Abstract
Background
We conducted a phase III trial of personalized peptide vaccination (PPV) for human leukocyte antigen (HLA)-A24+ recurrent glioblastoma to develop a new treatment modality.
Methods
We randomly assigned 88 recurrent glioblastoma patients to receive PPV (n = 58) or the placebo (n = 30) at a 2-to-1 ratio. Four of 12 warehouse peptides selected based on preexisting peptide-specific immunoglobulin G levels or the corresponding placebos were injected 1×/week for 12 weeks.
Results
Our trial met neither the primary (overall survival [OS]) nor secondary endpoints. Unfavorable factors for OS of 58 PPV patients compared with 30 placebo patients were SART2-93 peptide selection (n = 13 vs 8, hazard ratio [HR]: 15.9), ≥70 years old (4 vs 4, 7.87), >70 kg body weight (10 vs 7, 4.11), and performance status (PS)3 (8 vs 2, 2.82), respectively. Consequently, the median OS for PPV patients without SART2-93 selection plus one of these 3 favorable factors (<70 y old, ≤70 kg, or PS0–2) was significantly longer than that for the corresponding placebo patients (HR: 0.49, 0.44, and 0.51), respectively. Preexisting immunity against both all 12 warehouse peptides besides SART2-93 and the other cytotoxic T lymphocyte epitope peptides was significantly depressed in the patients with SART2-93 selection (n = 21) compared with that of the patients without SART2-93 selection (n = 67). Biomarkers correlative for favorable OS of the PPV patients were a lower percentage of CD11b+CD14+HLA-DRlow immunosuppressive monocytes and a higher percentage of CD4+CD45RA− activated T cells, the intermediate levels of chemokine C-C ligand 2 (CCL2), vascular endothelial growth factor, interleukin (IL)-6, IL-17, or haptoglobin, respectively.
Conclusion
This phase III trial met neither the primary nor secondary endpoints.
Keywords: biomarker for overall survival, personalized peptide vaccine, phase III trial, pre-existing immunity, recurrent glioblastoma
Key Points
This trial of personalized peptide vaccination did not meet the primary endpoint.
Personalized peptide vaccination shortened the OS of certain patients.
Intermediate CCL2 level was a biomarker correlative for favorable OS.
Importance of the Study
A new treatment modality to improve the OS of recurrent glioblastoma (rGBM) patients is needed, as they show extremely poor OS. Many clinical trials of immunotherapy in the past decades failed to provide clinical benefits for rGBM. Our randomized phase III trial of PPV did not meet either the primary or secondary endpoint for the enrolled patients. However, we found that PPV rather shortened the OS for patients with SART2-93 peptide selection, ≥70 years old, or >70 kg body weight, or PS3 compared with that for the corresponding placebo patients, respectively. Among these unfavorable factors, the SART2-93 selection was a predominant factor hampering the provision of clinical benefits of PPV for all the patients. We also found both the cellular and humoral biomarkers correlative for OS of both PPV patients and placebo patients. Furthermore, our results showed a contraindication of PPV for the patients whose pre-vaccination CCL2 level was not intermediate.
The overall survival (OS) of recurrent glioblastoma (rGBM) patients is very poor, although bevacizumab has been reported to improve the progression-free survival (PFS) of rGBM patients.1–3 Many clinical studies failed to provide clinical benefits for rGBM in the past decade.4–6 This failure may be partly due to the unique and diverse immunological features of GBM.4–10 GBM tumor cells produce many cytokines and chemokines as potential autocrine growth factors and subsequent immune regulators, which might in turn influence the self-proliferation in most patients.4–10 Among the GBM-producing cytokines, granulocyte-monocyte stimulating factor (GM-CSF) and the chemokine (C-C motif) ligand 2 (CCL2) are the two major factors for immune regulation.6–9 GM-CSF forms a cytokine network with interleukin (IL)-5, IL-6, IL-15, and IL-17, all of which are involved in inflammatory immune responses and subsequent angiogenesis. CCL2 recruits monocytes, memory T cells, and dendritic cells to the sites of inflammation.4–10 CCL2 produced by the glioma microenvironment was reported to be essential for the recruitment of T-regulatory cells (Tregs) and monocytic myeloid-derived suppressor cells (m-MDSC).10 Lower m-MDSC or higher Treg levels were reported to be favorable markers in a clinical study of ipilimumab for advanced melanoma patients.11
We previously reported the feasibility of personalized peptide vaccination (PPV), in which advanced GBM patients were vaccinated with different combinations of 4 peptides chosen from 48 warehouse peptides based on the individual patients’ human leukocyte antigen (HLA) type and preexisting peptide-specific immunoglobulin (Ig)G levels.12 We next conducted a phase I and follow-up study of a PPV consisting of 14 peptides for HLA-A24–positive rGBM patients, and the results confirmed the safety and potential clinical benefits of PPV.13 We describe herein the results of a randomized, double-blind, phase III trial of PPV for HLA-A24–positive rGBM patients.
Patients and Methods
The details of these sections are given in Supplementary Appendix 2.
Patients and Peptide Vaccination
HLA-A24–positive patients with supratentorial rGBM that had been diagnosed histologically and proven refractory after standard temozolomide and radiotherapy were eligible for this study. The other major criteria required for inclusion were as follows: age of 18 to 74 years; positive IgG responses to at least 2 of 12 warehouse peptides (ITK-1) (Supplementary Table 1) in pre-vaccination plasma; Eastern Cooperative Oncology Group performance status (PS) of 0, 1, or 2; neurological PS3. A redacted protocol is shown in Supplementary Appendix 1. Patients were allocated at a 2-to-1 ratio by computer-generated block randomization to receive PPV plus best supportive care (BSC) or the placebo plus BSC, with the stratifications by PS (0 to 1 vs 2 to 3), pathological diagnosis of GBM (primary vs secondary), and prior bevacizumab treatment (without vs with).
All study protocols were approved by the institutional review board at each participating hospital. After a full explanation of the protocol, written informed consent was obtained from all patients before enrollment.
