Abstract
Background
Meningioma incidence increases significantly with age. In the expanding elderly population, we lack complete understanding of population-based trends in meningioma incidence/survival. We provide an updated, comprehensive analysis of meningioma incidence and survival for individuals aged over 65.
Methods
Data were obtained from the Central Brain Tumor Registry of the United States (CBTRUS) from 2005–2015 for nonmalignant and malignant meningioma. Age-adjusted incidence rates per 100000 person-years were analyzed by age, sex, race, ethnicity, location, and treatment modalities. Survival was analyzed using Kaplan–Meier and multivariable Cox proportional hazards models for a subset of CBTRUS data.
Results
Nonmalignant meningioma incidence doubled from adults age 65–69 years to adults over age 85 years and was significantly greater in females than males for all ages. Malignant meningioma incidence did not differ by sex for any age grouping. Nonmalignant and malignant meningioma incidence was significantly greater in black populations versus others. Nonmalignant meningioma survival was worse with age, in black populations, and in males, including when analyzed by 5-year age groups. Surgical resection and radiation did not improve survival compared with resection alone in nonmalignant meningioma.
Conclusions
This study reports increasing nonmalignant meningioma incidence in the elderly, increased incidence in black populations, and in females. In contrast, malignant meningioma incidence did not differ between sexes. Risk of death was higher for black individuals and males. Additionally, radiation did not confer a survival advantage when combined with resection for nonmalignant meningioma. Thus, we identify clinically relevant discrepancies in meningioma incidence/survival that require further study.
Keywords: meningioma, incidence, survival, CBTRUS, SEER
Key Points
Meningioma incidence is highest in black populations, females, and increasingly the elderly
Nonmalignant meningioma survival is lowest in black populations, males, and increasingly the elderly
Adjuvant radiation following surgical resection does not confer a survival advantage in the elderly
Importance of the Study
Meningioma is the most common primary central nervous system tumor and incidence increases with age. Given the increasing age of the United States population, and the relative paucity of recent nationwide epidemiological reports addressing meningioma specifically in the elderly, we sought to provide an up-to-date, detailed analysis of incidence and survival trends in individuals over age 65 years. We examined nonmalignant and malignant meningioma from 2005–2015 using incidence data derived from the CBTRUS, which provides data for approximately 99% of the US population. Moreover, we report updated temporal trends in nonmalignant and malignant meningioma that potentially shed light on the effect of World Health Organization classification changes on incidence over time. We also examine the effects of demographic and clinical factors on survival. This includes the effect of combined resection and radiation, which remains a controversial issue in the treatment of meningioma in the elderly.
Meningioma is the most common primary neoplasm of the central nervous system (CNS), accounting for 36.4% of CNS tumors reported from 2010 to 2014.1 According to a recent study, nonmalignant meningioma has an incidence rate (IR) of 7.86 per 100000 people; a rate that has significantly increased from 2004 to 2010, with an annual percentage change (APC) of 3%.2 A report of atypical, World Health Organization (WHO) grade II meningioma revealed increasing incidence in 2004–2010, with a 3.6% APC.3 In fact, an estimated 29320 new meningioma diagnoses are projected in the United States for 2018 alone.1 Furthermore, there is clear evidence that incidence of meningioma increases with age, with a median age at diagnosis of 66 years old.1,4 However, few studies have taken a comprehensive approach to the descriptive epidemiology of malignant and nonmalignant meningioma in the elderly population.5,6
While few studies have addressed the descriptive epidemiology of meningioma in the elderly, many have examined the effects of patient characteristics and treatment modalities on survival.7–18 Several studies suggest that craniotomy for gross total or subtotal meningioma resection is associated with higher risk of morbidity and mortality in elderly patients compared with younger cohorts.10–12 Other studies, however, suggest that there is no association between age and overall survival, but rather between other prognostic factors such as male sex, comorbidity status, neurological deficits, and performance scales.13–15 Further analysis suggests comparable mortality rates between elderly and younger patients with meningioma but a greater number of minor complications and poorer functional outcomes for elderly patients undergoing treatment.16–18 Thus, there remains a general lack of consensus regarding the association between age and clinical outcomes for elderly patients with meningioma. Therefore, given the increasing life expectancy of the overall population, the concomitant increase in meningioma incidence, and paucity of descriptive epidemiological studies to address trends in this growing at-risk patient cohort, we aimed to provide an updated comprehensive analysis of meningioma incidence and survival trends specific to the elderly population.
Methods
This study was approved by the University Hospitals Case Medical Center Institutional Review Board. Data were obtained from the Central Brain Tumor Registry of the United States (CBTRUS), which includes incidence data from approximately 99% of the US population. CBTRUS data are derived from 50 state cancer registries and the cancer registry for the District of Columbia. Together, these include 46 National Program of Cancer Registration (NPCR) and 5 Surveillance, Epidemiology, and End Results (SEER) central cancer registries.2 Age-adjusted IRs were generated for nonmalignant and malignant meningiomas from 2005 to 2015. Nonmalignant meningiomas were specified by 9 specific ICD-O-3 codes: 9530/0 (Meningioma, NOS), 9530/1 (Meningiomatosis, NOS), 9531/0 (Meningothelial meningioma), 9532/0 (Fibrous meningioma), 9533/0 (Psammomatous meningioma), 9534/0 (Angiomatous meningioma), 9537/0 (Transitional meningioma), 9538/1 (Clear cell meningioma), and 9539/1 (Atypical meningioma, NOS), as previously reported.1 Malignant meningiomas were specified by 3 ICD-O-3 codes: 9530/3 (Meningioma, malignant), 9538/3 (Papillary meningioma), and 9539/3 (Meningeal sarcomatosis), also as previously reported.1 Age-adjusted IRs were standardized to the 2000 US population1 and reported per 100000 population.
