Abstract
Novel insights into the pathophysiology of primary central nervous system lymphoma (PCNSL) have identified the B-cell receptor and Toll-like receptor pathway as well as immune evasion and suppressed tumor immune microenvironment as a key mechanism in the pathogenesis of PCNSL. Small molecules and novel agents targeting these aberrant pathways have been introduced into clinical trials targeting the recurrent or refractory PCNSL patient population. Agents like the Bruton tyrosine kinase (BTK) inhibitor ibrutinib or immunomodulatory drugs (IMiDs) like pomalidomide and lenalidomide have shown promising high response rates in the salvage setting. Here, we give an overview about the recent, exciting developments in PCNSL and summarize the results of clinical trials using novel agents in the recurrent and refractory salvage setting, which include immune checkpoint inhibitors, IMiDs, as well as BTK, phosphatidylinositol-3 kinase, and mammalian target of rapamycin inhibitors.
Keywords: BTK, immune checkpoint inhibition, IMiD, PCNSL, targeted agents
For decades, there has been limited insight into the pathophysiology of primary central nervous system lymphoma (PCNSL), mainly due to the absence of comprehensive, multidimensional data, small clinical trials, and lack of correlation of histologic/genetic data with clinical outcomes. Moreover, tissue availability has been limited, since most patients undergo stereotactic biopsy at first diagnosis rather than surgical resection and the tumor tissue is often exhausted for histopathological diagnosis. Recently, there has been a surge of novel insights based on large-scale genomic investigations using archival tissue banks. These studies have identified novel drivers of PCNSL and these alterations are now being targeted with small molecular inhibitors and immune checkpoint inhibitors in clinical trials for patients with refractory and relapsed disease for which treatment options have been limited.
Pathophysiology of PCNSL
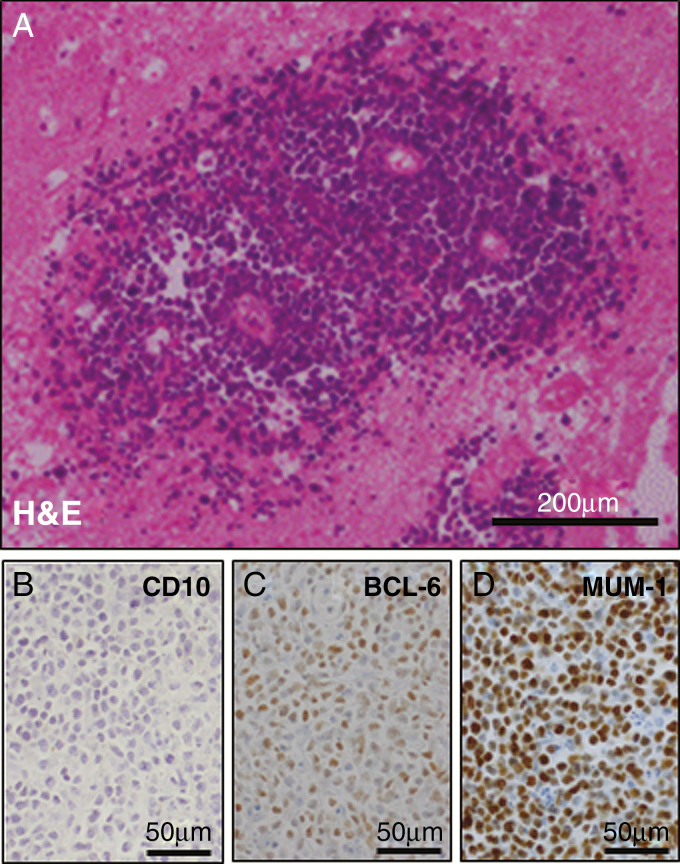
Pathologic review of PCNSLs reveals a diffuse large B-cell lymphoma (DLBCL) in most of the cases (90%). On rare occasions, Burkitt, low-grade, or T-cell lymphoma are observed.1 PCNSL cells are highly proliferative and grow in a perivascular growth pattern diffusely infiltrating the brain parenchyma (Fig. 1A). Gene expression profiling of systemic DLBCL tissue samples has established 3 molecular subgroups: (i) germinal center B-cell–like (GCB), (ii) activated B-cell–like (ABC)/nongerminal center (NGC), and (iii) type 3 subgroups.2 Immunohistochemical (IHC) markers (cluster of differentiation 10 [CD10], B-cell lymphoma 6 protein [BCL6], and multiple myeloma oncogene 1 [MUM1], also called interferon regulatory factor 4 [IRF4]) have been used to distinguish these DLBCL subtypes in tissue samples.3 Applying these markers on PCNSL specimens (Fig. 1B–D) demonstrates that the majority (>85%) of PCNSLs can be classified as NGC subtype,4,5 even though gene expression studies have identified an activated germinal center signature.6 Systemically, the NGC/ABC subtype is associated with inferior clinical outcome and frequent mutations within the B-cell receptor (BCR) pathway.7 Recently a large methylation study involving 95 PCNSLs, 73 systemic DLBCLs, and 7 DLBCLs with CNS involvement identified a significantly larger number of methylated cytosine-phosphate-guanine sites in PCNSL than in systemic DLBCL. Unsupervised clustering did not entirely separate PCNSL from DLBCL samples, but this study suggests that PCNSL might be a biologically distinct entity.
Fig. 1.
