To the editor
The cell cycle mechanisms coordinating proliferation with differentiation in epidermis remain intriguing and somewhat controversial. For decades it was assumed that differentiating keratinocytes underwent growth and cell cycle arrest in G0. This concept was supported by circumstantial data, namely the loss of proliferative capacity and the difficult detection of nucleotide incorporation in differentiating keratinocytes. However, older reports showing frequent mitotic figures in suprabasal layers of the skin and various other observations suggested otherwise (reviewed in [1]). We have obtained a large body of evidence for a role of mitotic checkpoints in squamous differentiation [2–5]. Our studies consistently showed that after a sustained block of mitosis keratinocytes undergo mitotic slippage or mitotic bypass, continue DNA replication and become tetraploid (4N) or polyploid (>4N) by a process known as endoreplication [1]. Whether the keratinocyte limiting factor is in G0/G1 or in G2/M is an important issue because it determines the way we understand homeostasis control and the targets that we should attack in disease. In addition, endoreplication provides a mechanism for the well known increase of cell size in differentiating keratinocytes. In a recent paper, Quek et al [6] report that two regulators of mitotic anaphase, CDC20 and CDH1, co-activators of the anaphase promoting complex (APC), influence differentiation in human keratinocytes. CDC20 is also a component of the mitotic checkpoint complex and participates in the spindle assembly checkpoint (SAC). This further supports a model in which mitosis is a limiting factor to keratinocyte cell fate. However, contrary to our findings, Quek et al state that differentiating keratinocytes stay in G0/G1 with no signs of polyploidy. Their results are at variance with considerable data obtained by ourselves and others that are incompatible with a G0/G1 arrest in differentiating keratinocytes (refs in [1]). Here, we aim to reconcile these apparent contradictory data. In 2000 we reported that the mitotic inhibitory drug Nocodazole (Nz) rapidly induces keratinocyte differentiation and a striking increase in the proportion of polyploid cells [7]. We suggested that this was a normal process during keratinocyte differentiation. While Quek et al reproduce Nz-induced differentiation, they do not detect polyploidy upon the mitosis blockade. Unfortunately, the DNA content profiles are not shown. They further report lack of polyploidy in keratinocytes isolated from human skin. As explained elsewhere [1], it is technically difficult to obtain a significant proportion of differentiating (ergo polyploid) cells from the skin due to their strong attachments to each other. Harsh trypsin treatments break cells and nuclei. An intermediate treatment however allows a certain population of polyploid cells. This fraction increases along with differentiation [2]. It is unclear whether Quek et al had a significant proportion of differentiating cells in their samples. We also found a fraction of polyploid nuclei and chromosomal amplifications in situ [2]. Like us, Quek et al made use of the Rheinwald method [8]. In these conditions keratinocytes are cultured in the presence of “high calcium” concentration (1,2 mM), serum and a fibroblastic feeder layer which allow stratification. Quek et al do not find polyploid cells even in differentiating colonies (“paraclones”). We cannot reconcile this with our results and with the fact that binucleated and multinucleated keratinocytes or a large nucleus are very frequently observed in Rheinwald primary cultures and also (less easily) on skin sections in situ (Figure 1). Moreover, binucleated and multinucleated keratinocytes are clearly visible in the cultures treated by Quek et al with Nz or left untreated (also upon inhibition of CDC20). A proportion of cells beyond 4N is also evident in dot-plot flow-cytometry analyses for DNA content in their Supplementary Figures, although