Abstract
Background
Pediatric cranial radiotherapy (CrRT) markedly increases risk of meningiomas. We studied meningioma risk factors with emphasis on independent and joint effects of CrRT dose, exposed cranial volume, exposure age, and chemotherapy.
Methods
The Dutch Cancer Oncology Group–Long-Term Effects after Childhood Cancer (DCOG-LATER) cohort includes 5-year childhood cancer survivors (CCSs) whose cancers were diagnosed in 1963–2001. Histologically confirmed benign meningiomas were identified from the population-based Dutch Pathology Registry (PALGA; 1990–2015). We calculated cumulative meningioma incidence and used multivariable Cox regression and linear excess relative risk (ERR) modeling.
Results
Among 5843 CCSs (median follow-up: 23.3 y, range: 5.0–52.2 y), 97 developed a benign meningioma, including 80 after full- and 14 after partial-volume CrRT. Compared with CrRT doses of 1–19 Gy, no CrRT was associated with a low meningioma risk (hazard ratio [HR] = 0.04, 95% CI: 0.01–0.15), while increased risks were observed for CrRT doses of 20–39 Gy (HR = 1.66, 95% CI: 0.83–3.33) and 40+ Gy (HR = 2.81, 95% CI: 1.30–6.08). CCSs whose cancers were diagnosed before age 5 versus 10–17 years showed significantly increased risks (HR = 2.38, 95% CI: 1.39–4.07). In this dose-adjusted model, volume was not significantly associated with increased risk (HR full vs partial = 1.66, 95% CI: 0.86–3.22). Overall, the ERR/Gy was 0.30 (95% CI: 0.03–unknown). Dose effects did not vary significantly according to exposure age or CrRT volume. Cumulative incidence after any CrRT was 12.4% (95% CI: 9.8%–15.2%) 40 years after primary cancer diagnosis. Among chemotherapy agents (including methotrexate and cisplatin), only carboplatin (HR = 3.55, 95% CI: 1.62–7.78) appeared associated with meningioma risk. However, we saw no carboplatin dose-response and all 9 exposed cases had high-dose CrRT.
Conclusion
After CrRT 1 in 8 survivors developed late meningioma by age 40 years, associated with radiation dose and exposure age, relevant for future treatment protocols and awareness among survivors and physicians.
Keywords: meningioma, cranial radiotherapy, childhood cancer survivors, radiation dose, radiation volume
Key Points
One in 8 childhood cancer survivors treated with cranial radiotherapy develops a late meningioma
Meningioma risk is dose dependent with no modification of the dose-response by age or exposed volume.
Our study contributes new evidence that can be useful for meningioma surveillance recommendations.
Importance of the study
There is considerable debate on the justification for or against active screening for meningioma among asymptomatic childhood cancer survivors. Cranial radiotherapy markedly increases the risk of meningioma; however, the roles of exposed cranial volume and age at radiotherapy are unclear. We studied a large, well-characterized cohort of CCSs with near-complete and unbiased assessment of late meningioma via PALGA. We found that 1 in 8 survivors developed a late meningioma by age 40 years after CrRT. We showed evidence for increased risk by radiation dose and among patients treated at the youngest ages; however, there was no significant modification of the radiation dose-response by age or by radiation-exposed cranial volume. Our study contributes new evidence to the key element of adequate risk stratification for surveillance recommendations by evaluating the modifying effects of exposed cranial volume and exposure age on the radiation dose-response association.
Among childhood cancer survivors (CCSs) who had cranial radiotherapy (CrRT), a markedly elevated incidence of subsequent central nervous system (CNS) neoplasms has been established.1 Meningiomas represent the most common type, and although mostly benign, meningiomas can cause serious neurologic morbidity.2 Meningiomas typically occur beyond 10 years after treatment; median/mean intervals from primary cancer diagnosis to meningioma diagnosis of more than 20 years have been reported in large cohort studies among CCSs.2,3 Furthermore, the excess risk does not seem to plateau over time.2,4 Meningioma risk appears to increase with increasing radiation dose,2–5 while the role of exposed cranial volume has not been studied. Some studies reported that a lower age at childhood cancer diagnosis was associated with an increased risk of meningioma,4,6,7 which may reflect a higher sensitivity to radiation, as observed for other tissues (eg, the thyroid gland).8 However, among studies that evaluated this hypothesis directly, by evaluating meningioma risk in CCSs3,5 and in children treated for tinea capitis,9 no clear variation was found in the strength of the radiation dose-response by exposure age.
Of all chemotherapy drugs evaluated in 2 large cohorts of CCSs, platinum agents and intrathecal methotrexate were the only ones for which some evidence of excess meningioma risk was reported.3,6,7 However, these initial findings have not been replicated.
Finding the right balance between benefits and drawbacks of active surveillance for CNS tumors, in particular meningioma, among asymptomatic individuals who had CrRT is challenging.2,10 Adequate risk stratification is one of several key elements to enable balanced decision making on surveillance recommendations, as currently is ongoing by the International Guideline Harmonization Group.11
We examined the independent and joint effects of CrRT dose, exposed cranial volume, and age at childhood cancer treatment to determine excess risk of meningiomas in the Dutch Cancer Oncology Group–Long-Term Effects after Childhood Cancer (DCOG-LATER) cohort of 5-year CCSs.
Methods
The full DCOG-LATER cohort includes 6165 individuals who were treated for childhood cancer between January 1, 1963 and December 31, 2001 in one of the 7 Dutch pediatric oncology and stem cell transplant centers before age 18 years and who survived at least 5 years after diagnosis. The study protocol was exempted from review by institutional review boards of all participating centers. More details were reported elsewhere.12
Cancer Diagnosis, Treatment Information
Information on prior cancer diagnosis, treatments for primary tumor and all recurrences, and cancer predisposition syndromes was collected by dedicated data managers.12 The 1440 survivors who received radiotherapy directed to the head—including those who received total body irradiation (TBI)—were assigned to one of 3 subgroups: full-cranial volume (full-CrRT; defined as 100% of the cranium in field), partial-cranial volume (partial-CrRT; defined as any CrRT with less than 100% of the cranium in field), and radiotherapy to the head without cranial involvement (no brain tissue in the field; not considered CrRT). For leukemia, CNS tumors, and retinoblastoma survivors (77.4% of 1440), 2 experienced radiation technologists (J.L.K. and A.v.E.) reviewed treatment protocols; for other childhood cancer types (19.1%) simulation films or anatomical diagrams in radiotherapy charts were used when available. When the radiotherapy record was missing or uninformative, volume was assigned by childhood cancer type and protocol (3.5%). The total dose for primary tumor and recurrences, including boost dose, was determined. We calculated the total maximum prescribed CrRT dose (in case of multiple CrRT treatments for primary tumor or recurrences) as follows: Dose was summed when the same location was irradiated (maximum dose to smallest CrRT field was assessed). In case of 2 or more non-overlapping CrRT fields, the dose to the field with the highest dose was assigned.