Information on patient survival outcomes was derived from SEER data, since NPCR registries do not provide follow-up data to CBTRUS. SEER data were analyzed to generate survival data for both nonmalignant and malignant meningiomas from 2005 to 2015. The current SEER registry system consists of 18 registries representing a subset of the population included in the CBTRUS dataset. Of note, NPCR and SEER dually provide funding for the 18 registries in the SEER subset. According to the US 2010 Census, data pulled from the 18 SEER registries provide population-based information for approximately 28% of the US population.19
Incidence rates and other relevant statistics were calculated using SEER*Stat 8.3.5. Figures were generated using GraphPad Prism 6, Adobe Illustrator and Photoshop, and R statistical software. Statistics were excluded for cells containing fewer than 16 counts as required by NPCR. Age-adjusted IRs and 95% CIs were estimated for nonmalignant and malignant meningiomas by sex, race, ethnicity, age groupings (65–69, 70–74, 75–79, 80–84, and 85+ y), and tumor location, including supratentorial (ICD-O-3 codes 700, 702–714), infratentorial (716–717), and spine (701, 720–721, 725). Race categories for this study included white, black, American Indian/Alaska Native (AIAN), and Asian/Pacific Islander (API). Incidence for Hispanic patients versus non-Hispanic patients was also analyzed. Unknown, unspecified, and other race categories were excluded from race-specific IR comparisons. However, these categories were included in statistics that were not race specific. Joinpoint Regression Program 4.6.0.0 software was used to compute APC in IRs from 2000 to 2015 to examine trends over time. Joinpoint software selects a minimum number of joinpoints to prohibit statistically significant improvement if one additional joinpoint is added (http://surveillance.cancer.gov/joinpoint).
SEER*Stat was used to generate survival outcomes by age, race, and sex. For survival data, race categories included white, black, API, and AIAN for nonmalignant meningioma. AIAN populations were excluded from malignant meningioma analysis due to insufficient sample size. Survival by age at diagnosis was examined using the age groupings described above. Given that the 18 SEER registries comprise only 28% of the CBTRUS dataset, an N > 50 was required for group-specific data inclusion to ensure sufficiently stable statistical analyses. Differences in survival were further analyzed in R using Kaplan–Meier and univariable and multivariable Cox proportional hazards models. Survival curves were generated by race, ethnicity, sex, age, tumor location, and treatment modality (gross total resection [GTR], subtotal resection [STR], GTR + radiotherapy [RT], and STR + RT), and compared using the log-rank test. However, for malignant meningioma, RT versus no RT was the only treatment modality we could include in the model, as the sample size was too small (N < 50) for statistical analyses pertaining to resection or combined resection and radiation. Adjusted estimates of survival by race, ethnicity, age, tumor location, and treatment modality were also performed in R using a multivariable Cox proportional hazards model. Adjusted estimates included all covariates a priori, regardless of individual significance level.
Results
Nonmalignant and Malignant Meningioma Incidence by Age and Sex, 2005–2015
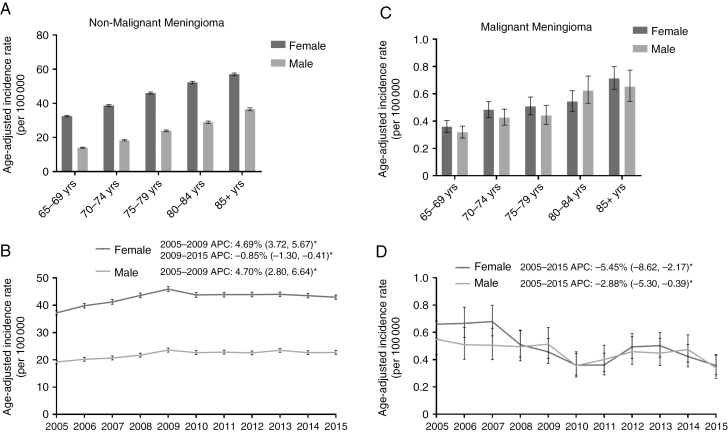
Age-adjusted IRs from 2005–2015 are depicted in Fig. 1 by 5-year age groups over the age of 65 years for males and females (Fig. 1). To note, the overall incidence of nonmalignant meningioma increased significantly for each 5-year age group, from 23.85 cases per 10000 in adults age 65–69 years (95% CI: 23.60, 24.11) to 50.33 cases per 100000 (95% CI: 49.77, 50.89) in individuals over age 85 (Supplementary Fig. 1A and Supplementary Tables 1–2). The incidence of nonmalignant meningioma was also significantly greater in females in every 5-year age grouping compared with males in the same age cohort (Fig. 1A, Supplementary Table 1). For males and females, in 2005–2009, there was a significant increase in nonmalignant meningioma incidence (female APC: 4.69% [95% CI: 3.72, 5.67], P < 0.0001; male APC: 4.70% [95% CI: 2.80, 6.64], P = 0.0009; Fig. 1B). However, in 2009–2015, there was a significant decrease in incidence in females (APC: −0.85% [95% CI: −1.30, −0.41], P = 0.0035), and no significant change in incidence for males (APC: −0.16% [95% CI: −1.01, 0.70], P = 0.66). Additionally, there was a significant increase in nonmalignant meningioma incidence in all age groups up to 2008–2009, followed by either no change in incidence or a significant decline in incidence in the case of 65–69 year olds in 2009–2015 (Supplementary Fig. 1B).
Fig. 1.
(A–D) Age-adjusted incidence rates and annual percent changes (APCs) for nonmalignant meningioma by (A) sex by 5-year age groupings, and by (B) sex over time from 2005–2015. Age-adjusted incidence rates and APCs for malignant meningioma by (C) sex by 5-year age groupings and (D) sex over time in 2005–2015. APCs are accompanied by 95% CIs in parentheses. *Only significant changes in APC are reported in the figures. (CBTRUS 2005–2015)
In malignant meningioma, IR increased for each 5-year grouping, though not significantly across all groups (Supplementary Fig. 1D, Supplementary Table 2). Contrary to nonmalignant meningioma IR, however, malignant meningioma incidence did not significantly differ by sex for any of the 5-year age groupings (Fig. 1C, Supplementary Table 2). From 2005 to 2015, there was a significant decrease in malignant meningioma incidence in females (APC: −5.45% [95% CI: −8.62, −2.17], P = 0.0048) and in males (APC: −2.88% [95% CI: −5.30, −0.39], P = 0.0028) (Fig. 1D). In most 5-year age groups, there was a significant decrease in incidence from 2005 to 2015 with the exception of 65–69 year olds, in whom the decreasing incidence did not reach significance (Supplementary Fig. 1F).