not shown in the corresponding profiles. Consistently, in their figures the cell cycle positive marker Ki67 coexists with the postmitotic differentiation marker keratin K10 in peribasal epidermal cells. Quek et al report that inhibition of CDC20 or CDH1 in keratinocytes induces differentiation or proliferation, respectively. Consistently, when we inhibited mitotic checkpoint kinases Cdk1, PLk1 or AURKB cells rapidly underwent terminal differentiation (Table 1 [9];). Treatment with genotoxic agents also induced differentiation within 48 h [2,9]. This suggests that keratinocyte differentiation responds to DNA damage through mitosis checkpoints [3]. All treatments above caused a striking increase in the proportion of polyploid cells (Table 1; Figure 1(g)). In contrast, blocking parallel cells in G1/S for 48 h neither induced polyploidisation nor differentiation. Quek et al also show this when inhibiting S phase by a thymidine block. Therefore we welcome their work as it further supports the role of mitosis checkpoints in epidermal cell fate that we find. However, some apparent discrepancies would need to be resolved. How cells can contain 2N DNA content upon Nz or inhibition of CDC20, treatments that impair mitosis and trigger terminal differentiation, therefore suppressing cell division. Or why, according to their report, mitotic (CDC20 expressing) cells are frequent in the peribasal differentiating layers of the epidermis while they find nearly no 4N cells in cell isolates. Or why they find on and off (cyclic) expression of CDH1 in suprabasal cells. We also found mitotic Cyclins A and B mainly in peribasal layers and heterogeneous expression of cell cycle proteins pH3, pRb, Cyclin E, CDC14A, Ndc80 or AURKB in more suprabasal layers [2]. These are observations further supporting that differentiating keratinocytes arrest in a special 4N state leading to intermittent DNA replication. This in turn explains the significant increase in cellular size of differentiating keratinocytes, a phenomenon hardly compatible with a G0/G1 arrest. Interestingly, we have found a similar DNA damage-induced mitosis-differentiation response involving polyploidy in keratinocytes of the skin and head and neck epithelia [5]. Other human cell types are well known to become polyploid (megakaryocytes, hepatocytes). Moreover, new cell types are progressively added to the list (cardiomyocytes, mammary gland, urothelium [3,10]). These tissues share important features with the epidermis, namely cell expansion, cell renewal and cell differentiation.
Figure 1.
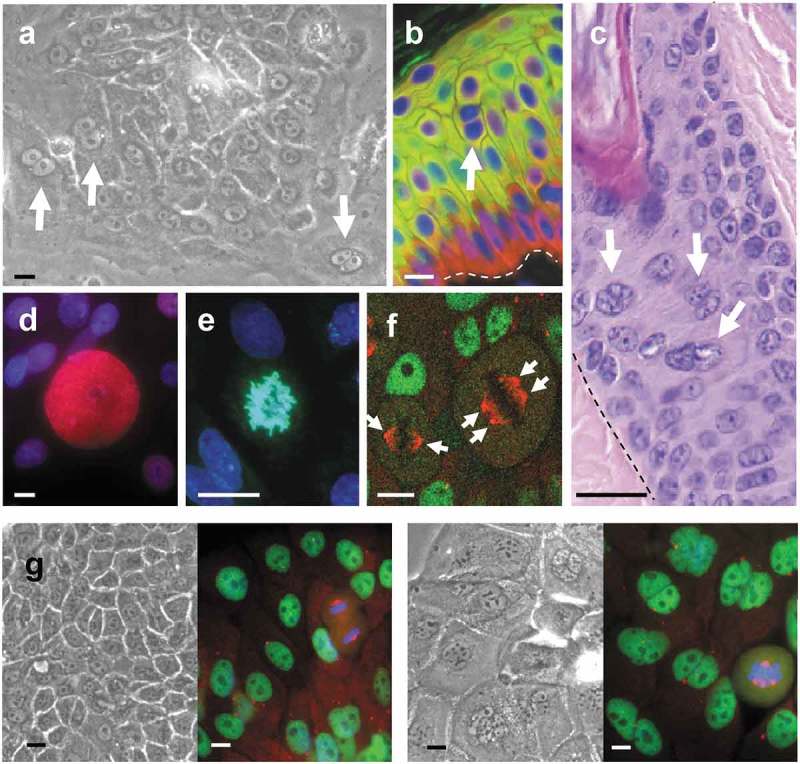
Keratinocytes often contain various or large nuclei. Cells with two or more nuclei (arrows) are frequent in keratinocyte cultures (a) and can be found in human (b) and mouse (c) epidermis in situ. Giant suprababasal nuclei (d) or aberrant polyploid mitosis (e,f) are also frequent in culture. (a) Phase contrast microscopy of freshly isolated keratinocytes of the skin in culture. (b) Epidermis in a microsection of human skin labeled by immunofluorescence for basal marker keratin K5 (red) and postmitotic differentiation marker keratin K1 (green). Dapi in blue for nuclear DNA. (c). Haematoxylin/eosin staining of a paraffin crosscut microsection of a mouse hair follicle. (d) Human primary keratinocytes (as in e-g) labeled by immmunofluorescence for DNA replication regulator Cyclin E (red) (Dapi in blue). Note the giant Cyclin E expressing suprabasal nucleus over smaller proliferative cells. (e) Aberrant polyploid metaphase. (Dapi in blue). (f) Double immunofluorescence for chromatin insulator CTCF (green, interphasic nuclei) and γ-tubulin (red, metaphases). Note a large metaphase with four centrosomes (arrows) next to a diploid metaphase with two centrosomes. (g) Phase contrast and immunofluorescence, as in f, of primary keratinocytes treated with vehicle only (DMSO; left) or with ZM447439 inhibitor of mitotic AURKB (right). Scale bars; 20 µm. Broken line in b and c: basement membrane.
