Abstract
Objectives
To provide accurate risk estimates of serious adverse events after elective shoulder replacement surgery for arthritis, including age and sex specific estimates of the lifetime risk of revision surgery.
Design
Population based cohort study.
Setting
Hospital episode statistics for NHS England, including civil registration mortality data.
Participants
58 054 elective shoulder replacements in 51 895 adults (aged ≥50 years) between April 1998 and April 2017.
Main outcome measures
The lifetime risk of revision surgery, calculated using an actuarial life table approach and the cumulative probability method. Rates of serious adverse events at 30 and 90 days post-surgery: pulmonary embolism, myocardial infarction, lower respiratory tract infection, acute kidney injury, urinary tract infection, cerebrovascular events, and all cause death. Secondary outcome measures were the number of surgeries performed each year and Kaplan-Meier estimates of revision risk at 3, 5, 10, and 15 years.
Results
The number of shoulder replacements performed each year increased 5.6-fold between 1998 and 2017. Lifetime risks of revision surgery ranged from 1 in 37 (2.7%, 95% confidence interval 2.6% to 2.8%) in women aged 85 years and older to 1 in 4 (23.6%, 23.2% to 24.0%) in men aged 55-59 years. The risks of revision were highest during the first five years after surgery. The risk of any serious adverse event at 30 days post-surgery was 1 in 28 (3.5%, 3.4% to 3.7%), and at 90 days post-surgery was 1 in 22 (4.6%, 4.4% to 4.8%). At 30 days, the relative risk of pulmonary embolism compared with baseline population risk was 61 (95% confidence interval 50 to 73) for women aged 50-64. Serious adverse events were associated with increasing age, comorbidity, and male sex. 1 in 5 (21.2%, 17.9% to 25.1%) men aged 85 years and older experienced at least one serious adverse event within 90 days.
Conclusions
Younger patients, particularly men, need to be aware of a higher likelihood of early failure of shoulder replacement and the need for further and more complex revision replacement surgery. All patients should be counselled about the risks of serious adverse events. These risks are higher than previously considered, and for some could outweigh any potential benefits. Our findings caution against unchecked expansion of shoulder replacement surgery in both younger and older patients. The more accurate age and sex specific estimates of risk from this study are long overdue and should improve shared decision making between patients and clinicians.
Study registration
ClinicalTrials.gov NCT03573765.
Introduction
Primary shoulder replacement surgery has been shown to provide early improvements in shoulder specific and overall quality of life measures when used for end stage glenohumeral arthropathies.1 2 3 Although the number of replacements do not yet match those of the hip and knee, recent trends from international joint replacement registries show a rapid increase in the incidence of primary shoulder replacements performed and the rapid adoption of newer implant technologies.4 5 Despite this growth, no large scale trials and very few published studies have been carried out on the long term outcomes across different age groups, including the risks of revision surgery. A small study with short follow-up suggested that the risk of early revision surgery might be greater in those aged less than 59 years.6
For all surgical procedures, shared decision making should be informed by evidence and include a full discussion of the risks, benefits, and alternatives to surgery.7 8 Information should be shared in a format that can be easily understood by patients.8 9 For joint replacement surgery, the risk of revision surgery is important and is commonly presented using time-to-event survival analyses at fixed time points, typically five and 10 years.10 A study of patient preferences found that patients favour risk description in terms of lifetime risk, and prefer longer over shorter follow-up for fixed time estimates.11 Lifetime risk statistics are commonly used in cancer epidemiology and have only recently been used to describe revision risk after leg joint replacement surgery, where the increased risks for younger patients have been starkly exposed.12
Besides being informed about the risks of revision surgery, patients should also be provided with high quality estimates of serious adverse events, including myocardial infarction, thromboembolic disease, and all cause death. Data on these and other events, such as pneumonia, acute kidney injury, and stroke, are sparse. The precision of event frequency estimates is limited by study sample size, and confidence intervals are rarely reported. The one study that provided some risk estimates on elective shoulder replacement used hospital episode statistics (HES) for England data and identified 12 pulmonary embolic events within 90 days from a sample of 10 229 shoulder replacements (0.1%) performed between 2005 and 2008.13 The sample size was small and considerably underpowered for both the number of associations tested and the interrupted time series analysis performed.
There therefore remains a lack of published evidence from large real world data on serious adverse events and the long term risks of primary elective shoulder replacement, including lifetime revision risk. We used a large HES dataset to calculate age and sex specific estimates for the lifetime risk of undergoing a revision joint replacement, or a reoperation procedure, together with precise estimates of the incidence of serious adverse events occurring within 30 and 90 days after a primary elective shoulder replacement.
Methods
Data source
We extracted individual participant data from the admitted patient care (APC) database within HES. HES is a data warehouse recording all activity carried out in National Health Service hospitals or funded by the NHS in England.14 Submission of records to HES is mandated for accurate remuneration of NHS hospitals, and as such HES provides universal coverage of day case and inpatient surgical care. It is also the standard tool against which the National Joint Registry of England, Wales, Northern Ireland, and the Isle of Man (NJR) audits its data submission compliance from NHS hospitals.15 HES APC data are stored according to financial year as “finished consultant episodes,” detailing procedures and dates when they were performed using the Office of Population Censuses and Surveys Classification of Interventions and Procedures, version 4 (OPCS-4) codes and diagnoses using World Health Organization International Classification of Diseases, 10th revision (ICD-10) codes. To avoid disclosure of personal identifiers, NHS Digital releases individual level data in a pseudonymised format with a restricted level of detail for demographic fields. Longitudinal follow-up of subsequent surgical interventions can be carried out by linking episodes through pseudonymised identifiers.16 HES data also contain accurate records for cause and date of death from civil registration mortality data (formerly provided by the Office for National Statistics). We obtained aggregate age and sex standardised mortality rates for England at five yearly age intervals from publicly available ONS tables generated for the year ending 2016.17
Participants
Based on the OPCS-4 code list (see supplementary file), we identified all patients in the HES APC database with a matching code for a primary elective shoulder replacement procedure between 6 April 1998 and 5 April 2017. We included patients in the study if they were aged 50 years or older at the time of surgery. Exclusions were presence of a primary diagnostic (ICD-10) code identifying the indication for surgery as acute shoulder trauma or a primary or secondary bone tumour. Mortality rates in the general population and in comparable studies of perioperative mortality in leg arthroplasty studies have both improved considerably over the study period.18 To account for this, we calculated mortality rates for a subset of patients undergoing surgery in the five years between April 2012 and April 2017.