Definition and Ascertainment of Subsequent Meningiomas
Histologically confirmed subsequent benign meningiomas diagnosed between January 1, 1990 and May 1, 2015 were identified by linkage based on family name, sex, and date of birth with the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA), which reached nationwide coverage in 1990.13 All pathology reports are summarized into short digital excerpts which contain one or more codes to classify the result of the pathologist review. These were manually reviewed by one author (J.L.K.) to identify eligible cases (morphology codes M9530-9539 and brain topography codes [Supplementary Table 1]). In case of doubt, excerpts were discussed with 2 experts, including a late effects outpatient clinic doctor (C.M.R., H.J.v.d.P). Cohort members were traced for vital status and emigration status as reported previously.12
Sibling Comparison Group
Because no reference rates on histologically confirmed benign meningiomas are available in the general population for this predominantly young population, we included a sibling comparison group to parallel the meningioma incidence in CCSs to the incidence in the general population. CCSs who participated in a 2013–2014 questionnaire survey (N = 3172) were asked to invite their siblings. These siblings (N = 1663) were approached, and after consent 883 (53%) siblings were linked with PALGA as described above.
Statistical Analyses
Survivors who declined usage of health care data (N = 152; 2.5%) and those who died, emigrated, or were lost to follow-up prior to 1990 (N = 170; 2.8%) were excluded. Follow-up started 5 years after childhood cancer diagnosis or January 1, 1990, whichever came last, and ended on the date of diagnosis of the first histologically confirmed meningioma, death, last known vital status (emigration/lost to follow-up), or end of study (May 1, 2015), whichever came first.
Cumulative incidence of benign meningiomas was estimated, considering death as a competing risk.14 Multivariable Cox regression models were used to estimate meningioma risks associated with prescribed CrRT dose (no CrRT, 1–19 Gy, 20–39 Gy, 40+ Gy). To construct a multivariable model, we first tested binary indicators for hematopoietic cell transplantation and single chemotherapeutic agents with at least 5 exposed meningioma cases (n = 15) in univariable models. Those with a univariable P-value < 0.1 were separately tested in models with CrRT dose, exposed cranial volume, and basic demographic factors. The final model included, in addition to CrRT dose, exposed cranial volume, and basic demographic factors, those binary indicators for hematopoietic cell transplantation and single chemotherapeutic agents that remained significantly associated with meningioma risk (P < 0.05) or that considerably changed the effect of the CrRT dose risk estimate if removed. In addition, we calculated the overall linear excess relative risk per Gy (ERR/Gy) among exposed individuals (see Supplementary material). Joint effect of CrRT and other characteristics were assessed in 2 ways. First, we estimated the joint effects of CrRT dose (≤25, >25 Gy) with exposed cranial volume (full-CrRT, partial-CrRT) and with age at childhood cancer diagnosis as a surrogate for CrRT age (<5, 5+ y). Since the prescribed CrRT doses show scattered peaks at standard-protocol doses (eg, 18–25 Gy, 50–54 Gy), we classified CCSs simultaneously according to CrRT dose (≤25 Gy vs >25 Gy), age at childhood cancer diagnosis (<5 vs 5+ y), and CrRT dose and exposed cranial volume. Second, we evaluated whether the effects of continuous CrRT dose is modified by age at diagnosis, volume, and sex by estimating separate ERRs/Gy for strata of the hypothesized effect modifiers; heterogeneity of ERRs/Gy was evaluated with likelihood ratio tests. Nonlinearity of the dose-response relationship was evaluated by testing whether a log-linear modification term for linear dose was significantly different from zero. We evaluated proportionality of hazards for each variable in the multivariable Cox regression model by adding interaction terms with attained age (the time scale) and found no evidence of non-proportionality. P-values < 0.05 were considered statistically significant and all statistical tests were 2-sided. Stata 13 (StataCorp) and Epicure softwares (Risk Sciences International) were used.
Results
This analysis includes 5843 five-year CCSs contributing 102937 person-years at risk during January 1, 1990–May 1, 2015. Median time since childhood cancer diagnosis was 23.3 years (range: 5.0–52.2 y) and median attained age at end of follow-up was 30.6 years (range: 5.8–67.5 y). Nearly half of the cohort was treated for either leukemia (33.2%) or CNS tumors (13.0%) (Table 1). In total, 1277 survivors received CrRT, including 956 full-CrRT and 321 partial-CrRT, and another 163 survivors received radiotherapy to the head without cranial involvement (Supplementary Figure 1A–C). The proportion of cohort members treated with CrRT strongly decreased over time (ie, 36.2%, 18.7%, and 12.5% for those diagnosed in 1963–1984, 1985–1994, and 1995–2001, respectively), largely attributed to a strong decline in proportion of patients, mainly leukemia survivors, treated with 20–39 Gy CrRT: 20.9% in 1963–1984, 2.4% in 1985–1994, 1.2% in 1995–2001 (data not shown).
Table 1.