Study Design and Treatment
This was a randomized, double-blind, phase III trial of the PPV for HLA-A24–positive rGBM patients. Four of the 12 warehouse peptides selected based on the patient’s preexisting peptide-specific IgG levels or the corresponding placebos were injected subcutaneously once weekly for 12 weeks during the first cycle, followed by biweekly vaccinations until disease progression. The primary endpoint was OS from the day of random assignment. Secondary endpoints were 1-year survival rate, antitumor responses, PFS, PFS at 6 months, peptide-specific IgG responses, and cytotoxic T lymphocyte (CTL) activity.
Toxicity and Patient Monitoring
Toxicity and general conditions were monitored at the time of each visit. Toxicity was evaluated using the Common Terminology Criteria for Adverse Events v4.0. MRI scans were obtained after the sixth and twelfth vaccinations during the first cycle followed by every fourth vaccination thereafter until progressive disease.
Immune Responses, Biomarker Studies
T-cell responses and IgG titers specific to the antigen peptides in peripheral blood were evaluated by interferon (IFN)-γ ELIspot (enzyme-linked immunosorbent spot) and bead-based multiplexed Luminex assay, respectively, before and after vaccination (at the end of each cycle or at the end of the trial), as described previously.12–14 The association between immune response to the vaccinated peptides and OS was evaluated by a landmark method.15
Statistical Analysis
Kaplan–Meier method, log-rank test, Cox proportional hazards analysis, Student’s t-test, the chi-square test, and Fisher’s exact test were used for the statistical analyses.
Results
Patient Characteristics
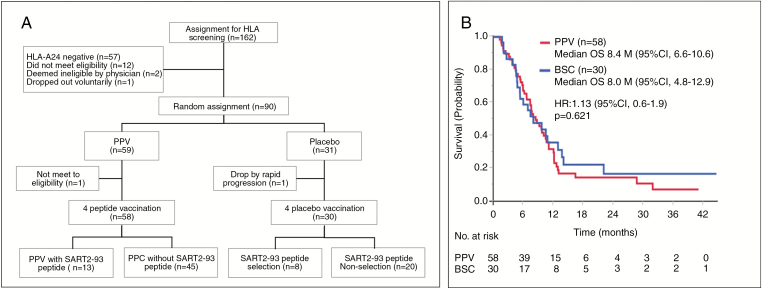
From January 2012 to March 2016, rGBM patients (N = 162) from 20 neurosurgical centers in Japan underwent HLA typing for the clinical study of PPV in which 4 of 12 warehouse peptides (ITK-1) were selected by preexisting peptide-specific IgG levels. Of these patients, 57 were HLA-A24–negative, 12 patients did not meet the eligibility criteria, 1 patient dropped out voluntarily, and 2 patients were determined to be unsuitable for the study by physicians. A Consolidated Standards of Reporting Trials (CONSORT) diagram is shown in Fig. 1A. We randomly assigned the 90 eligible HLA-A24–positive rGBM patients to receive PPV treatment with BSC (n = 59, PPV group) or the placebo injection with BSC alone (n = 31, placebo group) at a 2-to-1 ratio, respectively. The 2:1 allocation was performed by computer-generated block randomization, with stratifications by PS (0 to 1 vs 2 to 3), the pathological diagnosis of GBM (primary vs secondary), and the prior bevacizumab treatment (without vs with). Two of the 90 patients dropped out before the first treatment and were therefore excluded from the full analysis. The baseline characteristics of the remaining 88 patients (58 for PPV and 30 for placebo) are shown in Table 1. The clinical data cutoff date was December 16, 2016. The median follow-up time was 7.7 months (interquartile range: 7.3).
Fig. 1.
(A) CONSORT diagram. (B) Median overall survival (OS) in the PPV (n = 58) vs BSC (n = 30) groups.
Table 1.
Characteristics of the enrolled patients
| Treatment | |||
|---|---|---|---|
| ITK-1 (n = 58) | Placebo (n = 30) | P-value | |
| Age, y | 0.108a | ||
| Median (range) | 52.5 (20–74) | 59.0 (32–74) | |
| Gender | 1.000b | ||
| Male | 37 | 19 | |
| Female | 21 | 11 | |
| First diagnosis to entry | 0.798a | ||
| Median month | 12.0 | 13.0 | |
| Prior treatment | |||
| None | 20 | 15 | 0.353b |
| Chemotherapy | 15 | 4 | |
| Bevacizumab (Bmab) | 6 | 1 | |
| Bmab and chemotherapy | 12 | 7 | |
| Interferon (IFN) | 4 | 1 | |
| IFN and chemotherapy | 1 | 2 | |
| Performance status (PS) | 0.311b | ||
| 0 | 8 (13.8%) | 6 (20.0%) | |
| 1 | 22 (37.9%) | 7 (23.3%) | |
| 2 | 20 (34.5%) | 15 (50.0%) | |
| 3 | 8 (13.8%) | 2 (6.7%) | |
| Karnofsky performance status (KPS) | 1.000b | ||
| ≤80% | 43 (74.1%) | 23 (76.7%) | |
| 90% | 7 (12.1%) | 3 (10.0%) | |
| 100% | 8 (13.8%) | 4 (13.3%) | |
| Antipeptide IgG (FIU)d | 0.552a | ||
| Median | 84.6 | 81.2 | |
| Number of vaccinations | 0.950c | ||
| Median (range) | 9 (1–90) | 6 (1–84) | |
| % of peptides selected | |||
| EGF-R-800 | 8 (13.8%) | 6 (20.0%) | − |
| Lck-486 | 56 (96.6%) | 26 (86.7%) | |
| Lck-488 | 58 (100.0%) | 30 (100.0%) | |
| MRP3-503 | 18 (31.0%) | 4 (13.3%) | |
| MRP3-1293 | 17 (29.3%) | 12 (40.0%) | |
| PAP-213 | 1 (1.7%) | 0 (0.0%) | |
| PSA-248 | 0 (0.0%) | 0 (0.0%) | |
| PSMA-624 | 0 (0.0%) | 0 (0.0%) | |
| PTH-rP-102 | 12 (20.7%) | 7 (23.3%) | |
| SART2-93 | 13 (22.4%) | 8 (26.7%) | |
| SART2-161 | 11 (19.0%) | 9 (30.0%) | |
| SART3-109 | 38 (65.5%) | 18 (60.0%) |
aWilcoxon rank sum test, bFisher’s exact test, cStudent’s t-test, dfluorescence intensity unit.
