Abstract
Primary central nervous system lymphoma (PCNSL) is a rare form of non-Hodgkin lymphoma that affects the brain parenchyma, spinal cord, eyes, and cerebrospinal fluid without evidence of systemic, non-CNS involvement. PCNSL is uncommon and only a few randomized trials have been completed in the first-line setting. Over the past decades, the prognosis of PCNSL has improved, mainly due to the introduction and widespread use of high-dose methotrexate, which is now the backbone of all first-line treatment polychemotherapy regimens. Despite this progress, durable remission is recorded in only 50% of patients, and therapy can be associated with significant late neurotoxicity. Here, we overview the epidemiology, clinical presentation, staging evaluation, prognosis, and current up-to-date treatment of immunocompetent PCNSL patients.
Keywords: diagnosis, methotrexate, PCNSL, staging, therapy
Primary central nervous system lymphoma (PCNSL) is a highly aggressive non-Hodgkin lymphoma solely affecting the central nervous system, including the brain, eyes, spinal cord, and cerebrospinal fluid (CSF). Unlike other primary brain tumors, PCNSL respond favorably to chemo- and radiation therapy. Unfortunately, survival is usually inferior in comparison to systemic, non-CNS lymphomas. Moreover, the prognosis of some patients, like the elderly or patients relapsed or with refractory disease, remains very poor. Even though optimized polychemotherapy regimens have improved survival for most PCNSL patients, the management of this disease is still posing a significant challenge to the neuro-oncology community.
Epidemiology
PCNSL can be found in immunosuppressed patients (chronic use of immunosuppressive agents, HIV/AIDS patients, organ transplant recipients) or immunocompetent patients. Here, we will center on PCNSL found in immunocompetent patients. PCNSL is rare, representing 4% of intracranial neoplasms and 4–6% of extranodal lymphomas.1 It is diagnosed in approximately 1500 new patients in the US each year, generally in the fifth or sixth decade of life, with a male:female ratio of 1.2:1.7.2–4 There was an overall increase in PCNSL incidence in the 1970s through early 1980s, due to a growing elderly population caused by a longer life expectancy related to better lifestyles and lower comorbidity as well as a greater tendency to pursue diagnostic evaluation in older patients, improved neuro-imaging, and a more uniform nosology for the diagnosis of PCNSL. Since the initial increase, the incidence rate has remained stable since the mid-1980s,3 with only an intermittent sharp spike in the early 1990s, due to the HIV epidemic. However, in the last 10 years, a rising incidence has been observed in patients older than 60, and those who are 70–79 years of age have the highest incidence at 4.3 per 100000/year.3
Clinical Presentation
The diagnosis of PCNSL requires a high level of suspicion because clinical presentation varies depending on the involved CNS compartment. PCNSL involving the brain parenchyma or leptomeninges and causing focal neurologic deficits is observed in 70% of patients5 and leads to prompt diagnostic imaging. In up to 43% of patients, nonspecific behavioral or neurocognitive changes are the main presenting deficits and can result in a delayed diagnostic evaluation. Signs of increased intracranial pressure leading to headache, confusion, nausea, and vomiting are common (33%). Seizures are uncommonly observed (14%) because the cortex is less frequently involved by PCNSL lesions compared with primary brain tumors (gliomas) or brain metastases. Leptomeningeal dissemination is generally asymptomatic. Only in fewer than one-third of PCNSL patients with definite leptomeningeal involvement are the clinical signs suggestive of leptomeningeal dissemination.6 Leptomeningeal involvement without parenchymal brain lesions as well as isolated spinal cord lesions are almost never observed. If spinal cord lesions are found, they are usually discrete intramedullary nodules involving mainly the thoracic cord. Symptoms of lymphomatous spinal cord lesions parallel those observed with other intramedullary tumors and depend on the location within the spinal cord. Patients may experience often asymmetric sensory changes as well as weakness in arms and legs and bladder or bowel dysfunction. In some patients the peripheral nervous system, including peripheral nerves, nerve roots, plexus, or cranial nerves, can be involved by malignant lymphoma cells, a condition called neurolymphomatosis (NL). NL is rarely seen in PCNSL and is more commonly found in patients with systemic, non-CNS lymphoma (90%) as well as leukemia (10%).7 The most common presentation of NL is peripheral neuropathy or radiculopathy which is severe, relentless, and dysesthetic. NL can also present as cranial neuropathy, peripheral mononeuropathy, and painless polyneuropathy. The neuropathy is generally of the sensorimotor type on electromyographic evaluation, but pure motor neuropathies have been observed in ~20% of patients. The associated weakness frequently progresses, causing symmetric para- or quadriparesis with associated muscle atrophy. PCNSL patients with ocular involvement report decreased acuity, blurry vision, and/or floaters. Overall, these visual symptoms are rare at presentation (4%),5 even though ocular involvement is relatively high in newly diagnosed PCNSLs (20–25%).8,9 Patients are often asymptomatic or have only subtle symptoms, sometimes resembling uveitis, that may be misdiagnosed if visual symptoms are the sole clinical manifestation. Classic B-symptoms such as fever, sweats, and weight loss as observed in systemic, non-CNS lymphoma patients are uncommon in PCNSL.