Histologic Features of PCNSL. (A) Hematoxylin/eosin (H&E) staining demonstrating the typical perivascular growth of PCNSL. (B–D) Diffuse large B-cell lymphoma subgroup determination using 3 immunohistochemical markers (CD10, BCL6, and MUM1) and applying the Hans algorithm.3 The majority of PCNSLs are classified as nongerminal center/activated B-cell subtype and display a similar staining pattern as displayed in (B) CD10 negative, (C) BCL-6 positive, and (D) MUM1 positive.
Novel Insights
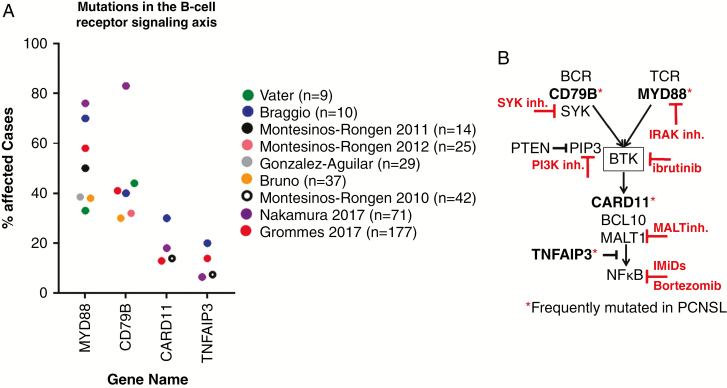
In PCNSL cohorts5,8–14 the BCR signaling axis, particularly MYD88, CD79B, and less frequently CARD11 and TNFAIP3 (Fig. 2A) are frequently affected by recurrent genomic alterations. The Toll-like receptor (TLR) signaling pathway is constitutively activated by MYD88 mutations.15 The BCR signaling pathway is activated by CD79B16 and CARD1117 mutations. Activation of both the TLR and BCR pathways leads to robust nuclear factor kappaB (NFκB) activity. Moreover, a negative regulator of NFκB, tumor necrosis factor alpha induced protein 3 (TNFAIP3) (also known as A20), is inactivated due to deletion or mutation, further amplifying NFκB activity.18MYD88 and CD79B mutations are enriched in ABC/NGC PCNSLs and are more frequently observed than in ABC DLBCL outside the CNS.19 Therefore, PCNSL more closely resembles lymphomas found in other immune-privileged organs like the testes, in which MYD88 and combined MYD88/CD79B mutations are reported in >70% of samples.20,21 Of note, MYD88 and/or CD79B mutations were also identified in PCNSL of the GCB subtype.5,22MYD88 and CD79B mutations are characterized as missense mutations and mainly found at hotspot locations (MYD88 at L265P and CD79B at Y196). IHC staining for MUM1, a transcriptional target of NFκB, is positive in 70–95%1,5,23 of PCNSL tissue samples, further suggesting that aberrant activation of the BCR signaling axis is a significant driver of PCNSL pathophysiology. The BCR signaling pathway can potentially be targeted at different signaling nodes (Fig. 2B). Upstream inhibition could target the spleen tyrosine kinase, phosphatidylinositol-3 kinase (PI3K), Bruton tyrosine kinase (BTK) or interleukin 1 receptor-associated kinase. Downstream, the pathway could be inhibited by immunomodulatory drugs (IMiDs) like thalidomide and its analogues lenalidomide and pomalidomide, which inhibit IRF4, or inhibitors of mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1). NFκB transcription factors are retained in cytoplasm by inhibitory kappaB (IkappaB). IkappaB kinase phosphorylates IkappaB, which then is degraded by proteasome. This allows NFκB transcription factors to enter the nucleus, resulting in alteration of gene expression. The proteasome-mediated hydrolysis of IkappaB, therefore, might be another aspect of the BCR signaling axis that could be targeted by proteasome inhibitors like bortezomib. Activity of current proteasome inhibitors might be limited due to poor CNS penetration, but novel agents with better blood–brain barrier penetration might be active in PCNSL.
Fig. 2.
Genomic alterations frequently target the BCR signaling axis. (A) Members of the BCR signaling axis are frequently mutated. Shown are the mutation frequency of BCR pathway members in PCNSL as identified by different sequencing projects and includes only single nucleotide variants but no copy number alterations (y-axis: percent affected cases in each study, range: 9–177 patients; x-axis: genes affected by genomic alteration). (B) Cartoon of the BCR/NFκB signaling axis. Genes affected by frequent genomic alterations in PCNSL are highlighted with red asterisks. Targetable signaling nodes are highlighted.