Table 1.
Mitotic impairment induces keratinocyte differentiation and polyploidy.
 |
Funding Statement
This work was supported by the Instituto de Salud Carlos III-FIS/FEDER [PI14/00900 and PI17/01307].
Acknowledgments
We apologise to authors we did not cite for lack of space. This work was funded by Instituto de Salud Carlos III-FIS/FEDER, grants PI14/00900 and PI17/01307 (Spain). NS is recipient of a scholarship (Personal Investigador en formación Predoctoral) from Universidad de Cantabria/IDIVAL/Council of Cantabria (Spain). We thank James T Elder and Paolo Dotto for critical reading of the manuscript and all the past and present members of the lab for their work.
References
- [1].Gandarillas A, Freije A.. Cycling up the epidermis: reconciling 100 years of debate. Exp Dermatol. 2014. February;23(2):87–91. [DOI] [PubMed] [Google Scholar]
- [2].Zanet J, Freije A, Ruiz M, et al. A mitosis block links active cell cycle with human epidermal differentiation and results in endoreplication. PLoS One. 2010;5(12):e15701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gandarillas A, Molinuevo R, Sanz-Gómez N.. Mammalian endoreplication emerges to reveal a potential developmental timer. Cell Death Differ. 2018. March;25(3):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Pedro I, Alonso-Lecue P, Sanz-Gomez N, et al. Sublethal uv irradiation induces squamous differentiation via a p53-independent, dna damage-mitosis checkpoint. Cell Death Dis. 2018 Oct 25;9(11):1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sanz-Gomez N, Freije A, Ceballos L, et al. Response of head and neck epithelial cells to a DNA damage-differentiation checkpoint involving polyploidization. Head Neck. 2018. Nov;40(11):2487–2497 [DOI] [PubMed] [Google Scholar]
- [6].Quek LS, Grasset N, Jasmen JB, et al. Dual role of the anaphase promoting complex/cyclosome in regulating stemness and differentiation in human primary keratinocytes. J Invest Dermatol. 2018 Aug;138(8):1851–1861. [DOI] [PubMed] [Google Scholar]
- [7].Gandarillas A, Davies D, Blanchard JM. Normal and c-Myc-promoted human keratinocyte differentiation both occur via a novel cell cycle involving cellular growth and endoreplication. Oncogene. 2000. July 6;19(29):3278–3289. [DOI] [PubMed] [Google Scholar]
- [8].Rheinwald JG. Methods for clonal growth and serial cultivation of normal human epidermal keratinocytes and mesothelial cells In: Baserga R, editor. Cell growth and division. Oxford: IRL Press; 1989. p. 81–94. [Google Scholar]
- [9].Freije A, Ceballos L, Coisy M, et al. Cyclin E drives human keratinocyte growth into differentiation. Oncogene. 2012. December 13;31(50):5180–5192. [DOI] [PubMed] [Google Scholar]
- [10].Wang J, Batourina E, Schneider K, et al. Polyploid superficial cells that maintain the urothelial barrier are produced via incomplete cytokinesis and endoreplication. Cell Rep. 2018. October 9;25(2):464–477 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]


