Abstract
Background
We assessed population-level changes in glioblastoma survival between 2000 and 2013 in Finland, with focus on elderly patients (>70 y) in order to assess if changes in treatment of glioblastoma are reflected also in population-based survival rates.
Methods
We identified all patients (age ≥18 y) from the Finnish Cancer Registry (FCR) with a histopathological diagnosis of primary glioblastoma in 2000–2013. Patients were followed up until December 2015. The accuracy of register-based search of glioblastoma patients was internally validated. We report age-standardized relative survival ratios and relative excess risks (RERs) of death in 2000–2006 (pre-period) and 2007–2013 (post-period).
Results
We identified 2045 glioblastoma patients from the FCR. The accuracy of the FCR-based search was 97%. Median age was 63.3 years, and 42% were women. Incidence increased on average by 1.6% (P = 0.004) and median age by 0.4 years per calendar year. Between the pre- and post-periods, the proportion of patients >70 years increased from 24% to 27%. In >70-year-old patients, the median survival time increased from 3.6 months in 2000–2006 to 4.5 months in 2007–2013 (RER 0.82, 95% CI: 0.68–0.98). In ≤70-year-old patients, the median survival time increased from 9.3 months in 2000–2006 to 11.7 months in 2007–2013 (RER 0.74, 95% CI: 0.67–0.82).
Conclusion
Despite the increased proportion of elderly glioblastoma patients, population-level survival of glioblastoma patients has improved since the year 2000. However, increasing incidence, increasing age of patients, and poor survival in elderly are alarming, and future studies should perhaps focus more on elderly.
Keywords: elderly, epidemiological study, glioblastoma, glioma, malignant glioma
Key Points
Glioblastoma incidence has increased consistently from 2000 to 2013.
Survival has improved more in younger glioblastoma patients.
Poor survival in elderly glioblastoma patients is alarming.
Importance of the Study
Following the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups and the National Cancer Institute of Canada (EORTC-NCIC) trial in 2005, the standard of care for patients with glioblastoma has consisted of tumor resection followed by radiotherapy and temozolomide. In this study, we estimated population-level survival changes following the EORTC-NCIC trial. We found that patients diagnosed with glioblastoma after the EORTC-NCIC trial had a 24% lower relative risk of death compared with patients diagnosed before the trial. The relative risk of death decreased more in glioblastoma patients ≤70 years compared with glioblastoma patients >70 years (26% vs 18%). Furthermore, median survival time increased only by 0.9 months in glioblastoma patients >70 years compared with 2.4 months in glioblastoma patients ≤70 years. We also found that the median age and incidence of glioblastoma, especially among the elderly, steadily increased. This study provides nationwide evidence showing that survival rates among glioblastoma patients have improved following the recent randomized controlled trial. Still, poor overall population-level survival in elderly glioblastoma patients raises concerns whether current treatment strategies for elderly patients are optimal. Given the rapidly aging population in many industrialized countries, studies focusing on elderly glioblastoma patients are urgently needed.
Glioblastoma, classified by the World Health Organization (WHO) as a grade IV glioma, is the most prevalent type of newly diagnosed primary malignant brain tumor in adults.1–4 To date, no curative treatment exists for these rapidly progressing tumors and there is no deadlier form of cancer than glioblastoma (5-y mortality rate of 97%).5 Since the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups and the National Cancer Institute of Canada (EORTC-NCIC) trial published in 2005 (conducted in 2000–2002),6 standard treatment consists of surgical tumor resection followed by concomitant radiotherapy and temozolomide (chemoradiation). In more detail, the EORTC-NCIC trial6 showed that by adding temozolomide to radiotherapy, the median survival improved from 12.1 months to 14.6 months in glioblastoma patients between 18 and 70 years. In 2017, another trial (conducted in 2007–2013) showed that in glioblastoma patients ≥65 years old, chemoradiation increased median survival time from 7.6 months to 9.3 months.7 Still, controversy exists whether elderly (>70 y) glioblastoma patients benefit from the more aggressive treatment.8
Nationwide results on cancer survival are among key indicators in assessing health care system effectiveness of care and management of cancer patients.5,9 Yet, nationwide epidemiological studies assessing the impact of these changes in treatment policies of glioblastoma reflected by survival are scarce. Finland has a government-subsidized social welfare and health care system, where socioeconomic factors and health inequalities are less likely to affect cancer treatments and treatment outcomes, which are shown to be among the best in the world.5 Since a nationwide cancer registry can be used to reliably estimate the population-level changes, including treatment changes, the primary aim of our register-based study was to explore if nationwide glioblastoma incidence and survival rates have changed after the EORTC-NCIC trial,6 with focus on elderly (>70 y) glioblastoma patients. We hypothesized that population-level glioblastoma survival has improved since standardization of chemotherapy.6
Materials and Methods
Ethical Considerations
According to Finnish law, individual-level data with identifiers from the Finnish Cancer Registry (FCR) can only be used for scientific research with approval from authorities or for statistics. The FCR follows strict regulations to ensure absolute confidentiality and protection of the individuals. The data processing procedures were evaluated and approved by Helsinki University Hospital’s research committee (HUS/356/2017 §107) and the National Institute for Health and Welfare (THL/1009/6.02.00/2018). We conducted the study according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (Electronic Supplementary Material 1).