Diagnosis and Staging
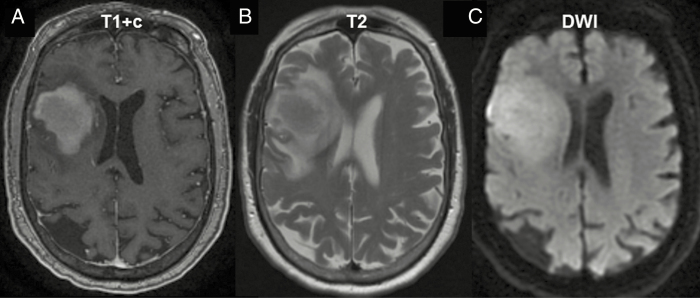
Patients who develop neurologic symptoms and deficits should undergo diagnostic brain imaging. Magnetic resonance imaging (MRI) is the imaging modality of choice and PCNSLs appear as a solitary lesion or multifocal disease. These lesions are often periventricular, involving the white matter of the centrum semiovale, the corpus callosum, or the basal ganglia. PCNSL lesions are isointense to hyperintense on T2-weighted MR images and enhance homogeneously (Fig. 1). The lesions frequently are surrounded by only a moderate amount of edema and are frequently restricted on diffusion weighted images.10 Less often, the eyes (20–25%),11 CSF (7–42%),12–15 and scarcely the spinal cord are involved. The International PCNSL Collaborative Group recommends baseline staging to determine the extent of disease, including contrast-enhanced MRI of the brain, contrast-enhanced MRI of the spine (if spinal symptoms are present), as well as ophthalmologic and CSF evaluation.16 The ophthalmologic exam should be conducted by an experienced ophthalmologist and must include a slit lamp evaluation to detect cellular vitreal or subretinal infiltrates. To detect non-CNS dissemination and the presence of systemic disease, a CT or fluorodeoxyglucose (FDG) body PET scan as well as a bone marrow biopsy should be performed. The yield of systemic staging with PET and/or CT is typically low and, when systemic disease is present, usually involves other extranodal sites, including the testis. Secondary malignancies which are not uncommon in PCNSL and involve the prostate, skin, and gastrointestinal tract17 can also be identified with PET/CT. The presence of concomitant monoclonal small-size population in peripheral and/or marrow blood has been identified in PCNSL patients,18,19 and with more sophisticated genomic detection tools we might be able to identify subclonal, systemic, non-CNS disease more frequently in the future. The role of a dedicated FDG PET of the brain for diagnosis and prognosis has not been established yet, and for now it should be used in only clinical studies.
Fig. 1.
PCNSL imaging pattern on MRI. (A) MRI T1 sequence with gadolinium contrast (T1+c) reveals homogeneously enhancing deep lesions. (B) Lesions are iso- to hyperintense on T2 imaging with a relatively small amount of edema. (C) Diffusion-weighted imaging (DWI) demonstrates restricted diffusion in the tumor.
Definitive diagnosis requires pathologic confirmation. The diagnostic procedure of choice is a stereotactic biopsy of the brain lesion, or, if ocular involvement is present, vitrectomy. CSF sampling demonstrating lymphoma cells on cytology or flow cytometry may also be sufficient. However, due to potential delay of diagnosis (and treatment) it is generally advisable to obtain a biopsy of an accessible brain lesion rather than obtaining CSF or vitreous fluid for assessment prior to biopsy. CSF and vitreous fluid can be used for cytological evaluation, immunophenotyping, and detection of immunoglobulin H or T-cell receptor rearrangements by PCR analysis indicating monoclonality.20 Vitreous fluid can be tested for interleukin (IL)10 and IL6 levels. High IL10 levels and/or a high IL10:IL6 ratio can be strongly suggestive of lymphomatous involvement but is not diagnostic.21 Recently, studies have suggested that MYD88 mutations are frequently found in vitreoretinal lymphomas and the detection of MYD88 increases the diagnostic yield of vitreous fluid.22,23 Corticosteroids are lymphotoxic and therefore can obscure the pathologic diagnosis.24,25 Whenever clinically possible, corticosteroid use should be deferred until tissue, CSF, or vitreous fluid is obtained, except for life-threatening mass effect and edema when corticosteroid use is necessary to stabilize rapidly worsening neurologic deficits.
Most commonly, PCNSLs are found to be diffuse large B-cell lymphomas (DLBCLs) (90%) and on rare occasions Burkitt, T-cell, or low-grade lymphomas.26 A higher incidence of PCNSL of the T-cell type is found in eastern Asia.27 Pathologic review reveals highly proliferating lymphoma cells that diffusely infiltrate the brain parenchyma in a typical perivascular growth pattern. Gene expression profiling of systemic, non-CNS DLBCL has identified 3 molecular subtypes: (i) germinal center B-cell–like (GCB), (ii) activated B-cell–like (ABC)/nongerminal center (NGC), and (iii) type 3 subgroup.28 The vast majority (>80%) of PCNSLs are of the ABC/NGC subtype.29,30 In systemic, non-CNS DLBCL, this subtype is correlated with inferior clinical outcomes and mutations affecting the B-cell receptor (BCR) signaling pathway.31 Next-generation sequencing has shed more light on the genetic alteration of PCNSL. The BCR signaling axis is frequent affected by recurrent mutations, particularly MYD88, CD79B, and less frequently CARD11 and TNFAIP3,30,32–38 activating the BCR downstream target, nuclear factor kappa B. MYD88 and CD79B mutations are enriched in ABC/NGC PCNSLs and are more frequently observed than in ABC DLBCL outside the CNS.39 Therefore, PCNSL more closely resembles lymphomas found in other immune-privileged sites like the testes, in which MYD88 and MYD88/CD79B mutations are reported in >70% of samples.40,41 Copy number alterations have been observed in PCNSL. A recently described gain at chromosome 9p24.1, which includes the programmed death ligands 1 and 2 locus, suggests that immune evasion and immune response modulation might play a role in PCNSL pathogenesis.42 Moreover, aberrant somatic hypermutation, a frequent genomic alteration observed in systemic, non-CNS DLBCL, has also been identified in PCNSL.32,43,44
Prognosis
Two prognostic scoring systems are widely applied to better predict clinical outcome and for patient stratification in clinical trials: (i) International Extranodal Lymphoma Study Group (IELSG) score45 and (ii) Memorial Sloan Kettering Cancer Center (MSKCC) score.46 The IELSG score includes Eastern Cooperative Oncology Group (ECOG) performance score, age, CSF protein concentration, serum lactate dehydrogenase (LDH) serum level, and deep brain involvement to determine prognosis. Two-year survival rates correlate with the presence of 0–1, 2–3, or 4–5 adverse risk factors and are 80%, 48%, or 15%, respectively. Three prognostic groups are defined by the MSKCC score using Karnofsky performance status (KPS) and age: (i) age ≤50, (ii) age >50 and KPS ≥70, (iii) age >50 and KPS <70, correlating with a median overall survival (OS) of 8.5, 3.2, and 1.1 years in an MSKCC population, respectively, and 5.2, 2.1, and 0.9 years in a Radiation Therapy Oncology Group validation cohort.
