ABSTRACT
It is now well established that antibodies have numerous potential benefits when developed as therapeutics. Here, we evaluate the technical challenges of raising antibodies to membrane-spanning proteins together with enabling technologies that may facilitate the discovery of antibody therapeutics to ion channels. Additionally, we discuss the potential targeting opportunities in the anti-ion channel antibody landscape, along with a number of case studies where functional antibodies that target ion channels have been reported. Antibodies currently in development and progressing towards the clinic are highlighted.
KEYWORDS: Ion channel, antibodies, biologics, therapeutic
Introduction
The human genome encodes at least 400 ion channel family members (~1.5%1), representing the second largest class of membrane proteins for drug discovery after G protein-coupled receptors (GPCRs) (Figure1(a)).2-5 Roughly 18% of small molecule drugs listed in the ChEMBL database are targeted towards ion channels,5 with global sales estimated to be $12 billion.6 Although it is well validated that ion channels are at the core of many diseases, approved drugs are available for only a small percentage of this protein class (approx. 8%) despite focused drug discovery efforts over the past 30 years.7 Ion channels function by transporting ions across cell membranes and play important roles in a broad range of physiological and pathophysiological processes. Mutations of single ion channel proteins have been demonstrated to be the cause of genetic diseases, collectively known as channelopathies.8 For example, mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) lead to cystic fibrosis, whereas various pain syndromes, including congenital indifference to pain and paroxysmal extreme pain disorder, are associated with either loss or gain of function mutations, respectively, in the SCN9A gene encoding the voltage-gated sodium channel Nav1.7. Along with direct effects on the functionality of ion channel subunits or the proteins that regulate them, channelopathies can also result from autoimmune responses to channel proteins.9
Figure 1.
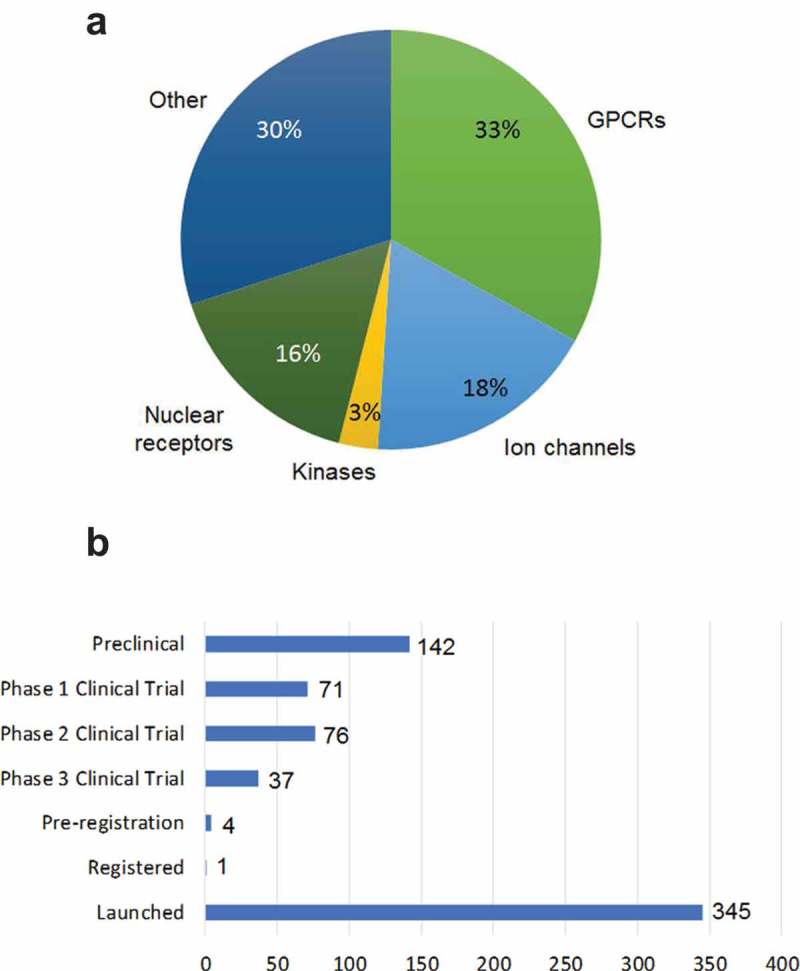
Market opportunities and global clinical pipeline for ion channel drug targets. (a) Market opportunities for targeting ion channels which represent the second largest membrane protein target class after GPCRs, adapted from Santos et al 2017.5 (b) Ion channel drugs in development and the clinical pipeline (sourced from Pharmaprojects as of March/April 2016).
To date, most ion channel drug development has focused on identifying and developing small molecule and peptide modulators, mainly through serendipitous discovery due to a lack of information on structure and function. Many ion channel modulators have been discovered from studies of naturally occurring substances, such as toxins from plants and venomous animals.10 The conotoxin family is the most well-known of the animal-derived toxins,11 with ziconotide, a selective Cav2.2 antagonist, a frequently cited example of a synthetic peptide analogue of cone snail ω-conotoxin used for the treatment of severe chronic pain.12 Despite the initial successes in identifying ion channel modulators, only two novel ion channel drugs have been approved by the US Food and Drug Administration (FDA) since the 1990s, despite vastly improved screening tools for small molecule/compound libraries.13 The most recently approved drugs are ivacaftor (Kalydeco), which potentiates the cystic fibrosis CFTR chloride channel14 and crofelemer (Mytesi), a proanthocyanidin oligomer, which inhibits both CFTR and the calcium-activated chloride channel TMEM16A.15 As with the vast majority of other drugs targeting ion channels, ivacaftor and crofelemer are both small molecule chemical entities.16
Alternative modalities for targeting ion channels have recently included monoclonal antibodies (mAbs), but their therapeutic potential has been vastly underexploited.17 An in-house analysis using information gleaned from the public domain revealed that only one antibody drug (a polyclonal or pAb) is in early clinical study among the > 650 ion channel targeting drugs under active development in the global pipeline (Figure 1(b)).
Advantages of targeting ion channels with antibodies
Although therapeutic antibodies are typically more expensive to develop, they generally attain higher approval success rates compared with their small molecule counterparts.18 As with antibodies targeting GPCRs,19,20 antibodies directed towards ion channels have the potential to offer many additional advantages relative to selectivity, bioavailability and effector function as summarized below.
Selectivity
Obtaining target selectivity in small molecule drug discovery is one of the foremost technical hurdles for drug development, regardless of the route from which the molecule is derived, i.e., rational design or random screening of large compound libraries. With respect to ion channels, this has been particularly challenging as ion channels within a given family often share high levels of homology, notably within the pore-forming domains where many channel blockers exert their effect, but have vastly different physiological roles. For example, the sodium channel isoforms Nav1.7, Nav1.8 and Nav1.9 have been identified as targets in nociceptor neurons where modulation ameliorates different pain states. However, stringent counter-screens are required to characterize potential modulators of these channels for effects on other Nav family members, such as Nav1.5, which initiates the cardiac action potential. Superior specificity and selectivity compared to small molecules are particularly relevant when the desire is to target specific ion channel isoforms, for example, the non-functional variant of P2X7 (nfP2X7),21 the neo-natal splice variant of Nav1.5 (nNav1.5),22 or isoforms of Kv11.1B that are up-regulated in certain tumors.23,24 An obvious alternative to small molecule promiscuity is the development of mAbs, where high levels of specificity would be expected to mitigate off-target effects, and therefore generate safer classes of drugs.
Biodistribution, half-life and effector functions
MAbs offer a number of potential benefits beyond selectivity, including 1) limiting central nervous system (CNS) penetration (when targeting a therapeutic to the periphery); 2) low variability in patient pharmacokinetics; and 3) longer duration of action leading to reduced dosing. The half-lives of native antibodies can be further extended through alterations to the variable domain that enhance FcRn-mediated recycling25 and for antibody fragments via modification with polyethelene glycol (PEG) (i.e., pegylation)26 or binding to human serum albumin.27 Other types of protein engineering apply approaches directed to the Fc domain that can be used to ablate or increase antibody-mediated effector functions, such as antibody-dependent cell-mediated cytotoxicity (ADCC), complement mediated cytotoxicity, or antibody-dependent cell-mediated phagocytosis,28 which are relevant in the case of autoimmune diseases and cancer. MAbs can also be conjugated to radioisotopes or toxic compounds, or linked to the T-cell receptor (so-called CAR-T technology) to directly kill tumors or elicit T-cell mediated tumor cell destruction, respectively. Given their exquisite specificity, it may also be possible to generate mAbs that recognize different conformational states of an ion channel, such as a depolarization-induced conformational change that may render an epitope more accessible to antibody binding.29 In addition, affinity and potency against a target can be further enhanced by well-established protein engineering methodologies for lead mAb optimization.
The challenges for ion channel antibody drug discovery
Despite considerable interest, only one polyclonal antibody, BIL010t (Biosceptre, Cambridge, UK), which recognizes a non-functional form of P2X7 and is formulated as a topical ointment for the treatment of basal cell carcinoma, has completed Phase 1 clinical trials.30 The lack of success in generating such antibodies, particularly mAbs, is attributable to a number of important challenges. For example, for many of the voltage-gated ion channels (VGICs), extracellular loops (where mAbs are most likely to elicit a modulating effect) are short and contain few potential epitopes (Figure 2). Additionally, these loops tend to be highly conserved at the primary amino acid sequence level, and thus lack sufficient immunogenicity to generate robust antibody responses in mammalian hosts. Even in cases where the extracellular domains are large, the proteins themselves are either poorly expressed or difficult to purify from conventional platforms used for recombinant protein production. This, in turn, can limit the starting material available for large-scale immunization and screening campaigns.
Figure 2.
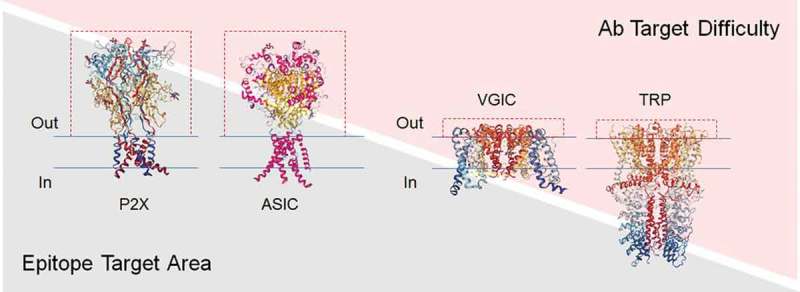
Ion channel extracellular domains can influence the difficulty in generating functional antibodies. A comparison of the structural topology of P2X, acid sensing (ASIC), voltage-gated (VGIC) and transient-receptor potential (TRP) ion channel families is shown with the relative mass of the extracellular domains (ECDs) highlighted by dashed red lines. Structural information was adapted from the Protein Data Bank (PDB) figures for P2X4 (3I5D)31, VGCC (4MTO)32, TRPA1 (3J9P)33 and ASIC1 (6AVE)34. The plasma membrane is represented by blue horizontal lines. Channels with large ECDs (e.g. P2X and ASIC) are expected to display a proportionally larger epitope target area than channels with much smaller ECDs (e.g. VGIC and TRP) and would therefore present less challenging targets in antibody discovery campaigns. Conversely, VGICs and TRP channels that display much smaller epitope target areas represent more challenging targets.
Despite these challenges, autoantibodies that bind ion channels (and presumably alter their activity) have been identified in patients with a number of diseases, including myasthenia gravis (nAChR),35 multiple sclerosis (Kir4.1),36,37 Lambert-Eaton Myasthenic Syndrome or LEMS (VGCC),38 neuromyotonia (Kv1 family) (voltage-gated potassium channels),39 melanoma retinopathy (TRPM1),40 autoimmune encephalitis (NMDA),41 progressive encephalo-myelitis with rigidity and myoclonus, also known as PERM (glycine receptor)42 and Morvan’s syndrome (the Kv1 family of voltage-gated potassium channels).43 Some autoantibodies44 and at least one mAb45 induce ion channel internalization, suggesting that antibody drug conjugates could also be a feasible therapeutic modality for targeting this drug class. Moreover, therapeutic antibodies generated in response to DNA immunization using an expression vector encoding the Kv1.3 potassium efflux channel have been shown to be effective in ameliorating autoimmune encephalomyelitis in rats, underscoring the validity of antibodies as ion channel drug candidates.46
Ion channel structural topology
The classification of ion channels can be based upon their ion selectivity, gating mechanism and/or sequence similarity. The ion channel gating mechanism system identifies three main groups, namely the voltage-gated channels, the extracellular ligand-gated channels and channel proteins utilizing other gating mechanisms, such as mechano-sensitive channels. The structural architecture associated with each family of ion channel has been described extensively elsewhere,47-52 and is not reviewed here.
Among the key factors governing successful discovery of antibodies that can modulate ion channel activity are the size, complexity, immunogenicity and mechanistic properties of the extracellular domains where antibodies are expected to bind. The topology of select ion channel family members, as shown in Figure 2, demonstrates stark differences in the size of the extracellular domains and loops relative to the whole ion channel, such as those observed between acid sensing and P2X channels (large extracellular domains) and voltage-gated and TRP channels (small extracellular loops). Owing to the paucity of potential immunogenic extracellular epitopes of the latter group, perhaps it is not surprising that the single antibody drug in clinical development targets an ion channel belonging to the former group (nfP2X7), and is actually polyclonal.
The challenges noted above have led to targeting specific ion channel extracellular domains with varying levels of success. For example, the E3 re-entrant loop of ion channels comprising six transmembrane (TM) motifs has held a particular interest since this region is thought to maintain positioning of the ion selectivity filter and, at least in some cases, appears to interact with toxins and physiological modulators.53 The length and accessibility of the E3 region between the fifth (S5) and sixth (S6) transmembrane domains (TMDs) presents a suitable targeting region for antibodies, and it is rarely post-translationally modified.50 The amino acid sequence of channel subtypes can be varied in this region, which also offers the opportunity for isoform-specific interactions to disrupt channel function. Many antibodies reported to have been generated to this region tend to be polyclonal, namely Kv1.2, Kv3.1, Kv10.1, TRPC1, TRPC5, TRPM3, TRPV1, Nav1.5, Cav2.1/Cav2.2,50 and exhibit functional activity, such as modulation of store-operated or agonist-evoked Ca2+ entry,54-59 promotion of oligodendrocyte proliferation and migration60 and inhibition of tumor growth.61,62 Additionally, NESOpAb is a polyclonal antibody that specifically recognizes a neonatal epitope presented on the second extracellular loop in Nav1.5 domain I and inhibits sodium currents up to 60% with an IC50 value of less than 25 nmol/L. Furthermore, it demonstrates selectivity, being able to distinguish between the neonatal and adult splice variant forms, which differ by seven amino acids.63
Sources of ion channels for generating and screening ion channel antibodies
Ion channels are typically low abundance proteins in the cells and tissues in which they are produced. Furthermore, when expressed as recombinant proteins in heterologous systems (e.g., mammalian, insect, yeast and bacterial cells) yields of purified functional protein are often low. Therefore, production of antibodies that can recognize and/or block channel activity has relied primarily on immunization of animals with either: 1) whole cells; 2) crude membrane fractions; 3) plasmid DNA expression vectors encoding channel protein subunits; or 4) peptide-based antigens that preferably mimic a targeted extracellular loop structure. As an alternative to immunization, antibody discovery can also be achieved by screening pre-existing libraries of antibody single-chain variable fragments (scFvs) from naïve or immunized animals via phage or yeast display.64 While this latter approach can preclude immunization altogether, the need for purified, correctly folded protein is generally required for the panning and screening phases of the process. Some of the sources of protein used as antigen and to screen for antibody identification are described briefy as follows.
Purified ion channels from native sources
Previous studies have shown that ion channels can be isolated from their native source in a way that maintains functionality of the purified protein following reconstitution into proteoliposomes. For example, a number of laboratories in the 1980s and 1990s purified voltage-gated sodium ion channels from rat brains,65 as well as from the electroplax of the electric eel, Electrophorus electricus,66 and were able to reconstitute functional activity from purified components.67-70 Given the high degree of conservation amongst ion channel orthologs, channel proteins from animal sources might therefore serve as antigens and screening reagents to identify antibodies that recognize and modulate their human counterparts. The obvious drawbacks here are the need to obtain sufficient amounts of material from sources whose channel proteins closely match their human orthologs, the typically complicated purifications required to generate that material, and the need to break tolerance in animal hosts being used for immunization.
Recombinant proteins expressed in mammalian cells
Mammalian cell lines (e.g., HEK293, CHO, U2OS) are arguably the gold standard for generating recombinant ion channels closest to their ‘native’ configurations and functionalities. However, as noted above, mammalian cells typically produce low levels of surface localized, functional recombinant ion channels, making them difficult to purify. The use of whole cells or crude cell fractions diminishes the antigenic load of the target protein and introduces additional contaminating proteins that are likely to be more abundant and more immunogenic than the recombinant ion channel of interest, making it difficult to generate an immune response or in vitro display output that is sufficiently enriched to effectively screen.
Chimeric channels expressed in E. coli
Despite the phylogenetic divergence between prokaryotes and eukaryotes, it has been possible to generate chimeric bacterial-human ion channels that facilitate protein expression and purification in bacterial host expression systems. For example, a functional chimera in which the extracellular domain (ECD) of the bacterial protein GLIC was fused to the transmembrane domain of the human α1 glycine receptor (α1GlyR) has been reported,71 as have functional pentameric ligand-gated ion channel chimeras containing large eukaryotic intracellular domains from nAchR-α7, GABAp1 and Glyα1 fused to the Gloeobacter violaceus GLIC channel.72 Similarly, the use of bacterial Nav channels has been elegantly exemplified in structural studies of VGICs73 and enabled crystallization of a chimeric voltage-gated sodium channel from Arcobacter butzleri fused to portions of the human Nav1.7 voltage-sensor 4 domain bound to aryl sulphonamide antagonists.74 Although chimeric channels could offer a possible solution to the generation of sufficient amounts of antigen and screening reagents to implement antibody discovery processes, they would nevertheless require extensive counter-screening. Moreover, the bacterial elements of the channel may present immunodominant epitopes that could overwhelm the response to the human components of the chimera.
Alternative platforms for recombinant protein expression
Cilated protozoa devote a large part of their metabolism towards membrane protein production and have expanded gene families for all four of the major classes of membrane transporters, including P-type ATPases, major facilitator superfamily members, ABC transporters and voltage-gated ion channels.75 Tetrahymena thermophila, in particular, has been identified as an attractive platform for over-expression of recombinant human ion channels based on the fact that its macronuclear genome encodes approximately three times as many voltage-dependent K+ channels as do human cells.75 Although a complex eukaryote, Tetrahymena shares many of the features of microbial expression hosts, including ease of growth in peptone-based media at scale with relatively short doubling times of 2 to 3 hours.76
TetraGenetics Inc., an early-stage biotechnology company in Arlington, MA, has demonstrated expression of approximately 20 recombinant human voltage-gated, ligand-gated and mechano-sensitive ion channels in Tetrahymena (unpublished data). Efficient recovery of purified channel proteins (in many cases, in the order of > 1mg/L culture) has enabled the development of antigen preparations and screening tools that have recently been used to generate a panel of blocking anti-Kv1.3 antibodies.77 Besides the propensity for this organism to encode hundreds of native ion channels, it remains unclear why it bypasses the limiting factors associated with low expression yields in mammalian cells where there appears to be an upper limit on how many functional recombinant channels can reach the plasma membrane before creating a toxic metabolic environment. No such toxicity is observed in Tetrahymena, allowing many more recombinant channels to reach the cell surface. In mammalian cells, plasma membrane channel number is likely to be regulated by a variety of mechanisms, including manipulation of various retention signals by auxiliary subunits. Tetrahymena does not encode any obvious orthologs of mammalian auxiliary subunits and, in the case of Na+-selective voltage gated channels, co-expression of the β subunits is not required for cell surface localization in the Tetrahymena system (unpublished data). While it is clear that Tetrahymena is well suited to the production of recombinant ion channel proteins, a number of other systems are being developed for this purpose, including virus-like particles,78 cell-free lysates,79-82 synthetic and semi-synthetic chemistries.83,84
Maintaining the native protein fold – SMALPs and nanodiscs
Characterization of modulating antibodies following immunization or in vitro display methods requires that a purified ion channel protein be maintained in its proper three-dimensional conformation regardless of its source. One approach towards stabiliizing the structure involves incorporation of detergent-solubilzed and purified membrane proteins into nanodiscs that utilize a supporting protein scaffold and lipids to generate an artificial bilayer into which the membrane protein of interest is embedded.85-87 Potential limitations to this approach are that transfer of proteins into nanodiscs requires initial solubilization by detergent, as well as reconstitution into a non-native lipid environment. In addition, solubilization of membrane proteins in detergents faces the technical challenge of maintaining a physiologically relevant conformation in addition to stability. An alternative strategy that provides a detergent-free route to membrane protein isolation with retention of the native lipid environment (as much as is possible) is to replace the detergent with amphipathic styrene-maleic acid (SMAs), where the polymer self-inserts into biological membranes and is capable of extracting small discs of the native lipid bilayer containing the membrane protein of interest, generating SMA-encapsulated lipid particles (SMALPs).88 More recently, the tetrameric potassium channel KcsA was isolated directly from the membranes of Escherichia coli without the need for detergent by using SMALPs.89 SMALPs have also been reconstituted into planar lipid bilayers directly from native nanodiscs, which enabled functional characterization of the TRPV1 channel by electrophysiology.90 The nanodisc approach was implemented in the reconstitution of tetrameric KirBac1.1 potassium channels into lipid nanodiscs, enabling single-molecule fluorescence resonance energy transfer confocal microscopy, which permitted the elucidation of structural changes that occur upon channel activation and inhibition.91
DNA immunization
Plasmid expression vectors encoding ion channel proteins are likely to produce correctly folded and functional antigens following immunization of mammals. Nevertheless, the yield of protein presented at the cell surface may still be low and the resulting immune response may not be sufficiently robust to generate an antibody titer high enough to identify potential modulators. The inclusion of adjuvants and the use of tailored expression vectors with strong promoters, such as the CMV promoter, are often applied in this instance.92 The use of T cell helper epitopes, such as PADRE, are also proving successful.46 The DNA immunization approach has been used to generate Kv1.3 nanobodies using the Ablynx platform,93 as well as in combination with purified antigen to generate conventional Kv1.3 antibodies.77 These are described in a later section.
Peptides
Peptide antigens have been used to generate functional polyclonal antibodies against multiple ion channels,50 as well as mAbs targeting select ion channels.61 Peptides usually do not suffer from issues of quantity or purity because they can typically be produced via chemical synthesis or robust cell expression systems, such as E. coli, to serve both antigen and screening requirements. However, the physiological relevance of peptide-based antigens, even those that are three-dimensionally accurate representations of surface loop structures, will always be limited as they lack the context of other molecular determinants associated with the ion channel surface ‘epitome’.
Ion channel antibody generation and screening – additional considerations
While the source of ion channel antigen is a critical consideration for any antibody discovery program, the approach to the generation and screening of ion channel antibodies should be scrutinized in the context of the challenges described above. The relative lack of success in the identification and clinical progression of ion channel antibodies suggests that therapeutically valuable antibodies are typically rare and difficult to identify in any given discovery program. Therefore, it would seem prudent to structure a discovery program that can either increase a specific immune response against the target ion channel and/or deeply mine an immune repertoire in an effort to capture as many potential hits as possible. In the case of the former, amino acid conservation and, depending on the ion channel, the topology of the ion channel can affect the generation of a robust immune response. Options that may mitigate this challenge include the use of phylogenetically diverse immune hosts; immunization strategies that break immune tolerance, such as DNA immunization; inclusion of T-helper cell epitopes; transgenic animals overexpressing the neonatal Fc receptor;94-96 and, related to this, transgenic animals where the target gene has been deleted. Of course, the latter approach will be dependent on the viability of the knock-out animal. With regards to mining the immune repertoire, a number of platform technologies, e.g., direct B-cell cloning and/or deep sequencing,97-99 have been developed recently that increase the probability of identifying rare antibodies and avoid standard hybridoma-based technologies where valuable antibodies may be missed owing to inefficient fusion events or the loss of rare B cell clones.100
Following the identification of antibodies that specifically recognize the ion channel target, it is important to select extracellular binders by some means to advance these clones into functional characterization. Typically, the method most commonly used to identify extracellular binders is flow cytometry using native or transfected mammalian cell lines expressing the ion channel of interest. This, however, is not necessarily straight-forward because many cell lines with confirmed electrophysiological activity may nevertheless express low surface channel numbers making definitive identification difficult. Alternatively, ELISA assays using peptide and protein fragments comprising various extracellular loops is relatively quick and simple. However, peptides applied in this manner are generally not conformational and false-negative results are likely for antibodies that recognize discontinuous or conformational epitopes.