Finnish Health Care System
Finland’s health care system offers equal and low-cost public health care for all citizens, also for those with or without income or insurance. For example, in 2017, the daily hospital fee for short-term institutional care in Helsinki University Hospital, one of the largest hospital organizations in industrialized countries, was €38.80. This out-of-pocket fee covers the whole treatment from all types of surgeries to intensive care stay, as medical care costs are tax-funded and paid by the municipality the patient lives in. The most specialized and high-level medical care is provided by 5 public and nonprofit university hospitals; for example, all intracranial neurosurgical operations, including brain tumor surgeries, are performed exclusively in these 5 university hospitals. In the Finnish health care payment model, employees in academic hospitals do not have, for example, procedure or capitation-based financial incentives.
Finnish Cancer Registry
The FCR is a nonprofit institute for statistical and epidemiological cancer research in Finland funded by the Cancer Society of Finland and the National Institute for Health and Welfare (THL, Terveyden ja Hyvinvoinnin Laitos). The FCR maintains a nationwide database covering all incident cancers since 1953. It covers 95% of all solid tumors.10 As mandated by law, health care organizations are required to report cancer cases to the FCR, which collects demographic, diagnostic, and treatment data on patients’ diagnoses and/or treatments in Finland. Accordingly, laboratories of pathology, cytology, and hematology send respective clinical (diagnostic) notifications (passive registration). Statistics Finland, which has archived all Finnish death certificates from 1936 onward, provides causes of death of individuals registered in the FCR. In addition, cases where the diagnosis of cancer is based solely on the death certificate will be received (death certificate only [DCO] cases). Coding of cancer cases is done by specifically qualified cancer coders and supervised by the FCR physician (pathologist). Since 2007, the FCR has used the International Classification of Diseases for Oncology Third Edition (ICD-O-3) nomenclature for cancer topography (primary site of cancer) and morphology (histological type). Older code combinations have been systematically converted into the ICD-O-3. For malignant gliomas, the conversion (ICD-O-3 nomenclature) is available from 2000 onward.
Identification of Glioblastoma Patients from the Finnish Cancer Registry
All patients (≥18 y of age) with primary glioblastoma were identified through the FCR. Using the topography codes (ICD-O-3) “C71.0–C71.9” and morphology code “9440” (glioblastoma), we identified persons with a histopathological diagnosis of first primary glioblastoma obtained through either biopsy or resection. We excluded morphology codes “9441” (giant cell glioblastoma, N = 25) and “9442” (gliofibroma/gliosarcoma, N = 58), as we presumed that including these rare malignancies might decrease, not increase, external validity (generalizability). Secondary or recurrent glioblastomas (ie, prior diagnosis of glioma or glioblastoma reoperation) were also excluded. As histopathological diagnosis was an inclusion criterion, we did not include DCO cases.
Identification of Glioblastoma Patients from the Hospital Registry and Treatment Strategies
In order to evaluate the internal validity of the FCR in glioblastoma identification, all patients operated on in 2 separate years (2005 and 2010) at Helsinki University Hospital were identified through a prospective surgical log book, which includes every neurosurgical patient operated on in the Department of Neurosurgery (which covers more than one-third of all glioblastoma patients in Finland). We then compared the number of glioblastoma patients identified through the FCR with the number of operated glioblastoma patients identified through the hospital registries. We further scrutinized electronic health record (EHR) data of these patients treated in 2005 and 2010 and collected data regarding type of surgery performed (resection or biopsy), and whether the patient received adjuvant chemotherapy including temozolomide.
Survival and Follow-up
Patients were followed up from the date of histopathological diagnosis until death, emigration, or the end of 2015. Due to the nationwide and obligatory registries including unique personal identifiers, follow-up was complete. Since the randomized trial results were published in 2005,6 a one-year transition period was taken into account, and therefore 2 time periods, namely 2000–2006 and 2007–2013, were used in the comparative analyses.
Statistical Analysis
We calculated age-standardized glioblastoma incidence per 100000 inhabitants using the European Standard Population 2013 (ESP2013). Incidence rates are also reported by age groups (five-year groups from 25–29 to 80–84, and ≥85 y). The relative annual change in age-standardized incidence rate was estimated by using the Poisson regression. The Davies’ test was used as a significance test to detect a change in the incidence trend.11
To estimate the accuracy of the FCR, we considered the hospital registry as the gold standard, and report the accuracy of the FCR-based search on persons with glioblastoma as percentage of cases found in FCR divided by cases found in the hospital registry.