Nonmalignant and Malignant Meningioma Incidence by Age and Race and Age and Ethnicity, 2005–2015
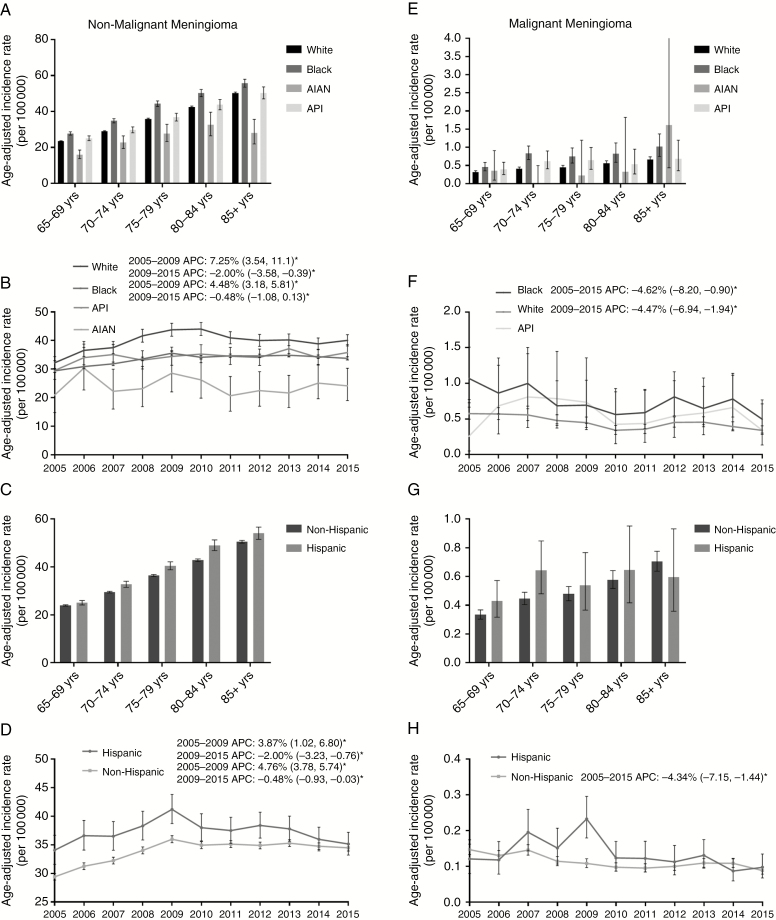
Overall IR for nonmalignant meningioma was significantly greater in black populations (39.7 cases per 100000 [95% CI: 40.34, 39.07]) compared with all other races, while the overall incidence for AIAN populations was significantly lower than all other races (Supplementary Fig. 1C). For every age group, nonmalignant meningioma IR was significantly greater in black populations, and significantly lower in AIAN populations, than all other races (Fig. 2A and Supplementary Table 1). From 2005 to 2009, the incidence of nonmalignant meningioma significantly increased in black and white populations (black: APC: 7.25% [95% CI: 3.54, 11.10], P = 0.0028, white: APC: 4.48% [95% CI: 3.18, 5.81], P = 0.0001). In 2009–2015, however, nonmalignant meningioma incidence decreased significantly in these two populations (black: APC: −2.00% [95% CI: −3.58, −0.39], P = 0.022; white: APC: −0.47% [95% CI: −1.08, 0.13]) (Fig. 2B). Hispanic populations had significantly higher IR than non-Hispanics for every age group except 65–69 year olds (Fig. 2C and Supplementary Table 1). In 2005–2009, the nonmalignant meningioma incidence significantly increased in non-Hispanic and Hispanic populations (APC: 4.76% [95% CI: 3.78, 5.74], P < 0.0001 vs APC: 3.87% [95% CI: 1.02, 6.80], P = 0.016) and decreased significantly in both populations in 2009–2015 (APC: −0.48% [95% CI: −0.93, −0.03], P < 0.0001 vs APC: −2.00% [95% CI: −3.23, −0.76], P = 0.016) (Fig. 2D).
Fig. 2.
(A–D) Age-adjusted incidence rates and annual percent changes (APCs) for nonmalignant meningioma by (A) age and race, including black, white, Asian/Pacific Islander (API), and American Indian Alaskan Native (AIAN), (B) race over time in 2005–2015, (C) age and ethnicity, and (D) ethnicity over time in 2005–2015. (E–F) Age-adjusted incidence rates and APCs for malignant meningioma by (E) age and race, (F) race over time in 2005–2015, (G) age and ethnicity, and (H) ethnicity over time in 2005–2015. APCs are accompanied by 95% CI in parentheses. *Only significant changes in APC are reported in the figures. (CBTRUS 2005–2015)
Overall IR for malignant meningioma was significantly greater in black populations (0.74 cases per 10000 [95% CI: 0.83, 0.65]) than in white populations (0.45 cases per 10000 [95% CI: 0.47, 0.43]) (Supplementary Fig. 1G). Similar to nonmalignant meningioma, IR in black populations trended higher than in all other races, with a significantly higher IR compared with whites in 70–79 year olds and 85+ year olds (Fig. 2E and Supplementary Table 2). Hispanic populations also trended toward higher IR for malignant meningioma, though no significant differences were identified for any age group (Fig. 2G and Supplementary Table 2). From 2005 to 2015, there was a significant decrease in malignant meningioma incidence in black and white populations (black: APC: −4.62% [95% CI: −8.20, −0.90], P = 0.021, white: APC: −4.47% [95% CI: −6.94, −1.94], P = 0.0033), but not in API populations (APC: −3.12% [95% CI: −9.64, 3.87], P = 0.33) (Fig. 2F). There was also a significant decrease in malignant meningioma incidence in non-Hispanic populations (APC: −4.34% [95% CI: −7.15, −1.44], P = 0.0083) but not in Hispanic populations (APC: −4.83% [95% CI: −11.4, 2.24], P = 0.15) (Fig. 2H). AIAN were excluded from race calculations in malignant meningioma due to insufficient sample size.