Patient characteristics of the DCOG-LATER cohort eligible for analyses (N = 5843) for survivors without meningioma (N = 5746) and survivors with meningioma (N = 97)
| Survivors without Meningioma | Survivors with Meningioma | |||
|---|---|---|---|---|
| N | % | N | % | |
| Childhood cancer type | ||||
| Leukemia | 1910 | 33.2 | 52 | 53.6 |
| Non Hodgkin lymphoma | 548 | 9.5 | 7 | 7.2 |
| Hodgkin lymphoma | 395 | 6.9 | 0 | 0 |
| Central nervous system non medulloblastoma | 615 | 10.7 | 13 | 13.4 |
| Medulloblastoma | 134 | 2.3 | 19 | 19.6 |
| Neuroblastoma | 313 | 5.5 | 0 | 0 |
| Retinoblastoma | 31 | 0.5 | 0 | 0 |
| Renal tumors | 578 | 10.1 | 0 | 0 |
| Hepatic tumors | 52 | 0.9 | 0 | 0 |
| Bone tumors | 343 | 6.0 | 0 | 0 |
| Soft tissue tumors | 423 | 7.4 | 3 | 3.1 |
| Germ cell tumors | 221 | 3.9 | 3 | 3.1 |
| Other and unspecified | 183 | 3.2 | 0 | 0 |
| Sex | ||||
| Male | 3222 | 56.1 | 47 | 48.5 |
| Female | 2524 | 43.9 | 50 | 51.5 |
| Age at childhood cancer diagnosis | ||||
| 0–4 y | 2595 | 45.2 | 46 | 47.4 |
| 5–9 y | 1557 | 27.1 | 26 | 26.8 |
| 10–17 y | 1594 | 27.7 | 25 | 25.8 |
| Calendar year of childhood cancer diagnosis | ||||
| 1963–1984 | 1721 | 30.0 | 70 | 72.2 |
| 1985–1994 | 2091 | 36.4 | 22 | 22.7 |
| 1995–2001 | 1934 | 33.7 | 5 | 5.1 |
| Attained age at end of follow-up | ||||
| <20 | 763 | 13.3 | 4 | 4.1 |
| 20–29 | 1975 | 34.4 | 36 | 37.1 |
| 30–39 | 1826 | 31.8 | 45 | 46.4 |
| 40+ | 1182 | 20.6 | 12 | 12.4 |
| Time since childhood cancer diagnosis | ||||
| <20 | 2106 | 36.7 | 24 | 24.7 |
| 20–29 | 2062 | 35.9 | 47 | 48.5 |
| 30–39 | 1257 | 21.9 | 25 | 25.8 |
| 40+ | 321 | 5.6 | 1 | 1.0 |
| Childhood cancer treatment a | ||||
| Surgery only | 573 | 10.0 | 0 | 0 |
| Chemotherapy, no radiotherapy | 2897 | 50.4 | 1 | 1.0 |
| Radiotherapy, no chemotherapy | 428 | 7.5 | 17 | 17.5 |
| Radiotherapy and chemotherapy | 1775 | 30.9 | 79 | 81.4 |
| No treatment/treatment unknown | 73 | 1.3 | 0 | 0 |
| CrRT (including TBI)a | ||||
| Nob | 4522 | 78.7 | 3 | 3.1 |
| Partial cranial volume | 307 | 5.3 | 14 | 14.4 |
| Full cranial volumec | 876 | 15.3 | 80 | 82.5 |
| CrRT dose (including TBI)a | ||||
| No head/cranium or TBI dose | 4522 | 78.7 | 3 | 3.1 |
| 1–19 Gy | 314 | 5.5 | 10 | 10.3 |
| 20–39 Gy | 397 | 6.9 | 48 | 49.5 |
| 40+ Gy | 458 | 8.0 | 35 | 36.1 |
| Carboplatin a | ||||
| No | 5308 | 92.4 | 88 | 90.1 |
| Yes | 400 | 7.0 | 9 | 9.3 |
| Hematopoietic cell transplantation a | ||||
| No | 5314 | 92.5 | 90 | 92.8 |
| Yes | 365 | 6.4 | 6 | 6.2 |
| WHO grade of first benign meningioma d | ||||
| 1 | NA | NA | 45 | 80.4 |
| 2 | NA | NA | 11 | 19.6 |
| Unknown | NA | NA | 41 | |
| Calendar period of first benign meningioma diagnosis | ||||
| 1990–1999 | NA | NA | 12 | 12.4 |
| 2000–2009 | NA | NA | 47 | 48.4 |
| 2010–2015e | NA | NA | 38 | 39.2 |
Abbreviations: CrRT = cranial radiotherapy; TBI = total body irradiation; NA = not applicable; WHO = World Health Organization.
Numbers do not always add up to 100% because of missing values or rounding.
aTreatment data include primary treatment and all recurrences. Chemotherapy (yes/no), radiotherapy (yes/no), and hematopoietic cell transplantation (yes/no) were missing for 32, 32, and 68 survivors, respectively.
bIncludes n = 163 (2.8%) patients irradiated to facial and other parts of head without cranial involvement, no meningioma cases. The 3 patients without a history of CrRT were diagnosed with a meningioma 14,37, and 26 years post–soft tissue sarcoma (n = 2) or post-ALL (n = 1), respectively.
cIncludes n = 210 patients treated with TBI, among which 4 developed a meningioma.
dPercentages were based on cases with a known WHO grade as reported by the pathologist, according to the WHO classification in use at the time of diagnosis.
eIncludes January to April 2015.
Characteristics of Survivors with Subsequent Meningioma
In total, 97 survivors (1.7%) developed at least one histologically confirmed benign meningioma. Among meningioma cases, median time since childhood cancer diagnosis was 24.9 years (range: 8.5–44.5 y, interquartile range [IQR]: 20.6–30.6) and median age at first meningioma diagnosis was 31.7 years (range: 15.5–49.9 y, IQR: 27.3–36.6). All but 3 patients with a subsequent benign meningioma had a history of CrRT, including 80/94 with full-CrRT for either acute lymphoblastic leukemia (ALL; n = 48), medulloblastoma (n = 19), non-Hodgkin lymphoma (n = 7), acute myeloid leukemia (n = 3), or a germ cell tumor (n = 3) (Table 1). Another 14/94 CrRT patients with meningioma had received partial-CrRT for other types of CNS tumors (n = 13) or soft tissue sarcoma (n = 1). Among meningioma patients who received any CrRT, 45.4% received a dose of 40 Gy or more compared with 10.6% of CrRT-treated patients in the total cohort. The dose distribution varied between the full-CrRT and partial-CrRT group; the majority of partial-CrRT (90.0%) received a dose of 40 Gy or more, while full-CrRT individuals were more equally distributed over the dose categories (Supplementary Figure 2). Two survivors who developed a meningioma after partial-CrRT had a confirmed neurofibromatosis diagnosis: one diagnosis of meningioma 13 years after a nerve sheath tumor and one diagnosis 25 years after a glioma. Three patients developed an intervening subsequent malignant neoplasm (SMN) before the meningioma was detected; only one received CrRT for the SMN, and that patient had already received full-CrRT for the childhood cancer. Our record linkages with the pathology registry (this paper) and the national cancer registry12 revealed 5 malignant meningiomas, including 4 survivors with a preceding benign meningioma and included in the analyses presented here.