Depending on the number of hits that are recovered from a given discovery program, it may be feasible to forego the antibody sorting described above and move directly to functional characterization. The accepted gold standard for ion channel functional characterization is patch-clamp electrophysiology, which allows real-time kinetic and pharmacological analysis of the effects of drug molecule candidates. Whilst electrophysiology is the most detailed analytical tool available for ion channel functional modulation and is key in making hit-to-lead candidate determinations, it is resource intensive and has suffered historically from low throughput. Progress has been attained with increasing screening throughput and maintaining accuracy with platforms that utilize robotic multi-patch clamp configurations (Patchliner®, Nanion Technologies; Qube384, Sophion; PatchXpress, Molecular Devices; IonFlux, Fluxion). Interestingly, some investigators and platform manufacturers have reported instances of compounds (including antibodies) that demonstrate functionality when analyzed by manual patch-clamp, but are inactive when analyzed by automated platforms (Colussi, personal communication). The reasons for this remain unclear, but the possibility of false-negative results may lead to the abandonment of potentially interesting antibodies. Moreover, antibodies may reasonably be assumed to exhibit slower binding and efficacy kinetics compared to small molecules, which should be considered when analyzing electrophysiological currents over the course of 5 to 10 minutes in which measurements are usually made.
In addition to electrophysiology, a number of other technologies are available that can offer effective screening of modulating compounds that each have their own advantages and disadvantages. These include flux-based assays that measure the cellular influx or efflux of radioactive Na+, Ca2+ and Rb+ for studying sodium, calcium and potassium channels, respectively, and fluorescence-based assays that utilize either voltage-sensitive dyes that measure cell membrane voltage changes or ion-specific fluorescent probes that measure changes in intracellular ion concentrations.101 A recent and more detailed review of ion channel antibody screening strategies can be found in Colley et al.102
Ion channel therapeutic opportunities
The wide range of physiological processes involving ion channels can be broadly summarized as follows: maintenance of cell resting potential, conductance of electrical signals, synaptic transmission at nerve terminals, intracellular transfer of ions and metabolites, cell volume regulation, excitation-contraction coupling and stimulation-secretion coupling, such as that involved in the release of insulin from the pancreas.
Using information in the public domain, we analyzed the ion channel target landscape against which antibody therapeutics could have potential in the treatment of disease, excluding those that would require CNS penetration or intracellular distribution. This analysis identified over 150 potential antibody targets, with over 35 of those possessing clinical or preclinical levels of validation from small molecule and peptide studies. Many opportunities fall within the oncology, autoimmune and inflammatory/neuropathic pain (including migraine) therapeutic areas (Figure 3), but there is also significant potential in the respiratory, metabolic and rare or neglected disease areas. Several ion channel targets fall into more than one therapeutic indication.103 Our findings are summarized in Table 1. The main subclasses of targets are voltage-gated and calcium-activated potassium ion channels, voltage-gated calcium and sodium channels, acid-sensing ion channels, transient receptor potential (TRP) channels, purinergic P2X channels, calcium-release activated channels and chloride channels, which are discussed below.
Figure 3.
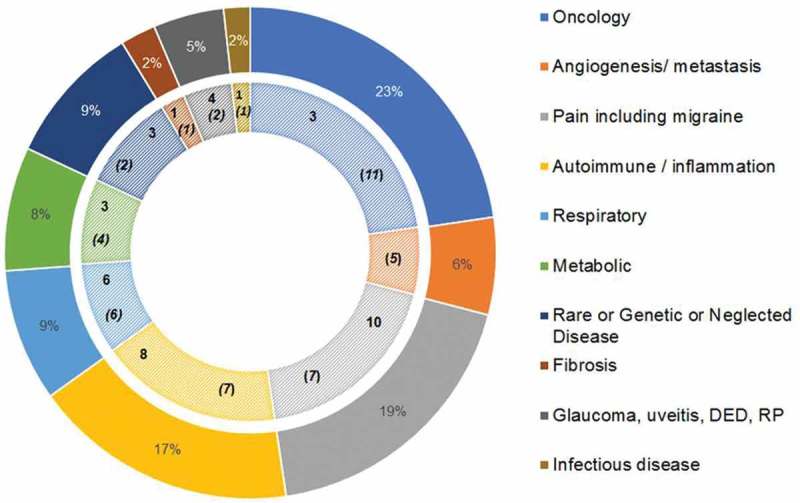
Therapeutic opportunities in the ion channel antibody target landscape shown by therapeutic area. The percentage values in the outer ring represent the number of ion channels implicated for that therapeutic area from the >150 potential antibody targets identified. The inner ring depicts each therapeutic area with the number of clinically (in Phase 2 or further development) validated targets in bold font and the number of preclinically validated targets indicated in bold italicized font and bracketed for distinction. In a few instances, an ion channel has presented targeting opportunities in multiple indications within a therapeutic area and therefore different levels of validation have been presented. Therefore, the highest level of validation is taken to avoid duplication, for example, P2X3 in different respiratory conditions. However, where there are ion channels representing a targeting opportunity in multiple therapeutic areas these have been treated separately and accordingly can demonstrate different levels of validation, for example, Kv1.3 (implicated in autoimmune conditions, such as type 1 diabetes, psoriasis, cutaneuous lupus; respiratory indications (asthma); inflammatory conditions (uveitis and dry eye disease), KCa3.1 (implicated in autoimmune condtions, such as IBD, multiple sclerosis, rheumatoid arthritis; oncology (glioma, renal cancer, NSCLC), respiratory indications (asthma), sickle cell anemia) and TRPC6 (pain; respiratory; metabolic; autoimmune/inflammation; oncology). For further details of the role of each of these ion channels in disease, refer to the main text. There are at least 35 ion channels with clinical or a preclinical level of validation provided by small molecule or peptidic approaches that are suitable for targeting with therapeutic antibodies. Abbreviations: DED dry eye disease; RP retinitis pigmentosa.
Table 1.
Examples of ion channel therapeutic opportunities with level of validation attained by different drug entities, or associated biology, including genetic evidence, knock-out models, etc.
| Ion channel | Therapeutic Area/Indication | Modality &/or Entity | In vitro validation | In vivo validation/preclinical | Clinical validation | Reference |
|---|---|---|---|---|---|---|
| Kv1.3 | MS, RA, T1D, atopic dermatitis, uveitis, DED, psoriasis myositis, cutaneous lupus, psoriatic arthritis, IBD, allergic asthma | Antagonist – peptide analogs of ShK toxin, e.g., dalazatide | Inhibition of TEM cell proliferation and migration, IL-2 secretion, Ca2+ signalling, inhibition of Kv1.3 currents, inhibition of CD3-antibody- and alloantigen-induced proliferation | Inhibition of TEM cell proliferation, blocking Kv1.3 in psoriasiform SCID mouse model, efficacy in DTH and EAE rat models. Clears viral and bacterial infections |
Validation in DED from T cells isolated from patient tissue; suppression of chemokine-induced migration of peripheral blood T cells isolated from healthy donors Dalazatide Ph1 & Ph2 | 77,93,104-112 |
| Atopic dermatitis, psoriasis | Antagonist – small molecule, e.g., PAP1 | Blocking of Kv1.3 currents. Significant dose-dependent inhibition of proliferation and suppression of IL-2 and IFN-γ production |
Potent suppression of oxazolone-induced inflammation by inhibiting the infiltration of CD8 + T cells in rat allergic contact dermatitis model; significant clinical and histological improvement of plaques in SCID mouse-psoriasis skin xenograft model with reduction in TEM cells | Patient psoriatic plaques enriched in TEM cells Ph1 (inactive) |
108-110,113 | |
| Kv10.2 | Brain cancer, lung and cervical | Antagonist – small molecule, e.g., TDZ | Induction of caspase-dependent apoptosis and cell cycle arrest | Reduction in xenografted MB growth and metastasis, inhibition of balbc/c nude mouse xenografts established using A549 sphere cells | Case report of MB patient demonstrated therapeutic efficacy although not without side effects | 114-116 |
| Kv11.1B | Some cancers (leukemias, gastric, colon) | Antagonist – small molecule, e.g., CD-60,130 | Reduction in cell proliferation of tumor cells and tumor cell invasiveness, reduction in VEGF secretion | Reduced leukemic cell infiltration in NOD/SCID and higher survival rates | Epigenetically silenced in ovarian cancer | 24,117-122 |
| KCa3.1 | Autoimmune, e.g., IBD, MS, RA, asthma, fibrosis, sickle cell anemia | Antagonist – small molecule, e.g., TRAM-34, NS6180, Senicapoc | Genetic knockdown of KCa3.1 suppresses T cell activation | No toxicities observed. KCa3.1 blockers validated in a number of animal models, e.g., rodent EAE and experimental colitis models | Restores corticosteroid sensitivity in cytokine-treated ASM cells from COPD and asthmatic patients Ph2 (inactive) |
123-133 |
| Breast, prostate, pancreatic, endometrial, GBM, HNSCC, leukemia, ICC, melanoma | Combined activation of KCa3.1 and inhibition of Kv11.1 – small molecule, e.g., Riluzole | Cisplatin-resistant CRC cells express higher levels of KCa3.1 and Kv11.1 channels; KCa3.1 activators and Kv11.1 inhibitors have a synergistic action with cisplatin in triggering apoptosis and inhibiting proliferation; TRAM-34 also potentiates response of TMZ |
In nude mice xenografted with human NSCLC, Senicapoc reduced tumor growth. In SCID mice xenografted with human GL-15 glioma cells, TRAM-34 reduced tumor infiltration and astrogliosis surrounding the tumor |
Ph1 NCT01303341 Ph2 NCT00866840 |
134-144 | |
| Cav3.1 | Breast and prostate cancer | Agonist | Tumor suppressor function | Expression inhibits proliferation and apoptosis of MCF7 cells. Inhibition of prostate cancer cell proliferation |
Mutations in Cav3.1 confer gain-of-function in adenomas | 145-147 |
| Nav1.7 | Pain | Antagonist – peptide | Blocking of Nav1.7 currents but also acts at Cav2.2 | Synthetic peptides based on spider-derived venom have reversed pain behaviours in mouse models of peripheral spontaneous pain | Genetic evidence provided by loss-of-function and gain-of-function channelopathies. | 9,148 |
| Antagonist – small molecule, e.g., PF-04856264, PF-05089771, CNV1014802/BIIB074 | Bind preferentially to slow inactivated state of Nav1.7, blocks TTX-induced current in DRG neurons | Less selective but more potent with respect to analgesia OD1 mouse model of Nav1.7 mediated pain |
Ph2 NCT01529346 NCT0156102, NCT02935608 |
52,149-151 | ||
| ASIC1 | Pain | Antagonist – small molecule, e.g, PPC-5650 | Inhibition of ASIC1 mediated currents | Preclinical cancer models demonstrate nociceptive neuronal expression of ASIC receptors, that respond to a significant increase in an acidic cancer-induced environment within the bone | Ph1 (inactive) NCT01818570 NCT01449487 Reduction of IBS-related pain |
152-154 |
| TRPA1 | Pain and inflammation | Antagonist | Small molecule in vitro inhibition of AITC-induced Ca2+ uptake | Functional upregulation in OVA-sensitized mice challenged with fine particulate matter Inhibition of TRPA1 eliminates the mechanical and cold allodynia induced by cisplatin and oxaplatin Endogenous agonists known to cause pain sensation TRPA1 knockout mice showed higher tolerance to heat |
Ph2 NCT01726413 Genetic evidence demonstrated by TRPA1 gain-of-function mutation linked to FEPS |
155-158 |
| TRPC3 | AP, SS, hypertension, atherosclerosis, COPD | Antagonist – small molecule, e.g., Pyr3, SalB | Mutated TRPC3 channels on Jurkat cells show decreased Ca2+ influx after TCR stimulation, which can be rescued by overexpression of wild-type TRPC3 | Rat model of sepsis demonstrated upregulation of TRPC3 in T cells enhancing T cell apoptosis TRPC3 inhibition protects salivary glands and pancreas cells from Ca2+ mediated toxicity by inhibiting the TRPC3-mediated component of Ca2+ influx |
Genetic evidence provided by TRPC6 mutations in FSGS resulting in excessive Ca2+ influx and subsequent injury or loss of podocytes | 159-162 |
| TRPV3 | Skin health, including inflammation and pain | Antagonist – small molecule, e.g., FTP-THQ, GRC15300 | Inhibits agonist-induced release of ATP and GM-CSF in m308 keratinocytes | Dose-dependently blocks histamine-induced itch in mouse models Reduction of CFA-induced thermal hypersensitivity |
Gain-of-function TRPV3 mutations identified in rodent and man that are associated with pain and Olmsted syndrome Ph2 (inactive) |
163,164 |
| P2X3 and P2X2/3 | Pain, fibrosis, chronic cough | Antagonist -small molecule, e.g., gefapixant, AF-219 | Block homo- and hetero-trimer forms | Blocks peripheral action in afferent neurons when ATP is released causing sensitization to pain signals | Ph2 and Ph3 NCT02477709 | 165 |
| P2X4 | Pain | Antagonist – small molecule, e.g., NC-2600, ivermectin | Inhibition of ATP-evoked intracellular Ca2+ influx | Efficacy demonstrated in CCI nerve neuropathic pain model and EAN. Dose-dependent inhibition of ATP-induced BDNF release | Ph1 https://adisinsight.springer.com/drugs/800038339 |
166-168 WO2010/093061 |
| P2X7 | Antagonist- – small molecule, e.g., EVT-401 | Blocks ATP-induced IL-1β release from monocytes. Blocks P2X7 mediated currents |
Efficacy demonstrated in CGN-induced model of inflammation, DNBS-induced model of distal colitis, EAE rodent model and CIA model | Ph1 https://adisinsight.springer.com/drugs/800025672 |
US20110118287 | |
| nfP2X7 | Basal cell carcinoma | Antagonist – antibody, e.g., pAb BIL010t | Expression of nfP2X7 in basal cell carcinoma confirmed by IHC | Lesion size reduced in a mouse model of melanoma | Ph1 NCT02587819 | 30 |
| Orai1 (CRAC) | Autoimmune/inflammatory disease, e.g., psoriasis, AP | Antagonist – small molecule, e.g., CM-4620 | Inhibits increase in intracellular Ca2+ in pancreatic acinar cells that leads to enzyme activation, mitochondrial dysfunction, ER stress and necrosis | Inhibits CRAC pathway in T cells, blocking the release of IL-2 and TNFα and reduces neutrophil activation | Ph1 NCT03401190 |
169 |
| Antagonist – mAb | Inhibition of T-cell effector function, T cell proliferation and cytokine release. Triggers internalization of Orai1 | Demonstrates efficacy in rodent T-cell mediated GvHD model | Loss-of-function mutations cause severe immunodeficiency with recurrent infections due to impaired T cell function | 170,171 |
Abbreviations and acronyms used in table:
AITC, allyl isothiocyanate; AP, acute pancreatitis; ASM, airway smooth muscle; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CCI, chronic constriction injury; CFA, complete Freund’s adjuvant; CGN, carrageenan; CIA, collagen-induced arthritis; COPD, chronic obstructive pulmonary disease; CRC, colorectal cancer; DED, dry eye disease; DNBS, 2,4-dinitrobenzene sulfonic acid; DTH, delayed type hypersensitivity; DRG, dorsal root ganglion; EAE, experimental autoimmune encephalitis; EAN, experimental neuritis; ER, endoplasmic reticulum; FEPS, familial episodic pain syndrome; FSGS, focal segmental glomerulosclerosis; GBM, glioblastoma; GM-CSF, granulocyte-macrophage colony-stimulating factor; GvHD, graft versus host disease; HNSCC, head and neck squamous cell carcinoma; IBD, inflammatory bowel disorder; IBS, irritable bowel syndrome; ICC, intrahepatic cholangiocarcinoma; IFN-γ, interferon gamma; IHC, immuno-histochemistry; IL-2, interleukin-2; MB, medullablastoma; MCF-7, Michigan Cancer Foundation-7 breast cancer cell line; MS, multiple sclerosis; NOD, non-obese diabetic; NSCLC, non-small-cell lung cancer; OD1, mouse model of Nav1.7-mediated pain based on intraplantar injection of the scorpion toxin OD1; OVA, ovalbumin; Ph1, Phase 1 clinical trial; Ph2, Phase 2 clinical trial; Ph3, Phase 3 clinical trial; RA, rheumatoid arthritis; SalB, salvianolic acid B; SCID, severe combined immunodeficiency; ShK, Stichodactyla toxin; SS, Sjögren’s syndrome; TCR, T cell receptor; T1D, type 1 diabetes; TEM, effector memory T lymphocytes; TNFα, tumor necrosis factor alpha; TDZ, Thioridazine; TTX, tetrodotoxin; VEGF, vascular endothelial growth factor.
Voltage-gated potassium ion channels
There are at least 40 voltage-gated potassium channels (Kv) in the human genome with physiological roles.172 Kv channels represent an ion channel subclass that offers substantial potential for drug development in a range of diseases, such as cancer, autoimmune diseases and metabolic, neurological and cardiovascular conditions. Their roles range from regulating calcium signalling, cell cycle progression, apoptosis and cell volume to driving cellular proliferation and migration, as well as repolarization of neuronal or cardiac action potentials,173-178 and thus present the potential for various pharmacological strategies to target KV channels with specific antibodies. The therapeutic potential of selected potassium channels is highlighted below.
Kv1.3
Kv1.3 is encoded by the gene KCNA3, expressed on human T cells and was initially recognized as a target for immunosuppression based on the observation that non-selective K+ channel blockers can inhibit T cell proliferation and interleukin (IL)-2 secretion.104,105 It has since been validated as a therapeutic target in numerous preclinical animal models using a variety of small molecule and peptide toxin blockers104,106-111 and, importantly, in the clinic through the development of the potent venom peptide analog Shk-186 (dalazatide).113
Kv1.3 is the predominant potassium channel expressed on effector memory T cells (TEM), which are implicated in a range of T-cell mediated diseases, such as, multiple sclerosis,106 rheumatoid arthritis,104 Type-1 diabetes mellitus,104 allergic contact dermatitis109 and psoriasis.108 Kv1.3 blockers selectively inhibit Ca2+ signalling, proliferation and in vivo migration of CCR7 negative and positive TEM cells; however, stronger effects are observed on CCR7− TEM cells compared to CCR7+ central memory CD4 T cells.179 More recently, Kv1.3 knock-down in memory T cells was shown to suppress CD40L expression and memory phenotype.180 CD40L is also a target for autoimmune disorders, and this finding provides further validation of the therapeutic potential of Kv1.3 blockade in immunomodulation.123
There are no significant intracellular calcium stores in T cells due to their small size, therefore Ca2+ influx through CRAC is paramount for NFAT translocation to the nucleus to elicit cytokine secretion and T cell proliferation. The T cell needs to retain a negative membrane potential through a counterbalancing K+ efflux via Kv1.3 and/or the other T cell K+ channel, Ca2+-activated channel KCa3.1, in order to be fully activated.172 Thus, pharmacological inhibition of Kv1.3 activity blocks activated T-cell proliferation and cytokine production by disrupting the driving force of sustained Ca2+ influx via CRAC.
Kv1.3 is also expressed in breast, prostate and colon cancer and is linked to resistance to apoptosis as observed by the upregulation of Kv1.3 expression in diffuse large B-cell lymphoma and glioma.181 Nevertheless, the role Kv1.3 plays in proliferation and apoptosis appears to be complex and context dependent, and further studies are required to validate Kv1.3 as a potential cancer target and biomarker.182
Kv10.1
A comprehensive overview of the biophysical and pharmacological roles of Kv10.1 and its potential mechanisms in disease has been described elsewhere.183,184 Kv10.1, also known as ether-a-go-go-related gene 1 (EAG1), is encoded by the gene KCNH1 and expression is predominantly restricted to the CNS in health. Most of the interest in Kv10.1 as a therapeutic target originates from the observation that it is aberrantly expressed in up to 70% of tumor cell lines and human cancers,185 including colon cancer,186 gastric cancer,187 breast cancer,188,189 soft tissue sarcoma,190 acute myeloid leukaemia (AML),62 adenoma,191 hepatocarcinoma,192 head and neck cancer,193 brain metastases and glioblastoma multiforme.194 Hence, Kv10.1 presents a good opportunity as an antibody target, in the context of disease association where targeting would be restricted to the periphery due to the inability of antibodies to cross the blood-brain-barrier. Kv10.1 expression also has potential as a biomarker in several of these tumor types and can correlate with a poor prognosis.187,195,196
As such, Kv10.1 has been extensively studied in terms of its role in aberrant cell proliferation and tumor growth, where expression has been reported to be activated by epidermal growth factor receptor (EGFR) tyrosine kinase197 and regulated by p53 and E2F1 that are also often altered in cancer.198 As well as presenting a potential therapeutic target, Kv10.1 is being explored as a diagnostic marker through tumor xenograft imaging in vivo studies.199 Kv10.1 also plays a key role in cytoskeletal organisation, which in turn affects cell viability, angiogenesis, migration and invasion,200 thereby conferring an advantage to tumor growth through increased vascularization and resistance to hypoxia. It has also been shown that Kv10.1 is constitutively and rapidly internalized by endocytosis and lysosomal sorting,201 and that recycling contributes to maintaining Kv10.1 surface level expression. This property is an important consideration for the potential of a drug’s mechanism of action, such as an antibody-drug conjugate or prodrug format, for the targeting of tumor cells. Kv10.1 knock-out mice show no apparent deleterious phenotype during embryogenesis and develop normally to adulthood with no behavioural abnormalities,202 suggesting that blockade or antagonism of aberrant ion channel expression is a feasible targeting strategy. Moreover, further validation is provided by experimental evidence generated from the specific inhibition of Kv10.1 by antisense technology203 and siRNA204 resulting in the reduction of tumor cell line proliferation in vitro.