Nonmalignant and Malignant Meningioma Incidence by Location, 2005–2015
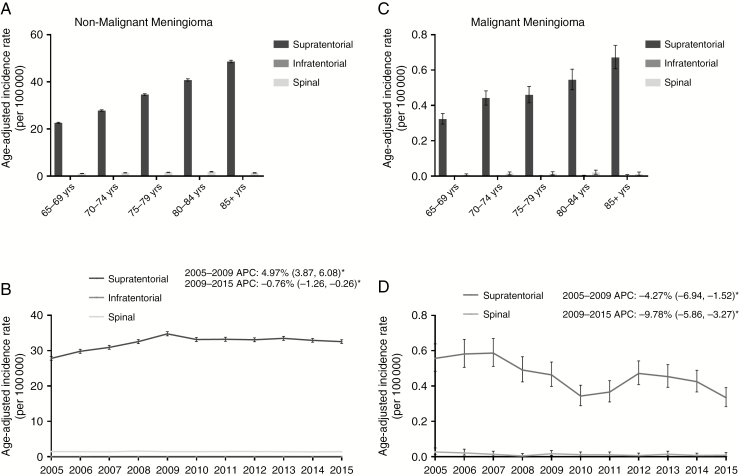
The vast majority (95.61%) of nonmalignant meningiomas were located in the supratentorial brain regions, with meningiomas of the spine accounting for 4.33% and meningiomas of infratentorial regions accounting for 0.07% (Supplementary Fig. 1D). Incidence of spine and supratentorial nonmalignant meningioma increased significantly for every 5-year increment in age, except for IR of spinal meningiomas in 85+ year olds, which decreased significantly compared with 75–84 year olds (85+: 1.42 [95% CI: 1.32, 1.52] vs 75–79: 1.66 [95% CI: 1.57, 1.75] and 80–84: 1.86 [95% CI: 1.75, 1.96]) (Fig. 3A and Supplementary Table 1). From 2005 to 2009, the incidence of supratentorial nonmalignant meningioma increased significantly (APC: 4.97% [95% CI: 3.87, 6.08], P < 0.0001) then declined significantly in 2009–2015 (APC: −0.76% [95% CI: −1.26, −0.26], P = 0.0099). In contrast, the incidence of nonmalignant spinal meningiomas remained stable over the 11-year study period (APC: −0.21 [95% CI: −0.91, 0.49], P = 0.51) (Fig. 3B, C).
Fig. 3.
(A–B) Age-adjusted incidence rates and annual percent changes (APCs) for nonmalignant meningioma by (A) age and location and (B) location over time in 2005–2015, including supratentorial, infratentorial, and spinal nonmalignant meningioma. (C, D) Age-adjusted incidence rates and APCs for malignant meningioma by (C) age and location and (D) location over time in 2005–2015, including supratentorial, infratentorial, and spinal malignant meningioma. APCs are accompanied by 95% CIs in parentheses. *Only significant changes in APC are reported in the figures. (CBTRUS 2005–2015)
The majority of malignant meningiomas also occurred in supratentorial brain regions (97.16%) (Supplementary Fig. 1H). Supratentorial IR increased significantly as age increased, while spinal IR trended upward with advancing age as well (Fig. 3C and Supplementary Table 2). Like nonmalignant meningiomas, IR of spinal malignant meningiomas decreased, though not significantly, in 85+ populations compared with 75–84 year olds (0.011 [95% CI: 0.005, 0.024] vs 0.02 [95% CI: 0.011, 0.035]) (Fig. 3C and Supplementary Table 2). For both spinal and supratentorial malignant meningiomas, incidence rates decreased significantly over time from 2005 to 2015 (spinal: APC: −9.78% [95% CI: −15.86, −3.27], P = 0.0087, supratentorial: APC: −4.27% [95% CI: −6.94, −1.52], P = 0.0068) (Fig. 3D). Infratentorial malignant meningioma was excluded from location analyses due to insufficient sample size.
Nonmalignant and Malignant Meningioma Survival by Age, Sex, Race, Ethnicity, Location, Treatment, 2005–2015
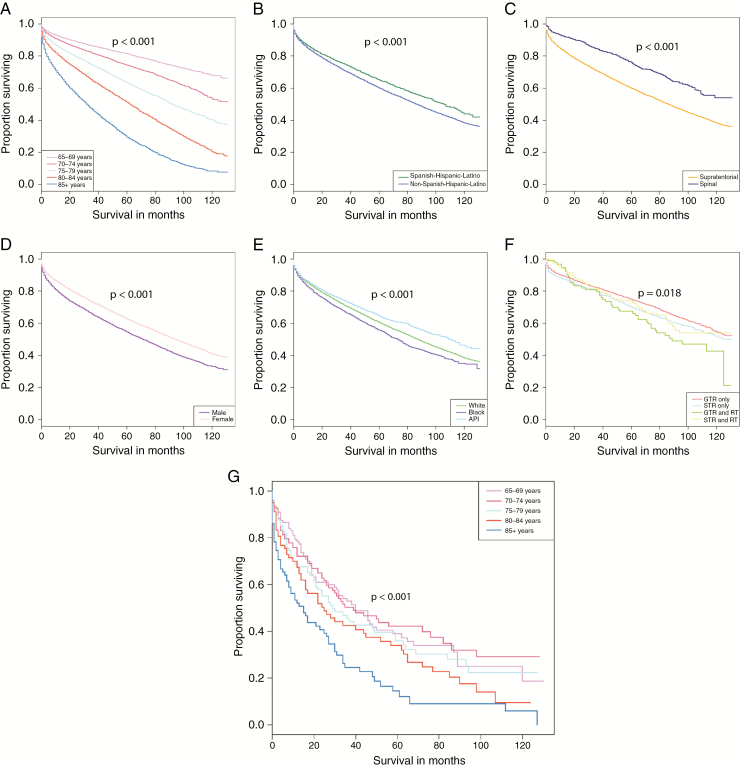
Kaplan–Meier estimates in nonmalignant meningioma showed significant differences in overall survival by age (P < 0.001), sex (P < 0.001), race (P < 0.001), ethnicity (P < 0.001), location (P < 0.001), adjuvant radiation treatment (P < 0.001), surgical resection status (P = 0.045), and combined surgical resection and radiation treatment (P = 0.018) (Fig. 4A–F). In contrast, age was the only variable that significantly affected survival in malignant meningioma (P < 0.001) (Fig. 4G). Multivariable Cox proportional hazards regression models were used to examine the association of clinical and demographic characteristics with overall survival in nonmalignant and malignant meningioma (Table 1). For nonmalignant meningioma, after controlling for all other variables in the model, age, sex, race, location, and combined resection and radiation treatment, all significantly affected survival, except for ethnicity (P = 0.053). For every increase in 5-year age group, the risk of death significantly increased compared with 65–69 year olds. Individuals age 70–74 years had 76% increase in risk of death (hazard ratio [HR]: 1.76 [95% CI: 1.49, 2.09], P < 0.001), and age 75–79 years had 2.71 times the risk of death (HR: 2.71 [95% CI: 2.29, 3.22], P < 0.001) compared with those age 65–69 years; 80–84 year olds had 4.48 times the risk of death (HR: 4.48 [95% CI: 3.76, 5.34], P < 0.001), and those age 85+ years had 5.91 times the risk of death (HR: 5.91 [95% CI: 4.82, 7.24], P < 0.001) compared with 65–69 year olds. Compared with females, males had a 42% increased risk of death (HR: 1.42 [95% CI: 1.26, 1.59], P < 0.001). Compared with white patients, black patients had a 21% increased risk of death (HR: 1.21 [95% CI: 1.00, 1.47], P = 0.049). Supratentorial location was associated with a 35% increase in risk of death compared with spinal location (HR: 1.35 [95% CI: 1.12, 1.63], P = 0.001). When surgery and radiation treatment were combined, GTR+RT and STR+RT were associated with a 57% and 43% increase in risk of death, respectively, compared with GTR alone (HR: 1.57 [95% CI: 1.16, 2.13], P = 0.004 and HR: 1.43 [95% CI: 1.06, 1.93], P = 0.019, respectively). In contrast to the multivariable Cox proportional hazards for nonmalignant meningioma, the only significant difference in survival in malignant meningioma was seen for patients age 85+ years whose risk of death was 2.06 times higher than those age 65–69 years (HR: 2.06 [95% CI: 1.45, 2.94], P < 0.001) (Fig. 4G) (Table 1). Multivariable Cox proportional hazards models by age group are provided in Supplementary Table 3. There were no clear statistically significant trends by age group for any analyzed variables.