Comparison with Sibling Cohort
In our sibling cohort (N = 883), 1 female developed a meningioma at the age of 45, whereas her sibling Hodgkin lymphoma survivor did not. After adjustment for attained age and sex, the incidence of meningiomas in the survivor cohort was significantly higher than among siblings (hazard ratio [HR] = 17.79; 95% CI: 2.48–127.76, P < 0.00001).
Independent Effects of Demographic and Treatment-Related Risk Factors
In multivariable models, having had any CrRT was a strongly influential factor for meningioma risk. Next, of the 3 related characteristics (dose, volume, age), CrRT dose appeared to be the most influential risk factor. Compared with survivors who received 1–19 Gy CrRT dose (10 cases/324 cohort members), survivors treated without CrRT had a strongly and significantly lower meningioma risk (HR = 0.04, 95% CI: 0.01–0.15; 3 cases/4525 cohort members), while CrRT doses 20–39 Gy (HR = 1.66, 95% CI: 0.83–3.33; 48 cases/445 cohort members) and 40+ Gy (HR = 2.81, 95% CI: 1.30–6.08; 35 cases/493 cohort members) inferred higher risk (Table 2). In other words, the reference group in this analysis (1–19 Gy CrRT) had a strongly increased risk compared with the group without CrRT. In the same dose-adjusted model, full-CrRT was not significantly associated with increased risk compared with partial-CrRT (HR = 1.66, 95% CI: 0.86–3.22). In addition to CrRT risk, survivors who had received carboplatin (9 cases/409 cohort members) versus no carboplatin had a statistically significantly increased risk (HR = 3.55, 95% CI: 1.62–7.78), without evidence for a carboplatin dose-response relationship. Individual chemotherapy agents other than carboplatin (including methotrexate [57 exposed cases] and cisplatin [2 exposed cases]) were not associated with risk of meningioma. Of note, of 9 patients with a meningioma after carboplatin-containing regimens, 1 had a prior ependymoma, while all 8 others received high-dose full-CrRT (primary 30 Gy, boost of >20 Gy to the fossa posterior) for medulloblastoma. Median CrRT doses were 55 Gy and 25 Gy for meningioma cases with and without carboplatin, respectively, and this distribution was similar to the difference in CrRT dose by carboplatin status in the entire cohort. Without adjustment for CrRT dose, the effect of carboplatin was stronger (HR = 5.79, 95% CI: 2.80–11.98; data not shown). The demographic factors of sex (HR = 1.36, 95% CI: 0.91–2.04 for females vs males) and age at childhood cancer diagnosis (HRs of 2.38, 95% CI: 1.39–4.07 and 1.09, 95% CI: 0.62–1.91 for ages 0–4 and 5–9 years, respectively, vs ages 10–17) were not statistically significantly associated with meningioma risk. Parallel analyses with time since rather than age at childhood cancer diagnosis in the model showed HRs of 2.18 (95% CI: 1.13–4.23) and 3.98 (95% CI: 1.57–10.11) for 20–29 years and >30 years since diagnosis, respectively, versus 5–19 years, while risk estimates for other covariates (Table 2, model 1) were not materially altered (not shown).
Table 2.
Multivariable Cox regression models for risk of meningioma by demographic and treatment-related risk factors (model 1) and by age at diagnosis/cranial radiotherapy dose (model 2), and cranial radiotherapy volume/dose (model 3) combinationsa
| N Total | N Cases | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Sex | ||||||||
| Male | 3269 | 47 | REF | REF | REF | |||
| Female | 2574 | 50 | 1.36 | 0.91–2.04 | 1.36 | 0.91–2.04 | 1.37 | 0.92–2.06 |
| Age at diagnosis | ||||||||
| 0–4 y | 2641 | 46 | 2.38 | 1.39–4.07 | 2.35 | 1.40–3.96 | ||
| 5–9 y | 1583 | 26 | 1.09 | 0.62–1.91 | 1.10 | 0.63–1.94 | ||
| 10–17 y | 1619 | 25 | REF | REF | ||||
| CrRT exposure | ||||||||
| No CrRT | 4525 | 3 | 0.04 | 0.01–0.15 | ||||
| 1–19 Gy | 324 | 10 | REF | |||||
| 20–39 Gy | 445 | 48 | 1.66 | 0.83–3.33 | ||||
| 40+ Gy | 493 | 35 | 2.81 | 1.30–6.08 | ||||
| Exposed cranial volume | ||||||||
| Partial CrRT | REF | |||||||
| Full CrRT | 1.66 | 0.86–3.22 | 1.40 | 0.73–2.66 | ||||
| Carboplatin | ||||||||
| No | 5396 | 88 | REF | REF | REF | |||
| Yes | 409 | 9 | 3.55 | 1.62–7.78 | 4.26 | 1.95–9.31 | 4.31 | 1.97–9.45 |
| CrRT exposure (age/dose) | ||||||||
| No CrRT | 4525 | 3 | 0.01 | 0.00–0.05 | ||||
| 0–4 y / ≤25 Gy | 313 | 31 | REF | |||||
| 0–4 y / >25 Gy | 153 | 15 | 1.84 | 0.95–3.56 | ||||
| 5+ y / ≤25 Gy | 382 | 23 | 0.47 | 0.27–0.81 | ||||
| 5+ y / >25 Gy | 414 | 24 | 0.61 | 0.33–1.13 | ||||
| CrRT exposure (volume/dose) | ||||||||
| No CrRT | 4525 | 3 | 0.01 | 0.00–0.05 | ||||
| Partial CrRTb / ≤25 Gy | 13 | 0 | REF | |||||
| Partial CrRTb / >25 Gy | 300 | 14 | ||||||
| Full CrRT / ≤25 Gy | 682 | 54 | 1.03 | 0.56–1.89 | ||||
| Full CrRT / >25 Gy | 267 | 25 | 1.45 | 0.75–2.83 | ||||
Abbreviations: CI = confidence interval; CrRT = cranial radiotherapy; Gy = Gray; HR = Hazard Ratio; N = number; REF = reference category.
aModels include only 96 meningioma cases due to missing values.
bDose groups were collapsed.