Adverse Events
Adverse events (AEs) during the study are shown in Supplementary Table 2, while those related to the vaccination (peptide plus adjuvant or placebo plus adjuvant) are shown in Supplementary Table 3. All AEs, number of positive patients, and percentage of event-positive patients (95% CI) in 58 PPV or 30 placebo patients were 340 or 120 events, 54 or 26 patients, and 93.1% (83.3–98.1) or 86.7% (69.3–96.2), respectively (P = 0.437 by Fisher’s exact test). All the severe AEs (grade 3 or higher), number of positive patients, and percentage of event-positive patients (95% CI) in 58 PPV or 30 placebo patients were 35 or 12 events, 23 or 11 patients, and 39.7% (27.0–53.4) or 36.7% (19.9–56.1), respectively (P = 0.821). Injection site skin reactions were 45 or 18 events, 41 or 16 patients, and 70.7% (57.3–81.9) or 53.3% (34.3–72.7), respectively (P = 0.157). An independent committee evaluated that one grade 3 AE (pulmonary embolism) was the only PPV-related AE of grade ≥3 in this trial.
Clinical Efficacy
The median OS for the PPV (n = 58) and the placebo patients (n = 30) was 8.4 months (95% CI: 6.6–10.6 mo) and 8.0 (95% CI: 4.8–12.9), respectively (hazard ratio [HR]: 1.13, 95% CI: 0.6–1.9, P = 0.621) (Fig. 1B). Then, our trial did not meet the primary endpoint. There was no significant difference of either the median PFS or 1 year survival rate between the PPV and the placebo patients (data not shown), indicating the secondary endpoints also failed.
Best clinical responses were evaluated by immune-related (IR) response criteria using a guideline for the evaluation of immune therapy in solid tumors, as shown in Supplementary Table 4 and Supplementary Appendix 2. They were 3 and 0 cases of IR partial response, 21 and 9 of IR stable disease, and 33 and 19 of IR progressive disease for the evaluable 57 PPV and 27 placebo patients, respectively (data not shown).
Immune Responses
Peripheral blood samples were available for the study from 56 of 58 PPV patients. CTL activities specific to at least 1 of the 4 vaccinated peptides were boosted at least once throughout the vaccination in the post-vaccination peripheral blood mononuclear cells (PBMCs) from 38 of 56 PPV patients tested (68%), while IgG boosting was observed in the post-vaccination plasma from 30 of 56 PPV patients (54%).
The association between immune response to the vaccinated peptides and OS for the PPV patients was evaluated by the landmark method in which the OS rates of the immune responders and nonresponders were compared by starting the clock at the end of the first cycle (twelfth vaccination), 13 weeks after randomization. Thirty-six of 58 PPV patients were dropped from the study at this time because of disease progression, and the remaining 22 patients were provided for measurement of peptide-specific IgG responses, with 17 patients showing IgG boosting and 5 patients showing no IgG boosting. The median for the responders or nonresponders was 10.3 months, 95% CI: 6.8–25.9 or 4.7, 2.2–not reached, respectively (HR: 0.59, 95% CI; 0.1–2.2, P = 0.418). CTL boosting was observed in 16 of 22 cases at that point.
Factors Involved in the Failure of Clinical Efficacy
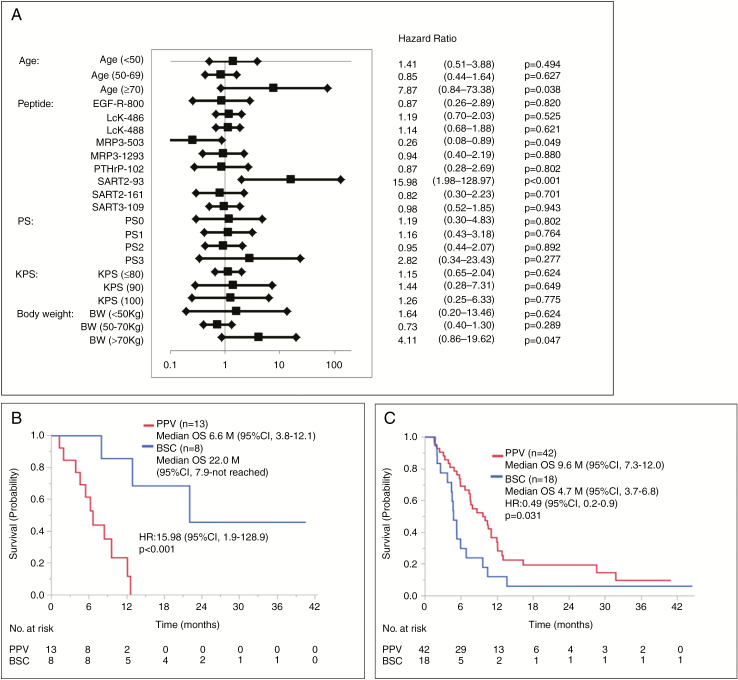
We then conducted Cox regression analyses for the factors designed in the protocol to better understand the major factors involved in the failure of clinical efficacy. By a univariate analysis, the unfavorable factors exceeding HRs of 2.0 for OS of PPV patients compared with those of placebo patients were SART2-93 peptide selection, ≥70 years old, >70 kg body weight, and PS3 (Fig. 2A). The median OS for the 13 of 58 PPV patients with the SART2-93 peptide selection (6.6 mo, 95% CI: 3.8–12.1) was significantly shorter than that of the 8 of 30 corresponding placebo patients (22.0, 7.9–not reached) (HR: 15.9, 95% CI: 1.9–128.9, P < 0.001) (Fig. 2B). Similar tendencies were obtained between the 4 PPV patients ≥70 years old and the corresponding 4 placebo patients (HR: 7.86, 95% CI: 0.8–73.3, P = 0.038) (Supplementary Fig. 1A), between 10 PPV patients with >70 kg weight, and the corresponding 7 placebo patients (HR: 4.11, 95% CI: 0.8–73.3, P = 0.047) (Supplementary Fig. 1B), as well as between the 8 PPV patients with PS3 and the corresponding 2 placebo patients (HR: 0.82, 95% CI: 0.3–23.4, P = 0.28) (data not shown). Consequently, the median OS for the PPV patients without SART2-93 selection plus one of the favorable 3 factors (<70 years old, ≤70 kg, or PS0–2) was significantly longer than that of the corresponding placebo patients, respectively. The median OS for the 42 PPV patients without SART2-93 selection and <70 years old (9.6 mo, 95% CI: 7.7–12.0) was significantly longer than that for the 18 corresponding placebo patients (4.7, 3.7–6.8) (HR: 0.49, 95% CI; 0.2–0.9, P = 0.031) (Fig. 2C). Similar significant differences were obtained in the patients without SART2-93 selection and ≤70 kg (Supplementary Fig. 1C) or the patients without SART2-93 selection and PS0–2 (Supplementary Fig. 1D). The significant difference, however, was not observed in any of the combination of 2 or 3 favorable factors if the factor “without SART2-93 selection” was absent. Collectively, these results suggest that a predominant factor involved in this failure was the SART2-93 selection.