Fig. 4.
Kaplan–Meier curves depicting variables with significant differences in survival for nonmalignant (A–F) and malignant meningioma (G). Significant associations with survival for nonmalignant meningioma included (A) 5-year age groups (P < 0.001), (B) ethnicity (P < 0.001), (C) location (P < 0.001), (D) sex (P < 0.001), (E) race (P < 0.001), and (F) surgical resection/radiation treatment (P = 0.018). The only significant association with survival for malignant meningioma was (G) 5-year age groups (P < 0.001).
Table 1.
Kaplan–Meier results and multivariable Cox proportional hazards models by race, ethnicity, sex, age, location, radiation, resection, and combined radiation and resection for nonmalignant and malignant meningioma
| Nonmalignant Meningioma | ||||
|---|---|---|---|---|
| Kaplan–Meier Results | N | Median | 95% CI | P-value |
| Race | ||||
| White | 34 341 | 88 | (85–89) | <0.001 |
| Black | 4119 | 73 | (68–77) | |
| API | 3141 | 110 | (103–121) | |
| AIAN | 213 | 104 | (84–--) | |
| Ethnicity | ||||
| Spanish-Hispanic-Latino | 3482 | 106 | (98–115) | <0.001 |
| Non-Spanish-Hispanic Latino | 38 625 | 87 | (85–88) | |
| Sex | ||||
| Male | 11 222 | 71 | (69–75) | <0.001 |
| Female | 30 885 | 94 | (92–96) | |
| Age | ||||
| 65–69 y | 9276 | -- | (--–--) | <0.001 |
| 70–74 y | 8655 | -- | (124–--) | |
| 75–79 y | 8291 | 92 | (89–96) | |
| 80–84 y | 7377 | 63 | (60–65) | |
| 85+ y | 8508 | 32 | (31–33) | |
| Location | ||||
| Supratentorial | 40 305 | 86 | (84–88) | <0.001 |
| Spinal | 1676 | -- | (119–--) | |
| Radiation | ||||
| No radiation | 41 402 | 88 | (86–89) | <0.001 |
| Radiation | 705 | 125 | (90–--) | |
| Resection | ||||
| GTR | 2612 | -- | (125–--) | 0.045 |
| STR | 896 | -- | (113–--) | |
| Surgery+Radiation | ||||
| GTR only | 2522 | -- | (126–--) | 0.018 |
| STR only | 778 | -- | (111–--) | |
| GTR + RT | 90 | 89 | (72–--) | |
| STR + RT | 118 | -- | (89–--) | |
| Multivariable Cox Proportional Hazards Model | Hazard Ratio | 95% CI | P-value | |
| black vs white* | 1.212 | (1.001–1.468) | 0.049 | |
| API vs white | 0.829 | (0.679–1.012) | 0.066 | |
| AIAN vs white | 0.794 | (0.255–2.469) | 0.690 | |
| Hispanic vs non-Hispanic | 0.821 | (0.672–1.002) | 0.053 | |
| Male vs female* | 1.415 | (1.263–1.585) | <0.001 | |
| 70–74 y vs 65–69 y* | 1.763 | (1.487–2.090) | <0.001 | |
| 75–79 y vs 65–69 y* | 2.712 | (2.287–3.216) | <0.001 | |
| 80–84 y vs 65–69 y* | 4.480 | (3.758–5.340) | <0.001 | |
| 85+ y vs 65–69 y* | 5.911 | (4.824–7.241) | <0.001 | |
| Supratentorial vs spinal* | 1.352 | (1.123–1.628) | 0.001 | |
| STR only vs GTR only | 1.123 | (0.986–1.280) | 0.082 | |
| GTR and RT vs GTR only* | 1.569 | (1.156–2.130) | 0.004 | |
| STR and RT vs GTR only* | 1.434 | (1.062–1.934) | 0.019 | |
| Malignant Meningioma | ||||
| Kaplan–Meier Results | N | Median | 95% CI | P-value |
| Age | ||||
| 65–69 y | 119 | 40 | (31–62) | <0.001 |
| 70–74 y | 119 | 39 | (27–82) | |
| 75–79 y | 88 | 29 | (21–59) | |
| 80–84 y | 77 | 25 | (16–52) | |
| 85+ y | 78 | 15 | (8–27) | |
| Multivariable Cox Proportional Hazard Model | Hazard Ratio | 95% CI | P-value | |
| Black vs white | 1.049 | (0.751–1.465) | 0.781 | |
| API vs white | 0.930 | (0.632–1.370) | 0.714 | |
| Male vs female | 1.227 | (0.972–1.549) | 0.085 | |
| 70–74 y vs 65–69 y | 0.906 | (0.639–1.287) | 0.583 | |
| 75–79 y vs 65–69 y | 1.117 | (0.777–1.605) | 0.550 | |
| 80–84 y vs 65–69 y | 1.326 | (0.924–1.903) | 0.126 | |
| 85+ y vs 65–69 y* | 2.061 | (1.446–2.936) | <0.001 | |
| Supratentorial vs spinal | 1.181 | (0.579–2.407) | 0.647 | |
| Radiation vs no radiation | 1.019 | (0.778–1.335) | 0.890 |
*Denotes statistical significance at <0.05 level.