We calculated age-standardized relative survival by using the Ederer II method.12,13 It summarizes patients’ excess risk of death due to the cancer by comparing the survival of patients with that of their reference population (the population of Finland stratified by sex, age, and calendar year). In the age standardization, the age group specific numbers of patients diagnosed in 2000–2013 were used as weights based on 6 groups (18–40, 41–50, 51–60, 61–70, 71–80, and 81 y and older at diagnosis). To compare differences in relative survival between 2000–2006 and 2007–2013, we estimated relative excess risk (RER) of death by using the Poisson regression model for relative survival.14 In age group specific analyses, the model included 7 intervals of follow-up time since diagnosis (0 to <2 mo, 2 to <6 mo, 6 to <12 mo, 12 to <18 mo, 18 to <24 mo, 2 to <3 y, 3 to <5 y) and the period of diagnosis. In analyses combining more than one age group, age at diagnosis (the same age groups as above) and interaction between follow-up time and age were also included in the model to allow for non-proportional excess risk of death by age. The first 5 years of follow-up were considered and longer survival times were censored at 5 years. An estimate of RER under 1 indicates that the excess risk of death among patients diagnosed in the 2007–2013 era was lower than that in the 2000–2006 era and vice versa. Subgroup-specific analysis was done by sex, age groups, and separately for patients >70 years. We also report median survival times. P-values for the differences in excess mortality between the two time periods were based on the likelihood-ratio test, which was adjusted for multiple comparisons by using the method of Benjamini and Hochberg.15 The adjustment was done for interactions between age and time period, and for the main effects of age and sex.
Results
Glioblastoma Incidence and Age at Diagnosis
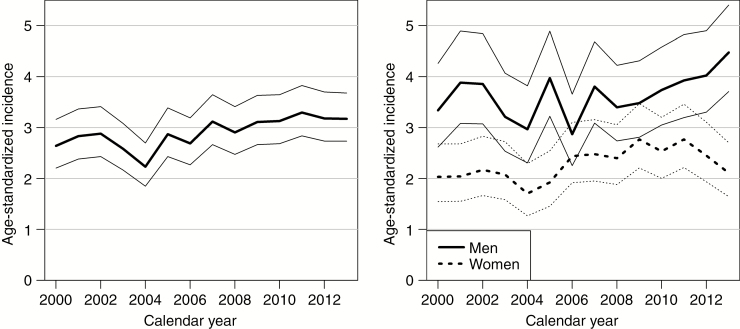
Between 2000 and 2013, a total of 2045 persons with a histopathological diagnosis of primary glioblastoma were recorded in the FCR. The median age at diagnosis was 63.3 years, and 42% were women (Table 1). The average increase in patient median age was 0.4 years per calendar year. The proportion of elderly (>70 y) increased from 23.6% in 2000–2006 to 26.7% in 2007–2013. The ESP2013 standardized mean incidence of glioblastoma during the study period was 2.9 per 100000 person-years. The age-standardized incidence of glioblastoma increased on average by 1.6% (95% CI: 0.5% to 2.7%) per calendar year (P = 0.004). The relative annual increase was 1.4% (95% CI 0.0% to 2.8%, P = 0.056) in men and 1.9% (95% CI 0.2% to 3.6%, P = 0.028) in women (Fig. 1). This increase was unchanged during the study period (P = 0.640). The incidence of glioblastoma increased sharply with age, being the highest among 65 to 79 year olds (Electronic Supplementary Material 2).
Table 1.
Patient characteristics in 2000–2006 and 2007–2013
| Variable | All Patients N = 2045 |
2000–2006 N = 893 |
2007–2013 N = 1152 |
|---|---|---|---|
| Age, y, median | 63.3 | 61.4 | 64.3 |
| Age, y, mean | 62.8 | 61.7 | 63.6 |
| 18–40 | 83 (4%) | 42 (5%) | 41 (4%) |
| 41–50 | 220 (11%) | 115 (13%) | 105 (9%) |
| 51–60 | 566 (28%) | 280 (31%) | 286 (26%) |
| 61–70 | 657 (32%) | 245 (27%) | 412 (36%) |
| 71–80 | 427 (21%) | 173 (20%) | 254 (22%) |
| >80 | 92 (4%) | 38 (4%) | 54 (5%) |
| Sex | |||
| Men | 1191 (58%) | 524 (59%) | 667 (58%) |
| Women | 854 (42%) | 369 (41%) | 485 (42%) |
Categorical variables shown as numbers with percentages.
Data from the Finnish Cancer Registry.
Fig. 1.
Age-standardized (the European Standardized Population in 2013) incidence of glioblastoma (per 100000 person-years) in Finland in 2000–2013 with 95% confidence intervals. To the left, the incidence for men and women and to the right the incidence for men and women separately. Data from the Finnish Cancer Registry.
Accuracy of Glioblastoma Patient Identification
Of all 2045 glioblastoma patients included in the study, 761 patients (37%) were operated in Helsinki. The FCR-based search identified 113 persons who were diagnosed with primary glioblastoma in 2005 and 2010 in the Helsinki University Hospital catchment area. The search, which was based on Helsinki University hospital registries and EHR, identified 116 glioblastoma patients operated on in the same years. Therefore, the accuracy of FCR-based search was 97% in identifying operated (burr-hole biopsy or craniotomy) glioblastoma patients.
Survival and Excess Risk of Death by Period and Age
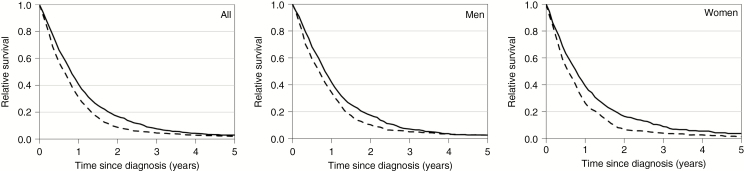
Age-standardized cumulative relative survival ratios are shown in Table 2 and Fig. 2. In 2000–2006, 1-year survival rate was 31%, 3-year survival rate was 5%, and 5-year survival rate was 2%. In 2007–2013, the 1-year survival rate increased to 41%, 3-year survival rate increased to 8%, and 5-year survival rate increased to 3%. Even though 1-year survival increased in all age groups from 2000–2006 to 2007–2013, 3-year and 5-year survival remained poor, particularly in people older than 60 years (Table 2). When comparing ≤70 and >70-year-old patients, 1-year survival for patients ≤70 years was approximately 3 times higher than in elderly. Three-year and 5-year survival remained overly poor in elderly patients. In fact, of elderly patients with diagnoses in 2007–2013, only 3% and 1% survived 3 and 5 years, respectively.