Fig. 2.
(A) Forest plot of the factors involved in OS. HR of the 58 PPV vs the 30 placebo group. (B) Median OS for 13 PPV patients with SART2-93 peptide selection vs the 8 corresponding placebo patients. (C) Median OS for 42 PPV patients without SART2-93 selection plus age <70 years old vs the 18 corresponding placebo patients.
In contrast to those 4 unfavorable factors, a favorable factor for OS of PPV patients compared with that of placebo patients were MRP3-503 peptide selection (Fig. 2A). The median OS for the 18 of 58 PPV patients with the MRP3-503 peptide selection (8.7 mo, 95% CI: 5.9–12.6) was significantly longer than that of the 4 of 30 corresponding placebo patients (4.9 mo, 2.6–7.9) (HR: 0.26, 95% CI: 0.08–1.8, P = 0.049) (data not shown). This could be mainly due to the very short survival of only 4 placebo patients, but not due to the MRP3-503 peptide vaccination of the PPV patients, since the median OS for 18 PPV patients was almost equal to that of the entire 58 PPV patients (8.4 mo).
Multivariate analysis was conducted for each of the factors listed in Fig. 2A using the discrete scale, but none of them tested significantly affected OS between the PPV and placebo patients (data not shown).
Mechanisms Involved in the Different Clinical Efficacy Caused by SART2-93 Peptide Selection
To better understand why the SART2-93 peptide selection, but not any other 11 peptides, was a predominant unfavorable factor for OS of PPV patients compared with that of placebo patients, we compared the patient characteristics, including those listed in Table 1 and Fig. 2A, and biomarkers between the group of 67 patients with SART2-93 selection (designated the SART2-93(−) group), and the group of the remaining 21 patients without the SART2-93 selection (the SART2-93(+) group). The percentage of patients receiving IFN prior to the entry or the selection of either of 2 peptides (SART2-161 or SART3-109), but not any others tested, was significantly lower or higher in the SART2-93(−) group compared with that in the SART2-93(+) group (Supplementary Tables 5 and 6), respectively.
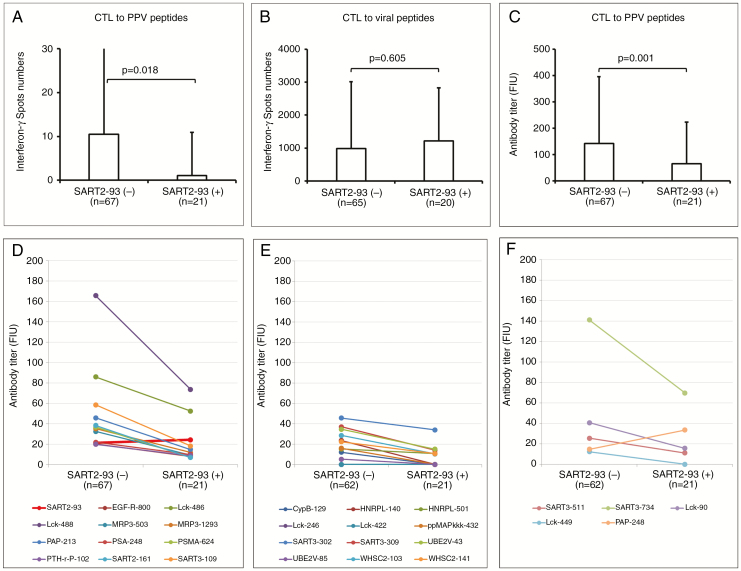
We then investigated the preexisting immune responses between these groups. The preexisting CTL responses against the peptides selected for PPV in rGBM patients entered in this study were very low, as expected from the results from the previously reported manuscripts.12,13 Under this circumstance, the positive CTL responses against each of the 4 peptides selected for the PPV were found in 13 of 268 wells of 11 of 67 patients from the SART2-93(−) group, with a total of 281 IFNγ spot numbers per 104 PBMCs, whereas these responses were found in only 1 of 84 wells of 1 of 21 patient from the SART2-93(+) group, with a total of 9 IFNγ spot numbers, respectively (P = 0.20, by Fisher’s exact test). Notably, the peptide used for this 1 positive well in the SART2-93(+) group was SART2-93 peptide, indicating that the PBMCs from the SART2-93(+) group showed no detectable CTL responses against any of the remaining 11 peptides selected for PPV. CTL responses, however, against a mix of the control viral epitope peptides were equally found in both the 25 patients of the SART2-93(−) group, with a total of 8848 spot numbers and the 10 patients of the SART2-93(+) group, with a total of 6417 spot numbers (P = 0.43). Subsequently, the mean spot number per 105 PBMCs from the SART2-93(−) group against the selected 4 peptides was significantly (P = 0.018 by Student’s t-test) higher than that from the SART2-93(+) group (Fig. 3A), whereas it was not the case for the control viral epitope peptides (P = 0.605) (Fig. 3B).