The median OS of patients with PCNSL in the US (from the Surveillance, Epidemiology, and End Results database) significantly increased from 12.5 months in the 1970s to 26 months in the 2010s.3 Five-year survival improved from 19% to 30% between 1990 and 2000.4 This survival benefit has been limited to patients <70 years of age. Conversely, the median survival of the elderly population, approximately 6 months, has not changed in the last 40 years,3 in part because at least 20% receive no treatment.
Tumor regression is achieved in about 85% of all patients, regardless of treatment type, but recurrence is common and is almost always restricted to the CNS compartment. PCNSLs only rarely metastasize outside the CNS.47,48 Advances in initial treatment have improved clinical outcome, but still up to half of patients relapse and 10–15% have primary refractory disease.49 Prognosis for primary refractory or relapsed PCNSL remains poor, with a median survival of 2 months without further treatment.50 Recurrent disease occurs at a median of 10–18 months after the initial treatment and most relapses develop within the first 2 years of diagnosis.49 In contrast to systemic, non-CNS DLBCL, relapsing disease has also been observed more than 5 and as long as 13 years after initial diagnosis and treatment.51 At relapse, prognostic factors for OS were age at relapse/progression (≥60 vs <60 y), KPS (≥70 vs <70), sensitivity to first-line therapy, duration of first remission (<1 y vs ≥1 y), administration of a salvage therapy, and use of rituximab as second-line therapy.47
Evolution of Standard Therapy for Newly Diagnosed PCNSL
Treatment for PCNSL has evolved over the last 40 years. No uniform gold standard regarding the optimal first-line chemotherapy regimen exists currently. Due to the diffusely infiltrating growth of PCNSL, surgery is usually restricted to stereotactic biopsy, and no survival benefit has been observed after gross total or subtotal resection in retrospective studies.5,52,53 The German PCNSL Study Group 1 trial54 challenged this view recently. The authors reported improved clinical outcomes in those undergoing gross total or subtotal resection in a subset analysis. This clinical survival benefit was lost after adjusting for the total number of lesions. A recent study by Rae et al55 showed a benefit of craniotomy over biopsy in patients with favorable prognostic markers. A clinical risk scale was defined and patients with a low risk profile benefited from resection, whereas those with a high-risk profile benefited from biopsy. The risk scale included presence of difficulties with activities of daily living, diabetes mellitus, lung disease, congestive heart failure, history of myocardial infarction or other cardiac disease, hypertension, encephalopathy, history of transient ischemic attack and stroke, peripheral vascular disease, age >55 years, multiple CNS lesions, and deep lesions. These comorbidities are frequently found in PCNSL patients and therefore most will fall into a high-risk group for which biopsy is the preferred surgical intervention. Currently, there is not sufficient evidence to support the recommendation for an aggressive surgical approach in PCNSL.
Historically whole brain radiotherapy (WBRT) was used for newly diagnosed PCNSL, as it was for all malignant brain tumors. In the 1980s, the first studies were performed with radiation, including evaluation of the need for the whole brain component of treatment, and increased relapses were observed in regions outside the radiation port.56 Dose intensification was tried using WBRT plus a boost to the sites of obvious diseases, but it was apparent that relapses occurred as frequently within and outside of the boosted field.57 Thus, WBRT at a dose of about 45 Gy was established as the necessary and sufficient dose and port for treatment of newly diagnosed PCNSL. WBRT was associated with an overall response rate (ORR) of 90%, but median OS was limited to 12–18 months, usually due to relapse at the primary site.57,58
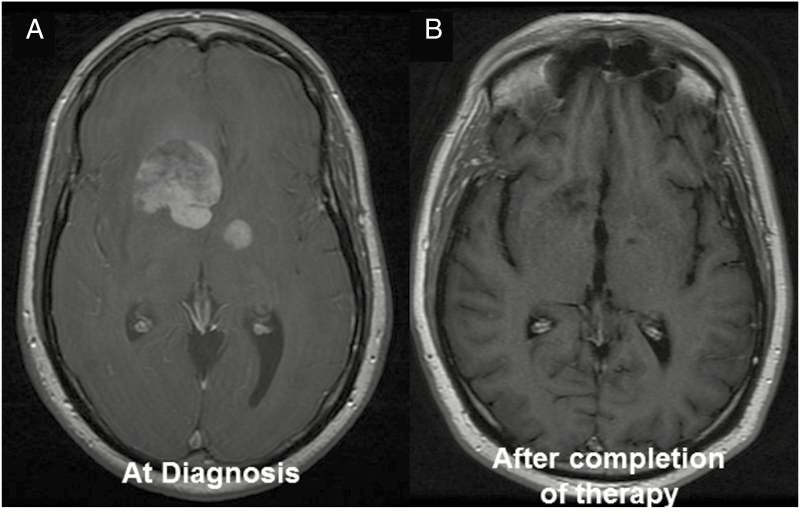
In the 1980s, chemotherapy was added to radiation in the hope of improving outcomes. Regimens used in systemic, non-CNS DLBCL, like CHOP (cyclophosphamide/doxorubicin/vincristine/prednisone), were ineffective,59–61 partly due to inadequate penetration of the blood–brain barrier. A significant advance was achieved with the introduction of high-dose methotrexate (HD-MTX) in combination with WBRT, leading to an improved median OS of 30–60 months with a 5-year survival rate of 30–50%.62–68 Responses to chemotherapy were dramatic and rapid (Fig. 2). Although the chemoradiation treatment prolonged survival, patients developed neurotoxicity, including psychomotor slowing, neurocognitive impairments, memory dysfunction, gait ataxia, behavioral changes, and incontinence associated with significant functional decline. Particular patients >60 years of age were affected by this delayed neurotoxicity.69 Subsequently, the only phase III randomized study completed in PCNSL thus far investigated whether omission of WBRT would affect survival. Trial participants were treated with HD-MTX (with or without ifosfamide). Those who achieved a complete response (CR) were randomized to 45 Gy WBRT or observation. Patients who failed to achieve CRs were randomized to treatment with 45 Gy WBRT or high-dose cytarabine.15 Even though patients receiving WBRT had a significantly longer progression-free survival (PFS) of 18 months compared with observation (12 months), no difference in OS was observed. Unfortunately, the interpretation of the study results is challenging due to caveats in study design and protocol adherence.70,71 The predetermined primary endpoint for non-inferiority was not met, one-third of the randomized trial population had major protocol violations, and the study was underpowered. Based on the results of this trial and the high risk of neurotoxicity, some clinicians no longer use WBRT as part of routine care of PCNSL patients, particularly in patients aged 60 and older. Ongoing clinical trials are further investigating the role of radiation in the first-line therapy of PCNSL. Currently, the role of WBRT is mainly in the salvage and palliative setting. Some centers still use WBRT as part of consolidation,72 delivered at a lower dose (23.4 Gy) and given to younger patients to avoid neurocognitive issues.