Table 2.
Relative excess risk (RER) of death between 2007–2013 and 2000–2006, and relative survival ratios (with 95% CIs) at 1, 3, and 5 years after diagnosis by age, sex, and diagnosis period
| Outcome | RER | One-Year Survival (%) | Three-Year Survival (%) | Five-Year Survival (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Time Period | 2007–13 vs 2000–06 | Adjusted P-value | 2000–2006 | 2007–2013 | 2000–2006 | 2007–2013 | 2000–2006 | 2007–2013 |
| All patients | 0.76 (0.69–0.83) | <0.001 | 31 (28–34) | 41 (38–43) | 5 (3–6) | 8 (6–9) | 2 (1–3) | 3 (2–4) |
| ≤70 y | 0.74 (0.67–0.82) | <0.001 | 39 (35–42) | 49 (46–53) | 6 (4–8) | 9 (7–12) | 3 (2–4) | 4 (2–6) |
| >70 y | 0.82 (0.68–0.98) | 0.053 | 8 (5–13) | 16 (12–20) | 1 (0–4) | 3 (1–5) | 1 (0–3) | 1 (0–3) |
| Age groups | ||||||||
| 18–40 y | 0.92 (0.57–1.48) | 0.735 | 64 (48–77) | 68 (52–80) | 24 (12–37) | 22 (11–36) | 12 (4–24) | 15 (6–29) |
| 41–50 y | 0.70 (0.53–0.92) | 0.021 | 49 (39–58) | 70 (60–78) | 11 (6–17) | 16 (10–24) | 4 (2–9) | 6 (2–12) |
| 51–60 y | 0.70 (0.59–0.83) | <0.001 | 40 (35–46) | 53 (47–58) | 3 (2–6) | 8 (5–12) | 1 (1–4) | 3 (2–6) |
| 61–70 y | 0.78 (0.66–0.92) | 0.007 | 31 (25–37) | 37 (32–42) | 4 (2–7) | 7 (4–10) | 2 (1–4) | 2 (1–5) |
| 71–80 y | 0.83 (0.68–1.01) | 0.092 | 10 (6–15) | 18 (14–23) | 1 (0–5) | 3 (1–6) | 1 (0–4) | 1 (0–3) |
| >80 y | 0.78 (0.51–1.20) | 0.335 | 3 (0–13) | 4 (1–12) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Sex and age | ||||||||
| Men | 0.79 (0.70–0.89) | <0.001 | 34 (31–38) | 42 (38–45) | 5 (3–7) | 7 (5–9) | 3 (1–4) | 2 (1–5) |
| ≤70 | 0.80 (0.70–0.92) | 0.004 | 43 (38–47) | 49 (45–54) | 6 (4–9) | 8 (6–11) | 3 (2–5) | 2 (1–4) |
| >70 | 0.75 (0.58–0.97) | 0.048 | 6 (3–12) | 16 (11–23) | 1 (0–5) | 3 (1–7) | 1 (0–5) | 1 (0–5) |
| Women | 0.71 (0.61–0.82) | <0.001 | 26 (22–31) | 39 (35–43) | 4 (2–6) | 9 (6–12) | 1 (1–3) | 4 (2–6) |
| ≤70 | 0.64 (0.54–0.76) | <0.001 | 33 (27–38) | 49 (43–54) | 5 (3–8) | 12 (8–15) | 2 (1–4) | 5 (3–9) |
| >70 | 0.88 (0.68–1.15) | 0.400 | 11 (6–19) | 16 (10–22) | 1 (0–7) | 3 (1–6) | 0 (0–0) | 0 (0–0) |
Relative excessive risk of death (RER) for patients with diagnoses glioblastoma in 2007–2013 compared with diagnoses in 2000–2006. RER less than 1 indicates that patients diagnosed in 2007–2013 had a lower excess risk of death compared with patients diagnosed in 2000–2006 and vice versa.
The estimates are age-standardized and can be compared between the two periods.
One-year, 3-year and 5-year survival rates are shown as percentages with 95% confidence intervals.
Adjusted P-values refer to P-values adjusted for multiple testing.
Data from the Finnish Cancer Registry.
Fig. 2.
Comparison of age-standardized cumulative relative survival ratios between persons diagnosed in 2000–2006 (dashed line) compared with 2007–2013 (solid line). To the left, all patients, men in the middle, and women to the right. Data from the Finnish Cancer Registry.