Data processing
To produce the raw study dataset, we extracted all episodes linkable to an included patient through a valid pseudonymised identifier, including the index episode and any prior or subsequent APC activity during the study period. We defined the index event as the first primary shoulder replacement procedure performed on either side, and the first subsequent shoulder replacement procedures per side as revision procedures. A set of non-arthroplasty procedures were recorded as reoperations (see supplementary file for OPCS-4 codes identifying revisions and reoperations). Duplicates were excluded, along with logically inconsistent operative sequences (ie, the first procedure had to be a primary one and could not be preceded by a revision procedure).
Primary diagnoses and serious adverse events were identified based on specific ICD-10 codes (see supplementary file). The Charlson comorbidity indices were calculated using the ICD-10 codes from HES APC records up to and including the index episode and using a previously validated algorithm.19
Statistical analysis
Data were grouped into five year age bands according to participant age and sex at the time of primary surgery, with the final group of participants aged 85 years and older. The unit for survival analyses was each primary procedure. For participants with both right and left shoulders replaced, we presumed the survival of the implant and censoring for death to be independent, in keeping with the standard practice employed by the NJR.20 We counted the number of valid primary cases by age, sex, and UK financial year (6 April to 5 April).
Using the actuarial life table method, we calculated revision-free and reoperation-free implant survival rates over 19 years of follow-up, applying revision, reoperation, and mortality events as multiple decrements at the end of each single year period. To reduce the leverage effect of rare late events, we excluded years of follow-up with fewer than 100 participants remaining at risk. We assumed that censored participants, through loss to follow-up at the end of the study, contributed half to the time at risk.21 From this we calculated person time incidence rates for each year of follow-up as the quotient of the number of revisions (or reoperations) performed divided by the total time at risk.
Estimates of lifetime risk were calculated using approaches established in the oncology literature, and recently applied to the lifetime risk of implant revision after total hip or knee replacement.12 21 22 23 24 The approach involves creating a standardised hypothetical population for each of the five year age bands from 50 to 54 years to 85 years and older, to which the age and sex specific person time incidence rates and ONS mortality rates were applied to calculate an estimate of lifetime risk as the sum of the “current probability” for each sequential year of follow-up.
The Kaplan-Meier method was used for time to event implant survival analyses, stratified where indicated by age and sex. We calculated confidence intervals for Kaplan-Meier survival estimates on a log-log scale.
Serious adverse events were reported as simple rates. Assuming a Poisson distribution, we used the delta method to calculate 95% confidence intervals. We compared risks rates for pulmonary embolism using a 2×2 contingency table to the background population risk calculated from a large UK prospective cohort of women aged 50-64 years (0.007% per 90 days, derived from 1547 events in 5 136 506 person years).25 We used the same method to compare the mortality risk with data from the ONS. The effect of age on the risk of any serious adverse event was modelled using a logistic regression model adjusted for sex and Charlson comorbidity index score. Age was handled as a continuous variable using restricted cubic splines (three knots) to account for a non-linear relation. Model performance was validated using 200 bootstrap samples.
Missing data and censoring
We censored participants at the end of the study period (5 April 2017). Incomplete records for any of age, sex, and date of surgery were present in a small number of cases (0.2%) and on the assumption that the data were missing completely at random, we excluded these records from the analysis.
Data cleaning and preprocessing was performed using Stata MP (release version 15.0; Statacorp, College Station, TX). Analyses and production of figures were carried out using R version 3.4.0 (R Core Team; R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
No patients were directly involved in setting the research question, nor were they involved in the design, conduct, or interpretation of the study. However, patients involved in the 2015 James Lind Alliance Priority Setting Partnership confirmed that the lack of evidence on outcome after elective shoulder replacement was an important research area and was subsequently made one of the top 10 research priorities.26
Results
Between 6 April 1998 and 5 April 2017, 58 054 eligible elective primary shoulder replacement procedures in 51 895 adults were identified for inclusion in the study. Figure 1 shows the flow of participants through the study, with exclusions. The mean age at primary surgery was 72.2 years (SD 8.9), 42 080 procedures (72%) were performed in women, the mean Charlson comorbidity index score was 1.05 (SD 1.49), and the most common indication for shoulder joint replacement was osteoarthritis or rotator cuff tear arthropathy (n=39 835, 69%), followed by inflammatory (n=6251) or other conditions (n=6241, 11% each), previous trauma (n=4784, 8%), and osteonecrosis (n=763, 1%). Shoulder replacement surgery was performed bilaterally in 6159 participants. Overall, 57 601 (99%) procedures were followed by at least one night in hospital: median stay 3 nights (interquartile range 2-5 nights). The maximum follow-up was 19 years (mean 5.6 (SD 4.1)), and the maximum time recorded to revision was 16.4 years, with 1015 procedures remaining at risk beyond this time. The annual case volume increased 5.6-fold over the study period (1018 cases in 1998 to 5691 cases in 2016), with increasing numbers of procedures performed across all age groups (table 1).
Fig 1.
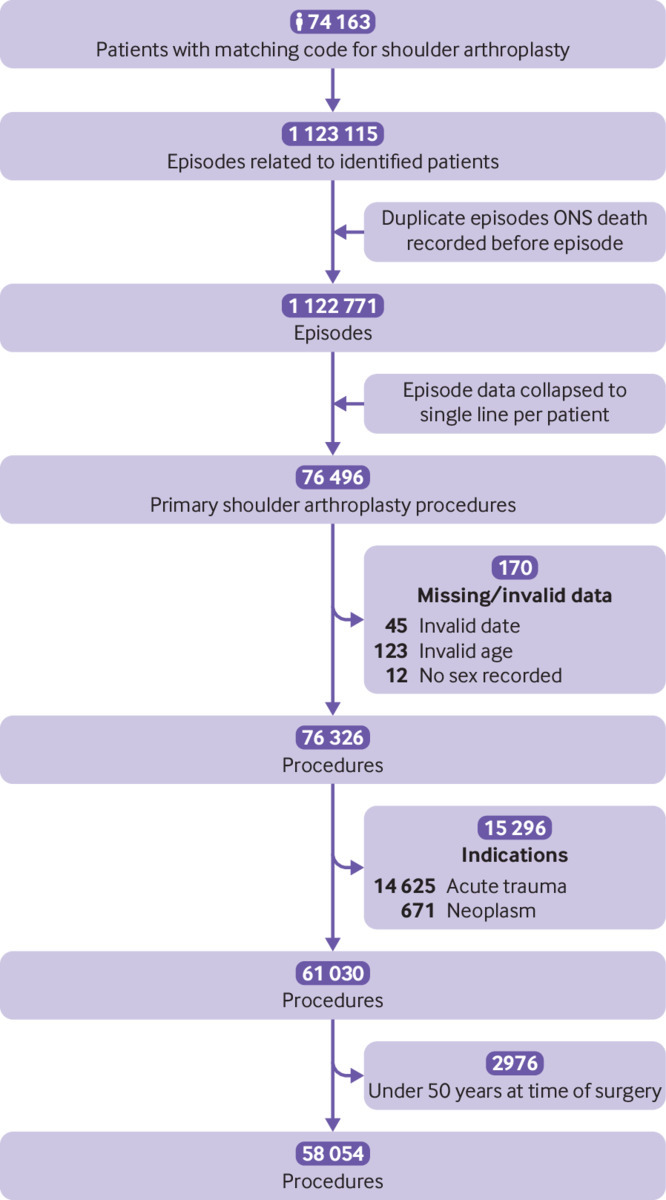
Study data flowchart
Table 1.