Fig. 2.
PCNSL is highly chemosensitive. (A) PCNSL is highly chemosensitive with dramatic response on T1 with gadolinium (B) after initiation of methotrexate combination therapy.
Chemotherapy-only trials were initiated to reduce neurotoxicity, using single-agent HD-MTX13,73 and HD-MTX–based polychemotherapy regimens (including the monoclonal antibody rituximab, which is directed against the B-cell surface antigen CD20).74–76 These trials demonstrated ORRs of 35–74% with a median OS of 25–50 months, similar to the results achieved in chemoradiation trials. A multicenter study using HD-MTX and WBRT with or without cytarabine showed that combination trials are adding clinical benefit.77
Different polychemotherapy regimens, consisting of induction and consolidation phases, have been established with variable responses and OS rates (Table 1). Currently, HD-MTX (dosed >3 g/m2 every 2–3 wk) combined with an alkylating agent and rituximab should be part of any first-line induction treatment.75,76,78 Of note, the addition of rituximab to an MTX-based chemotherapy regimen (HD-MTX, Lomustine [BCNU], teniposide, prednisone [MBTP]) in the HOVON 105/ALLG NHL 24 phase III study, which included 200 newly diagnosed PCNSLs, did not demonstrate a significant clinical benefit on response rate, event-free survival, and PFS. One-year event-free survival was 49% in the chemotherapy arm and 52% in the rituximab/chemotherapy arm. These data, only available in abstract form so far, is challenging the role of rituximab in newly diagnosed PCNSL, but longer follow-up will be needed to evaluate the effects on OS. Polychemotherapy regimens currently used are: rituximab/HD-MTX/vincristine/procarbazine (R-MVP),67,72,79 rituximab/HD-MTX/temozolomide (R-MT),76,80 rituximab/HD-MTX/thiotepa/cytarabine (MATRix),75 and rituximab/HD-MTX/BCNU/teniposide/prednisone (R-MBTP). Even though difficult to compare due to varying consolidation therapies, the 2-year PFS rates in these studies were similar, ranging from 57% (R-MT), 50–79% (R-MVP), to 61% (MATRix) (see also Table 1). In patients up to 70 years old, the MATRix combination has been associated with an ORR of 87%, with a 3-year OS of 67%. This combination is associated with grades 3–4 hematological toxicity in most patients, but treatment-related mortality is 4%, which is similar to those reported with other combinations. Patients who achieved tumor regression after MATRix and received consolidative radiotherapy of autologous stem cell transplant (ASCT) had a 4-year OS of over 80%.
Table 1.
Prospective upfront treatment trials in PCNSL
| Author | Year | Agents | Patients | Median Age | ORR (PR+CR) | Median PFS, mo | Median OS, mo |
|---|---|---|---|---|---|---|---|
| DeAngelis et al62 | 1992 | M(1)+RT(40 + 14 boost)+AraC(3) | 31 | 58 | 27/31 (87%) | 41 | 42.5 |
| Glass et al63 | 1994 | M(3.5)+RT(30–40) | 25 | 56 | 23/25 (90%) | 32 | 33 |
| O’Brien et al64 | 2000 | M(1)+RT (45 + 5.4 boost) | 46 | 58 | 44/46 (96%) | NR | 33 |
| Abrey et al65 | 2000 | M(3.5)+P(100)+V(1.4)+Ara-C(3)+IT M+IT A+RT(45) | 52 | 65 | 49/52 (94%) | NR | 60 |
| Ferreri et al66 | 2001 | M(3)+P(100)+V(1.4)+Ara-C(3)+RT(45) | 13 | 54 | 12/13 (92%) | 18 | 25+ |
| DeAngelis et al67 | 2002 | M(2.5)+V(1.4)+P(100)+AraC(3)+IT M+RT(45 or 36) | 102 (98 treated) | 56.5 | 47/50 (94%) | 24 | 37 |
| Herrlinger et al73 | 2002 | M(8) | 37 | 60 | 13/37 (35%) | 10 | 25 |
| Abrey et al85 | 2003 | M(3.5)+AraC(3); BEAM | 28 (14 transplanted) | 53 | Induction: 16/24 (57%), SCT 11/14 (77%) | 5.6 | Not reached |
| Batchelor et al13 | 2003 | M(8) | 25 | 60 | 17/23 (74%) | 12.8 | 22.8+ |
| Pels et al74 | 2003 | M(5)+AraC(3)+V(2)+ifos(800)+dex(10)+cyclo(200)+IT M+IT A+IT P | 65 | 62 | 43/61 (71%) | 21 | 50 |
| Poortmans et al68 | 2003 | M(3)+Ten(100)+B(100)+MP(60)+IT M+IT A+RT(40) | 52 | 51 | 42/52(81%) | NR | 46 |
| Colombat et al87 | 2006 | M(3)+B(100)+eto(100)+pred (60); BEAM+RT(30) | 25 (17 transplanted) | 52 | Induction: 21/25 (84%), SCT 16/16 (100%) | 40 | Not reached |
| Illerhaus et al86 | 2006 | M(8)+AraC(3)+thio (40 mg/m2); B(400)+thio(5 mg/kg)+RT(45) | 30 (23 transplanted) | 54 | Induction: 21/30 (70%), SCT 21/21 (100%) | NR | Not reached |
| Ferreri et al77 | 2009 | M(3.5)+/-AraC(2)+RT(45) | 79 | 59/58 | 27/39 (69%) vs 16/40 (40%) | 3 vs 18 | Nr |
| Thiel et al15 | 2010 | M(3;+ifos) +/- RT(45) | 526 (all)/318 (TPP) | 61 | 283/526 (53%) | 18.3 vs 11.9 | 32.4 vs 37.1 |
| Morris et al72 | 2013 | R(500)+M(3.5)+V(1.4)+P(100)+RT(23.4) | 52 | 60 | 41/52 (78%) | 92.4 | Not reached |
| Rubenstein et al76 | 2013 | R(375)+M(8)+T(150)+AraC(2) vs eto(40) | 44 | 61 | 34/47(72%) | 48 | Not reached |
| Omuro et al79 | 2015 | R(500)+M(3.5)+V(1.4)+P(100); thio(250)+cyclo(60)+bus(3.2) | 32 (26 transplanted) | 57 | Induction: 31/32 (97%), SCT 24/26 (92%) | Not reached | Not reached |
| Omuro et al78 | 2015 | M(3.5)+V(1.4)+P(100)+AraC(3) vs M(3.5)+T(150) | 95 | 72/73 | 37/45(82%) vs 34/42(74%) | 9.5 vs 6.1 | 31 vs 14 |
| Glass et al80 | 2016 | R(375)+M(3.5)+T(100)+RT(36) | 66 | 57 | 30/35 (86%) | 63 | 90 |
| Ferreri et al75 | 2016 | M(3.5)+AraC(2)+/-R(375)+/-thio(30) | 227 | 58/57/57 | 40/75(53%)/51/69(73%)/65/75 (86%) | 6/20/not reached | 12/30/not reached |
| Illerhaus et al86 | 2016 | R(375)+M(8)+AraC(3)+thio(40); R(375)+B(400)+thio(5 mg/kg) | 79 (73 transplanted) | 56 | Induction: 73/79 (92%), SCT: 72/79 (91%) | 74 | Not reached |
