borlaug BA, Haykowsky MJ, Kraus WE, Levine BD, Pfeffer MA, Pina IL, Poole DC, Reeves GR, Whellan DJ, Kitzman DW, National Heart L and Blood Institute Working G. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail. 2015;8:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilson JK, Wu SS, Cen SY, Feng Q, Rose DR, Behrman AL, Azen SP and Duncan PW. Characterizing and identifying risk for falls in the LEAPS study: a randomized clinical trial of interventions to improve walking poststroke. Stroke. 2012;43:446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Havakuk O, King KS, Grazette L, Yoon AJ, Fong M, Bregman N, Elkayam U and Kloner RA. Heart Failure-Induced Brain Injury. J Am Coll Cardiol. 2017;69:1609–1616. [DOI] [PubMed] [Google Scholar]
- 40.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK and Stewart S. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. 2010;12:508–15. [DOI] [PubMed] [Google Scholar]
- 41.Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJV and Quinn TJ. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J Card Fail. 2017;23:464–475. [DOI] [PubMed] [Google Scholar]
- 42.Havranek EP, Spertus JA, Masoudi FA, Jones PG and Rumsfeld JS. Predictors of the onset of depressive symptoms in patients with heart failure. J Am Coll Cardiol. 2004;44:2333–8. [DOI] [PubMed] [Google Scholar]
- 43.Lum HD, Carey EP, Fairclough D, Plomondon ME, Hutt E, Rumsfeld JS and Bekelman DB. Burdensome Physical and Depressive Symptoms Predict Heart Failure-Specific Health Status Over One Year. J Pain Symptom Manage. 2016;51:963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan MD, Newton K, Hecht J, Russo JE and Spertus JA. Depression and health status in elderly patients with heart failure: a 6-month prospective study in primary care. Am J Geriatr Cardiol. 2004;13:252–60. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK and Krishnan R. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blumenthal JA, Babyak MA, O’Connor C, Keteyian S, Landzberg J, Howlett J, Kraus W, Gottlieb S, Blackburn G, Swank A and Whellan DJ. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. 2012;308:465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedland KE, Carney RM, Rich MW, Steinmeyer BC and Rubin EH. Cognitive Behavior Therapy for Depression and Self-Care in Heart Failure Patients: A Randomized Clinical Trial. JAMA Intern Med. 2015;175:1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN and Alexander KP. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J Am Coll Cardiol. 2018;71:1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunlay SM, Roger VL and Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