Fig. 3.
Preexisting CTL responses against the 4 peptides selected for PPV (A) or against a mix of the control viral epitope peptides consisting of cytomegalovirus, Epstein–Barr virus, and influenza virus (B) in the patients of the 67 SART2-93(−) group vs those of the 21 SART2-93(+) group. Preexisting IgG responses against the 4 selected peptides for PPV (C), all the 12 HLA-A24–restricted peptides used in this study (D), 12 HLA-A2–restricted CTL peptides (E), or 5 HLA-A3 superfamily (A3, A11, A31, A33)–restricted CTL peptides (F) in these 2 groups, respectively.
The preexisting IgG responses in the SART2-93(−) group against the 4 peptides selected for the PPV were also significantly higher than those in the SART2-93(+) group (P = 0.001) (Fig. 3C). Moreover, the preexisting IgG levels to 11 of 12 warehouse peptides (ie, all but the SART2-93 peptide) were higher in the SART2-93(−) group (Fig. 3D).These results confirmed that the SART2-93 peptide was not selected in the SART2-93(−) group simply because of the higher IgG responses against the other 11 peptides, and indicated that PPV was more appropriate for the patients of SART2-93(−) group because of the higher immune responses to the peptides used for the PPV compared with the SART2-93(+) group. In contrast, PPV could not be recommended for the SART2-93(+) group due to the near absence of preexisting peptide-specific immunity against all 12 warehouse peptides besides the SART2-93 peptide.
We further investigated the preexisting IgG responses to the CTL epitope peptides other than 12 peptides shown above using 12 HLA-A2–restricted CTL peptides and 5 HLA-A3 super-type (A3, A11, A31, A33)–restricted CTL peptides. These peptides were used as warehouse peptides for PPV to cancer patients with HLA-A2 or HLA-A3, as reported in previous studies.14 Samples were collected from the 62 of 67 patients in the SART2-9 3(−) group and all 21 patients in the SART2-93(+) group who provided written approval to participate in this study. The preexisting IgG levels to all 12 HLA-A2+ peptides were higher in the SART2-93(−) group (Fig. 3E).Those to 4 of the 5 HLA-A3+ super-type peptides, with the exception being the PAP-248 peptide, were also higher in the SART2-93(−) group (Fig. 3F).
Biomarkers Predictive of OS for PPV or Placebo Patients
The following supplemental analyses were conducted additionally, apart from a protocol-based analysis shown above to provide novel information, if any, for the next step of clinical trial of PPV for rGBM patients. We at first investigated the changes in the immune cell subsets using the pre- and post-vaccination PBMCs from 37 of 58 PPV patients or 21 of 30 BSC patients for whom the cohort samples were available. There was no significant differences of the percentages of CD11b+CD14+HLA-DRlow immunosuppressive monocytes between the pre- and post-vaccination PBMCs of the PPV patients, whereas these cells significantly increased after the placebo injection in the BSC patients (data not shown). CD3+CD4+CD45RA− T cells with activated/memory T helper phenotype, CD3+CD8+CD45RA− T cells with activated/memory CTL phenotype, and CD4+CD25+FoxP3+ Treg cells were significantly decreased after PPV injection, suggesting that PPV induced these activated T cells to migrate from the circulation into tumor sites (data not shown).
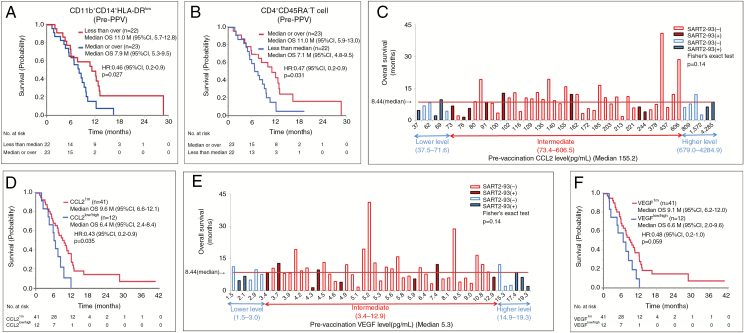
We then investigated the correlation between OS and the percentages of these immune cell subsets of pre-vaccination PBMCs in the 45 available PPV patients. The median OS for PPV patients with a lower (less than the median) percentage of CD11b+CD14+HLA-DRlow immunosuppressive monocytes was significantly longer than that with a higher (median or more) percentage of the corresponding cells (11.1 vs 8.0 mo, HR 0.46, 95% CI: 0.2–0.9, P = 0.027) (Fig. 4A). Similar results were obtained on CD11b+CD14+HLA-DR− immunosuppressive monocytes (data not shown). In contrast, the median OS for PPV patients with a higher percentage of CD3+CD4+CD45RA− T cells was significantly longer than that with a lower percentage (11.1 vs 7.1 mo, HR 0.47, 95% CI: 0.2–0.9, P = 0.031) of the corresponding cells (Fig. 4B). There was no significant correlation between OS of PPV patients and the other cell subsets tested (data not shown).
Fig. 4.