| Fritsch et al90 | 20 17 |
R(375)+M(3)+P(60)+L(110) | 107 (all)/(69 R-MPL) | 73 | 53/107 (50%); 32/69 (46% r-mpl) | 10.3/(9.6 r-mpl) | 20.7/(15.4 r-mpl) |
AraC: cytarabine (g/m2); B: BCNU (mg/m2); BEAM: carmustine, etoposide, cytarabine, melphalan; bus: busulfan (mg/kg); CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; cyclo: cyclophosphamide (mg/m2); eto: etoposide (mg/m2); ifos: ifosfamide (mg/m2); IT A: intrathecal cytarabine; IT M: intrathecal methotrexate; IT P: intrathecal prednisone; M: methotrexate (g/m2); L: lomustine (mg/m2) NR: not reported; P: procarbazine (mg/m2/day), SCT: stem cell transplant; pred: methylprednisone (mg/m2); R: rituximab (mg/m2); RT: whole brain radiation (dose used in Gy); T: temozolomide (mg/m2); Ten: teniposide (mg/m2); thio: thiotepa (mg/m2); V: vincristine (mg/m2).
The choice among different regimens is mostly based on geographical regions and drug availability, and with a single exception, randomized trials comparing these regimens do not exist. The exception is a multicenter phase II trial conducted in the pre-rituximab era, comparing 2 different MTX-based regimens in older patients (age ≥60): HD-MTX/temozolomide (MT) versus HD-MTX/vincristine/procarbazine (MVP). Toxicity profiles were similar between the treatment groups. ORR was 82% in the MVP group and 71% in the MT group, with a median OS of 31 and 14 months, respectively. Even though the predefined primary outcome did not reach statistical significance, the trend favored the MVP regimen.78 There has been no comparison study conducted in an unselected population of newly diagnosed PCNSL patients, and given the similar outcomes reported with these regimens, any one of them is a reasonable choice; the selection is dependent on geographic region, physician preference, and sometimes patient comorbidities.
The role of intrathecal (IT) therapy remains undefined. The rationale to include IT therapy is to achieve longer drug exposures at cytotoxic concentrations in the CSF,81 a potential reservoir for lymphoma cells. IT therapy has been part of the initial polychemotherapy regimens, and agents like MTX, cytarabine, thiotepa, and, more recently, rituximab82 have been demonstrated to be safe. However, increased toxicity (eg, MTX-associated leukoencephalopathy or myelopathy) and adverse events associated with the application of IT therapy (eg, Ommaya reservoir complications during placement and use; infections) have reduced the use of IT therapy as part of first-line treatment, even among recent and ongoing prospective trials. Likewise, retrospective studies have not demonstrated a benefit in adding IT therapy to first-line systemic chemotherapy.83,84
Conversely to chemotherapy-only strategies, several studies have used radiation therapy,72,75 conventional high-dose chemotherapy (HDC; cytarabine or etoposide/cytarabine),76 or myeloablative chemotherapy with ASCT85–88 as consolidation after induction chemoimmunotherapy. Age, performance status, response to induction and comorbidities undoubtedly play a role in limiting the available options for consolidation in any given patient. For instance, intensified, myeloablative chemotherapy has been more commonly used in younger patients and those with adequate organ function, but well-recognized recommendations to choose the best consolidation therapy are lacking. Importantly, some recent and ongoing trials are focused to establish the best candidates for each strategy. Low-dose WBRT (23.4 Gy) appears to offer improved disease control without cognitive impairment in a single-arm phase II trial, and data from a randomized phase II multicenter study are maturing, which should determine if this is a reasonable option for many, especially those unable to tolerate intensive chemotherapy. The IELSG32 randomized trial has demonstrated that both WBRT with 36 Gy and HDC-ASCT are safe and efficient as consolidation after 4 courses of the MATRix regimen89 (WBRT was associated with significant impairment in only some attention and executive functions). Two additional ongoing randomized phase II studies compare HDC-ASCT with conventional dose chemotherapy, respectively, with an infusional combination of etoposide/cytarabine and a high dose ifosfamide–based combination as experimental arms. Finally, there is growing interest in maintenance-based therapies, particularly for older patients who cannot tolerate aggressive chemotherapy or have severe renal compromise limiting the use of HD-MTX. Alkylating agents have been used as maintenance strategy in the elderly. In the PRIMAIN study90 patients ≥65 years received rituximab, HD-MTX, and procarbazine followed by 4 weeks of maintenance procarbazine with a 2-year PFS of 37.3%. In the NORDIC trial91 the 2-year PFS was even higher at 55.6% for patients ≥65 years who received polychemotherapy including rituximab, HD-MTX, and temozolomide followed by temozolomide maintenance. In comparison, a recent meta-analysis reported a median OS of 19 months in the elderly population.92 Observation can be applied for elderly patients or those unable to tolerate additional treatment. In addition, rituximab and lenalidomide have been used in such fashion, but this is mostly anecdotal and used in the setting of relapsed disease. This promising area requires further study and will hopefully shed more light on the optimal consolidation regimen.