Number of elective shoulder replacements included by UK financial year, stratified by age and sex
| Age groups | No of elective shoulder replacements by UK financial year | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
| Men: | |||||||||||||||||||
| 50-54 | 33 | 28 | 19 | 23 | 21 | 28 | 40 | 39 | 58 | 69 | 65 | 74 | 62 | 59 | 74 | 83 | 83 | 73 | 82 |
| 55-59 | 37 | 37 | 39 | 38 | 50 | 54 | 47 | 64 | 91 | 88 | 71 | 87 | 92 | 94 | 96 | 119 | 109 | 124 | 154 |
| 60-64 | 41 | 46 | 53 | 52 | 50 | 70 | 64 | 99 | 118 | 155 | 150 | 165 | 157 | 161 | 155 | 182 | 172 | 162 | 202 |
| 65-69 | 38 | 62 | 46 | 60 | 71 | 92 | 91 | 109 | 146 | 175 | 175 | 193 | 187 | 243 | 241 | 268 | 286 | 282 | 327 |
| 70-74 | 42 | 51 | 62 | 52 | 61 | 73 | 107 | 106 | 136 | 161 | 187 | 206 | 240 | 233 | 246 | 278 | 314 | 314 | 348 |
| 75-79 | 24 | 46 | 44 | 47 | 50 | 46 | 66 | 100 | 106 | 125 | 155 | 170 | 205 | 201 | 234 | 241 | 309 | 271 | 346 |
| 80-84 | 12 | 14 | 21 | 26 | 33 | 35 | 26 | 41 | 59 | 56 | 84 | 93 | 84 | 115 | 125 | 150 | 153 | 171 | 186 |
| ≥85 | 8 | 8 | 8 | 9 | 10 | 13 | 16 | 15 | 21 | 26 | 44 | 42 | 55 | 54 | 52 | 52 | 67 | 66 | 71 |
| Women: | |||||||||||||||||||
| 50-54 | 45 | 37 | 49 | 50 | 37 | 44 | 38 | 45 | 53 | 62 | 55 | 54 | 70 | 64 | 77 | 64 | 64 | 93 | 75 |
| 55-59 | 54 | 57 | 61 | 54 | 84 | 68 | 79 | 86 | 109 | 123 | 128 | 110 | 124 | 130 | 144 | 144 | 115 | 131 | 141 |
| 60-64 | 85 | 109 | 102 | 94 | 117 | 128 | 142 | 173 | 174 | 261 | 239 | 245 | 244 | 257 | 250 | 259 | 240 | 262 | 261 |
| 65-69 | 136 | 111 | 147 | 127 | 148 | 191 | 222 | 269 | 304 | 352 | 373 | 410 | 359 | 442 | 467 | 576 | 545 | 527 | 600 |
| 70-74 | 150 | 180 | 156 | 180 | 194 | 271 | 267 | 355 | 387 | 478 | 553 | 589 | 607 | 673 | 685 | 711 | 796 | 847 | 873 |
| 75-79 | 173 | 237 | 193 | 211 | 239 | 303 | 304 | 372 | 431 | 484 | 583 | 630 | 638 | 685 | 769 | 830 | 913 | 892 | 1015 |
| 80-84 | 99 | 102 | 120 | 137 | 171 | 218 | 239 | 262 | 312 | 348 | 406 | 397 | 475 | 500 | 513 | 555 | 639 | 641 | 680 |
| ≥85 | 41 | 62 | 80 | 82 | 81 | 78 | 85 | 143 | 167 | 199 | 212 | 270 | 230 | 266 | 282 | 285 | 285 | 268 | 330 |
| Total | 1018 | 1187 | 1200 | 1242 | 1417 | 1712 | 1833 | 2278 | 2672 | 3162 | 3480 | 3735 | 3829 | 4177 | 4410 | 4797 | 5090 | 5124 | 5691 |
The overall rate of serious adverse events at 30 days was 3.5% (95% confidence interval 3.4% to 3.7%) and at 90 days was 4.6% (4.4% to 4.8%). Table 2 shows the overall estimates for specific adverse events and table 3 the age and sex stratified estimates for any adverse event. The risk of any serious adverse event within 90 days for participants aged 85 years and older was high (1 in 9 women and 1 in 5 men). Supplementary table 1 presents the specific stratified rates for all cause death, pulmonary embolism, myocardial infarction, lower respiratory tract infection, acute kidney injury, urinary tract infection, and cerebrovascular events. Logistic regression confirmed that age, sex, and Charlson comorbidity index were all statistically significant predictors of the risk of serious adverse events at both 30 and 90 days (supplementary fig 1). For women aged 50-64, the relative risk of pulmonary embolism compared with baseline was 61 (95% confidence interval 50 to 73) from day 0 to day 30 and 28 (22 to 37) for the first 90 days overall. The likelihood of dying from any cause within 90 days of surgery was 0.47% (95% confidence interval 0.39% to 0.57%). Compared with overall baseline population mortality rates published by the ONS, the relative risk of all cause death at 30 days after shoulder replacement surgery was 0.93 (95% confidence interval 0.65 to 1.32) and at 90 days was 0.63 (0.50 to 0.79).
Table 2.