Investigations in copy number variations have been completed in cohorts of up to 22 PCNSL patients and reported chromosomal losses at 6q and gains at 12q, 18q, 19q, and 22q.24–28TNFAIP3 is located on chromosome 6q, and the frequent loss of this genomic region might further lead to NFκB activation. Frequent copy number gains at chromosome 9p24.1, which includes the programmed death ligand 1 and 2 (PD-L1/PD-L2) locus, have been observed.29 Moreover, the investigators identified novel chromosomal translocation involving the PD-L1 and PD-L2 loci in PCNSL samples, suggesting that immune evasion may play a role in PCNSL. A recent French study identified a novel recurrent gene fusion, E26 transformation-specific translocation variant 6–immunoglobulin heavy chain (ETV6-IgH) in 13 of 72 (18%) PCNSL samples and the gene fusion was associated with prolonged survival.30
Moreover, aberrant somatic hypermutation (aSHM), which has been found to be a prominent feature in systemic DLBCL, has also been identified in PCNSL.8,31,32 Aberrant SHM has been characterized by mutations within the first 2000 base pairs of the transcription start site that occur within a WGYR motif. The aSHM causes mainly transition (change from purine nucleotide to another purine) versus transversion (change from a purine to pyrimidine or vice versa) mutations and more frequently C/T versus A/G mutations. Aberrant SHM frequently affects PIM1, XBP1, BTG2, PRDM1, NFKBIE, TOX, and IRF4 in PCNSL.5,22 The common occurrence of aSHM, perhaps causing an increase in the mutational load, in combination with frequent copy number gain at chromosome 9p24.1 might increase the susceptibility of PCNSL to immune checkpoint inhibitors like pembrolizumab and nivolumab.
An additional molecular feature described in PCNSL is the frequent inactivation of CDKN2A through mutations or deletion.12,13,33 This genomic alteration could potentially be exploited therapeutically through cyclin-dependent kinase inhibitors (CDKNs), like abemaciclib, which has been FDA approved for the treatment of hormone-positive, human epidermal growth factor receptor 2–negative advance or metastatic breast cancer.
By using IHC, 41.8–93% of PCNSLs are found to express B-cell lymphoma 2 (BCL-2).1,23,34 One study suggests that high BCL-2 expression in PCNSL is associated with a poor prognosis.34 BCL-2 can be targeted by the small molecule venetoclax, a highly selective BCL-2 inhibitor that has been FDA approved for the treatment of chronic lymphocytic leukemia (CLL). Of note, response to venetoclax is not necessarily dependent on the degree of BCL-2 expression. Even though BCL-2 expression is higher in follicular lymphoma than in CLL, venetoclax treatment leads to better responses in CLL. In animal models, venetoclax seem to have limited CNS penetration.35
Current Salvage Therapy Options
Treatment of refractory and relapsed PCNSL has largely been based on the experience gathered in numerous small retrospective studies (Table 1). Whole brain radiation therapy (WBRT), in previously unirradiated patients, and high-dose methotrexate (HD-MTX) rechallenge have been used successfully. Rechallenging recurrent PCNSL with HD-MTX led to an overall response rate (ORR) of 85–91%,36,37 associated with a median overall survival (OS) of 41–62 months. A high ORR of 74–79% was also observed after salvage WBRT with a median OS of 10–16 months.38,39 Salvage WBRT might therefore be a treatment option for those recurrent PCNSLs that have not received radiation as a part of the initial treatment regimen. The clinical efficacy of HD-MTX rechallenge or WBRT has not been evaluated in prospective clinical trials. However, HD-MTX rechallenge is frequently used in relapsed PCNSL patients, especially if the time period between remission after initial HD-MTX therapy and recurrence is >1 year and the patient has responded well to the initial HD-MTX chemotherapy regimen. A French registry for PCNSL demonstrated that recurrent PCNSL have a better response to salvage therapy and improved OS if they had a good performance status (KPS ≥ 70), had been sensitive to the first-line treatment, had a longer than 1-year duration of first remission, and received salvage therapy at recurrence.40 In some centers, a high-dose ifosfamide-based chemotherapy regimen (ifosfamide, carboplatin, etoposide [ICE]41 or rituximab, ifosfamide, etoposide [R-IE])42 has been used at first recurrence. The R-IE combination led to an ORR of 41% in a multicenter series including 22 patients.42
Table 1.
Salvage regimen in PCNSL
| Author | Agents | # of Patients | ORR (PR+CR) | Median PFS, mo | Median OS, mo |
|---|---|---|---|---|---|
| Retrospective | |||||
| Herrlinger et al31 | PCV | 7 | 6/7 (86%) | NR | 39 |
| Arellano-Rodrigo et al32 | eto+ifos+AraC | 16 | 6/16 (37%) | 4.5 | 6 |
| Wong et al33 | Ritux+temozolomide | 7 | 7/7 (100%) | 6 | 8 |
| Enting et al34 | Ritux+temozolomide | 15 | 8/15 (53%) | 2.2 | 13.6 |
| Plotkin et al36 | HD-MTX | 22 | 20/22 (91%) | 25.8 | 61.9 |
| Nguyen et al39 | WBRT | 27 | 20/27 (74%) | 9.7 | 10.9 |