Isolated intraocular disease in patients is supported by low level of evidence. Currently, patients with brain lesions and intraocular disease are being treated like the others PCNSL patients, with the inclusion of posterior two-thirds of the orbits in the radiation volume in the case chemoradiotherapy is used, and with intraocular injection of MTX or rituximab in case of local residual disease among patients treated with chemoimmunotherapy. Patients with lymphoma exclusively involving the eyes at presentation may be treated with intravitreal drug delivery or radiation, whereas parameters to distinguish candidates for systemic treatment remain to be defined
Current Gold Standard of First-Line PCNSL Patients
Surgery
Biopsy only (stereotactic biopsy preferred)
To establish tissue diagnosis
Avoid corticosteroid use prior to biopsy
Surgical debulking can be indicated in selected cases to improve performance status fast and allows to start timely chemotherapy
Chemotherapy
Induction therapy: methotrexate-based polychemotherapy
Methotrexate combined with alkylating agent and rituximab should be preferred
The addition of high-dose cytarabine is advised in patients younger than 65 years
- Consolidation therapy:
- Depending on age, performance status, and comorbidities
- May use radiation, additional chemotherapy, high-dose chemotherapy with stem cell rescue
- Maintenance with oral alkylating agent is advised in elderly patients or patients who cannot receive consolidation with radiotherapy or myeloablative chemotherapy
Radiation
As part of consolidation (preferably low dose in responsive patients)
Ongoing randomized trials will hopefully soon add more clarity
Associated with increased neuro-cognitive deficits in elderly patients and at higher doses
Treatment of CSF Space
Increased risk of treatment-related adverse events (Ommaya reservoir infections)
Not currently part of standard first-line regimen
Considerations in the Elderly Population
Should receive treatment
Consider using methotrexate-based chemotherapy regimen
Methotrexate might need to be dose adjusted based on renal function
Future Directions
There has been significant progress in the treatment of PCNSL over the past decades. We now observe long-term disease control in up to half of the newly diagnosed PCNSL patients. In contrast to systemic, non-CNS DLBCL, relapses can develop even more than 10 years after the initial diagnosis and treatment.51 The current focus in the first-line setting is directed toward optimizing early diagnosis and upfront treatment to reduce the percentage of refractory patients, to prolong remission, and to increase treatment options for recurrent patients. Additionally, the increasing incidence of elderly PCNSL demands trials specifically targeting this patient population.
Funding
This research was supported by an NIH/NCI Cancer Center Support Grant (P30-CA008748) and by grants from Cycle for Survival Equinox (C.G.) and the Leukemia & Lymphoma Society (C.G.).
Conflict of interest statement. Research support: C.G., Pharmacyclics; Advisory affiliations: C.G., BTG pharmaceuticals; L.M.D, Sapience Therapeutics, Roche, and Juno; J.L.R., Celgene and Genentech.
Authorship statement. All authors contributed to the writing and final approval of the manuscript.
References
- 1. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43(3):199–201. [DOI] [PubMed] [Google Scholar]
- 3. Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol. 2018;20(5):687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92(2):261–266. [DOI] [PubMed] [Google Scholar]
- 6. Balmaceda C, Gaynor JJ, Sun M, Gluck JT, DeAngelis LM. Leptomeningeal tumor in primary central nervous system lymphoma: recognition, significance, and implications. Ann Neurol. 1995;38(2):202–209. [DOI] [PubMed] [Google Scholar]
- 7. Grisariu S, Avni B, Batchelor TT, et al. ; International Primary CNS Lymphoma Collaborative Group. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010;115(24):5005–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong JT, Chae JB, Lee JY, Kim JG, Yoon YH. Ocular involvement in patients with primary CNS lymphoma. J Neurooncol. 2011;102(1):139–145. [DOI] [PubMed] [Google Scholar]
- 9. DeAngelis LM, Yahalom J, Heinemann MH, Cirrincione C, Thaler HT, Krol G. Primary CNS lymphoma: combined treatment with chemotherapy and radiotherapy. Neurology. 1990;40(1):80–86. [DOI] [PubMed] [Google Scholar]
- 10. Coulon A, Lafitte F, Hoang-Xuan K, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol. 2002;12(2):329–340. [DOI] [PubMed] [Google Scholar]
- 11. Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Ann Oncol. 2007;18(11):1851–1855. [DOI] [PubMed] [Google Scholar]
- 12. Korfel A, Weller M, Martus P, et al. Prognostic impact of meningeal dissemination in primary CNS lymphoma (PCNSL): experience from the G-PCNSL-SG1 trial. Ann Oncol. 2012;23(9):2374–2380. [DOI] [PubMed] [Google Scholar]
- 13. Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21(6):1044–1049. [DOI] [PubMed] [Google Scholar]
- 14. Kiewe P, Fischer L, Martus P, Thiel E, Korfel A. Meningeal dissemination in primary CNS lymphoma: diagnosis, treatment, and survival in a large monocenter cohort. Neuro Oncol. 2010;12(4):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–1047. [DOI] [PubMed] [Google Scholar]
- 16. Abrey LE, Batchelor TT, Ferreri AJ, et al. ; International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Pulido JS, O’Neill BP, Johnston PB. Second malignancies in patients with primary central nervous system lymphoma. Neuro Oncol. 2015;17(1):129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCann KJ, Ashton-Key M, Smith K, Stevenson FK, Ottensmeier CH. Primary central nervous system lymphoma: tumor-related clones exist in the blood and bone marrow with evidence for separate development. Blood. 2009;113(19):4677–4680. [DOI] [PubMed] [Google Scholar]