Unadjusted all cause mortality and serious adverse events within 30 and 90 days of shoulder replacement surgery in 58 054 participants at risk
| Adverse event by follow-up time | No of events | Incidence: % (95% CI) |
|---|---|---|
| All cause death: | ||
| 30 days | 58* | 0.23 (0.18 to 0.30) |
| 90 days | 118* | 0.47 (0.39 to 0.57) |
| Pulmonary embolism: | ||
| 30 days | 106 | 0.18 (0.15 to 0.22) |
| 90 days | 156 | 0.27 (0.23 to 0.31) |
| Myocardial infarction: | ||
| 30 days | 121 | 0.21 (0.17 to 0.25) |
| 90 days | 161 | 0.28 (0.24 to 0.32) |
| Lower respiratory tract infection: | ||
| 30 days | 870 | 1.50 (1.40 to 1.60) |
| 90 days | 1110 | 1.91 (1.80 to 2.03) |
| Acute kidney injury: | ||
| 30 days | 475 | 0.82 (0.75 to 0.90) |
| 90 days | 565 | 0.97 (0.90 to 1.06) |
| Urinary tract infection: | ||
| 30 days | 593 | 1.02 (0.94 to 1.11) |
| 90 days | 809 | 1.39 (1.30 to 1.49) |
| Cerebrovascular event: | ||
| 30 days | 81 | 0.14 (0.11 to 0.17) |
| 90 days | 137 | 0.24 (0.20 to 0.28) |
| Any serious adverse event: | ||
| 30 days | 2045 | 3.52 (3.37 to 3.68) |
| 90 days | 2677 | 4.61 (4.44 to 4.79) |
Based on participants undergoing surgery in 2012-17 only (25 011 participants at risk).
Table 3.
Risk of any serious adverse event within 30 and 90 days of shoulder replacement surgery stratified by age and sex
| Age groups by sex | No at risk | 30 days | 90 days | |||
|---|---|---|---|---|---|---|
| No of events | Incidence: % (95% CI) | No of events | Incidence: % (95% CI) | |||
| Men: | ||||||
| 50-64 | 4758 | 75 | 1.6 (1.3 to 2.0) | 101 | 2.1 (1.7 to 2.6) | |
| 65-74 | 6309 | 147 | 2.3 (2.0 to 2.7) | 199 | 3.2 (2.7 to 3.6) | |
| 75-84 | 4270 | 252 | 5.9 (5.2 to 6.7) | 334 | 7.8 (7.0 to 8.7) | |
| ≥85 | 637 | 111 | 17.4 (14.5 to 21.0) | 135 | 21.2 (17.9 to 25.1) | |
| Women: | ||||||
| 50-64 | 6660 | 104 | 1.6 (1.3 to 1.9) | 145 | 2.2 (1.9 to 2.6) | |
| 65-74 | 15 258 | 351 | 2.3 (2.1 to 2.6) | 475 | 3.11 (2.8 to 3.4) | |
| 75-84 | 16 716 | 691 | 4.1 (3.8 to 4.5) | 903 | 5.4 (5.1 to 5.8) | |
| ≥85 | 3446 | 314 | 9.1 (8.2 to 10.2) | 385 | 11.2 (10.1 to 12.3) | |
The lifetime risk of further surgery was considerably higher in young participants compared with older participants and appears to decrease linearly (fig 2, supplementary table 2). In all age groups, the risk was slightly higher in men than in women. The lifetime risk of implant revision ranged from 2.7% (95% confidence interval 2.6% to 2.8%) in women aged 85 years and older to 23.6% (23.2% to 24.0%) in men aged 55 to 59 years. The estimates for the lifetime risk of any reoperation in the same groups were 3.7% (3.6% to 3.8%) and 31.9% (31.3% to 32.4%).
Fig 2.
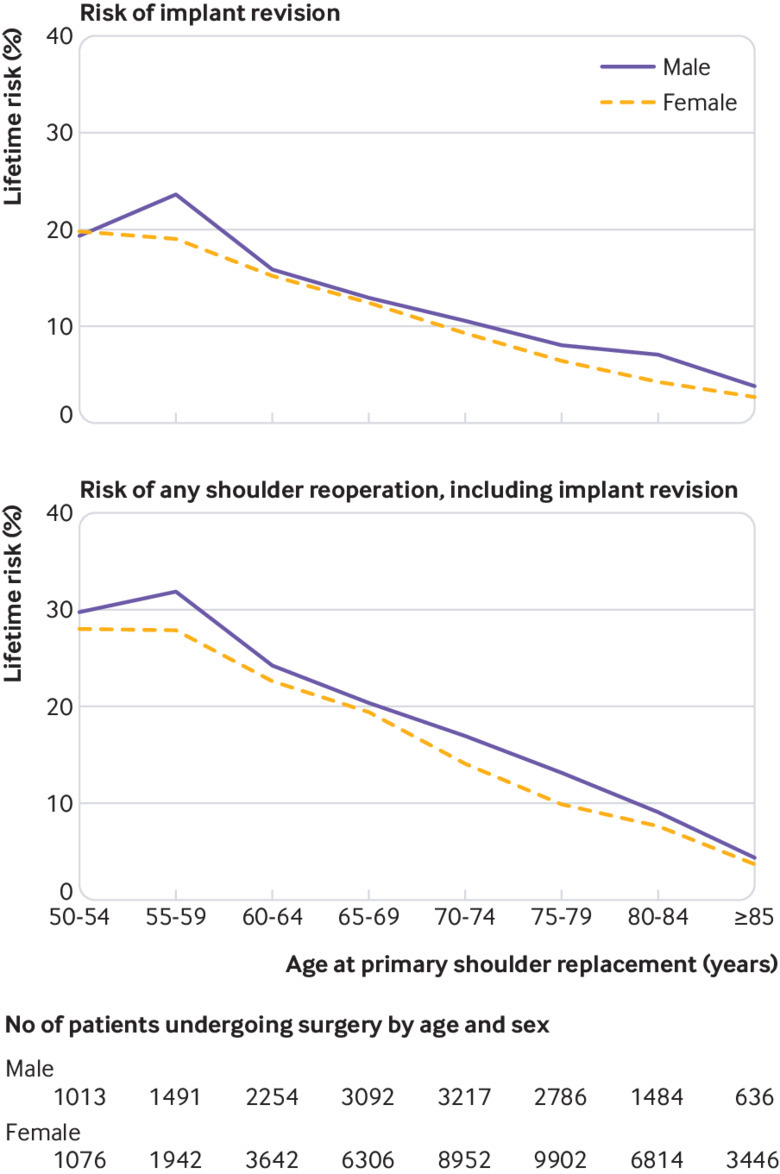
Lifetime risk of revision and reoperation after elective primary shoulder replacement, stratified by age and sex at time of primary procedure
In total, 3891 implants required revision, of which 456 were preceded by another reoperation. At the time of most recent follow-up, 1366 shoulders required other operations without implant revision. Overall revision-free implant survival rates were 90.0% (95% confidence interval 89.6% to 90.3%) at 10 years post-surgery and 87.8% (87.2% to 88.4%) at 18 years post-surgery (table 4, supplementary table 3). Nineteen per cent of participants undergoing one revision had at least one additional procedure on the same shoulder. The indication for the first recorded revision or reoperation was infection in 10.7% of cases (561/5257).