Combined Effects of Age/Dose and Volume/Dose Categories
Compared with young patients (<5 y) with a low CrRT dose (≤25 Gy), meningioma risk was nonsignificantly increased among young patients with a high CrRT dose (>25 Gy) (HR = 1.84, 95% CI: 0.95–3.56); those treated at older ages (5+ y), regardless of CrRT dose, had significantly lower meningioma risk (HR = 0.47, 95% CI: 0.27–0.81 for age 5+, ≤25 Gy, and HR = 0.61, 95% CI: 0.33–1.13 for age 5+, >25 Gy) (Table 2, model 2). In contrast, there was no clear effect of exposed cranial volume with HRs for survivors treated with full-CrRT at doses ≤25 Gy (HR = 1.03, 95% CI: 0.56–1.89) and full-CrRT at doses >25 Gy (HR = 1.45, 95% CI: 0.75–2.83) in comparison with any partial-CrRT (Table 2, model 3).
Effect Modification of Continuous CrRT Dose with Age, Volume, and Sex
When CrRT dose was analyzed as a continuous variable, adjusted for sex, age at diagnosis, and CrRT volume (no/partial/full), we observed a statistically significant linear dose-response among CrRT-exposed individuals (ERR/Gy of 0.30, 95% CI: 0.03‒unknown; Table 3, model 1). We found no evidence for nonlinearity of the dose-response relationship (P = 0.62). We did not observe significant modifications of the dose-response by age at diagnosis, exposed cranial volume, or sex (Table 3, models 2–5).
Table 3.
Effect modification of cranial radiotherapy dose-response for risk of meningiomaa
| N Total | N Cases | ERR/Gy | 95% CI | P Interaction | |
|---|---|---|---|---|---|
| Model 1: All patients | 1262 | 93 | 0.30 | 0.03–unknown | |
| Model 2: Age at diagnosis | 0.86 | ||||
| 0–4 y | 2641 | 46 | 0.31 | 0.10–1.36 | |
| 5–9 y | 1583 | 26 | 0.31 | 0.10–1.35 | |
| 10–17 y | 1619 | 25 | 0.27 | 0.08–1.19 | |
| Model 3: Volume | 0.98 | ||||
| Partial | 313 | 14 | 0.30 | 0.09–1.32 | |
| Full | 979 | 79 | 0.30 | 0.09–1.29 | |
| Model 4: Age at diagnosis and volume | 0.55 | ||||
| 0–4 y / partial | 86 | 6 | 0.52 | 0.13–2.49 | |
| 5–17 y / partial | 227 | 8 | 0.22 | 0.05–1.06 | |
| 0–4 y / full | 380 | 40 | 0.29 | 0.09–1.28 | |
| 5–17 y / full | 569 | 39 | 0.30 | 0.09–1.32 | |
| Model 5: Sex | 0.96 | ||||
| Male | 3269 | 47 | 0.30 | 0.10–1.29 | |
| Female | 2574 | 50 | 0.30 | 0.09–1.28 |
Abbreviations: ERR = excess relative risk; Gy = Gray; N = number; UNK = unknown.
aAll models adjusted for sex, age at diagnosis (<5, 5–9, 10+ y), cranial radiotherapy (no, partial, full). Coefficients for those variables were fixed at the values estimated in model 1 to improve stability of the model fitting. For likelihood ratio tests (P interaction), fixed parameters were counted as free, resulting in conservative P-values.
bDue to a very flat likelihood to the right of the maximum likelihood estimate, even much larger values are consistent with the data.
Cumulative Incidence of Subsequent Meningioma
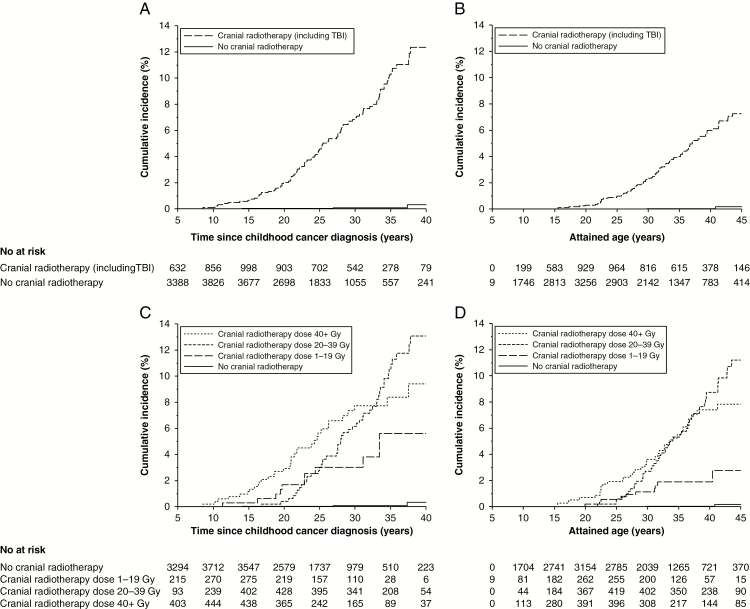
The cumulative incidence of benign meningiomas varied according to CrRT characteristics. For survivors treated with CrRT the cumulative incidence was 12.4% (95% CI: 9.8%–15.2%) 40 years after diagnosis (Fig. 1, panel A) and 7.3% (95% CI: 4.5%–10.8%) by age 45 (Fig. 1, panel B). For survivors without CrRT the cumulative incidence was much lower (0.3%, 95% CI: 0.1%–1.2% 40 years after diagnosis [Fig. 1, panel A] and 0.3%, 95% CI: 0.1%–1.2% by age 45 [Fig. 1, panel B]). By CrRT doses, the cumulative incidences 40 years after diagnosis were 5.6% (95% CI: 2.3%–11.0%), 13.1% (95% CI: 9.6%–17.1%), and 9.4% (95% CI: 6.3%–13.3%) for 1–19 Gy, 20–39 Gy, and 40+ Gy, respectively (Fig. 1, panel C). Similar patterns were observed by attained age (Fig. 1, panel D).