-- Denotes that the median was not achieved, indicating survival was greater than 50% at the end of the observation period
Discussion
Incidence
According to several studies, the incidence of CNS tumors as a whole has decreased over the last 20 years.2 However, when histology-specific analyses are performed, it is clear that incidence trends are far more heterogeneous.2,20 Furthermore, examining meningioma incidence and survival in the elderly specifically is necessary given that the median age at diagnosis is 66 years, 7 years older than the median age at diagnosis of primary CNS tumors in general.1 Recent addition of benign and uncertain tumors (ICD-O-3 behavior codes /0 and /1, respectively) to CBTRUS in 2004 has restricted longitudinal study of nationwide incidence and survival trends in nonmalignant meningioma.4 Thus, the purpose of this study was to provide an in-depth update regarding both nonmalignant and malignant meningioma incidence and survival in the elderly using the 11-year time period following the implementation of nonmalignant brain tumor collection in cancer registration databases in 2004.
Here, we report that the incidence of nonmalignant meningioma increased significantly for every 5-year age group from ages 65–69 years (23.85 cases per 100000 in adults) to 85+ year olds (50.34 cases per 100000) (Supplementary Fig. 1A). Similarly, for malignant meningioma, incidence increased significantly for every decade compared with the 65–69 year olds (Supplementary Fig. 1E). This is in contrast to other CNS tumors, such as oligodendrogliomas and anaplastic oligodendrogliomas, whose incidence tends to decrease in the oldest age groups.21 Interestingly, however, increases in nonmalignant meningioma with age parallel increased incidence in glioblastoma despite drastic differences in tumor cell origins, behavior, and molecular profiles.21 Recent research in glioblastoma and meningioma has highlighted the importance of epigenetic alterations such as DNA methylation patterns in tumorigenesis, which could explain the increased incidence in elderly populations for these 2 different tumor types.22–28 It will be necessary to conduct further research to explore this hypothesis and other alternative hypotheses relating to non-epigenetic molecular differences, environmental factors, and other potential contributors.
In addition to increased incidence with age, we also identified a significant female predominance in nonmalignant meningioma for every 5-year age group studied and a non-significant predominance for malignant meningioma (Fig. 1A and C). This finding in our elderly population is in contrast to reports indicating that the incidence of malignant meningioma was higher in males than females.3,4 These discrepancies are likely due to shorter time periods studied in previous reports along with differences in methodology for reporting age groupings. We also identified significant differences in meningioma incidence by race and ethnicity (Fig. 2A–G). For instance, for every age group, incidence was significantly higher in black populations, and significantly lower in AIAN populations, compared with other races, unlike previously reported incidence rates for oligodendrogliomas and medulloblastomas.21,29 Similarly, for Hispanics, nonmalignant meningioma incidence was significantly higher in all age groups compared with non-Hispanics. Finally, both nonmalignant and malignant meningiomas presented overwhelmingly in supratentorial locations, with a small minority presenting in spinal locations (Fig. 3A, D).
In a departure from previous reports citing an increased incidence of nonmalignant meningioma over the years,2 we find that extension of the study period to 2015 resulted in a more nuanced understanding of temporal trends. In particular, in all categories, including sex, age, race, ethnicity, and location, we found that APC increased to 2008 or 2009 and subsequently leveled off or significantly decreased up to 2015. This was true for: (i) males and females, (ii) every 5-year age group, (iii) black, white, and non-Hispanic populations, and (iv) supratentorial nonmalignant meningioma. In contrast, the incidence of spinal nonmalignant meningioma remained constant over the 11-year study period. It was previously hypothesized that the increase in nonmalignant meningioma was due to the adoption of the 2000 WHO guidelines, and later the 2007 WHO guidelines update, which downgraded cases of meningioma with brain invasion but without anaplasia from grade III (malignant) to grade II or I (nonmalignant).3,30,31 This hypothesis is supported by our findings that incidence generally increased only up to 2009, after which it plateaued or declined. This aligns well with the period after the adoption of the 2007 WHO guidelines, during which nonmalignant meningioma incidence may have increased simply due to classification changes. This hypothesis is further supported by the observation that supratentorial nonmalignant meningioma incidence increased from 2005 to 2009, a location which would be susceptible to reclassification due to brain invasion, compared with the stable incidence of spinal nonmalignant meningioma in which brain invasion is not a concern. Future studies should examine temporal trends extending beyond 2015 to determine whether incidence rates remain stable over the next several years. Additionally important to examine are other factors contributing to the high overall incidence of meningioma in the general population, including environmental factors such as ionizing radiation exposure, a known risk factor for meningioma occurrence, group differences in genetic and epigenetic tumorigenic processes, and/or greater detection biases due to increasing access to imaging technologies.3,4,32
In keeping with previous reports, the incidence of malignant meningioma decreased over time from 2005 to 2015 in both males and females, black, white, and non-Hispanic populations, as well as in spinal and supratentorial locations (Fig. 1D, 2F–H, 3D). The declines in malignant meningioma could be due to increasingly accurate meningioma reporting by CNS tumor registries, thereby shifting meningioma incidence from malignant to nonmalignant, as previously discussed.3,4,33 It is important to note, however, that temporal trends in malignant meningioma diverge from nonmalignant incidence given the continued decline up to 2015. Thus, particular attention should be paid to determining the root causes of these disparities in future research.
Survival
Our study also identified several clinical and demographic factors that are associated with decreased survival in nonmalignant and malignant meningioma in the elderly US population. For nonmalignant meningioma, the study data showed that black populations, males, and older age groups have a greater risk of death compared with white populations, females, and those age 65–69 years, respectively. It had been previously noted that black individuals have worse survival than whites in the general population,34 congruent with our findings in the elderly. As in this study, worse survival in both black populations and older ages was partially attributed to disparities in access to high-quality neuro-oncologic care.34,35 However, it is also possible that there are molecular and/or epigenetic differences among races and in aging individuals that contribute to tumor behavior. Interestingly, though the incidence of nonmalignant meningioma is greater in elderly females, elderly males had significantly worse survival outcomes. This previously reported dichotomy for spinal meningiomas36 was confirmed in the present study’s much larger patient cohort, highlighting the need for future research to address the disparity between the sexes.