Table 4.
Unadjusted Kaplan-Meier estimates for revision-free implant survival at 5, 10, 15, and 18 years post-surgery, including numbers lost to follow-up and death
| Years post-surgery | No still at risk | No of revisions | Reason for censoring | Implant survival: % (95% CI) | |
|---|---|---|---|---|---|
| Death | Study end | ||||
| 0 | 58 054 | ||||
| 3 | 36 783 | 2646 | 3757 | 14 868 | 94.7 (94.5 to 94.9) |
| 5 | 25 858 | 631 | 2674 | 7620 | 92.8 (92.6 to 93.0) |
| 10 | 8151 | 523 | 5032 | 12 152 | 90.0 (89.6 to 90.3) |
| 15 | 1664 | 87 | 1891 | 4509 | 88.1 (87.6 to 88.7) |
| 18 | 266 | 4 | 278 | 1116 | 87.8 (87.2 to 88.4) |
Participants were at greatest risk of revision in the first five years after surgery across all age groups. Kaplan-Meier estimates for cumulative revisions at 5, 10, and 15 years, stratified by age and implant type (figs 3 and 4) confirm the clear association between age and revision risk at all time points. These plots also show that revision risk is highest soon after surgery and diminishes over time—noticeably so in older participants. The period of ongoing risk for patients aged less than 60 years seems to extend beyond 10 years of follow-up, and there may be a peak of delayed revisions in these patients.
Fig 3.
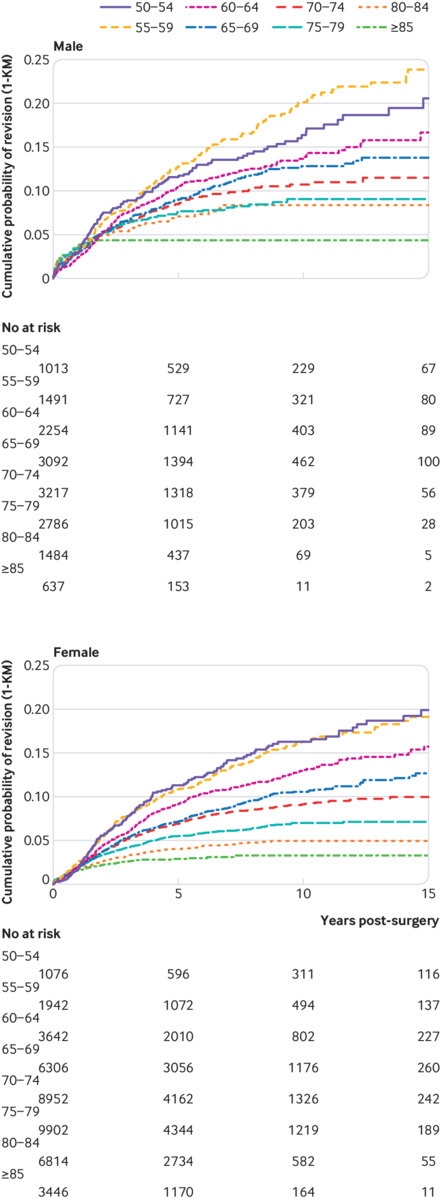
1 minus Kaplan-Meier estimates of revision risk, stratified by age and sex
Fig 4.
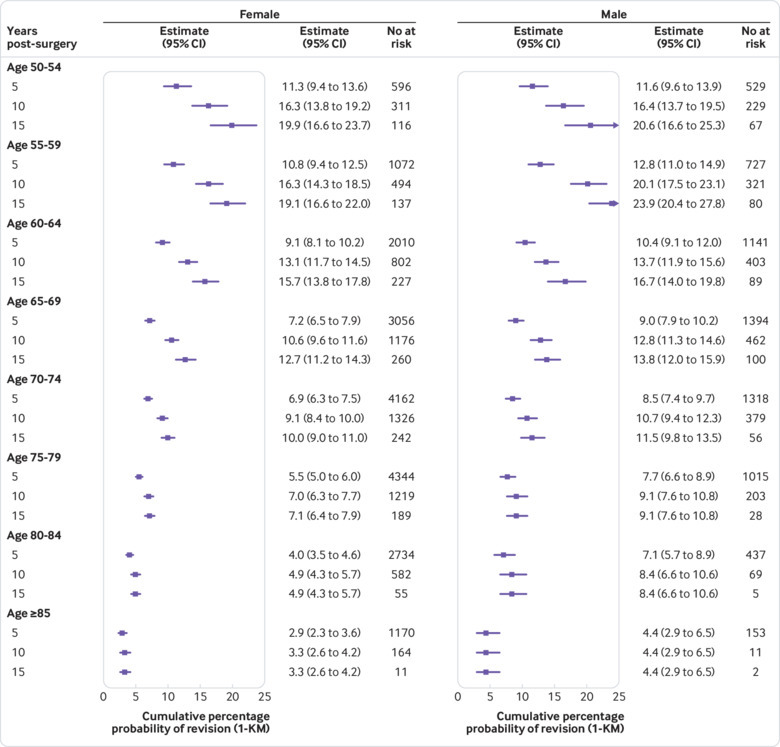
Point estimates of revision risk (95% confidence interval) at 5, 10, and 15 years, stratified by age and sex
The most common types of further non-revision surgery required after primary shoulder replacement were subacromial decompression or acromioclavicular joint excision (860 procedures, 1.5%). Manipulation under anaesthesia was also frequently performed (521 procedures, 0.9%) (table 5).
Table 5.
Most common reoperation procedures performed (excluding implant revision), grouped according to need for implant revision surgery
| Procedure type | No of revision surgeries | ||
|---|---|---|---|
| Reoperation only | Reoperation then revision | Revision then reoperation | |
| Subacromial decompression or acromioclavicular joint excision | 666 | 194 | 75 |
| Rotator cuff repair | 117 | 39 | 8 |
| Manipulation under anaesthesia | 362 | 159 | 46 |
| Washout/debridement/excision of tissue | 261 | 101 | 95 |
| Procedures for treatment of instability | 105 | 28 | 18 |
| Fixation of periprosthetic fracture | 41 | 9 | 6 |
Some participants underwent more than one additional procedure type.