Subsequently, a closely related family member, Kv10.2, has been identified as a potential target for brain, lung and cervical cancer where clinical proof-of-concept has been attained for the treatment of glioma using the re-purposed FDA-approved antipsychotic drug thioridazine as a Kv10.2 blocker achieving a reduction in tumor volume.114
Kv2.1
Kv2.1, encoded by the KCNB1 gene, mediates a classical delayed rectifier current that is involved in neuronal repolarization. In addition to their role in the CNS, Kv2.1 ion channels are also involved in cell differentiation and growth of non-excitable cells, and inhibition of Kv2.1 in pancreatic β–cells enhances insulin secretion, which offers a potential therapeutic strategy for the treatment of Type-2 diabetes.205 Over-expression and aberrant behaviour of Kv2.1 has also been reported in several tumor types including uterine cancers,206 gastric cancers207 and medullablastoma.208 Further evidence of the potential therapeutic benefit of targeting Kv2.1 is provided by studies with perifosine, which is a third generation alkylphospholipid analog with anti-tumor properties. The principal mechanism of action of perifosine is the inhibition of Akt signalling by disrupting lipid rafts to which Kv2.1 ion channels preferentially localize. A recent study demonstrated that perifosine induces a hyperpolarizing shift in the voltage dependence of Kv2.1 inactivation, accelerating the kinetics of closed-state inactivation but without altering the voltage dependence of activation.209
Kv11.1B
Kv11.1 (or hERG) is encoded by the KCNH2 gene and is the pore-forming α subunit of the voltage-gated inwardly rectifying potassium channel associated with cardiac arrhythmias and rhythmic excitability of the pituitary. Kv11.1 mediates the rapidly activating component of the delayed rectifying potassium current in heart (IKr)210 and its properties are modulated by cAMP and auxiliary subunit assembly. It was one of the first voltage-gated channels linked to cancer, and has been shown to be abundantly expressed in several leukemias, gastric and colon cancer, whereas it is epigenetically silenced in ovarian cancer, and thus does not seem required for tumorigenesis in all tumor types.24
As with some other ion channels, Kv11.1 is associated with the sigma-1 receptor (SigR1), a stress-activated transmembrane chaperone.211 SigR1 promotes the formation of Kv11.1/β1-integrin signalling complexes that trigger the activation of the PI3K/AKT pathways. The presence of Sig1R in tumor cells increases cell motility and vascular endothelial growth factor (VEGF) secretion. In vitro therapeutic validation has been illustrated by several experimental observations following blockade of Kv11.1, such as the reduction of cell proliferation in cultured tumor cells,117 ablation of the invasiveness of colorectal cancer cells118 and gastric cancer cells,119 as well as secretion of VEGF, a well-known driver of tumorigenesis and angiogenesis from glioma120 and myeloid leukaemia cells.117 NOD/SCID mice engrafted with acute lymphoid leukemia cells and treated with Kv11.1 channel blockers showed reduced leukemic infiltration and had higher survival rates, suggesting that potential therapeutic effects are relevant in an in vivo setting.121,122
However, the use of general Kv11.1 blockers in cancer therapy presents a risk for causing cardiotoxicity (by lengthening of the EGC QT interval), which would expose the patient to ventricular arrhythmias. The potential to circumvent this relies on the existence of at least 2 isoforms of Kv11.1, namely Kv11.1A and Kv11.1B.212 Tumor and cardiac cells express different ratios of the A and B Kv11.1 isoforms, thus side effects could be potentially avoided by specifically inhibiting the channel splice variant (Kv11.1B) that is highly expressed in certain tumors,121 such as, AML, neuroblastoma24 and acute lymphoblastic leukemia.122,213 Some progress towards achieving this goal has been made using the small molecule CD-160,130,121 which blocks the Kv11.1B isoform with higher specificity than the Kv11.1A isoform. MAbs targeting Kv11.1B could provide the wherewithal for superior selectivity, and thus provide a safer therapeutic strategy. This is discussed further in a later section.
Calcium-activated potassium ion channels
Calcium-activated potassium channels are potassium channels gated by calcium or that are structurally or phylogenetically related to calcium-gated channels.214 Intracellular calcium can also trigger potassium currents.215 These channels can be broadly categorized into three types based on their unitary conductance: large-, intermediate-, and small-conductance.216 Large-conductance channels are activated by both voltage and increases in cytosolic Ca2+ and represent a distinct gene family. Intermediate and small conductance K+ channels are activated exclusively by increases in intracellular Ca2+ and represent a distinct gene sub-family.217,218 Calcium-activated 6 or 7 TM K+ channels (KCa), represent another structural sub-type in the potassium channel family where KCa3.1 is the most well-characterized member.
Kca3.1
First described in the 1950s, KCa3.1, encoded for by the KCNN4 gene, is a voltage-independent potassium channel that is activated by intracellular calcium mediated by calmodulin.214 Activation triggers membrane hyperpolarization, which in turn promotes calcium influx. KCa3.1 is expressed on activated T and B cells, macrophages, microglia, vascular endothelium, epithelia, proliferating vascular smooth muscle cells and fibroblasts, and therefore presents a potential therapeutic target for inflammatory and autoimmune diseases, such as inflammatory bowel disease and multiple sclerosis.123 This ion channel is also expressed on erythrocytes, hence also has potential as a therapeutic target for sickle cell anemia.124 Elevated intracellular calcium activates KCa3.1, thereby maintaining a negative membrane potential, which is required for production of inflammatory chemokines and cytokines by T cells, macrophages and mast cells. Potassium efflux through KCa3.1 can be significant, resulting in efflux of > 50% of intracellular potassium content with the associated cell shrinkage being linked to apoptosis in certain circumstances.219,220 Functional cooperation between TRPC1 and KCa3.1 in the regulation of Ca2+ entry has been suggested as both these ion channels co-localize into lipid rafts, and knockdown of TRPC1 suppresses the Ca2+ entry induced by KCa3.1 activation.221
Preclinical proof-of concept studies in animal models have validated the therapeutic potential of KCa3.1 blockers, with no toxicities observed in KCa3.1 knock-out mice and inhibition from developing severe colitis in two mouse models of inflammatory bowel disease,125 a mouse model of experimental autoimmune encephalomyelitis,126 several models of cardiovascular diseases127 and unilateral ureteral obstruction-induced renal fibrosis in wild-type mice and rat.128 KCa3.1 blockers, such as senicapoc, have been evaluated in clinical trials for sickle cell anemia and exercise-induced asthma, but have so far not shown efficacy, although the results have confirmed that targeting KCa3.1 is safe.129,130 Although senicapoc did not reduce the number of painful sickling crises, which was the clinical endpoint that the sponsoring company, Icagen Inc., had selected for their trial,130-133 the compound did demonstrate a reduction in hemolysis with increasing hemoglobin and hematocrit levels, a non-significant reduction in late asthmatic response, and it was well tolerated. In addition, significant inter-patient variation was observed in senicapoc’s half-life, making dosing difficult. In a similar manner to Kv1.3 blockers, KCa3.1 blockade acts on specific T or B cell subsets, providing the wherewithal for targeted immunomodulation rather than whole-sale immunosuppression, and an antibody would provide a longer half-life, yielding a better pharmacokinetics/pharmacodynamics profile.
KCa3.1 has also been implicated in several cancers, presumably mediated by its role in the proliferative response of many cell types. Several reports describe successful inhibition of tumor cell proliferation and pro-invasive behaviour following KCa3.1 blockade in both in vitro and in vivo studies, including prostate,134 breast,135 pancreatic,136 endometrial,137 glioblastoma,138-140 head and neck squamous cell carcinoma (HNSCC)141 and leukemia.142 It has also been proposed that targeting KCa3.1 could provide a potential adjuvant therapy for the inhibition of tumor angiogenesis and tumor progression.139,143
Recently, the combined activation of KCa3.1 and inhibition of Kv11.1 has been identified as a potential alternative strategy to overcome cisplatin resistance in colorectal cancer (CRC) from studies using molecular and electrophysical approaches with the cisplatin-resistant CRC cell lines HCT-116 and HCT-8.144 Several previously characterized K+ channel modulators were tested in vitro individually and in combination for their action on K+ currents, cell viability, apoptosis, cell cycle, proliferation, intracellular signalling and platinum uptake. These effects were also analyzed in a mouse xenograft model that mimics chemoresistance. Cisplatin-resistant CRC cells express higher levels of KCa3.1 and Kv11.1 channels compared with cisplatin-sensitive CRC cells. In resistant cells, the KCa3.1 activator, SKA-31, and Kv11.1 inhibitor, E4031, revealed a synergistic action with cisplatin resulting in apoptosis and inhibition of cell proliferation. Similarly, riluzole is able to both activate KCa3.1 and inhibit Kv11.1, which suggests a combined approach or potential use of a bispecific antibody as a targeting strategy, for example, in patients with ovarian cancer where cisplatin resistance also presents a challenge in adjuvant therapy.
Voltage-gated calcium ion channels
Voltage-gated calcium channels (VGCC) are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g., muscle, glial cells, neurons) with selectivity for Ca2+. At resting membrane potential, VGCCs are normally closed. They are activated at depolarized membrane potentials and are key transducers of cell surface membrane potential changes into intracellular calcium influx that regulates intracellular processes such as contraction, secretion, neurotransmission and gene expression in many different cell types. There are 10 members of the voltage-gated calcium channel family that have been characterized in mammals, and these serve distinct roles in cellular signal transduction.222
Cav3.1 and Cav3.2
Cav3.1 and Cav3.2 are encoded by the CACNA1G and CACNA1H genes, respectively, and both belong to the T-type calcium channel subfamily. Although very closely related with similar biophysical properties, their functional effects are very different and emphasize the importance of being able to develop ion channel modulators with high selectivity. Cav3.1, but not Cav3.2, is thought to act as a tumor suppressor because it is involved in the inhibition of proliferation and promotes apoptosis in MCF-7 human breast cancer cells.145,146 Whereas overexpression of Cav3.1 suppresses cell proliferation and siRNA knockdown or treatment with ProTx-I, a selective inhibitor for Cav3.1, promotes cell proliferation of MCF-7 cells, gene knockdown or overexpression of Cav3.2 exhibits no effect on cell proliferation in this cancer cell line. Moreover, Cav3.1 expression has been shown to correlate with sensitivity to apoptosis145 and inhibition of prostate cancer cell proliferation.147
Cav3.2 has been suggested to promote a constitutive calcium entry influx due to the influence of KCa3.1.223 It is thought that Cav3.2 is responsible for the neuroendocrine differentiation associated with the increase in calcium-dependent secretion of mitogenic factors in prostate cancer,224 and has been nominated as a biomarker for breast cancer progression and treatment.225 Thus, a different mode of action would be required for modulating cancer drugs targeting each of these calcium channels,226 namely, one as a channel agonist (Cav3.1) and one as an antagonist (Cav3.2).
Voltage-gated sodium ion channels
The voltage-gated sodium channel family has 9 members (Nav1.1 to Nav1.9) that are encoded by the genes SCN1A to SCN11A. Nav sodium channels have key roles in the initiation and propagation of action potentials in excitable neuronal cells, muscles and heart tissues, and as such have historically been regarded as therapeutic targets for pain, arrhythmia and epilepsy. A range of inherited disorders affecting skeletal muscle, heart rhythm and the central and peripheral nervous systems have been linked to mutations in the Nav genes227 that confer loss-of-function or gain-of-function properties.228,229
Nav1.7, Nav1.8 and Nav1.9
Nav1.7, Nav1.8 and Nav1.9 are expressed in peripheral sensory neurons and hereditary gain of function mutations have been identified as the cause of pain disorders, including hereditary erythromelagia and paroxysmal extreme pain disorders (Nav1.7), as well as other painful peripheral neuropathies (Nav1.8, Nav1.9). Conversely, loss of function mutations in Nav1.7 lead to a congenital insensitivity to pain.230 It is not surprising then that these Nav isoforms, particularly Nav1.7, have generated substantial interest as targets for the development of non-opioid pain therapeutics. Additionally, recent evidence is growing that implicates Nav1.7 in cancer. Functional Nav1.7 expression has been found to be involved in EGF-mediated tumor cell invasion in non-small lung cancer cells231 and has also been reported to be abundantly expressed in prostate cancer146 and breast cancer.232
Nav1.5 and nNav1.5
In metastatic breast cancer cells, Nav1.5 is upregulated and found to potentiate tumor cell migration and invasion in both in vitro and in vivo experimental models,233 whereas stable down-regulation of Nav1.5 expression significantly reduces tumor growth, local invasion into surrounding tissue and metastasis to liver, lungs and spleen, thus providing a further body of evidence for its role in metastasis. Furthermore, a neonatal splice variant (nNav1.5) has been shown to be upregulated and associated with metastasis and breast cancer progression in vitro, in vivo and in clinical samples of patient lymph node tissue.234 In developmentally regulated D1:S3 splicing of the Nav1.5 gene, SCN5A, there are 31 nucleotide differences between the 5ʹ-exon (‘neonatal’) and the 3ʹ-exon (‘adult’) forms, resulting in seven amino-acid substitutions in the S3/S4 extracellular region of Domain 1 (D1:S3-S3/S4 linker). Functional activity of nNav1.5 can be suppressed by both siRNA and a specific polyclonal antibody, NESO-pAb.22 The siRNA rapidly reduced the level of nNav1.5 mRNA by ~ 90%, but not adult Nav1.5 mRNA; however, the effects on protein reduction were considerably less. NESO-pAb reduced metastatic activity of the breast cancer cell line, MDA-MB-231, in a dose-dependent manner. Other studies from the same group demonstrated that blockade of the Nav1.5 channel with small molecules or siRNA also inhibited the invasiveness of endocrine-resistant breast cancer cells.235 Recent work has shown that increasing the level of nNav1.5 cell surface expression increased the metastatic behavior of breast cancer cells236 due to a reduction in cell adhesion, and suggested a possible interaction with the Sig1R transmembrane chaperone. Further work is necessary to elucidate the nature of this interaction and provide further understanding of the role of nNav1.5 function in an oncology setting. The adult form of Nav1.5 is responsible for propagating the action potential in cardiac muscle, and therefore, like Kv11.1, targeting isoforms of these channels as a therapeutic strategy necessarily stresses the importance of selectivity.
ASIC channels
The acid-sensing ion channel (ASIC) family, encoded by ASCI1-5 genes, are part of the epithelial sodium channel superfamily and are voltage independent. Instead, ASICs are activated in response to reduced extracellular pH,237 particularly tissue acidosis and are Na+ permeable with an isoform, ASIC1a, showing low Ca2+ permeability. ASICs are expressed in the peripheral and central nervous system and are potential therapeutic targets for neurological conditions.238
ASIC1a
ASIC1a mediates Ca2+ overload and has been reported to contribute to ischemic nerve cell death and inflammation in multiple sclerosis.239 It has also been implicated in pain,240 migraine,241 pain associated with bone cancer,152 glioblastoma232,242 and breast cancer.243 Additionally, ASIC1 inhibitors have been shown to cause a significant reduction of tumor growth and load.243
TRP channels
The mammalian TRP channel family consists of six gene families comprising 28 members of cation channels activated by different stimuli and ligands with diverse physiological functions that range from pain and thermal perception to Ca2+-mediated cell processes and homeostatic reabsorption of calcium and magnesium. The mammalian TRP channel family can be broadly sub-divided into 6 sub-families that consist of TRPA (ankyrin), TRPV (vanilloid), TRPM (melastatin), TRPC (canonical), TRPP (polycystin) and TRPML (mucolipin). Known naturally occurring compounds that act as ligands include capsaicin (TRPV1) and menthol (TRPM8). Trp channels are thought to play a key role in several diseases encompassing inflammation, allergy, autoimmune, fibrosis, oncology and pain indications.244 Calcium entry through Trp channels may inhibit apoptosis, and is an effect that has been partly attributed to the stimulation of NF-kB.245 Examples of Trp channels in various disease settings are described below and have been reviewed in depth elsewhere with regards to targeting pharmacology strategies.155,246
TRPM7
TRPM7 is associated with proliferation, motility and metastasis of cancer cells. Inhibition of TRPM7-regulated PI3K/AKT and MEK/ERK signalling pathways has been demonstrated to suppress glioblastoma cell proliferation and migration in vitro.247,248 This inhibition is thought to enhance apoptosis induced by TRAIL,249 and induce replicative senescence and enhanced cytotoxicity with gemcitabine in pancreatic adenocarcinoma.250 Aldehyde dehydrogenase (ALDH1), which is a cytoplasmic stem cell marker in many malignancies, has recently been suggested to be a tumor stem cell marker in glioblastoma. The Notch signalling pathway plays a key role in cancer stem cell (CSC) survival, proliferation and maintenance of the CSC population. TRPM7 gene silencing down-regulates both the Notch and STAT3 pathways in glioma stem cells, whereas increased ALDH1 expression and activity is induced by TRPM7.251 Moreover, phosphorylated STAT3 binds and activates ALDH1 promoters in glioma cells. Thus, the authors concluded that these findings demonstrate that TRPM7 activates the Notch and STAT3 pathways leading to activation of ALDH1 and subsequent increases in cell proliferation and migration.251
TRPM8
TRPM8 is a receptor-activated non-cationic ion channel. In prostate epithelial cells, expression of TRPM8 is regulated by androgen, is elevated in androgen-sensitive cancerous cells compared with normal cells and has been confirmed as an ionotropic receptor for testosterone.252 As such, TRPM8 has been identified as a novel target for androgen-regulated prostate cancer,253 where overexpression is androgen-dependent and required for tumor cell survival. Although the precise mechanism involved is unknown,254 the influx of Ca2+ and Na+ in prostate cancer cells has been shown as necessary for survival and function,255 and it is well established that Ca2+ signaling regulates proliferation and apoptosis in cancer cells. In androgen-sensitive cell lines, such as LNCaP, testosterone activation of TRPM8 elevates basal Ca2+ levels252 whilst TRPM8 inhibition with a small molecule antagonist or siRNA results in cell death.255 Anti-androgen therapy also significantly reduces the expression of TRPM8.256
The tissue-specific function of TRPM8 in prostate physiology and carcinogenesis remain unknown. This is complicated by the different cellular locations for TRPM8, namely the cytoplasm (in the endoplasmic reticulum) and plasma membrane. Testosterone-induced plasma membrane TRPM8 activity elicits calcium uptake causing apoptotic cell death. In addition, the promoter region of trpm8 possesses a consensus p53 binding site, suggesting that TRPM8 may serve as a downstream target of tumor-suppressor genes potentially providing a protective role.
Conversely, TRPM8 expression is significantly lower in androgen-independent and metastatic prostate cancer.257 The androgen independent pathways do not require androgens, but can be activated by growth factors acting through kinase pathways. These initial observations suggested that TRPM8, as a therapeutic target, may only be suitable for androgen-sensitive prostate cancer. However, application of an adjuvant therapy that rescues TRPM8 expression or enhances its activity or acts as an agonist could pose a potential strategy for the treatment of androgen-independent prostate cancer.252,258,259 Such a hypothesis is substantiated by the observations that, whilst TRPM8 may not be essential for the survival of the androgen-independent prostate cancer cell line PC3, overexpression of this ion channel mediates a reduction in proliferation and migration, as well as facilitating apoptosis.258
Thus, androgen sensitivity would need to be taken into consideration when selecting the desired mechanism of action of an antibody targeting TRPM8 in prostate cancer. That is, an antibody with ADCC effector function targeting plasma membrane-associated TRPM8 might be preferred in the case of androgen-dependent prostate cancer, while an antibody with agonist activity combined with an adjuvant therapy to enhance expression might be more effective for androgen-independent prostate cancer.
TRPV1
TRPV1 is overexpressed in many tumor types, including endometrial,260 thyroid,261 breast,262 astrocytoma,263 prostate,264 pancreas,265 colon,266 melanoma267 and bladder.267 TRPV1 is activated by capsaicin and is probably the most well-known TRP channel targeted for pain with marketed products, such as Qutenza® (Acorda Therapeutics) and ZuactaTM (Sanofi), both of which are capsaicin-based. As an agonist, administration of capsaicin causes an initial enhanced stimulation of TRPV1-expressing nociceptors that may be associated with painful sensations, but this is followed by pain relief thought to be mediated by a reduction in TRPV1-expressing nociceptive nerve endings. However, there may be a gradual re-emergence of painful neuropathy over time, and this is thought to be due to TRPV1 nerve fibre reinnervation. This potentially could be circumvented by the use of an antagonist antibody. TRPV1 also plays a key role in deep tissue pain,268 joint pain in arthritis269 and bone cancer pain.152
TRPV3
The clinical significance of TRPV3 in non-small cell lung cancer (NSCLC) was recently reported270 where it was observed to be overexpressed in ~ 68% of lung cancer cases, correlating with the differentiation and tumor node metastasis stage of the tumor. Significantly, TRPV3 expression was associated with short overall survival. Blocking or knockdown of TRPV3 has been shown to inhibit lung cancer cell proliferation and arrest the cell cycle at the G1/S transition stage.270 The rate of proliferation of epithelial cells in TRPV3 knockout mice is less than that in wild-type mice and TRPV3 up-regulation has been shown to be associated with a high risk for development of CRC.271 TRPV3 has been proposed as a potential companion drug target for NSCLC.270
TRPV6
TRPV6 demonstrates higher calcium selectivity over other TRP channels and plays an important role in regulation of calcium homeostasis in the body. In cancer, evidence points to its upregulation and correlation with the advanced stages in prostate, colon, breast, thyroid and ovarian carcinomas where it translocates to the plasma membrane via an Orai1-mediated mechanism and promotes tumor cell survival by enhancing proliferation and conferring apoptosis resistance.272
TRPA1
TRPA1 is implicated in inflammatory pain and naturally derived compounds from plants, such as mustard oil, act as agonists on TRPA1 causing pain by excitation of nerve fibres.273 The closed-state structure of TRPA1 was recently solved,33 and further study using molecular dynamics simulations, in parallel with mutagenesis and functional evaluation by electrophysiology, explored conformational changes on the proposed open state for an informed understanding on the structure and function of this ion channel.274 However, selection of appropriate animal disease models requires careful consideration because cross-species variations in metabolic mechanisms and signal transduction pathways can lead to species-specific differences in TRPA1 function. Paclitaxel-induced neuropathy is thought to trigger the release of mast cell tryptase, which activates the protease-activated receptor 2 that in turn sensitizes TRPA1 (as well as TRPV1 and TRPV4) through the PLC, PKC and PKA signalling pathways.275 TRPA1 expression can be modulated by other GPCRs, including the bradykinin receptor, the bile acid receptor TGR5 and the MAS-related GPCR.156 Inhibition of TRPA1 eliminates the mechanical and cold allodynia induced by cisplatin and oxaplatin, which are commonly used chemotherapies.155 In addition, selective blockade of TRPA1 attenuates pain without altering body temperature regulation or the ability to feel cold.275 TRPA1 also presents a therapeutic opportunity in the treatment of migraine.276 Similar observations have been made for TRPM8 where it plays a major role in cold hypersensitivity277,278 and presents a therapeutic opportunity both in the treatment of pain and migraine.279
In addition to its role in pain signalling, TRPA1 is found in nerve fibers that innervate the respiratory tract, in the peripheral nervous system, as well as on non-neuronal cells, such as fibroblasts and epithelial cells,280 and it is an emerging target for respiratory conditions such as cystic fibrosis, asthma, allergic rhinitis, chronic cough and itch. A large body of evidence accumulated from in vitro experiments, animal disease models and patient data indicates that TRPA1 functions as a chemosensor for exogenous irritants and endogenous mediators of inflammation. Additionally, the presence of fine particulate matter in OVA-sensitized mice has been demonstrated to upregulate TRPA1 expression.157 Based on these observations, a therapeutic strategy targeting TRPA1 in respiratory disease would require blockade of this important ion channel.158
TRPC3
Excessive Ca2+ influx regulates cytotoxic processes associated with immune-mediated diseases, such as acute pancreatitis and Sjögren’s syndrome causing dry mouth and/or dry eye. Inhibition of TRPC3, and therefore Ca2+ influx, has been shown to protect pancreatic and salivary gland secretory cells from damage caused by Ca2+ cytotoxicity.159 TRPC3 also plays a role in airway smooth muscle proliferation associated with airway remodelling, a histological characteristic of chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD).160 Inhibition or knockdown of TRPC3 blocks increased activity of TRPC3 and membrane depolarization in OVA-sensitized/-challenged cells281 and suppresses airway smooth muscle cell proliferation and airway remodelling in mouse models of disease.282
TRPC6
The classical or canonical TrpC family (formerly short-TRPs, STRPs) encompasses channels presenting a large number of different activation modes. Some are store-operated, whereas others are receptor-operated channels activated by the production of diacylglycerol or redox processes. TrpC6 is amongst the latter subgroup of TrpC channels.283
Of clinical relevance, TrpC6 channels are upregulated in a wide range of cell types across a broad spectrum of disease indications, such as focal segmental glomerulosclerosis (FSGS),161 pulmonary fibrosis,284 cancer,285-290 hypertension291,292 and allergic asthma.293 Expression or over-expression of TrpC6 has been shown to have a pro-proliferative effect. For example, the presence of TrpC6 has been determined to be essential for the progression of gastric cancer in studies comparing normal and cancerous epithelial cells of humans, and TRPC6 inhibitors, and a dominant negative TRPC6 channel mutant, have been shown to promote cell cycle arrest in gastric cancer cell lines.294 There are no specific TrpC6 inhibitors in development that effectively suppress these processes, and therefore this ion channel presents a potential therapeutic opportunity. In the kidney, TrpC6 resides in podocyte membranes where it plays a role in maintaining glomerular function by acting as a non-selective cation channel that primarily transports Ca2+. TRPC6 has also been implicated in renal disease, such as primary forms of FSGS where circulating factors cause dysfunction or loss of podocytes via TrpC6 channel activation. TRPC6 mutations from both familial and sporadic cases of FSGS map to the N- and C-termini, often resulting in excessive calcium influx and subsequent injury or loss of podocytes.161 This in turn promotes glomerular mesangial cell apoptosis via calcineurin/NFAT and FasL/Fas signaling pathways.295 Additionally, mice with podocyte-specific overexpression of TrpC6 recapitulate many of the pathological features of FSGS.296 Despite the clear association of TrpC6 with FSGS, there are currently no clinical trials of therapeutics targeting TrpC6 for any condition.