Cumulative relative survival rates for patients ≤70 and >70 years are shown in Table 3 and Electronic Supplementary Material 3. In 2000–2006, median survival time for patients ≤70 years was 9.3 months compared with 11.7 months in 2007–2013. In men ≤70 years, median survival time increased from 9.8 months to 11.8 months; in women ≤70 years, median survival time increased from 8.9 months to 11.7 months. For patients >70 years, median survival time was 3.6 months in 2000–2006 compared with 4.5 months in 2007–2013. In men >70 years, median survival time increased from 3.6 months to 4.8 months; in women >70 years, median survival time increased from 3.7 months to 4.2 months.
Table 3.
Median survival times (point estimate with 95% CI) in months
| Outcome | Median Survival Time (95% CI) | |
|---|---|---|
| Time Period | 2000–2006 | 2007–2013 |
| All patients | 7.5 (6.8–8.2) | 9.6 (9.1–10.2) |
| ≤70 | 9.3 (8.6–10.1) | 11.7 (11.0–12.5) |
| >70 | 3.6 (3.2–4.1) | 4.5 (3.8–5.1) |
| Age group | ||
| 18–40 | 17.0 (11.1–22.0) | 19.7 (13.7–25.1) |
| 41–50 | 11.8 (10.2–13.2) | 17.3 (14.5–19.5) |
| 51–60 | 9.9 (9.2–11.2) | 12.4 (11.3–13.8) |
| 61–70 | 6.7 (5.6–7.8) | 9.3 (8.3–9.9) |
| 71–80 | 4.2 (3.6–5.2) | 5.2 (4.5–5.8) |
| >80 | 2.0 (1.3–2.6) | 2.3 (1.4–3.1) |
| Sex and age | ||
| Men | 8.1 (7.1–8.9) | 9.9 (9.2–10.8) |
| ≤70 | 9.8 (8.6–11.1) | 11.8 (10.9–12.7) |
| >70 | 3.6 (3.1–4.3) | 4.8 (3.8–5.5) |
| Women | 6.7 (5.7–7.8) | 9.2 (8.2–10.1) |
| ≤70 | 8.9 (7.8–9.8) | 11.7 (10.3–13.3) |
| >70 | 3.7 (2.8–4.6) | 4.2 (3.3–5.2) |
The estimates are age-standardized and can be compared between the two periods. Survival time shown in months.
Data from the Finnish Cancer Registry.
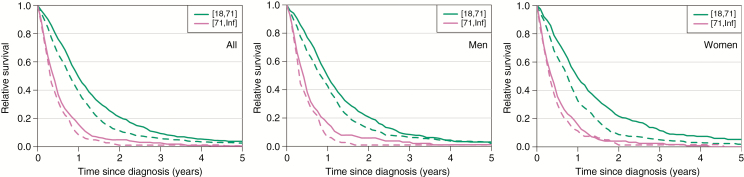
RERs of death by sex and age group are shown in Table 2. Patients diagnosed in 2007–2013 had a smaller relative excess risk of death (RER 0.76, 95% CI: 0.69–0.83, P < 0.001) as compared with patients diagnosed in 2000–2006. This reduction in RER of death between periods was evident for both patients ≤70 years (RER 0.74, 95% CI: 0.67–0.82, P < 0.001) and >70 years of age (RER 0.82, 95% CI: 0.68–0.98, P = 0.032). We found no evidence of the difference in the reduction of excess mortality from 2000–2006 to 2007–2013 between the age groups in men (adjusted P = 0.670) or in women (adjusted P = 0.073). When analyzed by sex, RER of death decreased significantly for men and women ≤70 years. Among patients >70 years, the decrease was significant in men only. The decrease was the largest in ≤70-year-old women (RER 0.64, 0.54–0.76), and significantly larger (P = 0.048) than that in older women (RER 0.88, 0.68–1.15). In an age group specific analysis, RER of death decreased significantly in patients aged 41–50 years (RER 0.70), 51–60 years (RER 0.70), and 61–70 years (RER 0.78) (Fig. 3).
Fig. 3.
Comparison of age-standardized cumulative relative survival ratios between patients over and under 70 years of age diagnosed during 2000–2006 (dashed line) and 2007–2013 (solid line). To the left, all patients, in the middle men, and to the left women. Data from the Finnish Cancer Registry.
Treatment Strategies in 2005 and 2010
Year 2005. Forty-eight glioblastoma patients were operated on in Helsinki University Hospital. Of these, 16 patients (33%) were >70 years. Of these 16 patients, 11 (69%) underwent craniotomy and tumor resection, and 5 (31%) underwent stereotactic tumor biopsy. Only 2 elderly patients (13%) received adjuvant chemoradiation including temozolomide. Of the 32 ≤70-year-old patients, 30 (94%) underwent craniotomy and tumor resection, and only 2 (6%) underwent stereotactic tumor biopsy. Of these 32 patients, 18 (56%) received adjuvant chemoradiation including temozolomide.
Year 2010. Of the 68 operated glioblastoma patients in Helsinki, 18 (26%) were >70 years. Of these 18 patients, 17 (94%) underwent craniotomy and tumor resection, and 1 patient (6%) underwent stereotactic tumor biopsy. Data on adjuvant radiation and chemotherapy of one elderly patient was missing. Six elderly patients (33%) received adjuvant chemoradiation including temozolomide. Of the 12 elderly patients who did not receive temozolomide, 4 (33%) were treated with whole brain radiotherapy. Chemotherapy in the elderly was omitted mainly due to fast progression or poor overall condition following surgery. Of the 50 <70-year-old patients, 46 (92%) received adjuvant chemoradiation including temozolomide, and 44 patients (88%) underwent craniotomy and tumor resection (6 patients [12%] underwent stereotactic tumor biopsy only).