Discussion
This study found that the estimated lifetime risk of revision surgery after an elective shoulder replacement is much higher in younger patients, particularly men (one in four for men aged 59 years and younger). The lifetime risk of revision varies greatly with age; patients aged 55-59 years have a fourfold risk compared with those aged 85 years and older. When all types of further shoulder surgery are considered, the lifetime risk of reoperation is as high as one in three for men aged 55-59 years. The secondary Kaplan-Meier analyses confirm that this is due to more than simply increased time at risk, with younger patients experiencing considerably higher revision rates within the first five years.
Serious adverse events have been considered low after this operation, but we found that they are more common than previously described. Inpatient care for serious medical adverse events or all cause death occurred in 46 per 1000 procedures. The most common events were lower respiratory tract infections, urinary tract infections, and acute kidney injury. Incidence rates for adverse events were considerably higher in older patients and men. Overall mortality was lower than the expected rates for the general population; however, one in 18 men aged more than 85 years had died within 90 days of surgery. Rates of pulmonary embolisms were much higher than in comparative baseline populations.
Strengths and weaknesses of this study
By using large volume population level data, we have been able to make precise estimates for both lifetime risk of revision surgery and incident rates of adverse events that are representative of real world national outcomes. The additional time-to-event estimates from routine survival analysis allow for direct comparison with other published data. This study benefits from universal coverage of a national healthcare system and has less risk of confounding from local geographical, socioeconomic, and commissioning factors than studies from single units or regions. Serious event rates and lifetime revision risks are presented here with sufficient granularity to support discussions relevant to age and sex specific patient groups.
The study methodology accounts for the competing risk of death by applying revision risk and all cause mortality to a standardised population. The estimates of lifetime risk are limited by the overall length of available follow-up; 19 years in this case. Longer follow-up may be expected to yield more accurate estimates for younger patients who are more likely to remain alive with their implant still in situ, thus remaining exposed to ongoing risk. Therefore, if anything, the lifetime risk estimates reported here represent a lower limit. Across age groups, however, the likelihood of revision surgery declines over time (figs 3 and 4), and no revisions were recorded between 16.4 and 19.0 years of follow-up. As such, we would expect any underestimate to be small. The possible exception is in men aged less than 55 years who had a slightly lower risk than the next age band. The factors influencing the likelihood of failure and the decision to undertake revision surgery in these younger patients are not fully understood.
Hospital episode statistics (HES) data rely on accurate coding of procedures by hospital trusts for them to receive correct remuneration. Although the information on procedure type might not be as detailed as that collected by dedicated joint replacement registries,27 HES data have been shown to provide a more complete record of revision arthroplasty procedures, with less risk of underreporting bias.28 Audit work from the North East Quality Observatory Service has found 96% agreement for the side of shoulder replacement surgery but only 87% agreement for the specific type of shoulder replacement performed. No procedures were, however, incorrectly identified as a shoulder replacement in a sample of 440 cases (L Lingard and A Rangan, personal communication, 2018). Stratification of results by procedure type and indication was specifically not performed within our study and could represent a source of confounding. The study results are not generalisable to patients aged less than 50 years, as we excluded them from the analysis. This patient group is considerably more heterogeneous, with more frequent inflammatory and post-traumatic indications.
No standard reference dataset exists for analysing adverse events; however, previous validation studies have found high agreement between HES data and primary care records, including rates of greater than 90% for myocardial infarction, pulmonary embolism, and cerebrovascular disease.29 Complication rates reported in this study are specifically those severe enough to warrant inpatient hospital care. We are not able to provide an estimate of the rate for all venous thromboembolism events, since many deep vein thromboses will be treated without hospital admission and not recorded, and therefore the results provide an underestimate. Lower respiratory tract infections in HES data might only represent 22% of all infections.30 Those treated in hospital, however, are likely to be more seriously ill. Likewise, chronic undiagnosed indolent prosthetic infections will lead to an underestimated prosthetic infection rate.
Comparison with other studies
The literature is dominated by unstratified time-to-event estimates of long term revision risk, which have limitations when applied across different patient groups. Revision risk is underestimated in young people, and overestimated in older patients, a group who may already be more risk averse.31 Previous studies have reported unadjusted estimates of revision-free implant survival ranging from 89% to 92% at 10 years.32 33 34 35 36 These published figures are similar to those found for our study population. The only published 20 year implant survival rates are from one centre (Mayo Clinic, MN) and indicate an 85% survival for 1431 humeral head replacements and 81% for 2588 total shoulder replacements.32 33 These estimates are lower than the mean long term implant survival estimates for our study, but the mean age of patients in these studies was 63 and 65 years, respectively; substantially lower than our study mean age of 73 years. The proportion of men was also considerably higher, which might account for the higher reported revision rates. More importantly, the Mayo Clinic report data from a single specialised centre, whereas we report more relevant and generalisable results by using national data from multiple specialist and general hospitals.
Our literature review did not identify any useful data on rates of acute kidney injury, cerebrovascular events, respiratory tract infection, or urinary tract infection. Several US studies using large nationwide discharge database samples report rates of inpatient pulmonary embolism as 0.1% to 0.3%, inpatient myocardial infarction as 0.1% to 0.6%, and all cause death as 0.1% to 0.2%.37 38 These study populations were, however, mixed and included large proportions of patients with trauma and acute fractures and those older than 85 years. These are all powerful risk factors for serious adverse events. Moreover, these studies did not capture the full period of increased risk, which is thought to extend for up to 90 days for thromboembolic disease.25 We report the incidence of pneumonia at 30 days to be three times higher than in another recent study.39 Studies that do present risk estimates at 30 and 90 days post-surgery are typically limited by small sample size and again are skewed by the inclusion of trauma and revision procedures.40 41 42 We did not include additional estimates of symptomatic deep vein thrombosis in our study.
The relative risk for pulmonary embolism compared with baseline risk was 61 in the first 30 days and 28 in the first 90 days after shoulder replacement surgery. Although this estimate is half that following hip and knee replacement surgery, it is at a surprisingly high level for previously considered lower risk surgery. In our study, mortality rates were lower than the population risk, which is similar to the findings for a study of hip replacement surgery within the same population.18 This suggests that patients undergoing elective surgery are likely to be a healthier subset.