Ligand-gated purinergic P2X channels
There are seven members of the P2X purinergic ligand-gated ion channel family (P2X1-P2X7) reported to date, which are encoded by the genes P2RX1-P2RX7 that have dispersed chromosomal locations.297 Of these, the homotrimers of P2X3, P2X4, P2X7 and heterotrimers of P2X2/3 offer the greatest potential for therapeutic targeting.298 On activation by ATP released by damaged cells, these ion channels open to allow the influx of calcium, leading to cell membrane depolarization.299 Whilst P2X channels have a wide tissue distribution, they are activated by ATP with differing affinities that trigger distinct physiological functions, such as central synapse transmission smooth muscle cell contraction, platelet aggregation, macrophage activation, cell proliferation and cell death.300
P2X3
Activation of P2X3 homomers contributes to acute nociception and activation of P2X2/3 heteromers that modulate nociceptive sensitivity associated with nerve injury or chronic inflammation. P2X3 receptors undergo rapid turnover that is dependent on their activity status (i.e., agonist-stimulated internalization), where expression and function are modulated by phosphorylation of intracellular domains in response to extracellular pain-causing molecules.301 Calcium/calmodulin-dependent serine protein kinase has been demonstrated to regulate purinergic nociceptive signalling of P2X3.302
P2X4
P2X4 has also been shown to play a role in neuropathic pain where knockdown, knockout and inhibition of P2X4 in rodent in vivo models reverses neuropathic pain.303 Studies show that the P2X4 channel expressed in the satellite glial cells of dorsal root ganglia is associated with neuropathic pain.304 Additionally, activation of P2X4 (and P2X7) on microglia is thought to maintain nociceptive sensitivity through neural-glial cell interactions as antagonists to these receptors have been observed to reduce neuropathic pain. These mechanisms are reviewed in depth elsewhere.305,306
P2X7
P2X7 is expressed on T cells and is implicated in the immune response where it is upregulated on macrophages and microglial cells following stimulation by IFNɤ or LPS driving T cell activation and proliferation with the release of IL-1β.307-310 P2X7 has also been directly implicated in systemic lupus erythematosus,311 arthritis312 and diabetic neuropathic pain.313
In cancer, P2X7 is thought to act as a danger “sensor” for high local ATP in inflammation314 and the tumor microenvironment,315 with overexpression of P2X7 linked to tumor growth and metastasis.316,317 It was recently demonstrated that the anti-parasitic agent ivermectin can modulate sensitivity of both P2X4 and P2X7 receptors to extracellular ATP in association with the pannexin-1 channel, resulting in the induction of a non-apoptotic and inflammatory form of cancer cell death.318 A non-functional conformational form of P2X7 (nfP2X7) has been described that is unable to form the large pore conformation associated with driving cell death and is specifically expressed on the surface of cancer cells sourced from a wide range of tumors.21 It is thought that this variant of P2X7 could result from rare splice isoforms or single nucleotide polymorphisms,319 and the resulting molecule only has one functional ATP binding site of the three available, thus providing a unique targeting epitope on nfP2X7 that is not present on functional P2X7.21 To date, this is the only ion channel target with an antibody (BIL010t) in clinical study, as described below in the “Antibodies in research and development” section.
Additionally, several P2X7 splice variants have been described, with the full-length and a truncated version (P2X7RB) specifically studied in osteosarcoma.320 In the case of glioma, Fang and co-workers have reported that suppression of P2X7 promotes tumor growth via the EGFR signalling pathway.321 The same group evaluated the potential of transplanted endothelial progenitor cells (EPCs) as a therapeutic strategy and imaging probe to overcome anti-angiogenic resistance of gliomas in the brain. This approach takes advantage of P2X7 receptor activation on EPCs prior to transplantation, which promotes their proliferation and homing to gliomas in vivo.322 P2X7 has also been shown to act as an upstream modulator of the VEGF signalling pathway with proof-of-concept demonstrated in experimental neuroblastoma323 using ACN human NB cells. In vivo studies also demonstrated ACN-derived tumor growth could be reduced in nude/nude mice, and an even stronger effect of P2X7 blockade was obtained in a syngeneic immune-competent neuroblastoma model where Neuro2A cells were injected in albino mice. Lastly, it has been reported that CD40 upregulates P2X7 in retinal endothelial cells, rendering them susceptible to ATP/P2X7–mediated programmed cell death.324
Calcium-release activated channels
Calcium-induced calcium release is a process that occurs in many cells and tissues whereby an increase in concentration of Ca2+ in the cytosol causes a further increase as Ca2+ is released from intracellular stores. In this case, Ca2+ release from the sarcoplasmic/endoplasmic reticulum occurs following activation of Ca2+ release channels in response to elevated levels of cytosolic Ca2+, inositol triphosphate (IP3), or changes in membrane potential.
On the other hand, Ca2+ release-activated Ca2+(CRAC) channels represent one of the main Ca2+ entry pathways into the cell. They are fully reconstituted via two proteins, the stromal interaction molecule 1 (STIM1), a Ca2+ sensor in the endoplasmic reticulum, and the Ca2+ ion channel Orai in the plasma membrane. After Ca2+ store depletion, STIM1 and Orai couple to each other, allowing Ca2+ influx.325
CRAC (Orai, STIM)
Calcium release-activated calcium (CRAC) channel protein 1 is a calcium selective ion channel that is encoded by the ORAI1 gene in humans. Four Orai1 subunits comprise the CRAC channel, which is indirectly activated by low intracellular Ca2+, whereby decreased calcium concentration in the endoplasmic reticulum is sensed by STIM1, which in turn aggregates and relocates near the plasma membrane, where it activates Orai1 via direct interaction with the ion channel.326,327 Sig1R inhibits store-operated Ca2+ entry (SOCE) by attenuating coupling of STIM1 to Orai1.328
CRAC channels are critical to the activation of T lymphocytes, mast cells and other hematopoietic cells, as they provide the primary route for the influx of calcium into these cells.329,330 CRAC channel inhibition could therefore provide a direct way of modulating the immune response for the treatment of multiple diseases and disorders. In addition, growth factors can stimulate CRAC channels (CRACs) to mediate Ca2+ entry and subsequent Ca2+ oscillations in proliferating cells. For example, tumor cell migration and metastases require Ca2+ influx and Orai and STIM have been shown to have a role in apoptosis resistance associated with proliferating and migrating tumor cells.331-334 In the context of metastatic CRC, where only 10–20% of patients receive a clinical benefit from the use of anti-EGFR mAbs, studies suggest that efficacy of these mAbs could depend on their ability to reduce SOCE that is known to promote cancer cell growth.335 Subsequent studies revealed that a lipid raft-ion channel complex, consisting of KCa2.3, TRPC1 and Orai1, regulates SOCE-dependent colon cancer cell migration,335 in agreement with previous observations.336 The formation of this lipid raft is triggered by STIM1 phosphorylation by EGF and activation of the AKT pathway. Additionally, evidence of an association of Orai and STIM with TRPC1 has been implicated in tumorigenesis.337
Chloride channels
Chloride channels are a superfamily of ion channels with diverse structures (e.g., voltage-gated and ligand-gated channels),338 but their biology is still poorly understood and as such remain a relatively under-exploited target class for drug discovery.339 These channels may conduct many different ions, but they are named for chloride (as this is the most abundant). They are involved in a wide range of biological functions, including epithelial fluid secretion, cell-volume regulation, neuroexcitation, smooth-muscle contraction and acidification of intracellular organelles. Mutations in several chloride channels cause human diseases, including cystic fibrosis, macular degeneration, myotonia, kidney stones, renal salt wasting and hyperekplexia. Chloride-channel modulators have potential applications in the treatment of some of these disorders, as well as in secretory diarrhoeas, polycystic kidney disease, osteoporosis and hypertension. The chloride ion channel family comprises CLCs (that contain 10 or 12 transmembrane helices), CLICs (comprising two TMDs linked by a large central pore loop that may be glycosylated and can switch from a soluble state to a membrane bound state), CFTR (an ABC transporter) and CACC (calcium activated chloride channels, e.g., TMEM16A). A number of chloride channels are intracellular in distribution, e.g., CLICs, and would therefore not be suitable for targeting with antibodies unless an “intrabody” approach could be successfully applied.
CLCs
CLCs have been implicated in osteopetrosis (CLC-7)340 and glioblastoma (CLC-2, CLC-3 and CLC-5),341 where in normal brain tissue only CLC-2 is expressed on the plasma membrane.
CACCs
CACCs, such as CLCA1 and CLCA2, are thought to have an apoptotic role, but are downregulated in cells resistant to detachment-induced apoptosis (knowns as anoikis), as shown in mammary gland cells,342 suggesting a role in cancer cells where reduction in expression is thought to contribute towards tumor cell survival.343,344 Moreover, the reduction in expression of CLCA2 by lentiviral shRNA causes cell overgrowth and focus formation, enhanced migration and invasion with an increased risk of metastasis.344 Overexpression of CLCA2, on the other hand, inhibits cell proliferation with increases in chloride current at the plasma membrane and accompanying reduced intracellular pH.345 CACCs are also thought to have a role in asthma, COPD, cystic fibrosis and neuropathic pain.346,347 Currently, there are no antibodies in discovery or development, and only 2 peptides targeting chloride channels, including lancovutide (duramycin) for the treatment of COPD and cystic fibrosis, were reported to be in development, but both have been discontinued (LanthiBio, TransMolecular).
Ion channel targeting antibodies in development
Given the advances made in targeting GPCRs over the past decade,20 there is now substantial interest from the biotechnology and pharmaceutical industries to extend these capabilities to therapeutic mAbs that target ion channels.348-351 Several efforts are ongoing to succeed with this target class, and, whilst the pipeline is in its infancy, the ion channel-antibody pipeline in 2016 was reminiscent of the early stage GPCR-antibody pipeline 10 years ago (Figure 4(a)). A review of information available in the public domain that includes company websites, publications, conferences and searches on commercial databases, such as Pharmaprojects, has identified over 20 ion channels that are the focus of research and development activities. However, the majority of these are in early discovery or preclinical development, with only one antibody that has recently completed Phase 1 studies for basal cell carcinoma. Nevertheless, it is evident that there is a continued and burgeoning interest in ion channels as therapeutic antibody targets (Figure 4(b)), where substantial progress has been made in this field recently, as reflected by the increase in the number of preclinical stage programs in 2018 (Figure 4(c)). The range of ion channel targets under investigation has broadened with 37 programs listed in 2016 directed to at least 17 ion channel targets compared to 56 programs directed to at least 23 ion channel targets in the research and development pipeline for 2018. This and several other ion channel-targeting antibodies are discussed below as case studies of antibodies with commercial interest.
Figure 4.
Ion channel targeting antibody programs in the R&D pipeline. Shown is a comparison between 2016 (a) and 2018 (b). Several ion channel targeting programs are undisclosed, such as Integral Molecular, Merck, Amgen, MedImmune, Theranyx, Ablynx and Innovative Targeting Solutions. The range of ion channel targets under investigation has broadened with 37 programs listed in 2016 compared to 56 programs in 2018. These antibody programs are directed to at least 17 targets in 2016 compared to at least 23 targets of interest in 2018, as can be observed by the increase in number of pie sectors. Selected targets of interest are denoted within the piechart layout with the number of programs indicated in brackets as the color coding of the pie sectors shifts due to the delisting of TRPM8 and the emergence of P2X2/P2X3. Since 2016, the number of programs underway for targeting Orai1 has decreased; there is a noticeable Increase in activity for Kv1.3 and P2X3; whereas Nav1.7 and Nav1.8 activity remains at a similar level. The P2X family is indicated by the black bracket line. Each target is color coded as depicted in the key to the right-hand side of each piechart. Information sourced from the public domain, such as scientific literature, company websites, etc. c. Shown are the ion channel antibodies in the R&D pipeline by stage depicting progress since 2016 to date. There is only 1 antibody program in clinical development (Ph1): nfP2X7 for basal cell carcinoma (Biosceptre). Some ion channel targets have more than one program for different therapeutic indications (for example, Kv1.3, P2X7 and CACNA2D1). Inactive programs include TRPA1 (Juno Therapeutics) and nAchR (NIH) and are listed as inactive (but not as terminated, unlike TRPM8 which is not currently listed).
Antibodies in research and development
Clinical development
nfP2X7
Biosceptre recently published Phase 1 study results for BIL010t, a polyclonal antibody that targets a non-functional form of P2X7 (nfP2X7) for the treatment of basal cell carcinoma.30 BIL010t is the first ion channel-targeting antibody to enter the clinic with the potential to become a first-in-class therapy. The company has built a pipeline focused on targeting nfP2X7 with various modalities in development (http://www.biosceptre.com/pipeline/) as outlined in further detail below.
P2X7 is an ATP-sensing pore-forming channel that can drive apoptosis by allowing rapid Ca2+ influx and downstream caspase activation.352 Non-functional variants of this channel exist, in particular, nfP2X7, which allows residual calcium flux but fails to form an apoptotic pore. This variant is expressed at high levels in many cancers, including melanomas, and presents a unique epitope, E200, at the cell surface which is not present on normal healthy cells.353 Selective exposure of this epitope makes it an ideal target for the development of therapeutic antibodies against a variety of different cancers.
BIL010t was generated by immunization of sheep using the E200 peptide sequence conjugated to keyhole limpet hemocyanin and then immunoglobulin G (IgG) was purified from the resulting sera. The disadvantage to this approach is that immune responses can differ from one individual host animal to another, leading to at least some batch-to-batch variation. Nevertheless, in vivo studies of BIL010t in a mouse melanoma preclinical model have demonstrated significant inhibition of tumor growth. BIL010t is being investigated as a topical therapy and has demonstrated safety and tolerability in this first clinical study,354 as well as providing an indication of efficacy that was confirmed by histopathological analysis of post-treatment biopsies.30 Biosceptre is building a pipeline of nfP2X7-targeting modalities: BIL03s is a human single domain antibody that has been developed for systemic administration for a number of solid tumors and will enter a Phase 1 trial imminently in Australia; a vaccine is in Phase 1 study for solid tumors (BIL06v), and an antibody-drug conjugate has progressed to preclinical development. The company’s pipeline presents the opportunity to target a broad range of other cancers, including breast, lung and prostate cancer.
Preclinical development
P2X7
P2X7 has been closely studied in cells of the hematopoietic lineage, particularly innate immune cells and lymphocytes. A collaboration between the University Medical Center Hamburg-Eppendorf and Ablynx has reported the generation of antagonist nanobodies (another type of single domain antibody) that can either block or potentiate P2X7 on T cells.355 ATP-induced gating leads to shedding of CD62L on T cells and IL-1β release, and antagonizing P2X7 may provide an alternative or complementary strategy to blocking IL-1β. With excellent specificity for P2X7, antagonist nanobodies in modular bivalent format significantly increased the potency of the nanobody (pM) to block the IL-1β inflammatory response in whole human blood, preventing pore formation, which in turn led to cell death in P2X7 transfected cells. In the presence of 100 µM ATP, these nanobodies demonstrate an IC50 of 0.1 nM with 1000-fold superior potency and efficacy over existing benchmark compounds (KN-62 and A438079). In vivo function was demonstrated using surrogate mouse nanobodies in an antibody-induced nephritis model, where administration of trivalent formated, half-life extended anti-P2X7 nanobodies could modulate P2X7 dependent pathology, as well as in a DNFB-sensitized mouse model for allergic dermatitis. The lead candidate, Dano1, is being progressed through preclinical development for the treatment of inflammation and neurological disease, such as rheumatoid arthritis, inflammatory bowel disease, COPD, multiple sclerosis, renal injury and graft-vs-host disease (GvHD), however no development has been reported since the acquisition of Ablynx by Sanofi in early 2018. It is noteworthy that agonist nanobodies were also identified, e.g., Dano5, which induced shedding of CD27 and cell death of P2X7-postive T cells, that could have utility for immuno-oncology applications.
P2X3 and P2X2/P2X3
Rinat (a subsidiary of Pfizer) recently described the modulation of P2X3 and P2X2/3 ion channels by mAbs.356 These ligand-gated ion channels have clinical relevance in pain sensation, such as inflammatory and visceral pain, cancer pain (particularly in bone and HNSCC), as well as chronic cough. Mouse mAbs were generated using standard hybridoma technology following immunization of balb/c mice with purified recombinant full-length P2X3 expressed in mammalian cells that was solubilized in dodecyl maltoside detergent and combined with Gerbu adjuvant to enhance the immune response.356 The resulting panel of hybridoma antibodies exhibited different functional effects depending on homomeric or heteromeric composition of the ion channel, as well as the kinetic state and the duration of antibody exposure. Binding to the native channel was confirmed by fluorescence-activated cell sorting (FACS) and immunocytochemistry with functional activity assessed by Ca2+ flux and electrophysiology using the whole cell voltage clamp technique. Short-term exposure with one mAb, 12D4, that bound the desensitized conformation was shown to block 80% of homomeric P2X3 αβ-meATP-activated inward currents at 0.3 µM with an IC50 of 16 nM, whereas the same antibody potentiated αβ-methylene ATP-evoked currents mediated by heteromeric P2X2/3. Interestingly, long-term exposure (24h) reversed the potentiating effect of 12D4 on P2X2/3 mediated current and led to potent inhibition. It was suggested that these differences are based on the composition of the channel (i.e., homomeric vs heteromeric) and the length of time for exposure, as well as differences in binding affinities and epitopes. In addition, mAb 12D4 rapidly internalizes and disappears from plasma membranes of P2X3 expressing cells. Efficacious in vivo activity was confirmed for reversing visceral pain in a 2,4,6-trinitrobenzene sulphonic acid-induced colitis rodent model, where the effects were reversible. However, no effect was seen in a complete Freunds adjuvant rodent model for inflammatory pain, nor in a 0.5% formalin test rodent model. Whilst showing promise for the therapeutic treatment of chronic pain, further work will be required to humanize mAb 12D4 and evaluate its effect in other models, including non-human primate.
Shark-derived VNAR (another type of single domain antibody) antagonists to P2X3 (OSX300) are being developed by Ossianix in collaboration with Lundbeck A/S for the treatment of chronic pain. The Ossianix discovery platform encompasses both semi-synthetic phage display libraries based on specific VNAR isoforms and immunized repertoires derived from nurse shark lymphocytes.357 Integral Molecular in collaboration with Crystal Bioscience (acquired in late 2017 by Ligand Pharmaceuticals for their transgenic chicken platform) presented data in 2016 indicating that they had also successfully raised chicken antibodies to P2X3, achieving sub-nanomolar affinities, potent inhibition of Ca2+ flux and in an ex vivo model for pain inhibition (90% dorsal root ganglion inhibition).358
Kv1.3
Kv1.3 is a well-validated therapeutic target for immune-modulation, and a number of companies have described preclinical data of Ig-based drug candidates in their pipelines. Ablynx has reported generating highly potent and selective nanobodies with in vivo proof-of-concept93 where the first extracellular loop (ECL1) has been shown to be essential for binding. Binding affinities are sub-nanomolar in bivalent/biparatopic format with greater than 10,000-fold selectivity for Kv1.3 than other related ion channels. Electrophysiology studies confirmed the lack of measurable off-target current blocking over the closest-related Kv1 family members and hERG, as well as functional activity that is comparable to the benchmark ShK toxin. Bivalent nanobody constructs demonstrated a fast onset of blocking activity on TEM cells, thereby halting T-cell activation, as well as an increased duration of effects. Both bivalent and trivalent constructs (anti-human serum albumin improved serum half-life) were assessed in vivo using a rodent delayed type hypersensitivity model and found to be comparable to ShK in efficacy. Whilst these nanobodies show promise as potential therapeutics, no further development has been reported recently.
The rational design of a bovine antibody has been used to generate a selective immunosuppressive mAb targeting Kv1.3.359 This was achieved by grafting the toxin peptide sequences for Moka-1 toxin and Vm24-toxin into the ultra-long bovine heavy chain complementarity-determining region 3 (CDR3). The resulting mAb, SVN-001, demonstrated good selectivity and potency against effector human TEM cells, a significantly improved plasma half-life and serum stability compared with the parent peptide, as well as potent in vivo efficacy.359 By targeting a unique subset of immune cells, SVN-001 is not broadly immunosuppressive, which improves the safety profile compared to typical immunosuppressants.
TetraGenetics, in collaboration with Crystal Biosciences (acquired by Ligand Pharmaceuticals) and argenx, has generated the first conventional light and heavy chain antagonist anti-Kv1.3 mAbs to be advanced into preclinical studies.77 Recombinant Kv1.3 was used for the immunization of chickens and llamas, as well as for antibody screening. The resulting panel of lead candidate antibodies show a high degree of potency (IC50 < 10 nM) in blocking Kv1.3 currents and the desired selectivity over related Kv family members that would be expected for mAbs. These antibodies are currently being developed for the treatment of Type 1 diabetes, although, as is common for Kv1.3 antagonists, the potential treatment of other autoimmune diseases by targeting Kv1.3 overexpression in TEM cells is being explored.
Kv10.1
The aggressive behavior of pituitary tumor cells has been shown to correlate with high expression levels of HER2 and the Kv10.1 channel.191 Therefore, it may be possible to target these cells via combination antibody therapy or possibly even a bispecific antibody format. Furthermore, Kv10.1 shares some homology with Kv11.1 in the inner vestibule area of the ion channel,360 and so antibodies with superior selectivity compared to small molecules (where cardiac safety is a major concern) would presumably present a significant advantage.
Until very recently, no specific Kv10.1 peptide toxin has been reported; however, a novel specific Kv10.1 inhibitor from the sea anemone Anthopleura elegantissima has now been identified.361 Prior to this, there was only one group (at the Max-Planck Institute of Experimental Medicine) who had described an antibody (mAb56) that demonstrates exquisite specificity for Kv10.1 mediated by binding to the E3 region61 and does not block Kv11.1 or the sub-family member Kv10.2. mAb56 was generated by immunization of mice using a fusion protein that incorporated the E3 region and tetramerizing domain of Kv10.1, followed by standard hybridoma methodology.185 Current inhibition in Kv10.1-expressing HEK293 and neuroblastoma cells has been demonstrated with mAb56 showing dose-dependent effects and yielding an IC50 value of 73 ± 47 nmol/L in HEK293 cells. Additionally, the antibody’s ability to inhibit tumor cell growth was confirmed both in vitro using anchorage-independent cancer cell growth assays and in vivo in both MDA-MB-435S human breast cancer and PAXF1657 human pancreas carcinoma xenograft models.61 It is not known, however, if this mAb will progress into clinical development.