| Hottinger et al38 | WBRT | 48 | 38/48 (79%) | 10 | 16 |
| Makino et al34 | Temozolomide | 17 | 8/17 (47%) | 1.9 | 6.7 |
| Wong et al55 | Temozolomide | 7 | 1/7 (14%) | 2 | 4 |
| Zhang et al56 | Pemetrexed | 30 (18 PCNSL) | 18/30 (60%) | 4.1 | 22.6 |
| Pentsova et al37 | HD-MTX | 39 | 33/39 (85%) | 16 | 41 |
| Chamberlain57 | Bendamustine | 12 | 6/12 (50%) | 3.5 | 5 |
| Houillier et al58 | Lenalidomide | 6 | 3/6 (50%) | 1.5 | 2.5 |
| Chamberlain59 | AraC | 14 | 5/14 (36%) | 3 | 12 |
| Chamoun et al60 | Ibrutinib | 14 (13 PCNSL) | 7/14 (50%) | NR | NR |
| Prospective | |||||
| Fischer et al46 | Topotecan | 27 | 9/27 (33%) | 2 | 8.4 |
| Voloschin et al47 | Topotecan | 15 | 6/15 (40%) | 2 (60 d) | 32.7 |
| Reni et al48 | Temozolomide | 36 | 11/36 (31%) | 2.8 | 3.9 |
| Soussain et al43 | CYVE+SCT | 43 | 20/40 (50%) | 11.6 | 18.3 |
| Batchelor et al49 | Ritux | 12 | 5/12 (42%) | 1.9 (57 d) | 20.9 |
| Raizer et al45 | Pemetrexed | 11 | 6/11 (55%) | 5.7 | 10.1 |
| Rubenstein et al61 | IT Ritux+ IT M | 14 (6 PCNSL) | 6/14 (43%) | 1.2 | NR |
| Nayak et al50 | Ritux+temozolomide+pred | 16 | 5/14 (36%) | 1.6 (7 wk) | NR |
| Korfel et al62 | Temsirolimus | 37 | 20/37 (54%) | 2.1 | 3.7 |
| Grommes et al5 | Ibrutinib | 20 (13 PCNSL) | 10/13 (77%) | 4.6 | 15 (PCNSL) |
| Rubenstein et al70 | Lenalidomide | 14 | 9/14 (64%) | 6 | NR |
| Tun et al71 | Pomalidomide | 25 | 12/25 (48%) | 5.3 | NR |
AraC: cytarabine; CYVE: cytarabine + etoposide; eto: etoposide; HD-MTX: high dose methotrexate; ifos: ifosfamide; IT Ritux: intrathecal rituximab, IT M: intrathecal methotrexate; PCV: procarbazine, CCNU, vincristine; pred: methylprednisolone; Ritux: rituximab; SCT: stem cell transplant; WBRT: whole brain radiation; NR: not reported; ORR: overall response; CR: complete response; PR: partial response.
A prospective French multicenter trial of high-dose etoposide/cytarabine followed by high-dose chemotherapy with autologous stem cell therapy (HDC-ASCT) demonstrated a median PFS of 11.6 months and 2-year OS of 45%.43 Moreover, patients rechallenged with methotrexate-based chemotherapy in a retrospective case series who achieved a partial and complete response were treated with HDC-ASCT consolidation and had an excellent outcome with a 3-year PFS of 93%.44 The age of HDC-ASCT recipients was limited to <65 years in both series, therefore limiting the applicability of this treatment approach to younger patients.
A limited number of prospective clinical trials have investigated single agents such as pemetrexed,45 topotecan,46,47 temozolomide,48 and rituximab49,50 (Table 1).These trials have demonstrated only modest response rates of 31–55% with associated limited median progression-free survival (PFS) of 1.6–5.7 months. An optimal salvage regimen has not been established for relapsed or refractory PCNSL patients. The choice of therapy in the salvage setting is dependent on the first-line regimen used (inclusion of WBRT), age, performance status, and duration of prior response. Hopefully, additional data from ongoing clinical trials and future, ideally randomized, clinical trials will identify the optimal salvage strategy for the refractory/relapsed PCNSL population.
Randomized trials have not been conducted so far in the salvage setting. In general, prospective trial in recurrent/refractory PCNSL patients have been challenging due to the limited pathophysiologic insights of this disease identifying suitable drug targets and a heterogeneous patient population with different anatomical sites of relapse (brain, CSF, eyes, or multiple sites), multiple prior relapses, and age at relapse.
The Use of Small Molecules and Immune Checkpoint Inhibitors in PCNSL
Based on the recent molecular insights into the underlying genomic alterations driving PCNSL, clinical trials for refractory or relapsed PCNSL assessing the efficacy of small molecules have been established. These agents mainly target the PI3K/mammalian target of rapamycin (mTOR) and BCR/TLR pathways as well as immune evasion and suppressed tumor immune microenvironment using IMiDs.
PI3K/mTOR Pathway
The first targeted agent ever used as salvage treatment was the mTOR inhibitor temsirolimus in a multicenter German phase II study.62 The investigators observed a response in 54% of patients, but median PFS was limited to only 2.1 months. An even lower response rate of 25% was seen in a clinical trial targeting the PI3K/mTOR axis using the pan-PI3K inhibitor buparlisib.63 A limited blood–brain barrier penetration by buparlisib might be the possible explanation for the low response rate and lack of clinical response. Pharmacokinetic assessments in blood and CSF demonstrated plasma concentrations that are similar to those reported in the literature,64 whereas the drug concentration in the CSF was below the half maximal inhibitory concentration needed to induce cell death in lymphoma cell lines. The use of PI3K inhibition in PCNSL is still under investigation in a multicenter phase I/II trial using the dual pan-PI3K/mTOR inhibitor PQR309 (NCT02669511).