- 19. Jahnke K, Hummel M, Korfel A, et al. Detection of subclinical systemic disease in primary CNS lymphoma by polymerase chain reaction of the rearranged immunoglobulin heavy-chain genes. J Clin Oncol. 2006;24(29):4754–4757. [DOI] [PubMed] [Google Scholar]
- 20. Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013;70(3):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cassoux N, Giron A, Bodaghi B, et al. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48(7):3253–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raja H, Salomão DR, Viswanatha DS, Pulido JS. Prevalence of Myd88 L265p mutation in histologically proven, diffuse large b-cell vitreoretinal lymphoma. Retina. 2016;36(3):624–628. [DOI] [PubMed] [Google Scholar]
- 23. Bonzheim I, Giese S, Deuter C, et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood. 2015;126(1):76–79. [DOI] [PubMed] [Google Scholar]
- 24. Gametchu B. Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science. 1987;236(4800):456–461. [DOI] [PubMed] [Google Scholar]
- 25. Weller M. Glucocorticoid treatment of primary CNS lymphoma. J Neurooncol. 1999;43(3):237–239. [DOI] [PubMed] [Google Scholar]
- 26. Camilleri-Broët S, Martin A, Moreau A, et al. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d’étude des Leucénies et Autres Maladies du Sang (GOELAMS). Am J Clin Pathol. 1998;110(5):607–612. [DOI] [PubMed] [Google Scholar]
- 27. Choi JS, Nam DH, Ko YH, et al. Primary central nervous system lymphoma in Korea: comparison of B- and T-cell lymphomas. Am J Surg Pathol. 2003;27(7):919–928. [DOI] [PubMed] [Google Scholar]
- 28. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. [DOI] [PubMed] [Google Scholar]
- 29. Camilleri-Broët S, Crinière E, Broët P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190–196. [DOI] [PubMed] [Google Scholar]
- 30. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pasqualucci L, Dalla-Favera R. The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vater I, Montesinos-Rongen M, Schlesner M, et al. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia. 2015;29(3):677–685. [DOI] [PubMed] [Google Scholar]
- 33. Braggio E, Van Wier S, Ojha J, et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res. 2015;21(17):3986–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011;122(6):791–792. [DOI] [PubMed] [Google Scholar]
- 35. Montesinos-Rongen M, Schäfer E, Siebert R, Deckert M. Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta Neuropathol. 2012;124(6):905–906. [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez-Aguilar A, Idbaih A, Boisselier B, et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res. 2012;18(19):5203–5211. [DOI] [PubMed] [Google Scholar]
- 37. Bruno A, Boisselier B, Labreche K, et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget. 2014;5(13):5065–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279–290. [DOI] [PubMed] [Google Scholar]
- 39. Khodabakhshi AH, Morin RD, Fejes AP, et al. Recurrent targets of aberrant somatic hypermutation in lymphoma. Oncotarget. 2012;3(11):1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kraan W, Horlings HM, van Keimpema M, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J. 2013;3:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Booman M, Szuhai K, Rosenwald A, et al. Genomic alterations and gene expression in primary diffuse large B-cell lymphomas of immune-privileged sites: the importance of apoptosis and immunomodulatory pathways. J Pathol. 2008;216(2):209–217. [DOI] [PubMed] [Google Scholar]
- 42. Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montesinos-Rongen M, Schmitz R, Courts C, et al. Absence of immunoglobulin class switch in primary lymphomas of the central nervous system. Am J Pathol. 2005;166(6):1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Montesinos-Rongen M, Van Roost D, Schaller C, Wiestler OD, Deckert M. Primary diffuse large B-cell lymphomas of the central nervous system are targeted by aberrant somatic hypermutation. Blood. 2004;103(5):1869–1875. [DOI] [PubMed] [Google Scholar]
- 45. Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–272. [DOI] [PubMed] [Google Scholar]
- 46. Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5715. [DOI] [PubMed] [Google Scholar]
- 47. Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ambady P, Fu R, Netto JP, et al. Patterns of relapse in primary central nervous system lymphoma: inferences regarding the role of the neuro-vascular unit and monoclonal antibodies in treating occult CNS disease. Fluids Barriers CNS. 2017;14(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jahnke K, Thiel E, Martus P, et al. ; German Primary Central Nervous System Lymphoma Study Group. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80(2):159–165. [DOI] [PubMed] [Google Scholar]
- 50. Reni M, Ferreri AJ, Villa E. Second-line treatment for primary central nervous system lymphoma. Br J Cancer. 1999;79(3-4):530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nayak L, Hedvat C, Rosenblum MK, Abrey LE, DeAngelis LM. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13(5):525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reni M, Ferreri AJ, Garancini MP, Villa E. Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol. 1997;8(3):227–234. [DOI] [PubMed] [Google Scholar]
- 53. Bellinzona M, Roser F, Ostertag H, Gaab RM, Saini M. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol. 2005;31(1):100–105. [DOI] [PubMed] [Google Scholar]
- 54. Weller M, Martus P, Roth P, Thiel E, Korfel A; German PCNSL Study Group. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol. 2012;14(12):1481–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rae AI, Mehta A, Cloney M, et al. Craniotomy and survival for primary central nervous system lymphoma. Neurosurg. 2018. Apr 4. doi: 10.1093/neuros/nyy096. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shibamoto Y, Hayabuchi N, Hiratsuka J, et al. Is whole-brain irradiation necessary for primary central nervous system lymphoma? Patterns of recurrence after partial-brain irradiation. Cancer. 2003;97(1):128–133. [DOI] [PubMed] [Google Scholar]