Median OS of the PPV patients with lower pre-vaccination percentages of CD11b+CD14+HLA-DRlow cells (A) or higher percentages of CD3+CD4+CD45RA− T cells (B) vs those with higher or lower percentages of these cells, respectively. (C) Relationship between pre-vaccination levels of CCL2 and OS in each of 53 PPV patients tested. (D) The median OS between the 2 groups by defining the 12 patients consisting of both the 6 patients from the lowest CCL2 level (lower tail 11th percentile) and the 6 patients from the highest level (upper tail 11th percentile) as CCL2low/high, and the remaining 41 patients (remaining 78th percentile) as CCL2im. (E) Relationship between pre-vaccination levels of VEGF and OS in each of 53 PPV patients tested. (F) The median OS between the 2 groups by defining the 12 patients consisting of both the 6 patients from the lowest VEGF level (lower tail 11th percentile) and the 6 patients from the highest level (upper tail 11th percentile) as VEGFlow/high, and the remaining 41 patients (remaining 78th percentile) as VEGFim.
We then investigated the correlation between pre-vaccination soluble factors and OS in 53 PPV patients from whom plasma samples were available. None of 34 soluble factors tested besides CCL4 significantly correlated with OS when the median level was used as an indicator to discriminate one from the other, as in the past cytokine studies.4–10 Exemption was CCL4. The median OS for patients with a higher CCL4 level (n = 26, the median or over) were significantly longer than that of patients with a lower CCL4 level (n = 27, less than the median) (10.1 mo, 95% CI: 5.9–12.2, vs 7.6 mo, 4.2–9.7, HR: 0.50, 95% CI: 0.2–0.9, P = 0.027) (data not shown).
To find more useful soluble biomarkers, if any, correlative to OS for PPV patients, the relationship between soluble factors and OS in each patient under PPV was investigated. The relations between these factors and the SART2-93 peptide selection, a risk factor for OS of the PPV patients, were also assessed to better understand the relation between soluble markers and peptide selection. As a result, the OS of both 9 of 10 patients from the lowest level and 4 of 6 PPV patients from the highest level of CCL2 had the OS below the median (Fig. 4C), suggesting that the OS of PPV patients with either very low or high CCL2 levels (CCL2low/high) was shorter than that of patients with an intermediate CCL2 level (CCL2im). Subsequently, we compared the median OS between these 2 groups by defining the 12 patients consisting of both the 6 patients from the lowest level (lower tail 11th percentile) and the 6 patients from the highest level (upper tail 11th percentile) as CCL2low/high, and the remaining 41 patients (remaining 78th percentile) as CCL2im. The median OS of the PPV patients with CCL2im (n = 41) was significantly longer than that of the PPV patients with CCL2low/high (n = 12) (9.7 mo, 95% CI: 6.6–12.1 vs 6.5 mo, 95% CI: 2.5–8.4; HR: 0.43, 95% CI: 0.2–0.9, P = 0.035) (Fig. 4D). A bell-shaped curve of OS was also observed in the case of vascular endothelial growth factor (VEGF) (Fig. 4E) with significantly longer survival in the PPV patients (n = 41) with VEGFim compared with the PPV patients (n = 12) with VEGF low/high (Fig. 4F). Similar results were also obtained in the case of IL-6 and IL-17, respectively (data not shown). Notably, the patients with the intermediate levels of CCL2 or VEGF mostly received PPV lacking the SART2-93 peptide, a favorable marker for OS of PPV patients. Furthermore, the bell-shaped curve was also observed in haptoglobin (Hp) with significantly longer survival in the PPV patients with Hpim than in the PPV patients with Hplow/high, when the median OS was compared between the 8 patients with Hplow/high (defined as the 8 patients consisting of both 4 patients from the lowest level [lower tail 8th percentile] and the 4 patients from the highest level [upper tail 8 percentile]), and the remaining 45 patients with Hpim (remaining 84th percentile), respectively (data not shown). The similar results were observed in IL-7 (data not shown). We then investigated the correlation coefficient among CCL2, VEGF, IL-6, IL-7, IL-17, and Hp (Supplementary Table 7). High correlation coefficients (r > 0.7) were observed among CCL2, VEGF, and IL-6. Furthermore, the 9 or 5 of 12 patients with CCL2low/high were also IL-6low/high or VEGF low/high, respectively. These results suggest that CCL2, VEGF, and IL-6 formed a close network that affected the peptide selection for PPV and the following clinical benefits. Relatively high correlation coefficients (0.4 < r < 0.5) were also observed between IL-17 and CCL2, VEGF, or IL-6. The 2, 1, or 2 of 12 patients with IL-17low/high were also CCL2low/high, IL-6low/high, or VEGF low/high, respectively.
In addition to these 6 factors mentioned above, the OS of all 5 patients from the highest level of GM-CSF was very short (Supplementary Fig. 2A), with significant differences of OS between these 5 patients and the remaining 48 patients (4.2 vs 9.2 mo, HR: 6.87, 95% CI: 2.1–18.7, P = 0.002) (Supplementary Fig. 2B). Similar results were found in the case of IL-1RA or IL-10 (data not shown). Collectively, these results indicated that the very high pre-vaccination levels of 9 of the 34 soluble factors were associated with a shorter OS of PPV patients.