BCR/TLR Pathway
The BCR has been identified as a central signaling pathway in PCNSL.5 The central signaling nodule BTK has been targeted with single-agent ibrutinib in a French retrospective case series reporting responses in 50% of patients leading to 2 investigator-initiated clinical trials which showed promising results in the recurrent/refractory setting. Fifty-two patients with refractory and relapsed PCNSL or ocular lymphoma were enrolled in a French study using 560 mg of daily dosed ibrutinib (NCT02542514). The investigators observed a radiographic response in 50% of patients after the first 2 cycles of ibrutinib.65 In the second study, ibrutinib was dosed at 840 mg daily in refractory and relapsed PCNSL and secondary CNS lymphoma (SCNSL) (NCT02315326). Twenty patients were treated and a radiographic response rate of 75% (77% in PCNSL and 71% in SCNSL) with a median PFS of 4.6 months in PCNSL has been reported.5 Finally, a study conducted by the National Cancer Institute (NCI) demonstrated that 15/18 (83%) PCNSL patients showed a radiographic response after 2 weeks of single-agent ibrutinib administration in a “window” study prior to the use of additional chemotherapy.66 The results of the latter study are difficult to interpret due to the inclusion of newly diagnosed PCNSL patients (5/18), the addition of multiple other chemotherapy agents after the initial 2 weeks of single-agent ibrutinib (rituximab, temozolomide, etoposide, doxorubicin, dexamethasone, and intrathecal cytarabine), and a much higher frequency of infectious complications (39% Aspergillus infections) than in single-agent ibrutinib studies (eg, 2/52 [3.8%] in 560 mg and 1/20 [5%] in the 840 mg study). The efficacy of ibrutinib is remarkable, with high response rates and a PFS that is promising. Remarkably, the response to ibrutinib observed in the brain is substantially higher5 than in patients with systemic DLBCL (25% ORR to single-agent ibrutinib; PFS: 2 mo67). This remarkable difference in response needs to be further investigated to better define the underlying differences between BCR activation and inhibition in the CNS and outside the CNS. This difference is also likely related to significant difference in incidence of MYD88 L265P mutation in CNS versus systemic lymphomas. Moreover, even patients without genomic alterations in the BCR pathway responded to ibrutinib.5 In contrast to systemic DLBCL, where those tumors with both CD79B and MYD88 mutations had a better response to ibrutinib,67CD79B mutations in PCNSL seem to provide redundant survival signals potentially promoting ibrutinib resistance.5 Based on these results the updated National Comprehensive Cancer Network (NCCN) 2018 guidelines for the treatment of recurrent/refractory PCNSL now include the use of ibrutinib. The ibrutinib resistance mechanism, causing the limited PFS, needs to be further investigated. Ibrutinib-based combination therapy trials are enrolling including HD-MTX (NCT02315326) and other targeted agents, like PI3K inhibitors (NCT03581942).
Immunomodulatory Drugs
The IMiDs lenalidomide and pomalidomide have been used in clinical trials for recurrent/refractory PCNSL patients alone or combined with rituximab. IMiDs inhibit NFκB68 but also block the PI3K/AKT pathway69 and therefore represent promising agents. Both agents have been demonstrated to cross the blood‒brain barrier and have been measured in the CSF.70,71 In preclinical model systems, pomalidomide was shown to have higher CNS penetration than lenalidomide (~40%72 vs 11%,73 respectively), but phase I pharmacokinetic data demonstrated that CNS penetration appears to be about the same for both agents.74
The third-generation IMiD, pomalidomide, has been tested in a phase I clinical trial.71 The trial is based on a preclinical study in which pomalidomide was shown to have significant therapeutic activity in 2 murine CNS lymphoma models.72 Therapeutic activity of IMiDs consists of direct cytotoxicity to lymphoma cells and indirect therapeutic activity via modulation of the lymphoma immune microenvironment. In particular, pomalidomide modulates tumor associated macrophages by converting their polarization status from M2 to M1. The trial consisted of a dose-escalation phase to determine the maximum tolerated dose (MTD) of pomalidomide and cohort expansion at the MTD. Twenty-nine patients were enrolled and 25 patients were eligible for assessment. The treatment consists of pomalidomide daily for 21 days every 28 days and dexamethasone 40 mg once a week. After the first 2 cycles, pomalidomide was continued alone until progression, intolerance, or patient’s withdrawal. The MTD of pomalidomide was determined to be 5 mg daily for 21 days every 28 days. The ORR for the study (10/25) was 40%. The ORR (whole study) was 48% (12/25; 95% CI: 27.8%, 68.7%) with 6 complete responses (CR), 2 complete responses‒unconfirmed (CRu), and 4 partial responses (PR). ORR (MTD cohort) was 50% (8/16; 95% CI: 24.7%, 75.4%) with 5 CR, 1 CRu, and 2 PR. Pseudoprogression was seen in 1 patient after 4 cycles of treatment. Median PFS was 5.3 months for the whole study and 9 months for responders. Overall, grade 3/4 toxicity included hematologic (neutropenia 21%, thrombocytopenia 8%) and nonhematologic events (lung infection 12%, fatigue 8%, syncope 4%, sepsis 4%, respiratory failure 8%, and rash 4%).
In a phase I study the MTD for single agent lenalidomide70 was defined as 15 mg daily dosing for 21 days out of 28. The radiographic response to single-agent lenalidomide was 64%, with a median PFS of 6 months. In a second study conducted in France, lenalidomide was combined with rituximab in a multicenter phase II trial for recurrent/ relapsed PCNSL or ocular lymphoma.75 Lenalidomide was dosed at 20 mg daily for 21 days out of 28 and combined with rituximab given at 375 mg/m2 at day 1. This treatment was followed by single-agent lenalidomide in those who responded to maintenance therapy at 10 mg. Forty-five patients have been enrolled and the investigators observed a radiographic response rate to the lenalidomide/rituximab combination in 63% of patients, with a PFS of 8.1 months. Based on these results the updated NCCN 2018 guidelines for the treatment of recurrent/refractory PCNSL now include the use of single-agent lenalidomide or the combination rituximab/lenalidomide.