The same group has also demonstrated the induction of tumor cell-selective apoptosis by targeting Kv10.1 via a bifunctional antibody that is a fusion protein comprising an anti-Kv10.1 scFv antibody fragment and the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).362 This antibody entity, scFv62-TRAIL, has also been reported to sensitize prostate cancer cells and other cancer cells to chemotherapy drugs, such as doxorubicin,362,363 which could provide a potential means to overcome drug resistance. The full-length IgG from which scFv62 was derived has been used for in vivo imaging of tumor xenografts, providing further evidence of the utility of these antibodies in targeted cancer therapy.199
Kv11.1B
KV11.1 (also known as hERG1) channels are often overexpressed in human cancers, but targeting them risks cardiotoxicity. CD-160,130 is a small molecule compound that blocks Kv11.1 channels with a higher efficacy for the Kv11.1 isoform B,121 and shows anti-tumor effects without the cardiovascular toxicity induced by Kv11.1 blockade. This validates the strategy to explore selective targeting of this isoform with antibodies for therapeutic benefit without the associated risk of cardiac arrhythmia, for example, by using a tumor-specific bifunctional antibody (similar to the scFv62-TRAIL strategy) or tumor-targeted nanoparticles.24,364 In the latter case, by linking antibodies that recognize tumor-specific cell surface receptors to nanoparticles, cellular uptake (and drug delivery) is dramatically enhanced,365 which could further improve the safety and efficacy of targeting Kv11.1 on cancer cells.24,366 Indeed, a proof-of-concept study has been described for the conjugation of a Kv11.1 mAb (specific against the S5 pore of Kv11.1)367 to dicarboxylic acid-terminated pegylated titanium oxide (PEG TiO2) nanoparticles for the targeting of pancreatic adenocarcinoma cells.364 Recent preclinical studies have demonstrated that antibody-targeted nanoparticles have better anti-tumor activity compared to non-targeted nanoparticles, due to more efficient localization and penetration into the tumor.365 It is also worth noting that neoplastic cells are often depolarized and their changes in membrane potential slow, even when these changes oscillate in phase with the cell cycle stages. Therefore, the proportion of time spent by a voltage-gated channel in a given state can be very different in tumors than in excitable cells.368 In another study, in vitro proof-of-concept was attained using doxorubicin-loaded, PEGylated gold nanoparticles conjugated to a commercially available anti-Kv11.1 polyclonal antibody preparation for the targeting of PANC1 cells.369 Preliminary results suggest that this approach has the potential to significantly enhance the therapeutic index of doxorubicin. The next logical step would be in vivo evaluation of these strategies using a mAb specific to the Kv11.1B isoform in order to assess targeting efficacy and safety, functional potency, biodistribution and bioavailability.
Discovery
Sodium channels, particularly members of the Nav family, have been the focus of intense drug discovery efforts. Notably, the key role that Nav1.7 plays in pain pathways and obtaining a selective subtype targeting molecule that avoids off-target related functions, such as cardiovascular side effects,52,370 has made this a priority in many neuroscience departments. Progress has been impeded due to the high sequence similarity with other Nav channels, especially in the pore region where the binding sites of small molecules are located.149 A selective neutralizing mAb to Nav1.7, SVmab1, was reported to have been generated by immunization with a peptide sequence corresponding to the loop between S3 and S4 using Abmart’s SEAL™ technology with standard hybridoma methodology.371 Subsequent characterization determined that SVmab1 bound to the voltage-sensor paddle of Nav1.7 and inhibited Nav1.7 in transiently expressing HEK cells as determined by whole cell voltage clamping.371 SVmab1 was also found to suppress acute and chronic itch in mouse models and inhibit chronic itch-potentiated synaptic transmission in spinal cord slices.371 However, following the excitement surrounding the initial discovery, subsequent work was unable to replicate the previously reported effects using a recombinant version of the mAb generated using published sequences.372 The lack of effectiveness of recombinant SVmab1 was recently confirmed by the group who initially discovered the antibody, but who also reaffirmed the activity of SVmab1 derived from two different batches of hybridoma-derived SVmab1, albeit with batch differences observed.373 The reasons offered for the apparent discrepancy in functionality between the different mAb preparations were potential differences in post-translational modifications between hybridoma- and HEK293- derived antibodies, batch-to-batch variability in hybridoma-generated antibody material and possible errors in antibody sequencing, although the authors indicate the latter possibility is remote.373
Whilst many small molecules have been investigated for their potential use for pain control, an interesting observation has been reported that the more specific the molecule, the less analgesic the effect.156 It remains to be seen if antibodies that target specific members of the Nav family can overcome this conundrum and provide an advantage, for example, by modulating channel activity in a therapeutically beneficial way. It appears the biopharmaceutical industry is not yet convinced, as many antibody programs highlighted only a few years ago have since been discontinued.374
At the same time, this challenging subclass of ion channel is still the target focus of many efforts with various modalities under investigation, including the insertion of toxin peptides or knottins (Cysteine knot mini-proteins) into the peripheral CDR loops of an IgG structure to gain specificity and potency, as shown by IONTAS in 2017.375 Similar proof-of-concept experiments that demonstrate the viability of this approach have been achieved with Kv1.3 (ShK toxin), KCa3.1 (kaliotoxin) and ASIC1a (psalmatoxin). A similar warhead approach using toxin analogs has been reported whereby the Nav1.7 GpTx-1 peptide toxin was tethered to antibodies to generate bifunctional molecules and utilizes FcRn-based antibody recycling to target Nav1.7 function.376
In the absence of purified ion channel protein that maintains a biologically relevant conformation, another approach that is under assessment by Visterra Inc. for the generation of mAbs directed to the voltage-sensing domain of Nav1.7 harnesses several strategies, namely, the use of yeast surface display and immunization using a chimeric format of the voltage-sensing domain fused to the prokaryotic NavAb channel, as described in 2017.377 The chimeric ion channel was purified and reconstituted into nanodiscs for use as antigen. Interestingly, in this study, single domain antibodies were found to engage the ion channel more efficiently. Immunizations of multiple host species that implement both DNA and nanodiscs with and without adjuvant are underway. The resulting immune repertoire will be deep-mined using microfluidics to identify specific mAbs, so further progress remains to be reported.
More encouragingly for antibody discovery, Amgen has reported the successful targeting of TRPA1 with antagonist murine mAbs that can block multiple modes of TRPA1 activation.378 Rather than the use of peptides as antigen, various other formats were utilized for immunization, including DNA, whole cells stably expressing TRPA1 and recombinant adenoviral vector expressing TRPA1 (which incorporated immune-modulating modifications to enhance the immune response). Standard hybridoma methodology was employed, and resulting stable clones were screened for binding to TRPA1 by FACS, followed by evaluation of the purified IgG molecules for functional activity in calcium uptake assays and blocking of TRPA1 activation. A panel of mAbs was identified that demonstrated dose-dependent inhibition of TRPA1 with the most potent mAb exhibiting IC50 values of 260 nM in the agonist (allyl isothiocyanate) blocking assay and 90 nM in the cold activation assay. Although not confirmed, it has been suggested that these mAbs bind to the pore region of TRPA1, which would be in keeping with other observations that antagonist ion channel targeting antibodies often bind to the third extracellular loop that forms the pore in many ion channels.50,348 The study also notes that only partial inhibition was achieved. Perhaps other antibody formats, such as nanobodies or Cowbodies that have an ultra-long heavy chain CDR3 loop, would bind epitopes otherwise inaccessible to full-length IgG antibodies and thereby achieve better inhibition.
Novo Nordisk has described the generation of antagonist antibodies to the Orai1 pore-forming subunits of CRAC in a study to assess antibody-mediated effects for SOCE in T cells.170 They have successfully identified a specific anti-human Orai1 mAb directed to the second extracellular loop that was generated by using a peptide sequence from the extracellular loop conjugated to bovine serum albumin for immunization and standard hybridoma generation. An ELISA- and FACS-positive antibody, 10F8, was able to inhibit T cell effector function in vitro, possibly through the contribution of antibody-mediated internalization of Orai1. Inhibition of effector function was also demonstrated in vivo by using a humanized GvHD mouse model, which confirmed a reduction in T cell proliferation and pro-inflammatory cytokine production. In addition, 10F8 was used to characterize Orai1 expression on immune cell subsets from blood and rheumatoid arthritis synovial fluid providing further validation of Orai1 as a target for autoimmune disease. Whilst the efficacy of this mAb has been demonstrated both in vitro and in vivo, no further information is currently available as to the progress of this molecule.
Amgen has described the targeting of Glycine Receptor α3 (GlyRα3), a Cys-loop receptor class ion channel implicated in pain. Glycine binding to the ECD triggers a conformational change, opening the ion channel to chloride ions. A specific antibody, AM-3607, directed to GlyRα3 was shown to enhance the response to exogenous glycine and was used in structural studies in complex with the pentameric GlyRα3 to further understand potentiator mechanism. The resulting crystal structure was resolved to 2.6Å, revealing that the mAb binding site was 10Å above the agonist binding site, suggesting a novel positive allosteric binding site.379 Whilst this provides valuable insights for structure-based drug discovery, no further therapeutic development is planned for this mAb.
Finally, Regeneron has generated mAbs to the ASIC1 ion channel using a DNA immunization approach and the VelocImmune transgenic mouse platform, as presented in 2016.380 Binders to cells expressing ASIC1 were identified from hybridoma clones and antibody diversity was evaluated by differential antigen disruption,381 where the effect of chemical disruption of the cell surface antigen is assessed by FACS analysis to produce a heat map that indicates the diversity of epitope coverage. Twelve individual mAbs were identified from 106 binders for further profiling.382 Select mAbs were shown to inhibit the pain response in vivo in a model of carrageen-induced muscle hyperalgesia, using a dose range of 10–40 mg/kg. However, no further development has recently been reported. More recently, the Shanghai Institute for Advanced Immunochemical Studies in collaboration with the Scripps Research Institute reported the successful isolation of ASIC1a antagonist mAbs by using the nanodisc antigen format. These mAbs were selective and potent in both in vitro testing and an in vivo middle cerebral artery occlusion (MCAO)-induced ischemia stroke model.383
Future directions
Currently, there are no marketed mAb-based therapeutics that target an ion channel, with only one polyclonal antibody targeting a non-functional form of P2X7 (nfP2X7) in Phase 1 clinical development for the treatment of basal cell carcinoma.30 Ion channels still remain significantly underexploited as antibody drug targets17 due mainly to the challenges of expressing sufficient protein in a biologically relevant conformation for antibody discovery purposes, as well as epitope accessibility and screening approaches. However, advances made in generating crystal structures and, more recently, cryo-electron microscopy structures, coupled with the deepening knowledge of ion channel gating and target biology validation now provide an informed base on which to progress and streamline antibody-based approaches in targeting ion channels to treat a variety of diseases.
Conclusions
Ion channels are widely recognized as important therapeutic targets in a range of diseases, but remain a challenge for drug discovery. There is still a paucity of functional ion channel mAbs described in the literature, and several of the examples cited here are drawn from recent conferences reflecting the most current advances and innovations enabling the discovery and development of therapeutic mAbs directed towards this important drug target subclass. Despite the significant focus on Nav channels, in particular Nav1.7, very little success has been reported. However, a review of the therapeutic pipeline suggests that the ligand-gated P2X family and Kv1.3 are likely to yield the initial successes for ion channel-targeting mAbs. The P2X family possesses a larger extracellular region than many other ion channels, and therefore is easier to target. The increasing number of ion channel structures being published will assist our understanding of this drug class and enable a deeper knowledge of the biology involved. Progress has been made in overcoming some of the technical challenges associated with ion channel expression and antibody screening, which are likely to facilitate the identification of new functional antibodies moving forward.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ALDH1
aldehyde dehydrogenase
- AML
acute myeloid leukaemia
- ASIC
acid-sensing ion channel
- CACC
calcium activated chloride channel
- Cav
voltage gated calcium channel family member
- CDR
Complementarity-determining region
- CNS
central nervous system
- CFTR
cystic fibrosis transmembrane conductance regulator
- CRAC
calcium release-activated calcium channel
- CRC
colorectal cancer
- CSC
cancer stem cell
- ECD
extracellular domain
- E. coli
Escherichia coli
- EPCs
endothelial progenitor cells
- FSGS
Focal segmental glomerulosclerosis
- GPCR
G protein-coupled receptor
- HNSCC
head and neck squamous cell carcinoma
- Kv
voltage-gated potassium channel family member
- mAb
monoclonal antibody
- Nav
voltage-gated sodium channel family member
- NSCLC
non-small cell lung cancer
- pAb
polyclonal antibody
- scFv
single-chain variable fragment
- ShK
Stichodactyla toxin
- SigR1
sigma-1 receptor
- SMALPs
styrene-maleic acid lipid particles
- SOCE
store-operated calcium entry
- STIM1
stromal interaction molecule 1
- TM
transmembrane
- TMD
transmembrane domain
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- VGIC
voltage gated ion channel
- VGCC
voltage gated calcium channels
Disclosure statement
Authors T.G. Clark and P. Colussi are employees of TetraGenetics Inc. and C.J. Hutchings provides consulting services to TetraGenetics Inc.
References
- 1.Clare JJ. Targeting voltage-gated sodium channels for pain therapy. Expert Opin Investig Drugs. 2010;19:45–62. doi: 10.1517/13543780903435340. [DOI] [PubMed] [Google Scholar]
- 2.Overington JP, Al-Lazikani B, Hopkins AL.. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 3.Finan C, Gaulton A, Kruger FA, Lumbers RT, Shah T, Engmann J, Galver L, Kelley R, Karlsson A, Santos R, et al. The druggable genome and support for target identification and validation in drug development. Sci Transl Med. 2017;9:383. doi: 10.1126/scitranslmed.aag1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terstappen GC, Roncarati R, Dunlop J, Peri R. Screening technologies for ion channel drug discovery. Future Med Chem. 2010;2:715–730. doi: 10.4155/fmc.10.180. [DOI] [PubMed] [Google Scholar]
- 5.Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, Karlsson A, Al-Lazikani B, Hersey A, Oprea TI, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16:19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickenden A, Priest B, Erdemli G. Ion channel drug discovery: challenges and future directions. Future Med Chem. 2012;4:661–679. doi: 10.4155/fmc.12.4. [DOI] [PubMed] [Google Scholar]
- 7.Krafte D, Erikson D. Ion Channels increasingly enticing targets for drug discovery. Drug Disc World. Summer 2016. http://www.ddw-online.com/drug-discovery/p315007-ion-channels-increasingly-enticing-targets-for-drug-discovery.html. [Google Scholar]
- 8.Imbrici P, Liantonio A, Camerino GM, De Bellis M, Camerino C, Mele A, Giustino A, Pierno S, De Luca A, Tricarico D, et al. Therapeutic approaches to genetic ion channelopathies and perspectives in drug discovery. Front Pharmacol. 2016;7:121. doi: 10.3389/fphar.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent A. Developments in autoimmune channelopathies. Autoimmune Rev. 2013;6:678–681. doi: 10.1016/j.autrev.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Kalia J, Milescu M, Salvatierra J, Wagner J, Klint JK, King GF, Olivera BM, Bosmans F. From foe to friend: using animal toxins to investigate ion channel function. J Mol Biol. 2015;427:158–175. doi: 10.1016/j.jmb.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degueldre M, Verdenaud M, Legarda G, Minambres R, Zuniga S, Leblanc M, Gilles N, Ducancel F, De Pauw E, Quinton L. Diversity in sequences, post-translational modifications and expected pharmacological activities of toxins from four Conus species revealed by the combination of cutting-edge proteomics, transcriptomics and bioinformatics. Toxicon. 2017;130:116–125. doi: 10.1016/j.toxicon.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Schmidtko A, Lötsch J, Freynhagen R, Geisslinger G. Ziconotide for treatment of severe chronic pain. Lancet. 2010;375:1569–1577. doi: 10.1016/S0140-6736(10)60354-6. [DOI] [PubMed] [Google Scholar]
- 13.Vincent D. Company report. 2015. June 9 http://www.bionomics.com.au/upload/investors/analystcoverage/Shaw%20Research%20Bionomics%20Limited%20(BNO)%20-%20A%20Paradigm%20Shift%20in%20Ion%20Channel%20….pdf.
- 14.Condren ME, Bradshaw MD. Ivacaftor: a novel gene-based therapeutic approach for cystic fibrosis. J Pediatr Pharmacol Ther. 2013;18:8–13. doi: 10.5863/1551-6776-18.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol. 2010;77:69–78. doi: 10.1124/mol.109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGivern JG. Ziconotide: a review of its pharmacology and use in the treatment of pain. Neuropsychiatr Dis Treat. 2007;3:69. doi: 10.2147/nedt.2007.3.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oprea TI, Bologa CG, Brunak S, Campbell A, Gan GN, Gaulton A, Gomez SM, Guha R, Hersey A, Holmes J, et al. Unexplored therapeutic opportunities in the human genome. Nat Rev Drug Discov. 2018;17:377. doi: 10.1038/nrd.2018.52. [DOI] [PubMed] [Google Scholar]
- 18.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- 19.Hutchings CJ, Koglin M, Marshall FH. Therapeutic antibodies directed at G protein-coupled receptors. mAbs. 2010;2:594–606. doi: 10.4161/mabs.2.5.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchings CJ, Koglin M, Olson WC, Marshall FH. Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat Rev Drug Discov. 2017;16:661. doi: 10.1038/nrd.2017.173. [DOI] [PubMed] [Google Scholar]
- 21.Barden JA, Yuksel A, Pedersen J, Danieletto S, Delprado W. Non-functional P2X7: a novel and ubiquitous target in human cancer. J Clin Cell Immunol. 2014;5:4. doi: 10.4172/2155-9899.1000237. [DOI] [Google Scholar]
- 22.Brackenbury WJ, Chioni AM, Diss JK, Djamgoz MB. The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2007;101:149–160. doi: 10.1007/s10549-006-9281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen AP, Bentzen BH, Grunnet M. Differential effects of Kv11.1 activators on Kv11.1a, Kv11.1b and Kv11.1a/Kv11.1b channels. Br J Pharmacol. 2010;161:614–628. doi: 10.1111/j.1476-5381.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arcangeli A, Becchetti A. Novel perspectives in cancer therapy: targeting ion channels. Drug Resist Updat. 2015;21-22:11–19. doi: 10.1016/j.drup.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S, Moriyama C, Watanabe T, Takubo R, Doi Y, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralisation. Nat Biotechnol. 2010;28:1203–1207. doi: 10.1038/nbt.1691. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Constantinou A, Deonarain M. Modulating antibody pharmacokinetics using hydrophilic polymers. Expert Opin Drug Deliv. 2011;8:1221–1236. doi: 10.1517/17425247.2011.602399. [DOI] [PubMed] [Google Scholar]
- 27.Constantinou A, Chen C, Deonarain MP. Modulating the pharmacokinetics of therapeutic antibodies. Biotechnol Lett. 2010;32:609–622. doi: 10.1007/s10529-010-0214-z. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9:63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sammar M, Spira G, Meiri H. Depolarization exposes the voltage sensor of the sodium channels to the extracellular region. J Membr Biol. 1992;125:1–11. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert SM, Gidley Baird A, Glazer S, Barden JA, Glazer A, Teh LC, King J. A phase I clinical trial demonstrates that nfP2X7 -targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br J Dermatol. 2017;177:117–124. doi: 10.1111/bjd.15364. [DOI] [PubMed] [Google Scholar]
- 31.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang L, Gamal El-Din TM, Payandeh J, Martinez GQ, Heard TM, Scheuer T, Zheng N, Catterall WA. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature. 2014;505:56–61. doi: 10.1038/nature12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoder N, Yoshioka C, Gouaux E. Gating mechanisms of acid-sensing ion channels. Nature. 2018;555:397–401. doi: 10.1038/nature25782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahn K, Franke C, Bufler J. Mechanism of block of nicotinic acetylcholine receptor channels by purified IgG from seropositive patients with myasthenia gravis. Neurology. 2000;54:474–479. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B, Rothhammer V, Chan A, Gold R, Berthele A. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367:115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marnetto F, Valentino P, Caldano M, Bertolotto A. Detection of potassium channel KIR4.1 antibodies in Multiple Sclerosis patients. J Immunol Methods. 2017;445:53–55. doi: 10.1016/j.jim.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Vincent A, Lang B, Newsom-Davis J. Autoimmunity to the voltage-gated calcium channel underlies the Lambert-Eaton myasthenic syndrome, a paraneoplastic disorder. Trends Neurosci. 1989;12:496–502. [DOI] [PubMed] [Google Scholar]
- 39.Park SB, Lin CS, Krishnan AV, Simon NG, Bostock H, Vincent A, Kiernan MC. Axonal dysfunction with voltage gated potassium channel complex antibodies. Exp Neurol. 2014;261:337–342. doi: 10.1016/j.expneurol.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Dhingra A, Fina ME, Neinstein A, Ramsey DJ, Xu Y, Fishman GA, Alexander KR, Qian H, Peachey NS, Gregg RG. Autoantibodies in melanoma-associated retinopathy target TRPM1 cation channels of retinal ON bipolar cells. J Neurosci. 2011;31:3962–3967. doi: 10.1523/JNEUROSCI.6007-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis in psychiatry. J Curr Psychiatry Rev. 2011;7:189–193. doi: 10.2174/157340011797183184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner MR, Irani SR, Leite MI, Nithi K, Vincent A, Ansorge O. Progressive encephalomyelitis with rigidity and myoclonus: glycine and NMDA receptor antibodies. Neurology. 2011;77:439–443. doi: 10.1212/WNL.0b013e318227b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misawa T, Mizusawa H. Anti-VGKC antibody-associated limbic encephalitis/Morvan syndrome. Brain Nerve. 2010;62:339–345. [PubMed] [Google Scholar]