Meaning of the study
The number of shoulder replacement procedures performed is expanding rapidly, and with growing numbers in patients aged less than 60 years as well as 80 years and older.6 43 44 All younger patients, particularly men, need to be aware of the high likelihood of early failure of their implant and the need for further and more complex revision replacement surgery. As the population ages, it is likely that demand for shoulder replacement in older people will continue to increase, and the high risk of adverse events described here should form part of shared decision making with patients. The risk levels in this study are higher than previously considered by clinicians. Patients need to be informed of these levels and carefully counselled about the potential risks of serious adverse events. The alarmingly high rates of adverse events in elderly patients with comorbidities suggests that better approaches to patient selection, preparation, and postoperative care might be required. The age and sex specific estimates in this study provide the best quality information to date for patients and clinicians to use when discussing elective shoulder replacement surgery.
Unanswered questions and future research
As future innovation will undoubtedly increase the possibilities of treatment for both younger and older patients, a pressing need remains to monitor serious adverse events and revision rates and develop prediction tools to help identify those patients most likely to benefit from surgery, while minimising such adverse events, revision surgery, and further healthcare costs. Revision shoulder replacement surgery is complex, challenging, and with greater risk, and so surgeons often encourage patients to live with shoulder disability and a failing implant. As such, revision rates might not be fully reflective of the true failure rates, which are likely to be higher, and the burden of patients living with an unsatisfactory shoulder replacement is largely unknown. The value of chemical thromboprophylaxis in shoulder surgery is uncertain and international and national variations in practice exist. This study should raise awareness further with its identified higher rates of thromboembolic events. We recommend further research to develop optimal thromboprophylaxis protocols to reduce thromboembolic events while limiting the secondary risk of bleeding complications in this patient group. If we are to continue to safely treat the growing population of older patients with multimorbidity, research is needed to aid selection, optimisation, and management of patients throughout their entire healthcare pathway to minimise the risk of serious adverse events.
What is already known on this topic
Rates of shoulder replacement surgery for arthritic conditions are increasing rapidly
No study has reported on the lifetime risk of further surgery after elective primary shoulder replacement surgery
Serious adverse events are considered rare after shoulder surgery, based on studies which typically lack sample size, adequate follow-up, or clear inclusion criteria
What this study adds
The estimated lifetime risk of revision surgery after an elective shoulder replacement is much higher in younger patients, particularly men (one in four for ages 59 years and younger)
The risks of serious adverse events within 90 days of shoulder replacement surgery are much higher than previously estimated
Rates were 4.6% overall, 0.3% for pulmonary embolism and for myocardial infarction, 1.9% for lower respiratory tract infection, 1.0% for acute kidney injury, 1.4% for urinary tract infection, 0.2% for cerebrovascular event, and 0.5% for all cause death
Acknowledgments
We thank Liz Lingard and Amar Rangan for sharing results from a 2015 Audit of the Quality of Shoulder Replacement Coding performed by the North East Quality Observatory Service, funded by Orthopaedic Research UK, and supported by the British Orthopaedic Association Patient Liaison Group and the British Elbow and Shoulder Society Council.
Web extra.
Extra material supplied by authors
Supplementary material: Additional tables, figures, and full code list
Contributors: RSC and JLR conceived the study. RSC, JLR, and GSC designed the study method. JCEL acquired the data. RSC, JCEL, and GSC performed the data analysis. RSC, AJC, DF, GSC, and JLR interpreted the data. RSC drafted the manuscript. All authors reviewed and edited the final manuscript. RSC and JLR had full access to all the data in the study. JLR is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. JCEL is a Versus Arthritis research fellow, and RSC is a Royal College of Surgeons (RCS)/National Joint Registry (NJR) research fellow. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, Department of Health, NJR steering committee, Healthcare Quality Improvement Partnership, RCS, or Versus Arthritis. The NIHR had no role in study design, data collection, data analysis, data interpretation, or report preparation.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work other than that listed above; no other financial relationships with organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the University of Oxford Research Services (project ID 12787), and the NHS Data Access Advisory Group. It was carried out in accordance with the NHS Digital data sharing agreement (DARS-NIC-29827-Q8Z7Q) and registered at ClinicalTrials.gov (NCT03573765). This study using non-identifiable records from hospital episode statistics is exempt from research ethics committee approval. Patients have the right to request that their data are not released by NHS Digital for use by researchers (register a “Type 2 opt-out”).
Data sharing: The study is based on NHS hospital episode statistics data and was provided within the terms of an NHS Digital data sharing agreement. The data do not belong to the authors and may not be shared by the authors, except in aggregate form for publication. Data can be obtained by submitting a research request through the NHS Digital Data Access Request Service.
Transparency: The guarantor (JLR) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1. Fevang B-TS, Lygre SHL, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Good function after shoulder arthroplasty. Acta Orthop 2012;83:467-73. 10.3109/17453674.2012.720118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fevang B-TS, Lygre SHL, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Pain and function in eight hundred and fifty nine patients comparing shoulder hemiprostheses, resurfacing prostheses, reversed total and conventional total prostheses. Int Orthop 2013;37:59-66. 10.1007/s00264-012-1722-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boorman RS, Kopjar B, Fehringer E, Churchill RS, Smith K, Matsen FA., 3rd The effect of total shoulder arthroplasty on self-assessed health status is comparable to that of total hip arthroplasty and coronary artery bypass grafting. J Shoulder Elbow Surg 2003;12:158-63. 10.1067/mse.2003.18. [DOI] [PubMed] [Google Scholar]
- 4. Lübbeke A, Rees JL, Barea C, Combescure C, Carr AJ, Silman AJ. International variation in shoulder arthroplasty. Acta Orthop 2017;88:592-9. 10.1080/17453674.2017.1368884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dillon MT, Chan PH, Inacio MCS, Singh A, Yian EH, Navarro RA. Yearly Trends in Elective Shoulder Arthroplasty, 2005-2013. Arthritis Care Res (Hoboken) 2017;69:1574-81. 10.1002/acr.23167. [DOI] [PubMed] [Google Scholar]
- 6. Dillon MT, Inacio MCS, Burke MF, Navarro RA, Yian EH. Shoulder arthroplasty in patients 59 years of age and younger. J Shoulder Elbow Surg 2013;22:1338-44. 10.1016/j.jse.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Good GMC. Medical Practice. www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/good-medical-practice (accessed 26 Jul 2018).
- 8.Consent GMC. Patients and doctors making decisions together. www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/consent (accessed 26 Jul 2018).