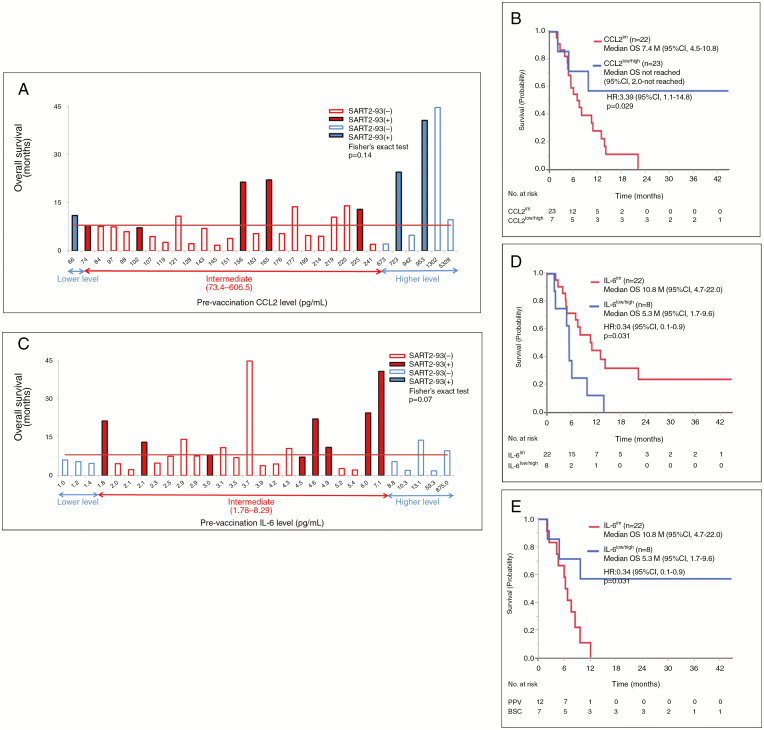
The same study shown above was conducted on the placebo patients. There was no correlation between OS and the percentages of the immune cell subsets tested (data not shown). None of 33 soluble factors tested besides IL-15 significantly correlated with OS when the median level was used as an indicator to discriminate one from the other (data not shown). However, the median OS of 7 of the patients with CCL2low/high (<73.4 or >606.5 pg/mL for PPV patients as shown in Fig. 4C) was significantly longer than that of the remaining 23 patients with CCL2im (73.4–606.5pg/mL) (not reached: 95% CI: 2.1–not reached vs 7.4 mo, 95% CI: 4.6–10.8; HR: 3.40, 95% CI; 1.1–14.8, P = 0.029) (Fig. 5A and B). On the contrary, the median OS of 8 patients with IL-6low/high (<1.78 or >8.29 pg/mL for PPV patients) was significantly shorter than the remaining 23 patients with IL-6im (1.78–8.29) (5.3 mo, 95% CI: 1.7–9.7 vs 10.8 mo, 95% CI: 4.8–22, HR: 0.35, 95% CI: 0.1–0.9, P = 0.031) (Fig. 5C and D).
Fig. 5.
(A) Relationship between pre-vaccination levels of CCL2 and OS in each of 30 placebo patients. (B) The median OS of 7 placebo patients with CCL2low/high vs that of the remaining 23 patients with CCL2im. (C) Relationship between pre-vaccination levels of IL-6 and OS in each of 30 placebo patients. (D) The median OS of 8 placebo patients with IL-6low/high vs the remaining 23 patients with IL-6im. (E) The median OS of 12 PPV patients with CCL2 low/high vs that of the 7 corresponding placebo patients.
These results suggested that PPV rather shortened the OS for the patients with CCL2 low/high, but not Il-6 low/high compared with that for corresponding placebo patients. Indeed, the median OS of 12 PPV patients with CCL2 low/high was significantly shorter than the 7 corresponding placebo patients (6.5 mo, 95% CI: 2.5–8.4 mo vs not reached, 2.1–not reached, HR 4.26, 95% CI: 1.2–19.9, P = 0.020) (Figure 5E). In contrast to these 2 factors, the median OS of the PPV patients with VEGF low/high, IL-7 low/high, or IL-17 low/high was not different from that of the corresponding placebo patients (data not shown).
Discussion
HLA-A24–positive rGBM patients are very few in Japan (about 500 patients per year). There is no approved drug to prolong OS of rGBM patients. In addition, no double-blind, randomized clinical study between a test reagent plus BSC and a placebo plus BSC was previously conducted for rGBM patients as far as searched. These circumstances genetically made it difficult to set up a protocol with a relatively large number of patients for the study with the appropriate stratifications. Although we made this protocol with a total of 110 patients—75 PPV and 35 placebo patients with the 3 stratifications (PS0–1 vs 2–3), pathological diagnosis of GBM (primary vs secondary), and prior bevacizumab treatment (without vs with)—the results revealed that the other factors (SART2-93 peptide, age, body weight, PS3) largely affected the primary endpoint (OS). Further, all the studies were based on the analyses in which each of them consisted of small groups, and thus the obtained results are at least not robust. This shall be a major limitation of this study, and the issues pointed to in this study shall be confirmed in the next clinical study of PPV for a relatively large number of rGBM patients.
Our trial met neither the primary nor secondary endpoint. Among the 4 unfavorable factors for OS of PPV patients compared with that of placebo patients, older age, obesity, and worse PS were reported as unfavorable factors for the OS of GBM patients.1–3,7,16,17 In contrast, the SART2-93 peptide selection was an unexpected unfavorable factor, primarily because this is the first clinical study of a double-blind, randomized clinical study between a test reagent plus BSC and a placebo plus BSC. Evaluation of pre-vaccination immunity against peptides used for PPV, however, revealed that the preexisting peptide-specific CTL and IgG responses in the SART2-93(−) group were significantly higher than those of the SART2-93(+) group. These results could partly explain the discrepancy of clinical outcomes that PPV shortened or lengthened the OS for PPV patients with or without SART2-93 peptide compared with that for placebo patients, respectively.
The median OS for the 22 placebo patients without SART2-93 selection (5.4 mo, 95% CI: 4.4–9.6) was significantly shorter than that of 8 placebo patients with SART2-93 selection (22, 7.9–not reached) (HR: 5.75, 95% CI: 1.8–24.9; P = 0.001), whereas the median OS for the 45 PPV patients without SART2-93 selection (9.1 mo, 95% CI: 7.3–11.0) was somewhat longer than that for 13 PPV patients with SART2-93 selection (6.6, 1.9–12.1) (HR 0.57, 95% CI: 0.3–11.0; P = 0.131) (data not shown). It is presently unclear why the OS for placebo patients with SART2-93 selection was significantly longer than that for placebo patients without SART2-93 selection. As far as we studied, a major difference between these groups was the near absence or presence of immune responses to CTL-epitope peptides against tumor-associated antigens provided for PPV, but not those against viral epitope peptides. Further studies with a relatively large number of rGBM patients shall be conducted to confirm whether the near absence of immune responses to the tumor-associated antigens is a favorable biomarker for OS of rGBM patients.