Discussion
Key Findings
In this nationwide study, we found that patients diagnosed in 2007–2013 had a 24% lower RER of death compared with patients diagnosed in 2000–2006. The RER of death decreased more in patients 70 years or younger (26%) compared with patients older than 70 years (18%). Median survival time increased by 2.4 months (from 9.3 to 11.7 mo) in patients 70 years or younger but only by 0.9 months (from 3.6 to 4.5 mo) in patients older than 70 years. Furthermore, the incidence of glioblastoma and median age of glioblastoma patients increased in time.
Comparison with Previous Studies
To estimate population-wide survival effects from controlled trials, one should turn to population-based studies.5,9 One of the first large epidemiological glioblastoma studies (before the EORTC-NCIC trial), including almost 17000 patients, came from the Surveillance, Epidemiology, and End Results (SEER) database and showed no improvement in survival rates for glioblastoma patients from the 1980s to 2001.16 A more recent study from the SEER database, including almost 7000 patients, showed that survival times for glioblastoma patients increased from 12 to 15 months between 2000–2001 and 2005–2006.17 Another study from the SEER database, including almost 14000 patients, showed an improvement in median survival time from 8.1 months in 2000–2003 to 9.7 months in 2005–2008.18 Still, it should be noted that the SEER database covers only 26% of the US population and that access to neuro-oncological treatment is unequal and dependent, for example, upon socioeconomic factors.19
A study from the nationwide Norwegian Cancer Registry (covering the whole Norwegian population like the FCR in Finland), including 1157 patients, showed that median survival time increased from 8.3 months in 2000–2003 to 10.1 months in 2004–2007.20 Median survival time was even higher among patients receiving adjuvant temozolomide compared with radiotherapy alone (16.2 mo vs 9.0 mo).20 A subgroup analysis showed that survival improved in patients 70 years or older as well. However, this subgroup analysis was compromised by the low number of patients, as only 22 out of 271 (8%) elderly patients received adjuvant temozolomide chemoradiation. In the present study, we could not assess the effect of temozolomide on survival times for the whole population, as the nationwide registries do not contain detailed data on individual treatments. However, the median survival time increased from 7.5 months in 2000–2006 to 9.6 months in 2007–2013 in Finland. These figures are in line with the previous population-based studies but are lower than results from the Danish Neuro-Oncology Register (including 1364 histopathological confirmed glioblastoma patients from 2009 to 2014), which showed a median overall survival time of 14.3 months for patients older than 70 years and a median survival of 18.6 months for glioblastoma patients between 18 and 59 years.21
As expected, we found a relationship between age and survival. For example, in 2007–2013, the 1-year survival rate in patients 70 years or younger was 49% but only 16% in patients older than 70 years. The difference in 1-year mortality was notable even in patients between 61–70 years and older than 70 years (37% vs 16%, respectively, in 2007–2013). If comparing survival in patients between 41–50 years and older than 70 years, survival differences seem even more evident (1-year survival 70% vs 18% in 2007–2013). In two studies from the SEER database, one reported a 1-year survival rate of 19% for glioblastoma patients older than 70 years (study years 1999–2010)22 and a median survival time of 4 months (study years 1994–2002).23 These figures are in line with our results.
We found that the incidence of glioblastoma seems to be increasing, especially among women. The finding is in line with a report from the Central Brain Tumor Registry of the United States and the SEER database.2,16 The increase in the incidence is probably linked to the higher life expectancy due to a reduction in cardiovascular deaths and other forms of cancer deaths in Finland.5,24 In the light of increasing incidence and population aging, one question is of increasing importance, namely how to optimally treat elderly glioblastoma patients today and in future. A French randomized controlled trial, showed that glioblastoma patients 70 years or older receiving adjuvant radiotherapy (50 Gy) had a median survival of 6.8 months compared with 3.9 months in patients receiving supportive care only.25 Importantly, there were no differences in quality of life between patients receiving radiotherapy compared with supportive care only, although almost half of the increased survival time was spent receiving the treatment.25 The NOA-08 trial showed no difference in overall survival for glioblastoma patients 65 years or older treated with adjuvant temozolomide compared with adjuvant conventional radiation therapy (60.0 Gy).26 On the other hand, the Nordic trial showed that conventional radiotherapy (60.0 Gy) was associated with poorer outcomes in comparison to temozolomide monotherapy or hypofractionated radiotherapy (34.0 Gy) in glioblastoma patients ≥60 years.27 Further, a recently published randomized controlled trial reported that glioblastoma patients 65 years or older had a survival advantage after receiving adjuvant radiotherapy (40.0 Gy) with concomitant temozolomide compared with adjuvant radiotherapy alone.7 Despite the fact that the above-mentioned studies were highly selected trials that excluded a number of potential elderly patients, median survival times for elderly were only 6.8 months,25 9.6 months,26 8.3 months,28 and 9.3 months.7 In contrast to these trials, the nationwide Norwegian Cancer Registry study reported that elderly patients (older than 70 years) receiving adjuvant temozolomide had an improved median survival time (13.4 mo).20 In comparison, glioblastoma patients older than 70 years not receiving any adjuvant therapy in the Norwegian study had a median survival of 2.8 months.20 Still, as only 8% of elderly patients received adjuvant temozolomide therapy, this survival benefit is highly selected. In our nationwide study, median survival time was 4.5 months (in 2007–2013) for patients older than 70 years. In the Helsinki University Hospital subgroup analysis, the median survival time of 6 out of 18 elderly (>70 years) patients who received temozolomide was 13.2 months, and 2.5 months for patients who underwent tumor resection but did not follow the temozolomide protocol (results not shown). This suggests that only a minority of elderly (>70 y) patients are pre and postoperatively fit to tolerate and benefit from chemoradiation. A recent population-based study supports this notion by showing that less than half of all glioblastoma patients complete the standard of care.29 Taken together, future studies should focus on defining the subpopulation of elderly, who have the best likelihood of benefiting from tumor resection and subsequent chemoradiation.