- 9. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med 2014;161:270-80. 10.7326/M14-0295. [DOI] [PubMed] [Google Scholar]
- 10.Khan T. Survival analysis of time-to-event data in orthopaedic surgery. Bone Jt 360 2017;6:37-9. https://online.boneandjoint.org.uk/doi/abs/10.1302/2048-0105.62.360517
- 11. Fortin JM, Hirota LK, Bond BE, O’Connor AM, Col NF. Identifying patient preferences for communicating risk estimates: a descriptive pilot study. BMC Med Inform Decis Mak 2001;1:2. 10.1186/1472-6947-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayliss LE, Culliford D, Monk AP, et al. Articles The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet. Published Online First, 2017, 10.1016/S0140-6736(17)30059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jameson SS, James P, Howcroft DWJ, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg 2011;20:764-70. 10.1016/j.jse.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 14.NHS Digital. Hospital Episode Statistics. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 26 Jul 2018).
- 15.NJR. Third Annual NJR Data Quality Audit launched. www.njrcentre.org.uk/njrcentre/NewsandEvents/201718DataQualityAuditlaunched/tabid/1450/Default.aspx (accessed 1 Feb 2018).
- 16. Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data Resource Profile: Hospital Episode Statistics Admitted Patient Care (HES APC). Int J Epidemiol 2017;46:1093-1093i. 10.1093/ije/dyx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Office for National Statistics. Mortality statistics - underlying cause, sex and age 2016. www.nomisweb.co.uk (accessed 4 Sep 2018).
- 18. Hunt LP, Ben-Shlomo Y, Clark EM, et al. National Joint Registry for England, Wales and Northern Ireland 90-day mortality after 409,096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis. Lancet 2013;382:1097-104. 10.1016/S0140-6736(13)61749-3. [DOI] [PubMed] [Google Scholar]
- 19. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676-82. 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 20.NJR. National Joint Registry for England, Wales and Northern Ireland. 14th Annual Report. 2017.
- 21. Esteve J, Benhamou E, Raymond L. Descriptive Epidemiology. Vol 128, Lyon, International Agency for Research on Cancer, 1994. (IARC Scientific Publications.). [PubMed] [Google Scholar]
- 22. Schouten LJ, Straatman H, Kiemeney LA, Verbeek AL. Cancer incidence: life table risk versus cumulative risk. J Epidemiol Community Health 1994;48:596-600. 10.1136/jech.48.6.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasieni PD, Adams J. Standardized lifetime risk. Am J Epidemiol 1999;149:869-75. 10.1093/oxfordjournals.aje.a009903 [DOI] [PubMed] [Google Scholar]
- 24.Cancer Research UK. Lifetime risk calculations. www.cancerresearchuk.org/sites/default/files/cs_lifetimerisk.xlsx (accessed 4 Apr 2018).
- 25. Sweetland S, Green J, Liu B, et al. Million Women Study collaborators Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 2009;339:b4583. 10.1136/bmj.b4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rangan A, Upadhaya S, Regan S, Toye F, Rees JL. Research priorities for shoulder surgery: results of the 2015 James Lind Alliance patient and clinician priority setting partnership. BMJ Open 2016;6:e010412. 10.1136/bmjopen-2015-010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NJR. National Joint Registry for England and Wales. 6th Annual Report. 2009.
- 28.NJR. National Joint Registry for England and Wales. 9th Annual Report. 2012.
- 29. Wright FL, Green J, Canoy D, Cairns BJ, Balkwill A, Beral V, Million Women Study Collaborators Vascular disease in women: comparison of diagnoses in hospital episode statistics and general practice records in England. BMC Med Res Methodol 2012;12:161. 10.1186/1471-2288-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet 1987;1:671-4. 10.1016/S0140-6736(87)90430-2 [DOI] [PubMed] [Google Scholar]
- 31. Mata R, Josef AK, Hertwig R. Propensity for Risk Taking Across the Life Span and Around the Globe. Psychol Sci 2016;27:231-43. 10.1177/0956797615617811. [DOI] [PubMed] [Google Scholar]
- 32. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br 2011;93:1513-7. 10.1302/0301-620X.93B11.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh JA, Sperling JW, Cofield RH. Risk factors for revision surgery after humeral head replacement: 1,431 shoulders over 3 decades. J Shoulder Elbow Surg 2012;21:1039-44. 10.1016/j.jse.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am 2006;88:1742-7. 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 35.Rothwell A, Hobbs T, Frampton C, et al. The New Zealand Joint Registry Eighteen Year Report - January 1999 to December 2016. 2017.
- 36. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res 2011;469:2469-75. 10.1007/s11999-011-1833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy AB, Menendez ME, Watson SL, Ponce BA. Metabolic syndrome and shoulder arthroplasty: epidemiology and peri-operative outcomes. Int Orthop 2016;40:1927-33. 10.1007/s00264-016-3214-3. [DOI] [PubMed] [Google Scholar]
- 38. Jiang JJ, Toor AS, Shi LL, Koh JL. Analysis of perioperative complications in patients after total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1852-9. 10.1016/j.jse.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 39. Anthony CA, Westermann RW, Gao Y, Pugely AJ, Wolf BR, Hettrich CM. What Are Risk Factors for 30-day Morbidity and Transfusion in Total Shoulder Arthroplasty? A Review of 1922 Cases. Clin Orthop Relat Res 2015;473:2099-105. 10.1007/s11999-014-4107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newman JM, Stroud SG, Yang A, et al. Total shoulder arthroplasty in octogenarians: Is there a higher risk of adverse outcomes? J Orthop 2018;15:671-5. 10.1016/j.jor.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cancienne JM, Brockmeier SF, Gulotta LV, Dines DM, Werner BC. Ambulatory Total Shoulder Arthroplasty: A Comprehensive Analysis of Current Trends, Complications, Readmissions, and Costs. J Bone Joint Surg Am 2017;99:629-37. 10.2106/JBJS.16.00287. [DOI] [PubMed] [Google Scholar]
- 42. Tashjian RZ, Lilly DT, Isaacson AM, et al. Incidence of and Risk Factors for Symptomatic Venous Thromboembolism After Shoulder Arthroplasty. Am J Orthop (Belle Mead NJ) 2016;45:E379-85. [PubMed] [Google Scholar]
- 43. Iriberri I, Candrian C, Freehill MT, Raiss P, Boileau P, Walch G. Anatomic shoulder replacement for primary osteoarthritis in patients over 80 years: outcome is as good as in younger patients. Acta Orthop 2015;86:298-302. 10.3109/17453674.2015.1006036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Churchill RS. Elective shoulder arthroplasty in patients older than ninety years of age. J Shoulder Elbow Surg 2008;17:376-9. 10.1016/j.jse.2007.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Additional tables, figures, and full code list