Immune Checkpoint Blockade
The use of immune checkpoint inhibitors in PCNSL might represent another promising treatment approach. In an immunocompetent preclinical model, immune checkpoint inhibition by anti-PD1 monoclonal antibodies had significant therapeutic activity against CNS lymphoma.76 Moreover, Nayak et al reported long-term responses in a small retrospective study77 of 4 patients with PCNSL. This observation in conjunction with the knowledge of 9p24.1 copy number alterations observed in PCNSL samples29 resulted in a multicenter trial investigating single-agent nivolumab in PCNSL and testicular lymphoma (NCT02857426). Moreover, a single institution trial using pembrolizumab (NCT02779101) is ongoing to further investigate the concept of immune evasion and PD-1 blockade in PCNSL. Additional clinical trials are planned combining checkpoint inhibitors with targeted agents, like ibrutinib or IMiDs.
Future Directions
Significant advances have been made in the treatment of PCNSL over the past decades. Novel insights into the pathophysiology of PCNSL led to the introduction of targeted agents into clinical trials in the salvage setting, which have shown promising clinical responses. Some of these agents have now been included in the NCCN guidelines for the treatment of recurrent/refractory PCNSL and will be more frequently used in the future. To overcome resistance to those agents, combination trials are on the way and hopefully will be able to overcome the limited PFS time observed with single-agent small inhibitors (Table 2). The role of immune checkpoint inhibition is not yet clearly defined but will be addressed by ongoing clinical studies in the salvage setting. Chimeric antigen receptor (CAR) T-cell therapy, which has recently been approved for the treatment of recurrent/refractory systemic DLBCL, hopefully will also be evaluated in PCNSL soon. CAR T cells have been found to penetrate the CNS, and responses have been observed in secondary CNS lymphoma patients.78 In the next years, we hopefully see the integration of small molecules into first-line treatment regimens and the emergence of additional maintenance treatment concepts, hopefully to reduce the number of refractory patients, further increase response rates, increase remission times, and further increase treatment options for recurrent patients who have a particularly poor clinical outcome. Small molecules might also help to improve outcomes in the elderly PCNSL population by reducing treatment associated comorbidities more commonly seen with conventional chemotherapy regimens.
Table 2.
Ongoing salvage trials
| Agents | ClinicalTrials. gov ID | Date Opened |
|---|---|---|
| Pembrolizumab | NCT027791014 | 5/2016 |
| PQR309 (dual PI3K/mTOR inhibitor) | NCT03127020 | 4/2017 |
| Abemaciclib | NCT03220646 | 6/2017 |
| R-CHOP preceded by blood–brain barrier permeabilization by t-NGR necrosis factor | NCT03536039 | 5/2018 |
| Rituximab+lenalidomide+nivolumab | NCT03558750 | 6/2018 |
| Copanlisib+ibrutinib | NCT03581942 | 9/2018 |
| Rituximab+lenalidomide+ibrutinib | NCT03703167 | 10/2018 |
Funding
This research was supported by NIH/NCI Cancer Center Support Grant no. P30-CA008748 and by grants from Cycle for Survival Equinox (C.G.) and the Leukemia & Lymphoma Society (C.G.).
Conflict of interest statement. Research support: C.G., Pharmacyclics; H. T., Celgene, Mundipharma, Spectrum, Bristol-Myers Squibb. Advisory affiliations: C.G., BTG Pharmaceuticals.
Authorship statement. All authors contributed in the writing and final approval of the manuscript.
References
- 1. Camilleri-Broët S, Martin A, Moreau A, et al. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d’étude des Leucénies et Autres Maladies du Sang (GOELAMS). Am J Clin Pathol. 1998;110(5):607–612. [DOI] [PubMed] [Google Scholar]
- 2. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. [DOI] [PubMed] [Google Scholar]
- 3. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 4. Camilleri-Broët S, Crinière E, Broët P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190–196. [DOI] [PubMed] [Google Scholar]
- 5. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tun HW, McKinney M. Differential gene expression of central nervous system lymphoma response. Blood. 2009;113(1):267–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasqualucci L, Dalla-Favera R. The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vater I, Montesinos-Rongen M, Schlesner M, et al. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia. 2015;29(3):677–685. [DOI] [PubMed] [Google Scholar]
- 9. Braggio E, Van Wier S, Ojha J, et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res. 2015;21(17):3986–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011;122(6):791–792. [DOI] [PubMed] [Google Scholar]
- 11. Montesinos-Rongen M, Schäfer E, Siebert R, Deckert M. Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta Neuropathol. 2012;124(6):905–906. [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez-Aguilar A, Idbaih A, Boisselier B, et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res. 2012;18(19):5203–5211. [DOI] [PubMed] [Google Scholar]
- 13. Bruno A, Boisselier B, Labreche K, et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget. 2014;5(13):5065–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279–290. [DOI] [PubMed] [Google Scholar]
- 15. Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–1679. [DOI] [PubMed] [Google Scholar]