Brain tumor resection can be considered as major surgery, particularly for elderly. The only randomized trial looking at the role of tumor resection versus diagnostic biopsy for glioblastoma patients 65 years or older showed a survival benefit for resection compared with diagnostic biopsy (5.7 mo vs 2.8 mo).30 In our subgroup analysis of Helsinki University Hospital patients, we found that in the years of 2005 and 2010, 82% of patients older than 70 years underwent craniotomy and tumor resection. Similar aggressive approaches have been reported elsewhere.31 On the other hand, some centers seem to have a more conservative approach. For example, at the Mayo Clinic during 2003–2008, only 50% of all glioblastoma patients 65 years or older underwent tumor resection.32 Further, a study using the SEER database from 1994–2002 showed that 61% of patients 65 years or older underwent tumor resection surgery.23 Studies including comprehensive nationwide data looking at the role of surgery in elderly glioblastoma patients are lacking.
Following glioblastoma resection, temozolomide treatment is undoubtedly an aggressive treatment approach for elderly, who often have functional impairments already prior to treatment. A study investigated the role of temozolomide in elderly patients with lowered functional status (the ANOCEF phase II trial) in 2007–2009.33 In the trial, glioblastoma patients 70 years or older with a Karnofsky performance score <70 (need of assistance) receiving adjuvant temozolomide had a median survival time of 5.8 months.33 Patient selection for adjuvant chemotherapy and/or radiation therapy is essential, considering that a notable number of elderly patients who receive chemoradiation, including temozolomide, experience significant toxicity.6,7,33,34 In the internal validation of the Helsinki University Hospital cohort in 2005 and 2010, the use of temozolomide increased from 56% to 92% in patients 70 years or younger and from 13% to 33% in patients older than 70 years. Thus, it is possible that the modest improvement in survival for patients older than 70 years is due to the increase of adjuvant temozolomide use. However, benefits of temozolomide in elderly should be weighed against treatment-related major side effects, modest increase in survival time, amount of time spent in the hospital, and the high costs.35 Furthermore, aggressively treating elderly glioblastoma patients may shift the attention away from end of life care, causing unnecessary suffering.36
Strengths and Limitations
Strengths of this study include that it was performed in a country with a government-subsidized social welfare and health care system where socioeconomic factors and personal insurance status do not affect availability and implementation of glioblastoma treatment. Thus, our assessments of incidence of glioblastoma and population-level mortality and survival of glioblastoma patients provide a somewhat unbiased picture of the very recent situation in Finland. Furthermore, we assessed the accuracy of FCR10 in identifying glioblastoma cases, and the accuracy was high. Combining nationwide cancer registry and hospital information of glioblastoma treatment provides unique possibility to evaluate implications of changes in treatment policy. Some limitations should be acknowledged. First, as the FCR does not contain detailed data regarding treatments, we are unable to directly compare pattern of care between groups. Still, the nested cohort analysis provides reliable data regarding treatment standards from one hospital, which can be applied to the other included hospitals as well. Second, the extent of glioblastoma resection is considered an important prognostic factor.37 Routine postoperative MRI studies after glioblastoma surgery were introduced in the middle of the study period. Hence, it was not possible or statistically sound to assess the role of surgery for all included patients. These are, however, common limitations of large epidemiological nationwide studies like this. Third, we only included patients with a histopathological diagnosis of glioblastoma. Thus, patients dying due to glioblastoma before a histopathological diagnosis could be obtained are not included in the present study (ie, DCO cases that comprise 4.2% of recorded malignant CNS tumors in the FCR38). Accordingly, the reported survival rates are probably slightly worse and incidence slightly higher than reported here. Fourth, due to the observational nature of the study (retrospective register-based cohort), we cannot establish any causation between changes in treatment between periods and excess mortality.
In conclusion, in this nationwide study we found that the incidences of glioblastoma and glioblastoma patient age are increasing and that the overall survival of glioblastoma patients has improved from 2000–2006 to 2007–2013. Survival improved only modestly in elderly glioblastoma patients compared with younger patients, and poor overall population-level survival in elderly glioblastoma patients raises concerns whether current treatment strategies for elderly patients are optimal. Given the rapidly aging population in many industrialized countries, studies focusing on elderly glioblastoma patients are urgently needed.