- 44.Tomimitsu H, Arimura K, Nagado T, Watanabe O, Otsuka R, Kurono A, Sonoda Y, Osame M, Kameyama M. Mechanism of action of voltage-gated K+ channel antibodies in acquired neuromyotonia. Ann Neurol. 2004;56:440–444. doi: 10.1002/(ISSN)1531-8249. [DOI] [PubMed] [Google Scholar]
- 45.Sun H, Luo L, Lal B, Ma X, Chen L, Hann CL, Fulton AM, Leahy DJ, Laterra J, Li M. A monoclonal antibody against KCNK9 K+ channel extracellular domain inhibits tumour growth and metastasis. Nat Comm. 2016;7:10339. doi: 10.1038/ncomms10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan C, Long R, You Y, Wang J, Yang X, Huang S, Sheng Y, Peng X, Liu H, Wang Z, et al. A novel PADRE-Kv1.3 vaccine effectively induces therapeutic antibodies and ameliorates experimental autoimmune encephalomyelitis in rats. Clin Immunol. 2018;193:98–109. doi: 10.1016/j.clim.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Anderson PA, Greenberg RM. Phylogeny of ion channels: clues to structure and function. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:17–28. [DOI] [PubMed] [Google Scholar]
- 48.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 49.Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- 50.Naylor J, Beech DJ. Extracellular ion channel inhibitor antibodies. The Open Drug Discovery Journal. 2009;1:36–42. doi: 10.2174/1877381800901010036. [DOI] [Google Scholar]
- 51.Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, Sidders B, Skerratt SE, Stevens EB, Storer RI, et al. Ion channels as therapeutic targets: a drug discovery perspective. J Med Chem. 2013;56:593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- 52.Bagal SK, Marron BE, Owen RM, Storer RI, Swain NA. Voltage gated sodium channels as drug discovery targets. Channels (Austin). 2015;9:360–366. doi: 10.1080/19336950.2015.1079674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beech DJ, Sukumar P. Channel regulation by extracellular redox protein. Channels (Austin). 2007;1:400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosado JA, Brownlow SL, Sage SO. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- 55.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.RES.88.1.84. [DOI] [PubMed] [Google Scholar]
- 56.Antoniotti S, Lovisolo D, Fiorio Pla A, Munaron L. Expression and functional role of bTRPC1 channels in native endothelial cells. FEBS Lett. 2002;510:189–195. doi: 10.1016/S0014-5793(01)03256-2. [DOI] [PubMed] [Google Scholar]
- 57.Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Cα phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- 58.Xu SZ, Boulay G, Flemming R, Beech DJ. E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol. 2006;291:H2653–9. doi: 10.1152/ajpheart.00495.2006. [DOI] [PubMed] [Google Scholar]
- 59.Naylor J, Milligan CJ, Zeng F, Jones C, Beech DJ. Production of a specific extracellular inhibitor of TRPM3 channels. Br J Pharmacol. 2008;155:567–573. doi: 10.1038/bjp.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiwari-Woodruff S, Beltran-Parrazal L, Charles A, Keck T, Vu T, Bronstein J. K+ channel KV3.1 associates with OSP/claudin-11 and regulates oligodendrocyte development. Am J Physiol Cell Physiol. 2006;291:C687–98. doi: 10.1152/ajpcell.00116.2006. [DOI] [PubMed] [Google Scholar]
- 61.Gómez-Varela D, Zwick-Wallasch E, Knötgen H, Sánchez A, Hettmann T, Ossipov D, Weseloh R, Contreras-Jurado C, Rothe M, Stühmer W. Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res. 2007;67:7343–7349. doi: 10.1158/0008-5472.CAN-07-0107. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal J, Griesinger F, Stuhmer W, Pardo L. The potassium channel Ether a go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. Mol Cancer. 2010;9:18. doi: 10.1186/1476-4598-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chioni AM, Fraser SP, Pani F, Foran P, Wilkin GP, Diss JK, Djamgoz MB. A novel polyclonal antibody specific for the Nav1.5 voltage-gated Na+ channel ‘neonatal’ splice form. J Neurosci Methods. 2005;147:88–98. doi: 10.1016/j.jneumeth.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 64.Ebersbach H, Geisse S. Antigen generation and display in therapeutic antibody drug discovery — a neglected but critical player. Biotechnol J. 2012;7:1433–1443. doi: 10.1002/biot.201100407. [DOI] [PubMed] [Google Scholar]
- 65.Hartshorne RP, Catterall WA. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984;259:1667–1675. [PubMed] [Google Scholar]
- 66.Ellisman MH, Miller JA, Agnew WS. Molecular morphology of the tetrodotoxin-binding sodium channel protein from Electrophorus electricus in solubilizaed and reconstituted preparations. J Cell Biol. 1983;97:1834–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenberg RL, Tomiko SA, Agnew WS. Reconstitution of neurotoxin-modulated ion transport by the voltage regulated sodium channel isolated from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1984;81:1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talvenheimo JA, Tamkun MM, Messner DJ, Hartshorne RP, Sharkey RM, Catterall WA. Structure and functional reconstitution of the sodium channel from rat brain. Biophys J. 1984;45:37–40. doi: 10.1016/S0006-3495(84)84098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamkun MM, Talvenheimo JA, Catterall WA. The sodium channel from rat brain. Reconstitution of neurotoxinactivated ion flux and scorpion toxin binding from purified components. J Biol Chem. 1984;259:1676–1688. [PubMed] [Google Scholar]
- 70.Trainer VL, Moreau E, Guedin D, Baden DG, Catterall WA. Neurotoxin binding and allosteric modulation at receptor sites 2 and 5 on purified and reconstituted rat brain sodium channels. J Biol Chem. 1993;268:17114–17119. [PubMed] [Google Scholar]
- 71.Duret G, Van Renterghem C, Weng Y, Prevost M, Moraga-Cid G, Huon C, Sonner JM, Corringer PJ. Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc Natl Acad Sci U S A. 2011;108:12143–12148. doi: 10.1073/pnas.1104494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mnatsakanyan N, Nishtala SN, Pandhare A, Fiori MC, Goyal R, Pauwels JE, Navetta AF, Ahrorov A, Jansen M. Functional chimeras of GLIC obtained by adding the intracellular domain of anion- and cation-conducting cys-loop receptors. Biochemistry. 2015;54:2670–2682. doi: 10.1021/acs.biochem.5b00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Payandeh J, Minor DL Jr.. Bacterial voltage-gated sodium channels (BacNa(V)s) from the soil, sea, and salt lakes enlighten molecular mechanisms of electrical signaling and pharmacology in the brain and heart. J Mol Biol. 2015;427:3–30. doi: 10.1016/j.jmb.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahuja S, Ahuja S, Mukund S, Deng L, Khakh K, Chang E, Ho H, Shriver S, Young C, Lin S, et al. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science. 2015;350:aac5464. doi: 10.1126/science.aad1815. [DOI] [PubMed] [Google Scholar]
- 75.Eisen JA, Coyne RS, Wu M, Wu D, Thiagarajan M, Wortman JR, Badger JH, Ren Q, Amedeo P, Jones KM, et al. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 2006;9:e286. doi: 10.1371/journal.pbio.0040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cassidy-Hanley DM. Tetrahymena in the laboratory: strain resources, methods for culture, maintenance, and storage. Methods Cell Biol. 2012;109:237–276. doi: 10.1016/B978-0-12-385967-9.00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bednenko J, Harriman R, Mariën L, Nguyen HM, Agrawal A, Papoyan A, Bisharyan Y, Cardarelli J, Cassidy-Hanley D, Clark T, et al. A multi-platform strategy for the discovery of conventional monoclonal antibodies that inhibit the voltage-gated potassium channel Kv1.3. MAbs. 2018;1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shirbaghaee Z, Bolhassani A. Different applications of virus-like particles in biology and medicine: vaccination and delivery systems. Biopolymers. 2016;105:11 3–32. doi: 10.1002/bip.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thoring L, Wüstenhagen DA, Borowiak M, Stech M, Sonnabend A, Kubick S. Cell-free systems based on CHO cell lysates: optimization strategies, synthesis of “Difficult-to-Express” proteins and future perspectives. PLoS One. 2016;11:e0163670. doi: 10.1371/journal.pone.0163670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Focke PJ, Hein C, Hoffmann B, Matulef K, Bernhard F, Dötsch V, Valiyaveetil FI. Combining in vitro folding with cell free protein synthesis for membrane protein expression. Biochemistry. 2016;55:4212–4219. doi: 10.1021/acs.biochem.6b00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Renauld S, Cortes S, Bersch B, Henry X, De Waard M, Schaack B. Functional reconstitution of cell-free synthesized purified Kv channels. Biochim Biophys Acta. 2017;1859:2373–2380. doi: 10.1016/j.bbamem.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Thoring L, Dondapati SK, Stech M, Wüstenhagen DA, Kubick S. High-yield production of “difficult-to-express” proteins in a continuous exchange cell-free system based on CHO cell lysates. Sci Rep. 2017;7:11710. doi: 10.1038/s41598-017-12188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Focke PJ, Valiyaveetil FI. Studies of ion channels using expressed protein ligation. Curr Opin Chem Biol. 2010;14:797–802. doi: 10.1016/j.cbpa.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 84.Li JB, Tang S, Zheng JS, Tian CL, Liu L. Removable backbone modification method for the chemical synthesis of membrane proteins. Acc Chem Res. 2017;50:1143–1153. doi: 10.1021/acs.accounts.7b00001. [DOI] [PubMed] [Google Scholar]
- 85.Chen Q, She J, Zeng W, Guo J, Xu H, Bai XC, Jiang Y.. Structure of mammalian endolysosomal TRPML1 channel in nanodiscs. Nature. 2017; 550:415–418. doi: 10.1038/nature24035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henrich E, Peetz O, Hein C, Laguerre A, Hoffmann B, Hoffmann J, Dötsch V, Bernhard F, Morgner N. Analyzing native membrane protein assembly in nanodiscs by combined non- covalent mass spectrometry and synthetic biology. eLife. 2017;6:e20954. doi: 10.7554/eLife.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Denisov IG, Sligar SG. Nanodiscs for structural and functional studies of membrane proteins. Nat Struct Mol Biol. 2016;23:481–486. doi: 10.1038/nsmb.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knowles TJ, Finka R, Smith C, Lin YP, Dafforn T, Overduin M. Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J Am Chem Soc. 2009;131:7484–7485. doi: 10.1021/ja810046q. [DOI] [PubMed] [Google Scholar]
- 89.Dörr JM, Koorengevel MC, Schäfer M, Prokofyev AV, Scheidelaar S, van der Cruijsen EA, Dafforn TR, Baldus M, Killian JA. Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: the power of native nanodiscs. Proc Natl Acad Sci U S A. 2014;111:18607–18612. doi: 10.1073/pnas.1416205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534:347–351. doi: 10.1038/nature17964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sadler EE, Kapanidis AN, Tucker SJ. Solution-based single-molecule FRET studies of K(+) channel gating in a lipid bilayer. Biophys J. 2016;110:2663–2670. doi: 10.1016/j.bpj.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fujimoto A. Enhancement of antibody responses to native G protein-coupled receptors using E.coli GroEL as a molecular adjuvant in DNA immunization. J Immunol Methods. 2012;375:243–251. doi: 10.1016/j.jim.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Stortelers C, Pinto-Espinoza C, Van Hoorick D, Koch-Nolte F. Modulating ion channel function with antibodies and nanobodies. Curr Opin Immunol. 2018;52:18–26. doi: 10.1016/j.coi.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 94.Kang TH, Mao CP, La V, Chen A, Hung CF, Wu TC. Innovative DNA vaccine to break immune tolerance against tumor self-antigen. Hum Gene Ther. 2013;24:181–188. doi: 10.1089/hum.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Percival-Alwyn JL, England E, Kemp B, Rapley L, Davis NH, McCarthy GR, Majithiya JB, Corkill DJ, Welsted S, Minton K, et al. Generation of potent mouse monoclonal antibodies to self-proteins using T-cell epitope “tags”. MAbs. 2015;7:129–137. doi: 10.4161/19420862.2014.985489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schneider Z, Cervenak J, Baranyi M, Papp K, Prechl J, László G, Erdei A, Kacskovics I. Transgenic expression of bovine neonatal Fc receptor in mice boosts immune response and improves hybridoma production efficiency without any sign of autoimmunity. Immunol Lett. 2015;137:62–69. doi: 10.1016/j.imlet.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 97.Adler AS, Mizrahi RA, Spindler MJ, Adams MS, Asensio MA, Edgar RC, Leong J, Leong R, Roalfe L, White R, et al. Rare, high-affinity anti-pathogen antibodies from human repertoires, discovered using microfluidics and molecular genomics. MAbs. 2017;9:1282–1296. doi: 10.1080/19420862.2017.1371383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Busse CE, Czogiel I, Braun P, Arndt PF, Wardemann H. Single-cell based high-throughput sequencing of full-length immunoglobulin heavy and light chain genes. Eur J Immunol. 2014;44:597–603. doi: 10.1002/eji.201343917. [DOI] [PubMed] [Google Scholar]
- 99.Rettig TA, Ward C, Bye BA, Pecaut MJ, Chapes SK. Characterization of the naive murine antibody repertoire using unamplified high-throughput sequencing. PLoS One. 2018;13:e0190982. doi: 10.1371/journal.pone.0190982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Starkie DO, Compson JE, Rapecki S, Lightwood DJ. Generation of recombinant monoclonal antibodies from immunised mice and rabbits via flow cytometry and sorting of antigen-specific IgG+ memory B Cells. PLoS One. 2016;11:e0152282. doi: 10.1371/journal.pone.0152282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu H-B, Li M, Wang W-P, Wang X-L. High throughput screening technologies for ion channels. Acta Pharmacologica Sinica. 2016;37:34–43. doi: 10.1038/aps.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colley CS, England E, Linley JE, Wilkinson TCI. Screening strategies for the discovery of ion channel antibodies. Curr Protoc Pharmacol. 2018;82:e44. doi: 10.1002/cpph.44. [DOI] [PubMed] [Google Scholar]
- 103.Leanza L, Managò A, Zoratti M, Gulbins E, Szabo I. Pharmacological targeting of ion channels for cancer therapy: in vivo evidences. Biochim Biophys Acta. 2016;1863:1385–1389. doi: 10.1016/j.bbamcr.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 104.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-dependent ion channels in T-lymphocytes. J. Neuroimmunol. 1985;10:71–95. [DOI] [PubMed] [Google Scholar]
- 106.Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Béraud E. Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci USA. 2001;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koo GC, Blake JT, Talento A, Nguyen M, Lin S, Sirotina A, Shah K, Mulvany K, Hora D Jr, Cunningham P, et al. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J Immunol. 1997;158:5120–5128. [PubMed] [Google Scholar]
- 108.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, et al. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Azam P, Sankaranarayanan A, Homerick D, Griffey S, Wulff H. Targeting effector memory T cells with the small molecule Kv1.3 blocker PAP-1 suppresses allergic contact dermatitis. J Invest Dermatol. 2007;127:1419–1429. doi: 10.1038/sj.jid.5700717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheong A, Li J, Sukumar P, Kumar B, Zeng F, Riches K, Munsch C, Wood IC, Porter KE, Beech DJ. Potent suppression of vascular smooth muscle cell migration and human neointimal hyperplasia by Kv1.3 channel blockers. Cardiovasc Res. 2011;89:282–289. doi: 10.1093/cvr/cvq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gilhar A, Bergman R, Assay B, Ullmann Y, Etzioni A. The beneficial effect of blocking Kv1.3 in the psoriasiform SCID mouse model. J Invest Dermatol. 2011;131:118–124. doi: 10.1038/jid.2010.245. [DOI] [PubMed] [Google Scholar]
- 112.Bose T, Lee R, Hou A, Tong L, Chandy KG. Tissue resident memory T cells in the human conjunctiva and immune signatures in human dry eye disease. Sci Rep. 2017;7:45312. doi: 10.1038/srep45312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tarcha EJ, Olsen CM, Probst P, Peckham D, Muñoz-Elías EJ, Kruger JG, Iadonato SP. Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: a randomized phase 1b trial. PLoS One. 2017;12:e0180762. doi: 10.1371/journal.pone.0180762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang X, He Y, Dubuc AM, Hashizume R, Zhang W, Reimand J, Yang H, Wang TA, Stehbens SJ, Younger S, et al. EAG2 potassium channel with evolutionarily conserved function as a brain tumor target. Nat Neurosci. 2015;18:1236–1246. doi: 10.1038/nn.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen J, Ma B, Zhang X, Sun X, Han J, Wang Y, Chu L, Xu H, Yang Y. Thioridazine has potent antitumor effects on lung cancer stem-like cells. Oncol Lett. 2017;13:1563–1568. doi: 10.3892/ol.2017.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Enkhtaivan G, Kim DH, Pandurangan M. Cytotoxic effect of TDZ on human cervical cancer cells. J Photochem Photobiol B. 2017;173:493–498. doi: 10.1016/j.jphotobiol.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 117.Pillozzi S, Brizzi MF, Bernabei PA, Bartolozzi B, Caporale R, Basile V, Boddi V, Pegoraro L, Becchetti A, Arcangeli A. VEGFR-1 (FLT-1), beta1 integrin, and hERG K+ channel for a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood. 2007;110:1238–1250. doi: 10.1182/blood-2006-02-003772. [DOI] [PubMed] [Google Scholar]
- 118.Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, Bencini L, Calistri M, Messerini L, Scatizzi M, et al. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–611. [DOI] [PubMed] [Google Scholar]
- 119.Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM. Expression and significance of HERG protein in gastric cancer. Cancer Biol Ther. 2008;7:45–50. [DOI] [PubMed] [Google Scholar]
- 120.Masi A, Becchetti A, Restano-Cassulini R, Polvani S, Hofmann G, Buccoliero AM, Paglierani M, Pollo B, Taddei GL, Gallina P, et al. hERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br J Cancer. 2005;93:781–792. doi: 10.1038/sj.bjc.6602775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gasparoli L, D’Amico M, Masselli M, Pillozzi S, Caves R, Khuwaileh R, Tiedke W, Mugridge K, Pratesi A, Mitcheson JS, et al. New pyrimido-indole compound CD-160130 preferentially inhibits the KV11.1B isoform and produces antileukemic effects without cardiotoxicity. Mol Pharmacol. 2015;87:183–196. doi: 10.1124/mol.114.094920. [DOI] [PubMed] [Google Scholar]
- 122.Pillozzi S, Masselli M, De Lorenzo E, Accordi B, Cilia E, Crociani O, Amedei A, Veltroni M, D’Amico M, Basso G, et al. Chemotherapy resistance in acute lymphoblastic leukemia requires hERG1 channels and is overcome by hERG1 blockers. Blood. 2011;117:902–914. doi: 10.1182/blood-2010-01-262691. [DOI] [PubMed] [Google Scholar]
- 123.Lam J, Wulff H. The lymphocyte potassium channels Kv1.3 and KCa3.1 as targets for immunosuppression. Drug Dev Res. 2011;72:573–584. doi: 10.1002/ddr.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wulff H, Castle NA. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert Rev Clin Pharmacol. 2010;3:385–396. doi: 10.1586/ecp.09.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di L, Srivastava S, Zhdanova O, Ding Y, Li Z, Wulff H, Lafaille M, Skolnik EY. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci. 2010;107:1541–1546. doi: 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reich EP, Cui L, Yang L, Pugliese-Sivo C, Golovko A, Petro M, Vassileva G, Chu I, Nomeir AA, Zhang LK. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 2005;35:1027–1036. doi: 10.1002/eji.200425954. [DOI] [PubMed] [Google Scholar]
- 127.Chou CC, Lunn CA, Murgolo NJ. KCa3.1: target and marker for cancer, autoimmune disorder and vascular inflammation? Expert Rev Mol Diagn. 2008;8:179–187. doi: 10.1586/14737159.8.2.179. [DOI] [PubMed] [Google Scholar]
- 128.Grgic I, Kiss E, Kaistha BP, Busch C, Kloss M, Sautter J, Müller A, Kaistha A, Schmidt C, Raman G, et al. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc Natl Acad Sci USA. 2009;106:14518–14523. doi: 10.1073/pnas.0903458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ataga KI, Reid M, Ballas SK, Yasin Z, Bigelow C, James LS, Smith WR, Galacteros F, Kutlar A, Hull JH, et al. ICA-17043-10 Study Investigators. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043). Br J Haematol. 2011;153:92–104. doi: 10.1111/j.1365-2141.2010.08520.x. [DOI] [PubMed] [Google Scholar]
- 131.Ataga KI, Orringer EP, Styles L, Vichinsky EP, Swerdlow P, Davis GA, Desimone PA, Stocker JW. Dose-escalation study of ICA-17043 in patients with sickle cell disease. Pharmacotherapy. 2006;26:1557–1564. doi: 10.1592/phco.26.11.1557. [DOI] [PubMed] [Google Scholar]
- 132.Ataga KI, Smith WR, De Castro LM, Swerdlow P, Saunthararajah Y, Castro O, Vichinsky E, Kutlar A, Orringer EP, Rigdon GC, et al. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111:3991–3997. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- 133.Castro OL, Gordeuk VR, Gladwin MT, Steinberg MH. Senicapoc trial results support the existence of different sub-phenotypes of sickle cell disease with possible drug-induced phenotypic shifts. Br J Haematol. 2011;155:636–638. doi: 10.1111/j.1365-2141.2011.08758.x. [DOI] [PubMed] [Google Scholar]
- 134.Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh CC. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur J Pharmacol. 2003;471:157–164. doi: 10.1016/S0014-2999(03)01825-9. [DOI] [PubMed] [Google Scholar]
- 135.Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004;287:C125–34. doi: 10.1152/ajpcell.00488.2003. [DOI] [PubMed] [Google Scholar]
- 136.Jäger H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol. 2004;65:630–638. doi: 10.1124/mol.65.3.630. [DOI] [PubMed] [Google Scholar]
- 137.Wang ZH, Shen B, Yao HL, Jia YC, Ren J, Feng YJ, Wang YZ. Blockage of intermediate-conductance-Ca(2+) -activated K(+) channels inhibits progression of human endometrial cancer. Oncogene. 2007;26:5107–5114. doi: 10.1038/sj.onc.1210308. [DOI] [PubMed] [Google Scholar]
- 138.Turner KL, Honasoge A, Robert SM, McFerrin MM, Sontheimer H. A proinvasive role for the Ca(2+)-activated K(+) channel KCa3.1 in malignant glioma. Glia. 2014;62:971–981. doi: 10.1002/glia.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.D’Alessandro G, Grimaldi A, Chece G, Porzia A, Esposito V, Santoro A, Salvati M, Mainiero F, Ragozzino D, Di Angelantonio S, et al. KCa3.1 channel inhibition sensitizes malignant gliomas to temozolomide treatment. Oncotarget. 2016;7:30781–30796. doi: 10.18632/oncotarget.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grimaldi A, D’Alessandro G, Golia MT, Grössinger EM, Di Angelantonio S, Ragozzino D, Santoro A, Esposito V, Wulff H, Catalano M, et al. KCa3.1 inhibition switches the phenotype of glioma-infiltrating microglia/macrophages. Cell Death Dis. 2016;7:e2174. doi: 10.1038/cddis.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yin MZ, Park SW, Kang TW, Kim KS, Yoo HY, Lee J, Hah JH, Sung MH, Kim SJ. Activation of K(+) channel by 1-EBIO rescues the head and neck squamous cell carcinoma cells from Ca(2+) ionophore-induced cell death. Korean J Physiol Pharmacol. 2015;20:25–33. doi: 10.4196/kjpp.2016.20.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grössinger EM, Weiss L, Zierler S, Rebhandl S, Krenn PW, Hinterseer E, Schmölzer J, Asslaber D, Hainzl S, Neureiter D, et al. Targeting proliferation of chronic lymphocytic leukemia (CLL) cells through KCa3.1 blockade. Leukemia. 2014;28:954–958. doi: 10.1038/leu.2014.37. [DOI] [PubMed] [Google Scholar]
- 143.Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Köhler R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:704–709. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- 144.Pillozzi S, D’Amico M, Bartoli G, Gasparoli L, Petroni G, Crociani O, Marzo T, Guerriero A, Messori L, Severi M, et al. The combined activation of KCa3.1 and inhibition of Kv11.1/hERG1 currents contribute to overcome Cisplatin resistance in colorectal cancer cells. Br J Cancer. 2018;118:200–221. doi: 10.1038/bjc.2017.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ohkubo T, Yamazaki J. T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int J Oncol. 2012;41:267–275. doi: 10.3892/ijo.2012.1422. [DOI] [PubMed] [Google Scholar]