- 18. Honma K, Tsuzuki S, Nakagawa M, et al. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood. 2009;114(12):2467–2475. [DOI] [PubMed] [Google Scholar]
- 19. Khodabakhshi AH, Morin RD, Fejes AP, et al. Recurrent targets of aberrant somatic hypermutation in lymphoma. Oncotarget. 2012;3(11):1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kraan W, Horlings HM, van Keimpema M, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J. 2013;3:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Booman M, Szuhai K, Rosenwald A, et al. Genomic alterations and gene expression in primary diffuse large B-cell lymphomas of immune-privileged sites: the importance of apoptosis and immunomodulatory pathways. J Pathol. 2008;216(2):209–217. [DOI] [PubMed] [Google Scholar]
- 22. Fukumura K, Kawazu M, Kojima S, et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol. 2016;131(6):865–875. [DOI] [PubMed] [Google Scholar]
- 23. Braaten KM, Betensky RA, de Leval L, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9(3):1063–1069. [PubMed] [Google Scholar]
- 24. Rickert CH, Dockhorn-Dworniczak B, Simon R, Paulus W. Chromosomal imbalances in primary lymphomas of the central nervous system. Am J Pathol. 1999;155(5):1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwindt H, Vater I, Kreuz M, et al. Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia. 2009;23(10):1875–1884. [DOI] [PubMed] [Google Scholar]
- 26. Weber T, Weber RG, Kaulich K, et al. Characteristic chromosomal imbalances in primary central nervous system lymphomas of the diffuse large B-cell type. Brain Pathol. 2000;10(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boonstra R, Koning A, Mastik M, van den Berg A, Poppema S. Analysis of chromosomal copy number changes and oncoprotein expression in primary central nervous system lymphomas: frequent loss of chromosome arm 6q. Virchows Arch. 2003;443(2):164–169. [DOI] [PubMed] [Google Scholar]
- 28. Harada K, Nishizaki T, Kubota H, Harada K, Suzuki M, Sasaki K. Distinct primary central nervous system lymphoma defined by comparative genomic hybridization and laser scanning cytometry. Cancer Genet Cytogenet. 2001;125(2):147–150. [DOI] [PubMed] [Google Scholar]
- 29. Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruno A, Labreche K, Daniau M, et al. Identification of novel recurrent ETV6-IgH fusions in primary central nervous system lymphoma. Neuro Oncol. 2018;20(8):1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montesinos-Rongen M, Schmitz R, Courts C, et al. Absence of immunoglobulin class switch in primary lymphomas of the central nervous system. Am J Pathol. 2005;166(6):1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montesinos-Rongen M, Van Roost D, Schaller C, Wiestler OD, Deckert M. Primary diffuse large B-cell lymphomas of the central nervous system are targeted by aberrant somatic hypermutation. Blood. 2004;103(5):1869–1875. [DOI] [PubMed] [Google Scholar]
- 33. Cobbers JM, Wolter M, Reifenberger J, et al. Frequent inactivation of CDKN2A and rare mutation of TP53 in PCNSL. Brain Pathol. 1998;8(2):263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makino K, Nakamura H, Shinojima N, et al. BCL2 expression is associated with a poor prognosis independent of cellular origin in primary central nervous system diffuse large B-cell lymphoma. J Neuro Oncol. 2018;140(1):115–121. [DOI] [PubMed] [Google Scholar]
- 35. Ackler S, Oleksijew A, Chen J, et al. Clearance of systemic hematologic tumors by venetoclax (Abt-199) and navitoclax. Pharmacol Res Perspect. 2015;3(5):e00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643–5646. [DOI] [PubMed] [Google Scholar]
- 37. Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol. 2014;117(1):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hottinger AF, DeAngelis LM, Yahalom J, Abrey LE. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69(11):1178–1182. [DOI] [PubMed] [Google Scholar]
- 39. Nguyen PL, Chakravarti A, Finkelstein DM, Hochberg FH, Batchelor TT, Loeffler JS. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23(7):1507–1513. [DOI] [PubMed] [Google Scholar]
- 40. Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi MK, Kang ES, Kim DW, et al. Treatment outcome of relapsed/refractory primary central nervous system diffuse large B-cell lymphoma: a single-center experience of autologous stem cell transplantation. Int J Hematol. 2013;98(3):346–354. [DOI] [PubMed] [Google Scholar]
- 42. Mappa S, Marturano E, Licata G, et al. Salvage chemoimmunotherapy with rituximab, ifosfamide and etoposide (R-IE regimen) in patients with primary CNS lymphoma relapsed or refractory to high-dose methotrexate-based chemotherapy. Hematol Oncol. 2013;31(3):143–150. [DOI] [PubMed] [Google Scholar]
- 43. Soussain C, Hoang-Xuan K, Taillandier L, et al. ; Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26(15):2512–2518. [DOI] [PubMed] [Google Scholar]
- 44. Welch MR, Omuro A, Deangelis LM. Outcomes of the oldest patients with primary CNS lymphoma treated at Memorial Sloan-Kettering Cancer Center. Neuro Oncol. 2012;14(10):1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raizer JJ, Rademaker A, Evens AM, et al. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer. 2012;118(15):3743–3748. [DOI] [PubMed] [Google Scholar]
- 46. Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17(7):1141–1145. [DOI] [PubMed] [Google Scholar]
- 47. Voloschin AD, Betensky R, Wen PY, Hochberg F, Batchelor T. Topotecan as salvage therapy for relapsed or refractory primary central nervous system lymphoma. J Neurooncol. 2008;86(2):211–215. [DOI] [PubMed] [Google Scholar]