A trend of the shorter OS for PPV patients with SART2-93 selection compared with that for PPV patients without SART2-93 selection might be explained in part by an idea that a therapeutic cancer vaccine without substantial preexisting immunity against the vaccinated peptides might shorten the OS of some advanced cancer patients by inducing inconvenient antitumor responses. Indeed, the results of our previously conducted nonpersonalized therapeutic peptide vaccine for advanced cancer patients supported such an assumption.18
Further clinical study of PPV with a relatively large number of rGBM patients shall also be conducted to solve this issue.
We could not clearly explain why pre-vaccination CCL-2 largely influenced the SART2-93(−) PPV patients using the small scale of the samples. This issue shall be reexamined in the next step of clinical trials.
Isocitrate dehydrogenase 1 (IDH1) mutation was examined by immune-histochemical staining with anti–IDH1-R132H monoclonal antibody in 83 patients (53 PPV and 30 placebo patients) from whom the written informed consents were obtained. IDH mutation was positive in 12 of 83 patients (6 of 53 PPV or 6 of 30 placebo patients), respectively. Pre-vaccination CTL (P = 0.16) or IgG (P < 0.01) levels used for the vaccination in IDH mutation(+) patients were lower than those of IDH mutation(−) patients, respectively. Post-vaccination CTL boosting was observed in 2 of 4 mutation(+) or 31 of 46 mutation(−) patients tested under PPV, while the post-vaccination IgG boosting was observed in 2 of 4 mutation(+) or 26 of 46 mutation(−) patients under PPV, respectively. Median OS of 6 mutation(+) or 47 mutation(−) PPV patients was 6.7 or 8.6 months, while that of 6 mutation(+) or 24 mutation(−) placebo patients was 4.6 or 8.0 months, respectively. These results suggest that IDH gene mutation did not affect the peptide-specific immune responses of the PPV patients. These results also did not support IDH mutation as a favorable marker for OS of PPV or placebo patients in this small scale of the study for recurrent GBM patients. We did not examine O6-methylguanine-DNA methyltransferase methylation status primarily because the available tumor samples were very limited, and all the patients failed therapy with temozolomide prior to the entry for PPV plus BSC or placebo plus BSC. These issues need to be confirmed (IDH1) or tested (MGMT) in the next study with relatively large numbers of patients.
Funding
This research was supported in part by a grant from the Japan Agency for Medical Research and Development, grant number 16ck0106086h0003.
Supplementary Material
Acknowledgments
We thank Dr Soichiro Shibui, Teikyo University Hospital, Tokyo, Japan, for acting as a scientific advisor to this phase III trial. We also thank BrightPath Biotherapeutics Co, Ltd. for the provision of ITK-1 and placebo for this phase III trial.
Conflict of interest statement. Kyogo Itoh received a grant from Taiho Pharmaceutical Co. Kyogo Itoh and Shigeki Shichijo gained income by selling stock of BrightPath Biotherapeutics Co., Ltd. Tetsuo Sasada received a grant from BrightPath Biotherapeutics Co. Akira Yamada is a part-time executive of BrightPath Biotherapeutics Co. The other authors have no competing interests to declare.
Authorship statement: Conception and design: M. Terasaki, K. Itoh, Y. Narita, S. Shichijo
Collection and assembly of data: M. Terasaki, K. Itoh, Y. Narita, S. Shichijo
Data analysis and interpretation: All authors
Manuscript writing: All authors
List of unpublished papers cited: None
References
- 1. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 2. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 3. Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8(53):91779–91794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cloughesy T, Finocchiaro G, Belda-Iniesta C, et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: efficacy, safety, and hepatocyte growth factor and O6-methylguanine-DNA methyltransferase biomarker analyses. J Clin Oncol. 2017;35(3):343–351. [DOI] [PubMed] [Google Scholar]
- 5. Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007;6(5):404–414. [DOI] [PubMed] [Google Scholar]
- 6. Vakilian A, Khorramdelazad H, Heidari P, Sheikh Rezaei Z, Hassanshahi G. CCL2/CCR2 signaling pathway in glioblastoma multiforme. Neurochem Int. 2017;103:1–7. [DOI] [PubMed] [Google Scholar]
- 7. Sims JS, Ung TH, Neira JA, Canoll P, Bruce JN. Biomarkers for glioma immunotherapy: the next generation. J Neurooncol. 2015;123(3):359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curran CS, Evans MD, Bertics PJ. GM-CSF production by glioblastoma cells has a functional role in eosinophil survival, activation, and growth factor production for enhanced tumor cell proliferation. J Immunol. 2011;187(3):1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohanbash G, McKaveney K, Sakaki M, et al. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-α. Cancer Res. 2013;73(21):6413–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang AL, Miska J, Wainwright DA, et al. CCL2 Produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5671–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martens A, Wistuba-Hamprecht K, Geukes Foppen M, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(12):2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yajima N, Yamanaka R, Mine T, et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res. 2005;11(16):5900–5911. [DOI] [PubMed] [Google Scholar]
- 13. Terasaki M, Shibui S, Narita Y, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen–A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol. 2011;29(3):337–344. [DOI] [PubMed] [Google Scholar]
- 14. Noguchi M, Matsumoto K, Uemura H, et al. An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin Cancer Res. 2016;22(1):54–60. [DOI] [PubMed] [Google Scholar]
- 15. van Houwelingen HC, Putter H. Dynamic predicting by landmarking as an alternative for multi-state modeling: an application to acute lymphoid leukemia data. Lifetime Data Anal. 2008;14(4):447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laperriere N, Weller M, Stupp R, et al. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2013;39(4):350–357. [DOI] [PubMed] [Google Scholar]
- 17. Erin M, Siegel L, Nabors B, et al. Pre-diagnostic body weight and survival in high grade glioma. Neurooncol. 2013;114(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mochizuki K, Sato Y, Tsuda N, et al. Immunological evaluation of vaccination with pre-designated peptides frequently selected as vaccine candidates in an individualized peptide vaccination regimen. Int J Oncol. 2004;25(1):121–131. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.