Fig. 1.
Cumulative incidence of meningiomas for survivors with and without cranial radiotherapy by time since childhood cancer diagnosis (panel A) and attained age (panel B) and according to cranial radiotherapy dose by time since childhood cancer diagnosis (panel C) and attained age (panel D), accounting for death as competing risk.
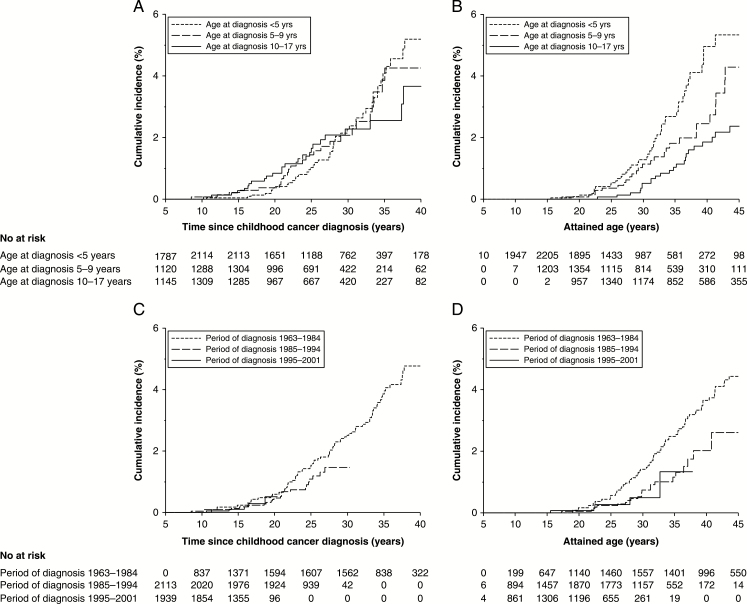
When evaluated separately by age at childhood cancer diagnosis, the cumulative incidences of meningioma diagnosed for survivors at ages 0–4 years, 5–9 years, and 10–17 years were 5.2% (95% CI: 3.7%–7.1%), 4.3% (95% CI: 2.6%–6.4%), and 3.7% (95% CI: 2.1%–5.9%) 40 years after diagnosis (Fig. 2, panel A) and 5.3% (95% CI: 3.7%–7.3%), 4.3% (95% CI: 2.6%–6.6%), and 2.4% (95% CI: 1.5%–3.5%) by age 45 (Fig. 2, panel B), respectively. Fifteen-year cumulative incidences were similar across periods of diagnosis (0.2%, 95% CI: 0.1%–0.7% for 1963–1984, 0.1%, 95% CI: 0.0%–0.4% for 1985–1994, and 0.1%, 95% CI: 0.0%–0.4% for 1995–2001, Fig. 2, panel C).
Fig. 2.
Cumulative incidence of meningiomas for 5-year survivors according to age at diagnosis categories by time since childhood cancer diagnosis (panel A) and attained age (panel B) and according to period of childhood cancer diagnosis by time since childhood cancer diagnosis (panel C) and attained age (panel D), accounting for death as competing risk.
Discussion
Our nationwide study with complete information on histologically confirmed benign meningiomas after childhood cancer shows that, after cranial radiotherapy, 1 in 8 survivors developed a late meningioma 40 years after primary cancer diagnosis. We found evidence for increased risk by radiation dose and among patients treated at the youngest ages—however, no significant modification of the radiation dose-response by age or by radiation-exposed cranial volume.
CrRT is the most important risk factor for meningioma risk among CCSs; nearly all cases (97%) occurred among survivors who were treated with CrRT. Consistent with our findings of a dose-related excess risk, CrRT has frequently been reported to linearly increase risk of meningioma in a dose-related fashion among young CCSs,3–5 atomic bomb survivors,15 and children treated for tinea capitis,9 as summarized in Supplementary Table 2. While a main effect of age at childhood cancer diagnosis has been reported by others,4,6,7 data are inconsistent as to whether age modifies the radiation dose-response curve. We hypothesized that younger exposure age, as a crude indicator of vulnerability during brain development, is related to higher radiation sensitivity, in other words, that the effect of radiation dose on meningioma risk varies by age at radiotherapy. Our results did not firmly support this hypothesis, consistent with earlier reports by Neglia et al (66 meningioma cases) and Taylor et al (137 meningioma cases) for the US/Canadian and UK childhood cancer survivor studies.3,5 Our analyses are based on prescribed CrRT dose, whereas the latter 2 studies3,5 used absorbed CrRT dose in a case-control setting. Although it can be assumed that the prescribed CrRT dose is almost similar to the absorbed dose at the organ at risk, the meninges, for meningioma cases treated with full-CrRT (n = 80 cases), the prescribed CrRT dose may represent an overestimation of the true absorbed dose at the meningioma location for partial-CrRT treated patients (n = 14 cases). A new aspect of our study is that we analyzed the potential modifying effect of exposed cranial volume on the relation between radiation dose and meningioma risk. Our second hypothesis was that a higher volume of brain tissue exposed will increase the radiation dose-related risk of meningioma. However, we did not find evidence of a stronger dose-response among patients treated with full-CrRT compared with those treated with partial-CrRT; there was a suggestive but nonsignificant main effect of full-CrRT (HR = 1.66 for full vs partial). Of note, these results need to be interpreted with caution owing to a combination of factors: (i) the lack of statistical significance; (ii) the probability that CrRT volume may be a surrogate of other patient characteristics that influence meningioma risk (eg, other risk factors such as neurofibromatosis 1 status) or early detection (eg, head MRIs or CTs for other side effects of CNS tumors or their treatment) not covered in our study variables. Also, there is a strong correlation between CrRT dose and exposed cranial volume (higher doses with partial volumes) as such that no meningioma cases were observed among the few patients treated with less than 40 Gy partial-CrRT, as illustrated in Supplementary Figure 2.