Funding
The study had no funding. R.R. has received personal research grants from Svenska Kulturfonden, Medicinska Understödsföreningen Liv and Hälsa, Finska Läkaresällskapet, Maud Kuistilan Säätiö, Eemil Aaltosen Säätiö, Ella and Georg Ehrnroothin Säätiö, Suomalais-Norjalainen Lääketieteen Säätiö, Suomen Lääketieteen Säätiö, and Maire Taposen Säätiö.
Supplementary Material
Conflict of interest statement. None declared.
Authorship statement. MK had the main responsibility for study design, interpretation of data, and coordinating the study. MK and RR had the main responsibility for writing the manuscript. MK, RR, KS, TL, and JP contributed to acquisition of data, data analysis, interpretation of data, and revision of the manuscript. KS, TL, and JP were in charge of the statistical analyses. MS, HM, and NM contributed to interpretation of data and critical revision of the manuscript.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 5. Allemani C, Matsuda T, Di Carlo V, et al. ; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 7. Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 8. Zarnett OJ, Sahgal A, Gosio J, et al. Treatment of elderly patients with glioblastoma: a systematic evidence-based analysis. JAMA Neurol. 2015;72(5):589–596. [DOI] [PubMed] [Google Scholar]
- 9. Coleman MP. Cancer survival: global surveillance will stimulate health policy and improve equity. Lancet. 2014;383(9916):564–573. [DOI] [PubMed] [Google Scholar]
- 10. Leinonen MK, Miettinen J, Heikkinen S, Pitkäniemi J, Malila N. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31–39. [DOI] [PubMed] [Google Scholar]
- 11. Davies RB. Hypothesis testing when a nuisance parameter is present only under the alternatives. Biometrika. 1987;74(1):33. [Google Scholar]
- 12. Seppä K, Hakulinen T, Läärä E, Pitkäniemi J. Comparing net survival estimators of cancer patients. Stat Med. 2016;35(11):1866–1879. [DOI] [PubMed] [Google Scholar]
- 13. Ederer F, Heise H.. Instructions to IBM 650 programmers in processing survival computations. Methodological note no. 10. Bethesda, MD: End Results Eval Sect Natl Cancer Inst; 1959. [Google Scholar]
- 14. Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23(1):51–64. [DOI] [PubMed] [Google Scholar]
- 15. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 16. Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: surveillance, epidemiology, and end results program, 1973 to 2001. Neurosurg Focus. 2006;20(4):E1. [DOI] [PubMed] [Google Scholar]
- 17. Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107(1):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. [DOI] [PubMed] [Google Scholar]
- 19. Mukherjee D, Zaidi HA, Kosztowski T, Chaichana KL, Brem H, Chang DC. Disparities in access to neuro-oncologic care in the United States. Arch Surg. 2010;145(3):247. [DOI] [PubMed] [Google Scholar]
- 20. Rønning PA, Helseth E, Meling TR, Johannesen TB. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol. 2012;14(9):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hansen S, Rasmussen BK, Laursen RJ, et al. Treatment and survival of glioblastoma patients in Denmark: the Danish Neuro-Oncology registry 2009–2014. J Neurooncol. 2018. [DOI] [PubMed] [Google Scholar]
- 22. Shah BK, Bista A, Sharma S. Survival trends in elderly patients with glioblastoma in the United States: a population-based Study. Anticancer Res. 2016;36(9):4883–4886. [DOI] [PubMed] [Google Scholar]
- 23. Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. [DOI] [PubMed] [Google Scholar]
- 24. Jousilahti P, Laatikainen T, Peltonen M, et al. Primary prevention and risk factor reduction in coronary heart disease mortality among working aged men and women in eastern Finland over 40 years: population based observational study. BMJ. 2016;352:i721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keime-Guibert F, Chinot O, Taillandier L, et al. ; Association of French-Speaking Neuro-Oncologists. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. [DOI] [PubMed] [Google Scholar]
- 26. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 27. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 28. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 29. Lwin Z, MacFadden D, AL-Zahrani A, et al. A population-based study of glioblastoma multiforme (GBM) in the new stupp paradigm: have we improved outcome?J Clin Oncol. 2011;29(15 Suppl):2012. [Google Scholar]
- 30. Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir (Wien). 2003;145(1):5–10. [DOI] [PubMed] [Google Scholar]
- 31. Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE. Glioblastoma in the elderly: glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center experience (1997–2007). Cancer. 2009;115(16):3758–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF. Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg. 2013;118(4):786–798. [DOI] [PubMed] [Google Scholar]
- 33. Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–3055. [DOI] [PubMed] [Google Scholar]
- 34. Sijben AE, McIntyre JB, Roldán GB, et al. Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol. 2008;89(1):97–103. [DOI] [PubMed] [Google Scholar]
- 35. Raizer JJ, Fitzner KA, Jacobs DI, et al. Economics of malignant gliomas: a critical review. J Oncol Pract. 2015;11(1):e59–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sizoo EM, Taphoorn MJ, Uitdehaag B, et al. The end-of-life phase of high-grade glioma patients: dying with dignity?Oncologist. 2013;18(2):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma. JAMA Oncol. 2016;2(11):1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leinonen MK, Miettinen J, Heikkinen S, Pitkäniemi J, Malila N. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.