- 57. Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23(1):9–17. [DOI] [PubMed] [Google Scholar]
- 58. Shibamoto Y, Ogino H, Hasegawa M, et al. Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int J Radiat Oncol Biol Phys. 2005;62(3):809–813. [DOI] [PubMed] [Google Scholar]
- 59. O’Neill BP, O’Fallon JR, Earle JD, Colgan JP, Brown LD, Krigel RL. Primary central nervous system non-Hodgkin’s lymphoma: survival advantages with combined initial therapy?Int J Radiat Oncol Biol Phys. 1995;33(3):663–673. [DOI] [PubMed] [Google Scholar]
- 60. Schultz C, Scott C, Sherman W, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol. 1996;14(2):556–564. [DOI] [PubMed] [Google Scholar]
- 61. Mead GM, Bleehen NM, Gregor A, et al. A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer. 2000;89(6):1359–1370. [PubMed] [Google Scholar]
- 62. DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10(4):635–643. [DOI] [PubMed] [Google Scholar]
- 63. Glass J, Gruber ML, Cher L, Hochberg FH. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg. 1994;81(2):188–195. [DOI] [PubMed] [Google Scholar]
- 64. O’Brien P, Roos D, Pratt G, et al. Phase II multicenter study of brief single-agent methotrexate followed by irradiation in primary CNS lymphoma. J Clin Oncol. 2000;18(3):519–526. [DOI] [PubMed] [Google Scholar]
- 65. Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18(17):3144–3150. [DOI] [PubMed] [Google Scholar]
- 66. Ferreri AJ, Reni M, Dell’Oro S, et al. Combined treatment with high-dose methotrexate, vincristine and procarbazine, without intrathecal chemotherapy, followed by consolidation radiotherapy for primary central nervous system lymphoma in immunocompetent patients. Oncology. 2001;60(2):134–140. [DOI] [PubMed] [Google Scholar]
- 67. DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ; Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20(24):4643–4648. [DOI] [PubMed] [Google Scholar]
- 68. Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. ; European Organization for Research and Treatment of Cancer Lymphoma Group. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21(24):4483–4488. [DOI] [PubMed] [Google Scholar]
- 69. Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24(28):4570–4574. [DOI] [PubMed] [Google Scholar]
- 70. Ferreri AJ, DeAngelis L, Illerhaus G, et al. Whole-brain radiotherapy in primary CNS lymphoma. Lancet Oncol. 2011;12(2):118–9; author reply 119. [DOI] [PubMed] [Google Scholar]
- 71. DeAngelis LM. Radiotherapy: has the role of WBRT in primary CNS lymphoma been settled?Nat Rev Clin Oncol. 2011;8(4):196–198. [DOI] [PubMed] [Google Scholar]
- 72. Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Herrlinger U, Küker W, Uhl M, et al. ; Neuro-Oncology Working Group of the German Society. NOA-03 trial of high-dose methotrexate in primary central nervous system lymphoma: final report. Ann Neurol. 2005;57(6):843–847. [DOI] [PubMed] [Google Scholar]
- 74. Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21(24):4489–4495. [DOI] [PubMed] [Google Scholar]
- 75. Ferreri AJ, Cwynarski K, Pulczynski E, et al. ; International Extranodal Lymphoma Study Group (IELSG). Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217–e227. [DOI] [PubMed] [Google Scholar]
- 76. Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ferreri AJ, Reni M, Foppoli M, et al. ; International Extranodal Lymphoma Study Group (IELSG). High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512–1520. [DOI] [PubMed] [Google Scholar]
- 78. Omuro A, Chinot O, Taillandier L, et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251–e259. [DOI] [PubMed] [Google Scholar]
- 79. Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Glass J, Won M, Schultz CJ, et al. Phase I and II study of induction chemotherapy with methotrexate, rituximab, and temozolomide, followed by whole-brain radiotherapy and postirradiation temozolomide for primary CNS lymphoma: NRG oncology RTOG 0227. J Clin Oncol. 2016;34(14):1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bleyer WA, Poplack DG, Simon RM. “Concentration x time” methotrexate via a subcutaneous reservoir: a less toxic regimen for intraventricular chemotherapy of central nervous system neoplasms. Blood. 1978;51(5):835–842. [PubMed] [Google Scholar]
- 82. Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25(11):1350–1356. [DOI] [PubMed] [Google Scholar]
- 83. Ferreri AJ, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58(10):1513–1520. [DOI] [PubMed] [Google Scholar]
- 84. Khan RB, Shi W, Thaler HT, DeAngelis LM, Abrey LE. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma?J Neurooncol. 2002;58(2):175–178. [DOI] [PubMed] [Google Scholar]
- 85. Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21(22):4151–4156. [DOI] [PubMed] [Google Scholar]
- 86. Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24(24):3865–3870. [DOI] [PubMed] [Google Scholar]
- 87. Colombat P, Lemevel A, Bertrand P, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38(6):417–420. [DOI] [PubMed] [Google Scholar]
- 88. Montemurro M, Kiefer T, Schüler F, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18(4):665–671. [DOI] [PubMed] [Google Scholar]
- 89. Ferreri AJM, Cwynarski K, Pulczynski E, et al. ; International Extranodal Lymphoma Study Group (IELSG). Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4(11):e510–e523. [DOI] [PubMed] [Google Scholar]
- 90. Fritsch K, Kasenda B, Schorb E, et al. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia. 2017;31(4):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pulczynski EJ, Kuittinen O, Erlanson M, et al. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: results from a phase II study by the Nordic Lymphoma Group. Haematologica. 2015;100(4):534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kasenda B, Ferreri AJ, Marturano E, et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)—a systematic review and individual patient data meta-analysis. Ann Oncol. 2015;26(7):1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]