Owing to clinical reality the radiation exposure metrics we applied tend to overestimate the volume of the brain exposed to the summed doses of main field and boost, since the sum of doses is assigned to the entire cranium. This is particularly true for medulloblastoma patients. Therefore, the true risk may be slightly overestimated in this study. Sensitivity analyses based on the full CrRT dose of approximately 30 Gy (ie, disregarding the additional boost dose of around 24 Gy used in medulloblastoma protocols) provide a lower boundary for the estimated dose-related risk. Of note, CCS cohorts include individuals with several characteristics (eg, radiotherapy, volume, age, demographics) that are quite correlated. For example, the proportion of survivors who were treated with CrRT declined over time (from 36% prior to 1985 to 13% during 1995–2001), since 1985 prophylactic CrRT was eliminated from the DCOG-ALL protocols.16 Moreover, although CrRT remained indicated for ALL patients with CNS involvement up to 2004,17 the dose was reduced from 24 to 18 Gy in 1988.18
We did not find an effect of sex on meningioma risk, nor was there significant variation in the radiation dose-response according to sex, unlike some other studies reporting higher risks among women, after adjustment for CrRT dose.2,4,6
Only 3 previous studies showed some effects of chemotherapy: 2 reports of the North American Childhood Cancer Survivor Study indicated increased meningioma risk after treatment with platinum agents without a clear dose-response.6,7 At face value, these findings seem consistent with our finding of elevated risks associated with carboplatin (but not with cisplatin). However, the multivariable models from the cited studies6,7 included only a radiotherapy yes/no indicator, which is likely insufficient to fully adjust for the radiotherapy dose effects. Importantly, carboplatin can be part of medulloblastoma protocols, a patient group considered at highest risk for meningioma because they all received full-CrRT and boosts up to total doses exceeding 50 Gy. We question the causality of the carboplatin-meningioma association, due to lack of dose-response relation, collinearity with high-risk radiotherapy characteristics, no observed meningioma cases among survivors with carboplatin exposure without CrRT, no relation of cisplatin with meningioma risk, and no clear evidence on carboplatin carcinogenicity from in vitro and in vivo studies. Nonetheless, we cannot entirely discard a true, albeit small, effect of carboplatin either.
In the British Childhood Cancer Survivor Study cohort, meningioma risk among individuals receiving 1–39, 40–69, and 70 or more mg/m2 of intrathecal methotrexate was increased by 15-fold, 11-fold, and 36-fold, respectively, compared with unexposed survivors.3 The authors added a strong cautionary note to their findings: few survivors were treated with intrathecal methotrexate without CrRT and no effects were observed for non-intrathecal methotrexate. These findings have not been confirmed by other studies, including our results reported here.
Strengths of our study are the large cohort size with detailed individual treatment information and objective and near complete data on histologically confirmed benign meningiomas from linkage to the nationwide registry of histo- and cytopathology (PALGA)13 for more than 95% of the total cohort. Other studies relied on initial self-report and/or linkage with tumor registries.2,3 In addition, a comparison with a sibling group was performed, to obtain more insight into the incidence of meningiomas, which enabled us to parallel the meningioma incidence in CCSs to the general population. PALGA is an internationally unique resource to ascertain benign meningiomas, because benign CNS tumors are not typically recorded completely in cancer registries, although this is changing in more recent years.
Several weaknesses of our approach deserve attention as well. PALGA has complete national coverage from 1990 onward; tumors occurring during 1968–1989 were not recorded reliably. By including only follow-up time since 1990 and the fact that most meningiomas occur >20 years after childhood cancer, a follow-up interval which nearly all surviving cohort members completed after 1990, we are confident that the underestimation of the true cumulative incidence caused by left truncation is minimal. Secondly, as in most studies on meningioma, true incidence is likely not captured; we report on histologically confirmed benign meningiomas, while a certain proportion of such tumors can remain asymptomatic/indolent for some time. Other factors may have increased the meningioma detection rate: medical care has changed, including access to and indications for brain imaging as well as indications for surgery of cranial masses suspect for meningioma. Also, between 1996 and 2006, Dutch late effects outpatient clinics were implemented in which CCSs were followed up according to evidence-based Dutch guidelines. These guidelines do not recommend active screening for CNS tumors among asymptomatic individuals. Nevertheless, all survivors have received guideline-based follow-up at fixed intervals, which is more frequent for those with high-intensity treatment (including those who received radiotherapy). It is quite possible that more intensive medical attention in the outpatient clinics slightly increased the detection rates of asymptomatic meningioma—for example, among patients with a history of seizures, headaches, or other neurologic problems owing to a brain tumor or CNS metastases, but also brain surgery, hydrocephalus, or high-dose radiotherapy. Longer follow-up of available cohorts with complete treatment information and adequate follow-up methods to detect these benign tumors, as well as pooled analyses of these studies, are needed to shed light on the influence of these issues on meningioma incidence rates.
The results of this study can be used to inform surveillance recommendations for long-term CCSs who had CrRT, such as those currently being formulated by the International Guideline Harmonization Group.19 Although risk estimation in terms of prescribed CrRT dose is necessary in light of surveillance guideline development, further studies are forthcoming to express excess risk in terms of cranial volume–based absorbed dose distributions. These will be useful to inform future pediatric radiotherapy treatment guidelines. To fully disentangle effects of dose, volume, age, and the potential role of chemotherapy agents, an international pooling effort is warranted to achieve sufficient statistical power.
In conclusion, 1 in 8 CCSs exposed to cranial radiotherapy develops a late meningioma 40 years after childhood cancer diagnosis, and this risk is dose and exposure-age related. We did not find significant modifications of the radiation dose-response by age or by exposed cranial volume. While the proportion of patients in need of (full) CrRT with curative intent has decreased over time, this treatment cannot be abolished without compromising cancer survival for children with intracranial tumors or other indications for CrRT. These findings are important to raise awareness among survivors, their parents, and care providers about these long-term sequelae and to support ongoing efforts to reduce the radiation exposure to healthy tissues, where feasible, without compromising treatment efficacy.
Funding
This work was supported by the Dutch Cancer Society (grant numbers DCOG2011-5027 and UVA2012-5517). Judith Kok is appointed on a Flexible Onderzoeker in Opleiding (OiO) grant awarded by the Academic Medical Center (AMC) Executive Board to C. M. Ronckers/L. C. Kremer.