- 146.Rao VR, Perez-Neut M, Kaja S, Gentile S. Voltage-gated ion channels in cancer cell proliferation. Cancers (Basel). 2015;7:849–875. doi: 10.3390/cancers7020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Díaz-Lezama N, Hernández-Elvira M, Sandoval A, Monroy A, Felix R, Monjaraz E. Ghrelin inhibits proliferation and increases T-type Ca2+ channel expression in PC-3 human prostate carcinoma cells. Biochem Biophys Res Commun. 2010;403:24–29. doi: 10.1016/j.bbrc.2010.10.100. [DOI] [PubMed] [Google Scholar]
- 148.Deuis JR, Dekan Z, Wingerd JS, Smith JJ, Munasinghe NR, Bhola RF, Imlach WL, Herzig V, Armstrong DA, Rosengren KJ, et al. Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a. Sci Rep. 2017;7:40883. doi: 10.1038/srep40883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vetter I, Deuis JR, Mueller A, Israel MR, Starobova H, Zhang A, Rash LD, Mobli M. NaV1.7 as a pain target - From gene to pharmacology. Pharmacol Ther. 2017;172:73–100. doi: 10.1016/j.pharmthera.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 150.Yang Y, Adi T, Effraim PR, Chen L, Dib-Hajj SD, Waxman SG. Reverse pharmacogenomics: carbamazepine normalizes activation and attenuates thermal hyperexcitability of sensory neurons due to Nav 1.7 mutation I234T. Br J Pharmacol. 2018;175:2261–2271. doi: 10.1111/bph.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alexandrou AJ, Brown AR, Chapman ML, Estacion M, Turner J, Mis MA, Wilbrey A, Payne EC, Gutteridge A, Cox PJ, et al. Subtype-selective small molecule inhibitors reveal a fundamental role for Nav1.7 in nociceptor electrogenesis, axonal conduction and presynaptic release. PLoS One. 2016;11:e0152405. doi: 10.1371/journal.pone.0152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW. Cancer-induced bone pain: mechanisms and models. Neurosci Lett. 2013;557:52–59. doi: 10.1016/j.neulet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nielsen LM, Olesen AE, Andresen T, Simrén M, Törnblom H, Drewes AM. Efficacy and safety of PPC-5650 on experimental rectal pain in patients with irritable bowel syndrome. Basic Clin Pharmacol Toxicol. 2015;116:140–145. doi: 10.1111/bcpt.12294. [DOI] [PubMed] [Google Scholar]
- 154.Olesen AE, Nielsen LM, Larsen IM, Drewes AM. Randomized clinical trial: efficacy and safety of PPC-5650 on experimental esophageal pain and hyperalgesia in healthy volunteers. Scand J Gastroenterol. 2015;50:138–144. doi: 10.3109/00365521.2014.966319. [DOI] [PubMed] [Google Scholar]
- 155.Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716:61–76. doi: 10.1016/j.ejphar.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 156.Chen J, Hackos DH. TRPA1 as a drug target–promise and challenges. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:451–463. doi: 10.1007/s00210-015-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu H, Fan X, Wang N, Zhang Y, Yu J. Exacerbating effects of PM2.5 in OVA-sensitized and challenged mice and the expression of TRPA1 and TRPV1 proteins in lungs. J Asthma. 2017;54:807–817. doi: 10.1080/02770903.2016.1266495. [DOI] [PubMed] [Google Scholar]
- 158.Mukhopadhyay I, Kulkarni A, Khairatkar-Joshi N. Blocking TRPA1 in respiratory disorders: does it hold a promise?. Pharmaceuticals (Basel). 2016;9:E70. doi: 10.3390/ph9040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115. doi: 10.1053/j.gastro.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen XX, Zhang JH, Pan BH, Ren HL, Feng XL, Wang JL, Xiao JH. TRPC3-mediated Ca(2+) entry contributes to mouse airway smooth muscle cell proliferation induced by lipopolysaccharide. Cell Calcium. 2016;60:273–281. doi: 10.1016/j.ceca.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 161.Dietrich A, Chubanov V, Gudermann T. Renal TRPathies. J Am Soc Nephrol. 2010;21:736–44. doi: 10.1681/ASN.2009121291. [DOI] [PubMed] [Google Scholar]
- 162.Wu QY, Sun MR, Wu CL, Li Y, Du JJ, Zeng JY, Bi HL, Sun YH. Activation of calcium-sensing receptor increases TRPC3/6 expression in T lymphocyte in sepsis. Mol Immunol. 2015;64:18–25. doi: 10.1016/j.molimm.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 163.Broad LM, Mogg AJ, Eberle E, Tolley M, Li DL, Knopp KL. TRPV3 in drug development. Pharmaceuticals (Basel). 2016;9:E55. doi: 10.3390/ph9030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33. doi: 10.1186/s13023-015-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, Smith JA. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385:1198–1205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- 166.Bhattacharya A. Clinical optimism for antagonists targeting certain ion channels. Drug Target Review. 2016;Winter:10–13. [Google Scholar]
- 167.Jurga AM, Piotrowska A, Makuch W, Przewlocka B, Mika J. Blockade of P2X4 receptors inhibits neuropathic pain-related behavior by preventing MMP-9 activation and, consequently, pronociceptive interleukin release in a rat model. Front Pharmacol. 2017;8:48. doi: 10.3389/fphar.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. P2X4 receptors and neuropathic pain. Front Cell Neurosci. 2013;7:191. doi: 10.3389/fncel.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wen L, Voronina S, Javed MA, Awais M, Szatmary P, Latawiec D, Chvanov M, Collier D, Huang W, Barrett J, et al. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology. 2015;149:481–492. doi: 10.1053/j.gastro.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cox JH, Hussell S, Søndergaard H, Roepstorff K, Bui JV, Deer JR, Zhang J, Li ZG, Lamberth K, Kvist PH, et al. Antibody-mediated targeting of the Orai1 calcium channel inhibits T cell function. PLoS One. 2013;8:e82944. doi: 10.1371/journal.pone.0082944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vaeth M, Zee I, Concepcion AR, Maus M, Shaw P, Portal-Celhay C, Zahra A, Kozhaya L, Weidinger C, Philips J, et al. Ca2+ signaling but not store-operated Ca2+ entry is required for the function of macrophages and dendritic cells. J Immunol. 2015;195:1202–1217. doi: 10.4049/jimmunol.1403013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Szabò I, Zoratti M, Gulbins E. Contribution of voltage-gated potassium channels to the regulation of apoptosis. FEBS Lett. 2010;584:2049–2056. doi: 10.1016/j.febslet.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 175.Bortner CD, Cidlowski JA. Ion channels and apoptosis in cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130104. doi: 10.1098/rstb.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol. 2014;206::151–62. doi: 10.1083/jcb.201404136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chandy KG, Norton RS. Immunology: channelling potassium to fight cancer. Nature. 2016;537:497–499. doi: 10.1038/nature19467. [DOI] [PubMed] [Google Scholar]
- 178.Han J, Lee SH, Giebisch G, Wang T. Potassium channelopathies and gastrointestinal ulceration. Gut Liver. 2016;10:881–889. doi: 10.5009/gnl15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fung-Leung WP, Edwards W, Liu Y, Ngo K, Angsana J, Castro G, Wu N, Liu X, Swanson RV, Wickenden AD. T Cell subset and stimulation strength-dependent modulation of T Cell activation by Kv1.3 blockers. PLoS One. 2017;12:e0170102. doi: 10.1371/journal.pone.0170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chimote AA, Hajdu P, Kottyan LC, Harley JB, Yun Y, Conforti L. Nanovesicle-targeted Kv1.3 knockdown in memory T cells suppresses CD40L expression and memory phenotype. J Autoimmun. 2016;69:86–93. doi: 10.1016/j.jaut.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bose T, Cieślar-Pobuda A, Wiechec E. Role of ion channels in regulating Ca2⁺ homeostasis during the interplay between immune and cancer cells. Cell Death Dis. 2015;6:e1648. doi: 10.1038/cddis.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Comes N, Bielanska J, Vallejo-Gracia A, Serrano-Albarrás A, Marruecos L, Gómez D, Soler C, Condom E, Ramón Y, Cajal S, et al. The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front Physiol. 2013;4:283. doi: 10.3389/fphys.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ouadid-Ahidouch H, Ahidouch A, Pardo LA. Kv10.1 K(+) channel: from physiology to cancer. Pflugers Arch. 2016;468:751–762. doi: 10.1007/s00424-015-1784-3. [DOI] [PubMed] [Google Scholar]
- 184.Pardo LA, Stühmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14:39. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- 185.Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME, Martin S, Schliephacke T, Jenke M, Heinz-Joachim-Radzun, et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41. doi: 10.1186/1476-4598-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Spitzner M, Martins JR, Soria RB, Ousingsawat J, Scheidt K, Schreiber R, Kunzelmann K. Eag1 and Bestrophin 1 are up-regulated in fast-growing colonic cancer cells. J Biol Chem. 2008;283:7421–7428. doi: 10.1074/jbc.M703758200. [DOI] [PubMed] [Google Scholar]
- 187.Ding XW, Luo HS, Jin X, Yan JJ, Ai YW. Aberrant expression of Eag1 potassium channels in gastric cancer patients and cell lines. Med Oncol. 2007;24:345–350. [DOI] [PubMed] [Google Scholar]
- 188.García-Becerra R, Díaz L, Camacho J, Barrera D, Ordaz-Rosado D, Morales A, Ortiz CS, Avila E, Bargallo E, Arrecillas M, et al. Calcitriol inhibits Ether-à go-go potassium channel expression and cell proliferation in human breast cancer cells. Exp Cell Res. 2010;316:433–442. doi: 10.1016/j.yexcr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 189.Hammadi M, Chopin V, Matifat F, Dhennin-Duthille I, Chasseraud M, Sevestre H, Ouadid-Ahidouch H. Human ether à-gogo K(+) channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J Cell Physiol. 2012;227:3837–3846. doi: 10.1002/jcp.24095. [DOI] [PubMed] [Google Scholar]
- 190.Wu J, Zhong D, Wei Y, Wu X, Kang L, Ding Z.. Potassium channel ether à go-go1 is aberrantly expressed in human liposarcoma and promotes tumorigenesis. Biomed Res Int. 2014;2014:345678. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Del Pliego MG, Aguirre-Benítez E, Paisano-Cerón K, Valdovinos-Ramírez I, Rangel-Morales C, Rodríguez-Mata V, Solano-Agama C, Martín-Tapia D, de la Vega MT, Saldoval-Balanzario M, et al. Expression of Eag1 K+ channel and ErbBs in human pituitary adenomas: cytoskeleton arrangement patterns in cultured cells. Int J Clin Exp Pathol. 2013;6:458–468. [PMC free article] [PubMed] [Google Scholar]
- 192.Chávez-López MG, Zúñiga-García V, Pérez-Carreón JI, Avalos-Fuentes A, Escobar Y, Camacho J. Eag1 channels as potential early-stage biomarkers of hepatocellular carcinoma. Biologics. 2016;10:139–148. doi: 10.2147/BTT.S87402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Menendez ST, Villaronga MA, Rodrigo JP, Alvarez-Teijeiro S, García-Carracedo D, Urdinguio RG, Fraga MF, Pardo LA, Viloria CG, Suárez C, et al. Frequent aberrant expression of the human ether à go-go (hEAG1) potassium channel in head and neck cancer: pathobiological mechanisms and clinical implications. J Mol Med (Berl). 2012;90:1173–1184. doi: 10.1007/s00109-012-0893-0. [DOI] [PubMed] [Google Scholar]
- 194.Martínez R, Stühmer W, Martin S, Schell J, Reichmann A, Rohde V, Pardo L. Analysis of the expression of Kv10.1 potassium channel in patients with brain metastases and glioblastoma multiforme: impact on survival. BMC Cancer. 2015;15:839. doi: 10.1186/s12885-015-1584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ding XW, Yan JJ, An P, Lü P, Luo HS. Aberrant expression of ether à go-go potassium channel in colorectal cancer patients and cell lines. World J Gastroenterol. 2007;13:1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rodriguez-Rasgado JA, Acuna-Macias I, Camacho J. Eag1 channels as potential cancer biomarkers. Sensors (Basel). 2012;12:5986–5995. doi: 10.3390/s120505986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wu W, Dong MQ, Wu XG, Sun HY, Tse HF, Lau CP, Li GR. Human ether-à-go-go gene potassium channels are regulated by EGFR tyrosine kinase. Biochim Biophys Acta. 2012;1823:282–289. doi: 10.1016/j.bbamcr.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 198.Urrego D, Movsisyan N, Ufartes R, Pardo LA. Periodic expression of Kv10.1 driven by pRb/E2F1 contributes to G2/M progression of cancer and non-transformed cells. Cell Cycle. 2016;15:799–811. doi: 10.1080/15384101.2016.1138187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Napp J, Pardo LA, Hartung F, Tietze LF, Stühmer W, Alves F. In vivo imaging of tumour xenografts with an antibody targeting the potassium channel Kv10.1. Eur Biophys J. 2016;45:721–733. doi: 10.1007/s00249-016-1152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Toral C, Mendoza-Garrido ME, Azorín E, Hernández-Gallegos E, Gomora JC, Delgadillo DM, Solano-Agama C, Camacho J. Effect of extracellular matrix on adhesion, viability, actin cytoskeleton and K+ currents of cells expressing human ether à go-go channels. Life Sci. 2007;81:255–265. doi: 10.1016/j.lfs.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 201.Kohl T, Lörinczi E, Pardo LA, Stühmer W. Rapid internalization of the oncogenic K+ channel K(V)10.1. PLoS One. 2011;6:e26329. doi: 10.1371/journal.pone.0026329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ufartes R, Schneider T, Mortensen LS, de Juan Romero C, Hentrich K, Knoetgen H, Beilinson V, Moebius W, Tarabykin V, Alves F, et al. Behavioural and functional characterization of Kv10.1 (Eag1) knockout mice. Hum Mol Genet. 2013;22:2247–2262. doi: 10.1093/hmg/ddt076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pardo LA, Del Camino D, Sánchez A, Alves F, Brüggemann A, Beckh S, Stühmer W. Oncogenic potential of EAG K+ channels. Embo J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Weber C, Mello de Queiroz F, Downie BR, Suckow A, Stühmer W, Pardo LA. Silencing the activity and proliferative properties of the human EagI potassium channel by RNA interference. J Biol Chem. 2006;281:13030–13037. doi: 10.1074/jbc.M600883200. [DOI] [PubMed] [Google Scholar]
- 205.Li XN, Herrington J, Petrov A, Ge L, Eiermann G, Xiong Y, Jensen MV, Hohmeier HE, Newgard CB, Garcia ML, et al. The role of voltage-gated potassium channels Kv2.1 and Kv2.2 in the regulation of insulin and somatostatin release from pancreatic islets. J Pharmacol Exp Ther. 2013;344:407–416. doi: 10.1124/jpet.112.199083. [DOI] [PubMed] [Google Scholar]
- 206.Suzuki T, Takimoto K. Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Int J Oncol. 2004;25:153–159. [PubMed] [Google Scholar]
- 207.Han Y, Shi Y, Han Z, Sun L, Fan D. Detection of potassium currents and regulation of multidrug resistance by potassium channels in human gastric cancer cells. Cell Biol Int. 2007;31:741–747. doi: 10.1016/j.cellbi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 208.Al-Owais MM, Scragg JL, Dallas ML, Boycott HE, Warburton P, Chakrabarty A, Boyle JP, Peers C. Carbon monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in medulloblastoma DAOY cells via K+ channel inhibition. J Biol Chem. 2012;287:24754–24764. doi: 10.1074/jbc.M112.357012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Delgado-Ramírez M, Morán-Zendejas R, Aréchiga-Figueroa IA, Toro-Castillo C, Ramírez-Martínez JF, Rodríguez-Menchaca AA. Modulation of the voltage-gated potassium channel Kv2.1 by the anti-tumor alkylphospholipid perifosine. Pharmacol Rep. 2016;68:457–461. doi: 10.1016/j.pharep.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 210.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. [DOI] [PubMed] [Google Scholar]
- 211.Crottès D, Rapetti-Mauss R, Alcaraz-Perez F, Tichet M, Gariano G, Martial S, Guizouarn H, Pellissier B, Loubat A, Popa A, et al. SIGMAR1 regulates membrane electrical activity in response to extracellular matrix stimulation to drive cancer cell invasiveness. Cancer Res. 2016;76:607–618. doi: 10.1158/0008-5472.CAN-15-1465. [DOI] [PubMed] [Google Scholar]
- 212.Lees-Miller JP, Kondo C, Wang L, Duff HJ. Electrophysiological characterization of an alternatively processed ERG K1 channel in mouse and human hearts. Circ Res. 1997;81:719–726. doi: 10.1161/01.RES.81.5.719. [DOI] [PubMed] [Google Scholar]
- 213.Pillozzi S, Accordi B, Rebora P, Serafin V, Valsecchi MG, Basso G, Arcangeli A. Differential expression of hERG1A and hERG1B genes in pediatric acute lymphoblastic leukemia identifies different prognostic subgroups. Leukemia. 2014;28:1352–1355. doi: 10.1038/leu.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958;30:653–654. [DOI] [PubMed] [Google Scholar]
- 215.Meech RW, Strumwasser F. Intracellular calcium injection activates potassium conductance in Aplysia nerve cells. Fed Proc. 1970;29:834. [DOI] [PubMed] [Google Scholar]
- 216.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 217.Dutertre S, Lewis RJ. Use of venom peptides to probe ion channel structure and function. J Biol Chem. 2010;285:13315e13320. doi: 10.1074/jbc.R109.076596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729e749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 219.Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol Cell Physiol. 2003;285:C1553–1560. doi: 10.1152/ajpcell.00083.2003. [DOI] [PubMed] [Google Scholar]
- 220.Taylor SR, Gonzalez-Begne M, Dewhurst S, Chimini G, Higgins CF, Melvin JE, Elliott JI. Sequential shrinkage and swelling underlie P2X7-stimulated lymphocyte phosphatidylserine exposure and death. J Immunol. 2008;180:300–308. doi: 10.4049/jimmunol.180.1.300. [DOI] [PubMed] [Google Scholar]
- 221.Faouzi M, Hague F, Geerts D, Ay AS, Potier-Cartereau M, Ahidouch A, Ouadid-Ahidouch H. Functional cooperation between KCa3.1 and TRPC1 channels in human breast cancer: role in cell proliferation and patient prognosis. Oncotarget. 2016;7:36419–36435. doi: 10.18632/oncotarget.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 223.Gackière F, Warnier M, Katsogiannou M, Derouiche S, Delcourt P, Dewailly E, Slomianny C, Humez S, Prevarskaya N, Roudbaraki M, et al. Functional coupling between large-conductance potassium channels and Cav3.2 voltage-dependent calcium channels participates in prostate cancer cell growth. Biol Open. 2013;2:941–951. doi: 10.1242/bio.20135215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gackière F, Bidaux G, Delcourt P, Van Coppenolle F, Katsogiannou M, Dewailly E, Bavencoffe A, Van Chuoï-Mariot MT, Mauroy B, Prevarskaya N, et al. CaV3.2 T-type calcium channels are involved in calcium-dependent secretion of neuroendocrine prostate cancer cells. J Biol Chem. 2008;283:10162–10173. doi: 10.1074/jbc.M707159200. [DOI] [PubMed] [Google Scholar]
- 225.Pera E, Kaemmerer E, Milevskiy MJG, Yapa KTDS, O’Donnell JS, Brown MA, Simpson F, Peters AA, Roberts-Thomson SJ, Monteith GR. The voltage gated Ca(2+)-channel Cav3.2 and therapeutic responses in breast cancer. Cancer Cell Int. 2016;16:24. doi: 10.1186/s12935-016-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dziegielewska B, Casarez EV, Yang WZ, Gray LS, Dziegielewski J, Slack-Davis JK. T-Type Ca2+ channel inhibition sensitizes ovarian cancer to carboplatin. Mol Cancer Ther. 2016;15:460–470. doi: 10.1158/1535-7163.MCT-15-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.George AL., Jr. Inherited disorders of voltage-gated sodium channels. J Clin Invest. 2005;115:1990–1999. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, Ostman J, Klugbauer N, Wood JN, Gardiner RM, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52:767–774. doi: 10.1016/j.neuron.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 229.Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Eberhardt MJ, Leffler A. Pain and analgesia: mutations of voltage-gated sodium channels. Schmerz. 2017;31:14–22. doi: 10.1007/s00482-016-0139-0. [DOI] [PubMed] [Google Scholar]
- 231.Campbell TM, Main MJ, Fitzgerald EM. Functional expression of the voltage-gated Na⁺-channel Nav1.7 is necessary for EGF-mediated invasion in human non-small cell lung cancer cells. J Cell Sci. 2013;126:4939–4949. doi: 10.1242/jcs.130013. [DOI] [PubMed] [Google Scholar]
- 232.Litan A, Langhans SA. Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Front Cell Neurosci. 2015;9:86. doi: 10.3389/fncel.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nelson M, Yang M, Millican-Slater R, Brackenbury WJ. Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget. 2015;6:32914–32929. doi: 10.18632/oncotarget.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327 [DOI] [PubMed] [Google Scholar]
- 235.Mohammed FH, Khajah MA, Yang M, Brackenbury WJ, Luqmani YA. Blockade of voltage-gated sodium channels inhibits invasion of endocrine-resistant breast cancer cells. Int J Oncol. 2016;48:73–83. doi: 10.3892/ijo.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Aydar E, Stratton D, Fraser SP, Djamgoz MB, Palmer C. Sigma-1 receptors modulate neonatal Nav1.5 ion channels in breast cancer cell lines. Eur Biophys J. 2016;45:671–683. doi: 10.1007/s00249-016-1135-0. [DOI] [PubMed] [Google Scholar]
- 237.Benarroch EE. Acid-sensing cation channels: structure, function, and pathophysiologic implications. Neurology. 2014;82:628–635. doi: 10.1212/WNL.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 238.Sherwood TW, Frey EN, Askwith CC. Structure and activity of the acid-sensing ion channels. Am J Physiol Cell Physiol. 2012;303:C699–710. doi: 10.1152/ajpcell.00188.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kellenberger S, Schild L. International Union of Basic and Clinical Pharmacology. XCI. structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol Rev. 2015;67:1–35. doi: 10.1124/pr.114.009225. [DOI] [PubMed] [Google Scholar]
- 240.Chu XP, Xiong ZG. Acid-sensing ion channels in pathological conditions. Adv Exp Med Biol. 2013;961:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Holland PR, Akerman S, Andreou AP, Karsan N, Wemmie JA, Goadsby PJ. Acid-sensing ion channel 1: a novel therapeutic target for migraine with aura. Ann Neurol. 2012;72:559–563. doi: 10.1002/ana.23653. [DOI] [PubMed] [Google Scholar]
- 242.Rooj AK, McNicholas CM, Bartoszewski R, Bebok Z, Benos DJ, Fuller CM. Glioma-specific cation conductance regulates migration and cell cycle progression. J Biol Chem. 2012;287:4053–4065. doi: 10.1074/jbc.M111.311688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gupta SC, Singh R, Asters M, Liu J, Zhang X, Pabbidi MR, Watabe K, Mo YY. Regulation of breast tumorigenesis through acid sensors. Oncogene. 2016;35:4102–4111. doi: 10.1038/onc.2015.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 245.Thippegowda PB, Singh V, Sundivakkam PC, Xue J, Malik AB, Tiruppathi C. Ca2+ influx via TRPC channels induces NF-kappaB-dependent A20 expression to prevent thrombin-induced apoptosis in endothelial cells. Am J Physiol Cell Physiol. 2010;298:C656–64. doi: 10.1152/ajpcell.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Marwaha L, Bansal Y, Singh R, Saroj P, Bhandari R, Kuhad A. TRP channels: potential drug target for neuropathic pain. Inflammopharmacology. 2016;24:305–317. doi: 10.1007/s10787-016-0288-x. [DOI] [PubMed] [Google Scholar]
- 247.Chen WL, Barszczyk A, Turlova E, Deurloo M, Liu B, Yang BB, Rutka JT, Feng ZP, Sun HS. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget. 2015;6:16321–16340. doi: 10.18632/oncotarget.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen WL, Barszczyk A, Turlova E, Deurloo M, Liu B, Yang BB, Rutka JT, Feng ZP, Sun HS. Xyloketal B suppresses glioblastoma cell proliferation and migration in vitro through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling pathways. Mar Drugs. 2015;13:2505–2525. doi: 10.3390/md13042505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lin CM, Ma JM, Zhang L, Hao ZY, Zhou J, Zhou ZY, Shi HQ, Zhang YF, Shao EM, Liang CZ. Inhibition of transient receptor potential melastain 7 enhances apoptosis induced by TRAIL in PC-3 cells. Asian Pac J Cancer Prev. 2015;16:4469–4475. [DOI] [PubMed] [Google Scholar]