- 48. Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96(6):864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76(10):929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nayak L, Abrey LE, Drappatz J, et al. ; North American Brain Tumor Consortium. Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk Lymphoma. 2013;54(1):58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herrlinger U, Brugger W, Bamberg M, Kuker W, Dichgans J, Weller M. PCV salvage chemotherapy for recurrent primary CNS lymphoma. Neurology. 2000;54(8):1707–1708. [DOI] [PubMed] [Google Scholar]
- 52. Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, Nomdedeu B, Montserrat E, Graus F. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70(4):219–224. [DOI] [PubMed] [Google Scholar]
- 53. Wong ET, Tishler R, Barron L, Wu JK. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer. 2004;101(1):139–145. [DOI] [PubMed] [Google Scholar]
- 54. Enting RH, Demopoulos A, DeAngelis LM, Abrey LE. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63(5):901–903. [DOI] [PubMed] [Google Scholar]
- 55. Wong SF, Gan HK, Cher L. A single centre study of the treatment of relapsed primary central nervous system lymphoma (PCNSL) with single agent temozolomide. J Clin Neurosci. 2012;19(11):1501–1505. [DOI] [PubMed] [Google Scholar]
- 56. Zhang JP, Lee EQ, Nayak L, et al. ; Retrospective study of pemetrexed as salvage therapy for central nervous system lymphoma. J neuro-oncol. 2013;115(1):71–77. [DOI] [PubMed] [Google Scholar]
- 57. Chamberlain MC. Salvage therapy with bendamustine for methotrexate refractory recurrent primary CNS lymphoma: a retrospective case series. J neuro-oncol. 2014;118(1):155–162. [DOI] [PubMed] [Google Scholar]
- 58. Houillier C, Choquet S, Touitou V, et al. ; Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology. 2015;84(3):325–326. [DOI] [PubMed] [Google Scholar]
- 59. Chamberlain MC. High-dose cytarabine salvage therapy for recurrent primary CNS lymphoma. J neuro-oncol. 2016;126(3):545–550. [DOI] [PubMed] [Google Scholar]
- 60. Chamoun K, Choquet S, Boyle E, et al. ; Ibrutinib Monotherapy in Relapsed/Refractory Cns Lymphoma: A Retrospective Case Series. Neurology. 2017;88(1):101–102. [DOI] [PubMed] [Google Scholar]
- 61. Rubenstein JL, Li J, Chen L, et al. ; Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013;121(5):745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Korfel A, Schlegel U, Herrlinger U, et al. Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol. 2016;34(15):1757–1763. [DOI] [PubMed] [Google Scholar]
- 63. Grommes C, Pentsova E, Nolan C, Wolfe J, Mellinghoff IK, Deangelis L. Phase II study of single agent buparlisib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Ann Oncol. 2016;27(suppl 6):335. [Google Scholar]
- 64. Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30(3):282–290. [DOI] [PubMed] [Google Scholar]
- 65. Choquet S, Houillier C, Bijou F, et al. Ibrutinib monotherapy in relapse or refractory primary CNS lymphoma (PCNSL) and primary vitreo-retinal lymphoma (PVRL). Result of the interim analysis of the iLOC phase II study from the Lysa and the French LOC Network. Blood. 2016;128(22):784. [Google Scholar]
- 66. Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833–843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oliva EN, Cuzzola M, Aloe Spiriti MA, et al. Biological activity of lenalidomide in myelodysplastic syndromes with del5q: results of gene expression profiling from a multicenter phase II study. Ann Hematol. 2013;92(1):25–32. [DOI] [PubMed] [Google Scholar]
- 69. Dredge K, Horsfall R, Robinson SP, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1-2):56–63. [DOI] [PubMed] [Google Scholar]
- 70. Rubenstein JL, Geng H, Fraser EJ, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018;2(13):1595–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tun HW, Johnston PB, DeAngelis LM, et al. Phase I study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreo-retinal lymphoma. Blood. 2018;132(21):2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li Z, Qiu Y, Personett D, et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS One. 2013;8(8):e71754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Muscal JA, Sun Y, Nuchtern JG, et al. Plasma and cerebrospinal fluid pharmacokinetics of thalidomide and lenalidomide in nonhuman primates. Cancer Chemother Pharmacol. 2012;69(4):943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rubenstein JL. Biology of CNS lymphoma and the potential of novel agents. Hematology Am Soc Hematol Educ Program. 2017;2017(1):556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ghesquieres H, Houillier C, Chinot O, et al. Rituximab-lenalidomide (REVRI) in relapse or refractory primary central nervous system (PCNSL) or vitreo retinal lymphoma (PVRL): results of a “proof of concept” phase II study of the French LOC network. Blood. 2016;128(22):785. [Google Scholar]
- 76. Qiu Y, Li Z, Pouzoulet F, et al. Immune checkpoint inhibition by anti-PDCD1 (anti-PD1) monoclonal antibody has significant therapeutic activity against central nervous system lymphoma in an immunocompetent preclinical model. Br J Haematol. 2018;183(4):674–678. [DOI] [PubMed] [Google Scholar]
- 77. Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Abramson JS, McGree B, Noyes S, et al. Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. N Engl J Med. 2017;377(8):783–784. [DOI] [PubMed] [Google Scholar]