In keeping with the whole cohort survival analyses, 5-year age group–specific subanalyses revealed similar trends toward worsened survival in males, black populations, supratentorial locations, and STR/GTR with RT, though statistical significance was only occasionally attained (Supplementary Table 3). It is difficult to ascertain whether statistical significance in certain age groups is indicative of age-specific tumor behavior versus sample size reduction–related increases in estimate uncertainty (ie, increased confidence interval span). Thus, these results should be interpreted with caution and further research conducted to examine the impact of age.
Beyond demographic prognostic factors, we also found that the addition of adjuvant radiation following either GTR or STR resulted in increased risk of death compared with GTR alone despite radiation and GTR improving survival when analyzed independently using the Kaplan–Meier method. Studies in atypical meningioma, which are included in our nonmalignant group based on the most recent CBTRUS report classification by ICD-O-3 codes,1 have noted either a trend toward worse outcomes in patients receiving adjuvant radiation37 or no difference in survival with adjuvant radiation.38,39 Thus, our findings in the elderly seem to corroborate previous reports in the general population, though these results should be interpreted with caution given the previous studies’ focus on atypical meningioma alone. Furthermore, conclusions should be reserved until publication of results from the Radiation versus Observation following surgical resection of Atypical Meningioma /European Organisation for Research and Treatment of Cancer 1308: a multicenter randomized controlled trial examining outcomes in patients with atypical meningioma treated with GTR and adjuvant radiation versus GTR and active MRI monitoring for recurrence.40
In contrast to nonmalignant meningioma, there were no significant differences in malignant meningioma survival by race, sex, location, or radiation therapy. The only 2 groups with significantly worse survival were the 85+ category, with increasing age being a well-established risk factor for poorer outcomes,1 likely due to increased risk of complications,12,13 and males aged 65–69 years old (Supplementary Table 3). Encouragingly for 65–84 year olds, after controlling for other variables in the model, age was not an independent predictor of increased mortality following malignant meningioma diagnosis. This finding may help mitigate fears of surgical treatment for malignant meningioma in elderly individuals based on age alone. Additionally, radiation had no significant protective effect in spite of the supporting evidence for its use in anaplastic meningioma.41,42 However, the supporting literature came from noncontrolled and/or reports analyzing small cohorts and thus far has limited definite conclusions. In response to the paucity of Level 1 evidence, a large-scale study is currently under way in Europe to assess the effect of post-resection adjuvant radiation on survival following anaplastic meningioma diagnosis.43 In the meantime, our results should be interpreted with caution given the insufficient sample size to perform analyses of combined resection and radiation.
Limitations
There are important limitations of epidemiological studies such as this that are noteworthy. First, we chose to classify nonmalignant and malignant meningioma according to the ICD-O-3 codes used in the CBTRUS Statistical Report to ensure standardization of comparisons.1 However, in the literature, meningioma is often grouped according to other factors, such as WHO grade, which does not exactly align with the statistical report’s ICD-O-3–based categorization. Second, as is the case with the majority of large-scale epidemiological studies, we were unable to examine incidence and survival trends by molecular signatures of nonmalignant and malignant meningioma. In the future, when molecular data collection becomes more widespread and complete, the epidemiology of tumor behavior will be better addressed. For now, we were able to assess the demographic and clinical factors contributing to incidence from CBTRUS for approximately 99% of the US population, thereby affording us a thorough, in-depth analysis of nationwide trends over the 1-year study period. It is also important to specify that survival data obtained from SEER includes only 28% of the US population, thereby limiting generalization of survival results to the rest of the US population. Finally, we were unable to include important prognostic factors beyond the demographic and treatment data available in the CBTRUS and SEER registries such as functional status, comorbid diseases, and performance scales. Future studies should examine the intersection of demographic and function/performance information.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under contract no. 2016-M-9030, the American Brain Tumor Association, The Sontag Foundation, Novocure, AbbVie, the Musella Foundation, the National Cancer Institute (NCI) under contract no. HHSN261201800176P, as well as from private and in kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Supplementary Material
Conflict of interest statement. There are no conflicts of interest to report.
Authorship statement. Rebecca L. Achey—study design, data collection, data analysis/interpretation, figure preparation, manuscript drafting, reviewing/editing manuscript
Haley Gittleman—study design, data collection, data analysis/interpretation, manuscript drafting, reviewing/editing manuscript
Julia Schroer—data analysis/interpretation, manuscript drafting, reviewing/editing manuscript
Vishesh Khanna—study design, data analysis/interpretation, manuscript drafting, reviewing/editing manuscript
Carol Kruchko—data integrity, manuscript drafting, reviewing/editing manuscript
Jill S Barnholtz-Sloan—study design, data collection, data analysis/interpretation, manuscript drafting, reviewing/editing manuscript
References
- 1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gittleman HR, Ostrom QT, Rouse CD, et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015;121(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol. 2015;17(8):1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikawa F, Kinoshita Y, Takeda M, et al. Review of current evidence regarding surgery in elderly patients with meningioma. Neurol Med Chir (Tokyo). 2017;57(10):521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ambekar S, Sharma M, Madhugiri VS, Nanda A. Trends in intracranial meningioma surgery and outcome: a nationwide inpatient sample database analysis from 2001 to 2010. J Neurooncol. 2013;114(3):299–307. [DOI] [PubMed] [Google Scholar]
- 7. Dobran M, Marini A, Nasi D, et al. Surgical treatment and outcome in patients over 80 years old with intracranial meningioma. Clin Neurol Neurosurg. 2018;167:173–176. [DOI] [PubMed] [Google Scholar]