Supplementary Material
Acknowledgments
We thank all data managers in the 7 participating centers and Aslihan Mantici and Anja van Eggermond for obtaining the data for this study. Furthermore, we thank the following other members of the DCOG-LATER group for their contributions: Lilian Batenburg, Margreet Veening, Marloes Louwerens, Gea Huizinga, Lideke van der Steeg, Hanneke de Ridder-Sluiter, and Andrica de Vries. We thank the staff of the PALGA Foundation for providing record linkage data on meningiomas from their registry.
DCOG-LATER Study Group authors. The DCOG-LATER Study Group for benign CNS tumors includes the listed authors and the following persons:
MH van den Berg (Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam)
AH Bruggink (PALGA Foundation, Houten)
HN Caron (Emma Children’s Amsterdam UMC, University of Amsterdam, Amsterdam)
WV Dolsma (University of Groningen/University Medical Center Groningen)
MA Grootenhuis (Princess Máxima Center for Pediatric Oncology, Utrecht)
JG den Hartogh (Dutch Childhood Cancer Parent Organisation [VOKK], Nieuwegein)
N Hollema (Dutch Childhood Oncology Group, Utrecht)
MC Jongmans (Radboud University Medical Center, Nijmegen, University Medical Center Utrecht, Utrecht, and Princess Máxima Center for Pediatric Oncology, Utrecht)
MWM Jaspers (Amsterdam UMC, University of Amsterdam, Amsterdam)
A Postma (Dutch Childhood Oncology Group, Utrecht)
MJ van de Vijver (Amsterdam UMC, University of Amsterdam, Amsterdam)
Conflict of interest statement. The authors declare no potential conflicts of interest.
Authorship statement. Conception and design: J. L. Kok, J. C. Teepen, F. E. van Leeuwen, L. C. M. Kremer, C. M. Ronckers
Development of methodology. J. L. Kok, J. C. Teepen, F. E. van Leeuwen, M. Hauptmann, L. C. M. Kremer, C. M. Ronckers
Acquisition of data. W. J. E. Tissing, H. J. van der Pal, J. J. Loonen, D. Bresters, B. Versluys, M. M. van den Heuvel-Eibrink, E. van Dulmen-den Broeder, M. van der Heiden-van der Loo
Analysis and interpretation of data. All authors
Writing, review and/or revision of the manuscript. All authors
Administrative, technical, or material support. M. van der Heiden-van der Loo, C. M. Ronckers
Study supervision. L. C. M. Kremer, F. E. van Leeuwen, C. M. Ronckers
Final approval of the version to be published. All authors
Contributor Information
DCOG-LATER Study Group:
M H van den Berg, A H Bruggink, H N Caron, W V Dolsma, M A Grootenhuis, J G den Hartogh, N Hollema, M C Jongmans, M W M Jaspers, A Postma, and M J van de Vijver
References
- 1. Bowers DC, Nathan PC, Constine L, et al. . Subsequent neoplasms of the CNS among survivors of childhood cancer: a systematic review. Lancet Oncol. 2013;14(8):e321–e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bowers DC, Moskowitz CS, Chou JF, et al. . Morbidity and mortality associated with meningioma after7 cranial radiotherapy: a report from the childhood cancer survivor study. J Clin Oncol. 2017:35(14): 1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor AJ, Little MP, Winter DL, et al. . Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(36):5287–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patterson BC, Chen Y, Sklar CA, et al. . Growth hormone exposure as a risk factor for the development of subsequent neoplasms of the central nervous system: a report from the childhood cancer survivor study. J Clin Endocrinol Metab. 2014;99(6):2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neglia JP, Robison LL, Stovall M, et al. . New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21):1528–1537. [DOI] [PubMed] [Google Scholar]
- 6. Friedman DL, Whitton J, Leisenring W, et al. . Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turcotte LM Liu Q, Yasui Y, et al. . Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970–2015. Jama. 2017; 317(8):814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ronckers CM, Sigurdson AJ, Stovall M, et al. . Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166(4):618–628. [DOI] [PubMed] [Google Scholar]
- 9. Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424–432. [DOI] [PubMed] [Google Scholar]
- 10. Sugden E, Taylor A, Pretorius P, Kennedy C, Bhangoo R. Meningiomas occurring during long-term survival after treatment for childhood cancer. JRSM Open. 2014;5(4):2054270414524567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. International Guideline Harmonization Group. International Guideline Harmonization Group for late effects of childhood cancer http://www.ighg.org/international-guideline-harmonization-group/. Accessed April 3, 2018.
- 12. Teepen JC, van Leeuwen FE, Tissing WJ, et al. ; DCOG LATER Study Group. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER Study Cohort: Role of Chemotherapy. J Clin Oncol. 2017;35(20):2288–2298. [DOI] [PubMed] [Google Scholar]
- 13. Casparie M, Tiebosch AT, Burger G, et al. . Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. [DOI] [PubMed] [Google Scholar]
- 15. Preston DL, Ron E, Yonehara S, et al. . Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 2002;94(20):1555–1563. [DOI] [PubMed] [Google Scholar]
- 16. Veerman AJ, Hählen K, Kamps WA, et al. . High cure rate with a moderately intensive treatment regimen in non-high-risk childhood acute lymphoblastic leukemia. Results of protocol ALL VI from the Dutch Childhood Leukemia Study Group. J Clin Oncol. 1996;14(3):911–918. [DOI] [PubMed] [Google Scholar]
- 17. Veerman AJ, Kamps WA, van den Berg H, et al. . Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004). Lancet Oncol. 2009; 10(10):957–966. [DOI] [PubMed] [Google Scholar]
- 18. Kamps WA, Bokkerink JP, Hahlen K, et al. . Intensive treatment of children with acute lymphoblastic leukemia according to ALL-BFM-86 without cranial radiotherapy: results of Dutch Childhood Leukemia Study Group Protocol ALL-7 (1988–1991). Blood. 1999; 94(4):1226–1236. [PubMed] [Google Scholar]
- 19. Kremer LC, Mulder RL, Oeffinger KC, et al. ; International Late Effects of Childhood Cancer Guideline Harmonization Group. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60(4):543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.