- 8. McCarthy BJ, Davis FG, Freels S, et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88(5):831–839. [DOI] [PubMed] [Google Scholar]
- 9. Slot KM, Peters JVM, Vandertop WP, Verbaan D, Peerdeman SM. Meningioma surgery in younger and older adults: patient profile and surgical outcomes. Eur Geriatr Med. 2018;9(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barthélemy E, Loewenstern J, Konuthula N, et al. Primary management of atypical meningioma: treatment patterns and survival outcomes by patient age. J Cancer Res Clin Oncol. 2018;144(5):969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konglund A, Rogne SG, Lund-Johansen M, Scheie D, Helseth E, Meling TR. Outcome following surgery for intracranial meningiomas in the aging. Acta Neurol Scand. 2013;127(3):161–169. [DOI] [PubMed] [Google Scholar]
- 12. Steinberger J, Bronheim RS, Vempati P, et al. Morbidity and mortality of meningioma resection increases in octogenarians. World Neurosurg. 2018;109:e16–e23. [DOI] [PubMed] [Google Scholar]
- 13. Connolly ID, Cole T, Veeravagu A, Popat R, Ratliff J, Li G. Craniotomy for resection of meningioma: an age-stratified analysis of the marketscan longitudinal database. World Neurosurg. 2015;84(6):1864–1870. [DOI] [PubMed] [Google Scholar]
- 14. Galhom AE, Madawi AA, Ellabban MM. Surgical outcomes and predictors of complication in elderly patients with meningiomas. Egypt J Neurol Psychiatry Neurosurg. 2018;54(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen-Inbar O, Soustiel JF, Zaaroor M. Meningiomas in the elderly, the surgical benefit and a new scoring system. Acta Neurochir (Wien). 2010;152(1):87–97; discussion 97. [DOI] [PubMed] [Google Scholar]
- 16. Boviatsis EJ, Bouras TI, Kouyialis AT, Themistocleous MS, Sakas DE. Impact of age on complications and outcome in meningioma surgery. Surg Neurol. 2007;68(4):407–11; discussion 411. [DOI] [PubMed] [Google Scholar]
- 17. Poon MT-C, Fung LH-K, Pu JK-S, Leung GK-K. Outcome comparison between younger and older patients undergoing intracranial meningioma resections. J Neurooncol. 2013;114(2):219–227. [DOI] [PubMed] [Google Scholar]
- 18. Brokinkel B, Holling M, Spille DC, et al. Surgery for meningioma in the elderly and long-term survival: comparison with an age- and sex-matched general population and with younger patients. J Neurosurg. 2017;126(4):1201–1211. [DOI] [PubMed] [Google Scholar]
- 19. Number of Persons by Race and Hispanic Ethnicity for SEER Participants - SEER Registries http://seer.cancer.gov/registries/data.html.
- 20. Ostrom QT, Gittleman H, de Blank PM, et al. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2016;18 Suppl 1(suppl 1):i1–i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Achey RL, Khanna V, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Incidence and survival trends in oligodendrogliomas and anaplastic oligodendrogliomas in the United States from 2000 to 2013: a CBTRUS Report. J Neurooncol. 2017;133(1):17–25. [DOI] [PubMed] [Google Scholar]
- 22. Shaikh N, Dixit K, Raizer J. Recent advances in managing/understanding meningioma. F1000Research. 2018;7. pii: F1000 Faculty Rev-490. doi:10.12688/f1000research.13674.1 [Google Scholar]
- 23. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 24. Olar A, Wani KM, Wilson CD, et al. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017;133(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Majchrzak-Celińska A, Paluszczak J, Szalata M, Barciszewska A-M, Nowak S, Baer-Dubowska W. DNA methylation analysis of benign and atypical meningiomas: correlation between RUNX3 methylation and WHO grade. J Cancer Res Clin Oncol. 2015;141(9):1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferreira WAS, Pinheiro D do R, Costa Junior CA da, et al. An update on the epigenetics of glioblastomas. Epigenomics. 2016;8(9):1289–1305. [DOI] [PubMed] [Google Scholar]
- 27. Gusyatiner O, Hegi ME. Glioma epigenetics: From subclassification to novel treatment options. Semin Cancer Biol. 2018;51:50–58. [DOI] [PubMed] [Google Scholar]
- 28. Liao P, Ostrom QT, Stetson L, Barnholtz-Sloan JS. Models of epigenetic age capture patterns of DNA methylation in glioma associated with molecular subtype, survival, and recurrence. Neuro Oncol. 2018;20(7):942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khanna V, Achey RL, Ostrom QT, et al. Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neurooncol. 2017;135(3):433–441. [DOI] [PubMed] [Google Scholar]
- 30. Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–25; discussion 226–9. http://www.ncbi.nlm.nih.gov/pubmed/11895036. Accessed September 12, 2016. [DOI] [PubMed] [Google Scholar]
- 31. Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007;23(4):E3. [DOI] [PubMed] [Google Scholar]
- 32. Murnyák B, Bognár L, Klekner Á, Hortobágyi T. Epigenetics of meningiomas. Biomed Res Int. 2015;2015:532451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evans DGR, Maher ER, Baser ME. Age related shift in the mutation spectra of germline and somatic NF2 mutations: hypothetical role of DNA repair mechanisms. J Med Genet. 2005;42(8):630–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cahill KS, Claus EB. Treatment and survival of patients with nonmalignant intracranial meningioma: results from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. J Neurosurg. 2011;115(2):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mukherjee D, Zaidi HA, Kosztowski T, et al. Disparities in Access to Neuro-oncologic Care in the United States. Arch Surg. 2010;145(3):247. [DOI] [PubMed] [Google Scholar]
- 36. Westwick HJ, Shamji MF. Effects of sex on the incidence and prognosis of spinal meningiomas: a Surveillance, Epidemiology, and End Results study. J Neurosurg Spine. 2015;23(3):368–373. [DOI] [PubMed] [Google Scholar]
- 37. Hammouche S, Clark S, Wong AH, Eldridge P, Farah JO. Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Wien). 2014;156(8):1475–1481. [DOI] [PubMed] [Google Scholar]
- 38. Yang S-Y, Park C-K, Park S-H, Kim DG, Chung YS, Jung H-W. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79(5):574–580. [DOI] [PubMed] [Google Scholar]
- 39. Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46(1):57–61. http://www.ncbi.nlm.nih.gov/pubmed/10656373. Accessed June 15, 2018. [DOI] [PubMed] [Google Scholar]
- 40. Jenkinson MD, Javadpour M, Haylock BJ, et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: study protocol for a randomised controlled trial. Trials. 2015;16(1):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Modha A, Gutin PH. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57(3):538–50; discussion 538–50. http://www.ncbi.nlm.nih.gov/pubmed/16145534. Accessed June 15, 2018. [DOI] [PubMed] [Google Scholar]
- 42. Dziuk TW, Woo S, Butler EB, et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998;37(2):177–188. http://www.ncbi.nlm.nih.gov/pubmed/9524097. Accessed June 15, 2018. [DOI] [PubMed] [Google Scholar]
- 43. Combs SE, Edler L, Burkholder I, et al. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer. 2010;10:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.