- 250.Yee NS, Zhou W, Lee M, Yee RK. Targeted silencing of TRPM7 ion channel induces replicative senescence and produces enhanced cytotoxicity with gemcitabine in pancreatic adenocarcinoma. Cancer Lett. 2012;318:99–105. doi: 10.1016/j.canlet.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu M, Inoue K, Leng T, Guo S, Xiong ZG. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell Signal. 2014;26:2773–2781. doi: 10.1016/j.cellsig.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Asuthkar S, Demirkhanyan L, Sun X, Elustondo PA, Krishnan V, Baskaran P, Velpula KK, Thyagarajan B, Pavlov EV, Zakharian E. The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J Biol Chem. 2015;290:2670–2688. doi: 10.1074/jbc.M114.610873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yee NS. Roles of TRPM8 ion channels in cancer: proliferation, survival, and invasion. Cancers (Basel). 2015;7:2134–2146. doi: 10.3390/cancers7040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kulkarni P. TRPM8 and prostate cancer: to overexpress or repress, that is the question-comment on “Effects of TRPM8 on proliferation and motility of prostate cancer PC-3 cells” by Yang ZH et al. in Asian Journal of Andrology. Asian J Androl. 2009;11:150–151. doi: 10.1038/aja.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- 256.Asuthkar S, Velpula KK, Elustondo PA, Demirkhanyan L, Zakharian E. TRPM8 channel as a novel molecular target in androgen-regulated prostate cancer cells. Oncotarget. 2015;6:17221–17236. doi: 10.18632/oncotarget.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Prevarskaya N, Skryma R, Bidaux G, Flourakis M, Shuba Y. Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ. 2007;14:1295–1304. doi: 10.1038/sj.cdd.4402162. [DOI] [PubMed] [Google Scholar]
- 258.Yang ZH, Wang XH, Wang HP, Hu LQ. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J Androl. 2009;11:157–165. doi: 10.1038/aja.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang Y, Wang X, Yang Z, Zhu G, Chen D, Meng Z. Menthol inhibits the proliferation and motility of prostate cancer DU145 cells. Pathol Oncol Res. 2012;18:903–910. [DOI] [PubMed] [Google Scholar]
- 260.Fonseca BM, Correia-da-Silva G, Teixeira NA. Cannabinoid-induced cell death in endometrial cancer cells: involvement of TRPV1 receptors in apoptosis. J Physiol Biochem. 2018;74:261–272. doi: 10.1007/s13105-018-0611-7. [DOI] [PubMed] [Google Scholar]
- 261.Xu S, Zhang L, Cheng X, Yu H, Bao J, Lu R. Capsaicin inhibits the metastasis of human papillary thyroid carcinoma BCPAP cells through the modulation of the TRPV1 channel. Food Funct. 2018;9:344–354. doi: 10.1039/c7fo01295k. [DOI] [PubMed] [Google Scholar]
- 262.Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP channels: diagnostic markers and therapeutic targets for breast cancer? Trends Mol Med. 2013;19:117–124. doi: 10.1016/j.molmed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 263.Stock K, Kumar J, Synowitz M, Petrosino S, Imperatore R, Smith ES, Wend P, Purfürst B, Nuber UA, Gurok U, et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat Med. 2012;18:1232–1238. doi: 10.1038/nm.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Alvarez-Berdugo D, Jiménez M, Clavé P, Rofes L. Pharmacodynamics of TRPV1 agonists in a bioassay using human PC-3 cells. Scientific World Journal. 2014;2014:184526. doi: 10.1155/2014/184526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mergler S, Skrzypski M, Sassek M, Pietrzak P, Pucci C, Wiedenmann B, Strowski MZ. Thermo-sensitive transient receptor potential vanilloid channel-1 regulates intracellular calcium and triggers chromogranin A secretion in pancreatic neuroendocrine BON-1 tumor cells. Cell Signal. 2012;24:233–246. doi: 10.1016/j.cellsig.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 266.Santoni G, Farfariello V. TRP channels and cancer: new targets for diagnosis and chemotherapy. Endocr Metab Immune Disord Drug Targets. 2011;11:54–67. [DOI] [PubMed] [Google Scholar]
- 267.Mistretta F, Buffi NM, Lughezzani G, Lista G, Larcher A, Fossati N, Abrate A, Dell’Oglio P, Montorsi F, Guazzoni G, et al. Bladder cancer and urothelial impairment: the role of TRPV1 as potential drug target. Biomed Res Int. 2014;2014:987149. doi: 10.1155/2014/987149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol. 2011;704:615–636. doi: 10.1007/978-94-007-0265-3_33. [DOI] [PubMed] [Google Scholar]
- 269.Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005;388:75–80. doi: 10.1016/j.neulet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 270.Li X, Zhang Q, Fan K, Li B, Li H, Qi H, Guo J, Cao Y, Sun H. Overexpression of TRPV3 correlates with tumor progression in non-small cell lung cancer. Int J Mol Sci. 2016;17:437. doi: 10.3390/ijms17040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hoeft B, Linseisen J, Beckmann L, Müller-Decker K, Canzian F, Hüsing A, Kaaks R, Vogel U, Jakobsen MU, Overvad K, et al. Polymorphisms in fatty-acid-metabolism-related genes are associated with colorectal cancer risk. Carcinogenesis. 2010;31:466–472. doi: 10.1093/carcin/bgp325. [DOI] [PubMed] [Google Scholar]
- 272.Raphaël M, Lehen’kyi V, Vandenberghe M, Beck B, Khalimonchyk S, Vanden Abeele F, Farsetti L, Germain E, Bokhobza A, Mihalache A, et al. TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc Natl Acad Sci U S A. 2014;111:E3870–9. doi: 10.1073/pnas.1413409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gerhold KA, Bautista DM. TRPA1: irritant detector of the airways. J Physiol. 2008;586:3303. doi: 10.1113/jphysiol.2008.160390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Marsakova L, Barvik I, Zima V, Zimova L, Vlachova V. The first extracellular linker is important for several aspects of the gating mechanism of human TRPA1 channel. Front Mol Neurosci. 2017;10:16. doi: 10.3389/fnmol.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–451. doi: 10.1016/j.neuroscience.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 276.Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol. 2014;167:1–43. doi: 10.1007/112_2014_18. [DOI] [PubMed] [Google Scholar]
- 277.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Basso L, Altier C. Transient Receptor Potential Channels in neuropathic pain. Curr Opin Pharmacol. 2017;32:9–15. doi: 10.1016/j.coph.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 279.Lehto SG, Weyer AD, Zhang M, Youngblood BD, Wang J, Wang W, Kerstein PC, Davis C, Wild KD, Stucky CL, et al. AMG2850, a potent and selective TRPM8 antagonist, is not effective in rat models of inflammatory mechanical hypersensitivity and neuropathic tactile allodynia. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:465–476. doi: 10.1007/s00210-015-1090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mukhopadhyay I, Gomes P, Aranake S, Shetty M, Karnik P, Damle M, Kuruganti S, Thorat S, Khairatkar-Joshi N. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Recept Signal Transduct Res. 2011;31:350–358. doi: 10.3109/10799893.2011.602413. [DOI] [PubMed] [Google Scholar]
- 281.Xiao JH, Zheng YM, Liao B, Wang YX. Functional role of canonical transient receptor potential 1 and canonical transient receptor potential 3 in normal and asthmatic airway smooth muscle cells. Am J Respir Cell Mol Biol. 2010;43:17–25. doi: 10.1165/rcmb.2009-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang L, Li J, Zhang J, He Q, Weng X, Huang Y, Guan M, Qiu C. Inhibition of TRPC3 downregulates airway hyperresponsiveness, remodeling of OVA-sensitized mouse. Biochem Biophys Res Commun. 2017;484:209–217. doi: 10.1016/j.bbrc.2016.12.138. [DOI] [PubMed] [Google Scholar]
- 283.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381−404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Dietrich A, Steinritz D, Gudermann T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium. 2017;67:123–137. doi: 10.1016/j.ceca.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 285.Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol Ther. 2011;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sozucan Y, Kalender ME, Sari I, Suner A, Oztuzcu S, Arman K, Yumrutas O, Bozgeyik I, Cengiz B, Igci YZ, et al. TRP genes family expression in colorectal cancer. Exp Oncol. 2015;37:208–212. [PubMed] [Google Scholar]
- 287.Zhang Z, Wang J, He J, Zeng X, Chen X, Xiong M, Zhou Q, Guo M, Li D, Lu W. Identification of TRPCs genetic variants that modify risk for lung cancer based on the pathway and two-stage study. Meta Gene. 2016;9:191–196. doi: 10.1016/j.mgene.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yang LL, Liu BC, Lu XY, Yan Y, Zhai YJ, Bao Q, Doetsch PW, Deng X, Thai TL, Alli AA, et al. Inhibition of TRPC6 reduces non-small cell lung cancer cell proliferation and invasion. Oncotarget. 2017;8:5123–5134. doi: 10.18632/oncotarget.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Gkika D, Prevarskaya N. TRP channels in prostate cancer: the good, the bad and the ugly? Asian J Androl. 2011;13:673–676. doi: 10.1038/aja.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ding X, He Z, Zhou K, Cheng J, Yao H, Lu D, Cai R, Jin Y, Dong B, Xu Y, et al. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst. 2010;102:1052–1068. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- 291.Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang JY, et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Álvarez-Miguel I, Cidad P, Pérez-García MT, López-López JR. Differences in TRPC3 and TRPC6 channels assembly in mesenteric vascular smooth muscle cells in essential hypertension. J Physiol. 2017;595:1497–1513. doi: 10.1113/JP273327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sel S, Rost BR, Yildirim AO, Sel B, Kalwa H, Fehrenbach H, Renz H, Gudermann T, Dietrich A. Loss of classical transient receptor potential 6 channel reduces allergic airway response. Clin Exp Allergy. 2008;38:1548−1558. doi: 10.1111/j.1365-2222.2008.03043.x. [DOI] [PubMed] [Google Scholar]
- 294.Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G, Jin Y, Wang Y. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int J Cancer. 2009;125:2281–2287. doi: 10.1002/ijc.24551. [DOI] [PubMed] [Google Scholar]
- 295.Soni H, Adebiyi A. TRPC6 channel activation promotes neonatal glomerular mesangial cell apoptosis via calcineurin/NFAT and FasL/Fas signaling pathways. Sci Rep. 2016;6:29041. doi: 10.1038/srep29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, Carpio JD, Ruiz P, Mezzano SA, Li J, Wei C, et al. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One. 2010;5:e12859. doi: 10.1371/journal.pone.0012859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Jacobson KA, Müller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2016;104:31–49. doi: 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83:759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiologica. 2010;199:93–147. [DOI] [PubMed] [Google Scholar]
- 301.Fabbretti E, Nistri A. Regulation of P2X3 receptor structure and function. CNS Neurol Disord Drug Targets. 2012;11:687–698. [DOI] [PubMed] [Google Scholar]
- 302.Gnanasekaran A, Sundukova M, Hullugundi S, Birsa N, Bianchini G, Hseuh YP, Nistri A, Fabbretti E. CASK is a new intracellular modulator of P2X3 receptors. J Neurochem. 2013;126:102–112. doi: 10.1111/jnc.12272. [DOI] [PubMed] [Google Scholar]
- 303.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ying M, Liu H, Zhang T, Jiang C, Gong Y, Wu B, Zou L, Yi Z, Rao S, Li G, et al. Effect of artemisinin on neuropathic pain mediated by P2X4 receptor in dorsal root ganglia. Neurochem Int. 2017;108:27–33. doi: 10.1016/j.neuint.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 305.Stojilkovic SS, Yan Z, Obsil T, Zemkova H. Structural insights into the function of P2X4: an ATP-gated cation channel of neuroendocrine cells. Cell Mol Neurobiol. 2010;30:1251–1258. doi: 10.1007/s10571-010-9568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Burnstock G. Purinergic mechanisms and pain. Adv Pharmacol. 2016;75:91–137. doi: 10.1016/bs.apha.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 307.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Monif M, Reid CA, Powell KL, Drummond KJ, O’Brien TJ, Williams DA. Interleukin-1β has trophic effects in microglia and its release is mediated by P2X7R pore. J Neuroinflammation. 2016;13:173. doi: 10.1186/s12974-016-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Thomas LM, Salter RD. Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on TLR4. J Immunol. 2010;185:3740–3749. doi: 10.4049/jimmunol.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Barberà-Cremades M, Baroja-Mazo A, Pelegrín P. Purinergic signaling during macrophage differentiation results in M2 alternative activated macrophages. J Leukoc Biol. 2016;99:289–299. doi: 10.1189/jlb.1A0514-267RR. [DOI] [PubMed] [Google Scholar]
- 311.Savio LEB, Coutinho-Silva R. Purinergic signaling in infection and autoimmune disease. Biomed J. 2016;39:304–305. doi: 10.1016/j.bj.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Aeschlimann D, Knäuper V. P2X7 receptor-mediated TG2 externalization: a link to inflammatory arthritis? Amino Acids. 2017;49:453–460. doi: 10.1007/s00726-016-2319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Liu S, Zou L, Xie J, Xie W, Wen S, Xie Q, Gao Y, Li G, Zhang C, Xu C, et al. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol Brain. 2016;23(9):44. doi: 10.1186/s13041-016-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 315.Bianchi G, Vuerich M, Pellegatti P, Marimpietri D, Emionite L, Marigo I, Bronte V, Di Virgilio F, Pistoia V, Raffaghello L. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment. Cell Death Dis. 2014;5:e1135. doi: 10.1038/cddis.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jelassi B, Chantôme A, Alcaraz-Pérez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A, Roger S. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30:2108–2122. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 317.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 318.Draganov D, Gopalakrishna-Pillai S, Chen YR, Zuckerman N, Moeller S, Wang C, Ann D, Lee PP. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci Rep. 2015;5:16222. doi: 10.1038/srep16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sluyter R, Stokes L. Significance of P2X7 receptor variants to human health and disease. Recent Pat DNA Gene Seq. 2011;5:41–54. [DOI] [PubMed] [Google Scholar]
- 320.Giuliani AL, Colognesi D, Ricco T, Roncato C, Capece M, Amoroso F, Wang QG, De Marchi E, Gartland A, Di Virgilio F, et al. Trophic activity of human P2X7 receptor isoforms A and B in osteosarcoma. PLoS One. 2014;9:e107224. doi: 10.1371/journal.pone.0107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Fang J, Chen X, Zhang L, Chen J, Liang Y, Li X, Xiang J, Wang L, Guo G, Zhang B, et al. P2X7R suppression promotes glioma growth through epidermal growth factor receptor signal pathway. Int J Biochem Cell Biol. 2013;45:1109–1120. doi: 10.1016/j.biocel.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 322.Fang J, Chen X, Wang S, Xie T, Du X, Liu H, Wang S, Li X, Chen J, Zhang B, et al. The expression of P2X₇ receptors in EPCs and their potential role in the targeting of EPCs to brain gliomas. Cancer Biol Ther. 2015;16:498–510. doi: 10.1080/15384047.2015.1016663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, Raffaghello L, Pistoia V, Varesio L, Adinolfi E. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34:5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 324.Portillo JC, Lopez Corcino Y, Dubyak GR, Kern TS, Matsuyama S, Subauste CS. Ligation of CD40 in human müller cells induces P2X7 receptor-dependent death of retinal endothelial cells. Invest Ophthalmol Vis Sci. 2016;57:6278–6286. doi: 10.1167/iovs.16-20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Derler I, Butorac C, Krizova A, Stadlbauer M, Muik M, Fahrner M, Frischauf I, Romanin C. Authentic CRAC channel activity requires STIM1 and the conserved portion of the Orai N terminus. J Biol Chem. 2018;293:1259–1270. doi: 10.1074/jbc.M117.812206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Gudlur A, Zhou Y, Hogan PG. STIM-ORAI interactions that control the CRAC channel. Curr Top Membr. 2013;71:33–58. doi: 10.1016/B978-0-12-407870-3.00002-0. [DOI] [PubMed] [Google Scholar]
- 327.Lang F, Münzer P, Gawaz M, Borst O. Regulation of STIM1/Orai1-dependent Ca2+ signalling in platelets. Thromb Haemost. 2013;110:925–930. doi: 10.1160/TH13-02-0176. [DOI] [PubMed] [Google Scholar]
- 328.Srivats S, Balasuriya D, Pasche M, Vistal G, Edwardson JM, Taylor CW, Murrell-Lagnado RD. Sigma1 receptors inhibit store-operated Ca2+ entry by attenuating coupling of STIM1 to Orai1. J Cell Biol. 2016;213:65–79. doi: 10.1083/jcb.201506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Shaw PJ, Feske S. Regulation of lymphocyte function by ORAI and STIM proteins in infection and autoimmunity. J Physiol. 2012;590:4157–4167. doi: 10.1113/jphysiol.2012.233221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Flourakis M, Lehen’kyi V, Beck B, Raphaël M, Vandenberghe M, Abeele FV, Roudbaraki M, Lepage G, Mauroy B, Romanin C, et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 333.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Fiorio Pla A, Kondratska K, Prevarskaya N. STIM and ORAI proteins: crucial roles in hallmarks of cancer. Am J Physiol Cell Physiol. 2016;310:C509–19. doi: 10.1152/ajpcell.00364.2015. [DOI] [PubMed] [Google Scholar]
- 335.Gueguinou M, Crottès D, Chantôme A, Rapetti-Mauss R, Potier-Cartereau M, Clarysse L, Girault A, Fourbon Y, Jézéquel P, Guérin-Charbonnel C, et al. The SigmaR1 chaperone drives breast and colorectal cancer cell migration by tuning SK3-dependent Ca2+ homeostasis. Oncogene. 2017;36:3640–3647. doi: 10.1038/onc.2016.501. [DOI] [PubMed] [Google Scholar]
- 336.Chantôme A, Potier-Cartereau M, Clarysse L, Fromont G, Marionneau-Lambot S, Guéguinou M, Pagès JC, Collin C, Oullier T, Girault A, et al. Pivotal role of the lipid Raft SK3-Orai1 complex in human cancer cell migration and bone metastases. Cancer Res. 2013;73:4852–4861. doi: 10.1158/0008-5472.CAN-12-4572. [DOI] [PubMed] [Google Scholar]
- 337.Jardin I, Rosado JA. STIM and calcium channel complexes in cancer. Biochim Biophys Acta. 2016;1863:1418–1426. doi: 10.1016/j.bbamcr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 338.Accardi A. Structure and gating of CLC channels and exchangers. J Physiol. 2015;593:4129–4138. doi: 10.1113/JP270575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kornak U, Ostertag A, Branger S, Benichou O, de Vernejoul MC. Polymorphisms in the CLCN7 gene modulate bone density in postmenopausal women and in patients with autosomal dominant osteopetrosis type II. J Clin Endocrinol Metab. 2006;91:995–1000. doi: 10.1210/jc.2005-2017. [DOI] [PubMed] [Google Scholar]
- 341.Simon OJ, Müntefering T, Grauer OM, Meuth SG. The role of ion channels in malignant brain tumors. J Neurooncol. 2015;125:225–235. doi: 10.1007/s11060-015-1896-9. [DOI] [PubMed] [Google Scholar]
- 342.Gruber AD, Pauli BU. Tumorigenicity of human breast cancer is associated with loss of the Ca2+-activated chloride channel CLCA2. Cancer Res. 1999;59:5488–5491. [PubMed] [Google Scholar]
- 343.Elble RC, Pauli BU. Tumor suppression by a proapoptotic calcium-activated chloride channel in mammary epithelium. J Biol Chem. 2001;276:40510–40517. doi: 10.1074/jbc.M104821200. [DOI] [PubMed] [Google Scholar]
- 344.Walia V, Yu Y, Cao D, Sun M, McLean JR, Hollier BG, Cheng J, Mani SA, Rao K, Premkumar L, et al. Loss of breast epithelial marker hCLCA2 promotes epithelial-to-mesenchymal transition and indicates higher risk of metastasis. Oncogene. 2012;31:2237–2246. doi: 10.1038/onc.2011.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Walia V, Ding M, Kumar S, Nie D, Premkumar LS, Elble RC. hCLCA2 Is a p53-Inducible Inhibitor of Breast Cancer Cell Proliferation. Cancer Res. 2009;69:6624–6632. doi: 10.1158/0008-5472.CAN-08-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Brett TJ. CLCA1 and TMEM16A: the link towards a potential cure for airway diseases. Expert Rev Respir Med. 2015;9:503–506. doi: 10.1586/17476348.2015.1081064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kamaleddin MA. Molecular, biophysical, and pharmacological properties of calcium-activated chloride channels. J Cell Physiol. 2018;233:787–798. doi: 10.1002/jcp.25823. [DOI] [PubMed] [Google Scholar]
- 348.Sun H, Li M. Antibody therapeutics targeting ion channels: are we there yet? Acta Pharmacol Sin. 2013;34:199–204. doi: 10.1038/aps.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wilkinson TC, Gardener MJ, Williams WA. Discovery of functional antibodies targeting ion channels. J Biomol Screen. 2015;20:454–467. doi: 10.1177/1087057114560698. [DOI] [PubMed] [Google Scholar]
- 350.Wilkinson TC. Discovery of functional monoclonal antibodies targeting G-protein-coupled receptors and ion channels. Biochem Soc Trans. 2016;44:831–837. doi: 10.1042/BST20160028. [DOI] [PubMed] [Google Scholar]
- 351.Douthwaite JA, Finch DK, Mustelin T, Wilkinson TC. Development of therapeutic antibodies to G protein-coupled receptors and ion channels: opportunities, challenges and their therapeutic potential in respiratory diseases. Pharmacol Ther. 2017;169:113–123. doi: 10.1016/j.pharmthera.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 352.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumour sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Barden JA, Sluyter R, Gu BJ, Wiley JS. Specific detection of non-functional human P2X(7) receptors in HEK293 cells and B-lymphocytes. FEBS Lett. 2003;538:159–162. [DOI] [PubMed] [Google Scholar]
- 354.US National Library of Medicine ClinicalTrials.gov. 2016. https://clinicaltrials.gov/ct2/show/NCT02587819.
- 355.Danquah W, Meyer-Schwesinger C, Rissiek B, Pinto C, Serracant-Prat A, Amadi M, Iacenda D, Knop JH, Hammel A, Bergmann P, et al. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci Transl Med. 2016;8:366ra162. doi: 10.1126/scitranslmed.aaf0746. [DOI] [PubMed] [Google Scholar]
- 356.Shcherbatko A, Foletti D, Poulsen K, Strop P, Zhu G, Hasa-Moreno A, Melton Witt J, Loo C, Krimm S, Pios A, et al. Modulation of P2X3 and P2X2/3 receptors by monoclonal antibodies. J Biol Chem. 2016;291:12254–12270. doi: 10.1074/jbc.M116.722330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ossianix 2018. https://www.ossianix.co.uk. [Google Scholar]
- 358.Chambers R. Integral molecular presentation: new approaches for mAb discovery against GPCRs, ion channels, and transporters. CHI 14th Annual Discovery on Target; 2016. September 19–22; Boston, USA. [Google Scholar]
- 359.Wang RE, Wang Y, Zhang Y, Gabrelow C, Zhang Y, Chi V, Fu Q, Luo X, Wang D, Joseph S, et al. Rational design of a Kv1.3 channel-blocking antibody as a selective immunosuppressant. Proc Natl Acad Sci U S A. 2016;113:11501–11506. doi: 10.1073/pnas.1612803113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Gómez-Varela D, Contreras-Jurado C, Furini S, García-Ferreiro R, Stühmer W, Pardo LA. Different relevance of inactivation and F468 residue in the mechanisms of hEag1 channel blockage by astemizole, imipramine and dofetilide. FEBS Lett. 2006;580:5059–5066. doi: 10.1016/j.febslet.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 361.Moreels L, Peigneur S, Galan DT, De Pauw E, Béress L, Waelkens E, Pardo LA, Quinton L, Tytgat J. APETx4, a novel sea anemone toxin and a modulator of the cancer-relevant potassium channel KV10.1. Mar Drugs. 2017;15:E287. doi: 10.3390/md15090287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Hartung F, Stühmer W, Pardo LA. Tumor cell-selective apoptosis induction through targeting of K(V)10.1 via bifunctional TRAIL antibody. Mol Cancer. 2011;10:109. doi: 10.1186/1476-4598-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Hartung F, Pardo LA. Guiding TRAIL to cancer cells through Kv10.1 potassium channel overcomes resistance to doxorubicin. Eur Biophys J. 2016;45:709–719. doi: 10.1007/s00249-016-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sette A, Spadavecchia J, Landoulsi J, Casale S, Haye B, Crociani O, Arcangeli A. Development of novel anti-Kv 11.1 antibody-conjugated PEG-TiO2 nanoparticles for targeting pancreatic ductal adenocarcinoma cells. J Nanopart Res. 2013;15:2111. doi: 10.1007/s11051-013-2111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S, Florindo HF, Barata TS. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front Chem. 2014;2:105. doi: 10.3389/fchem.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Arcangeli A, Becchetti A. hERG channels: from antitargets to novel targets for cancer therapy. Clin Cancer Res. 2017;23:3–5. doi: 10.1158/1078-0432.CCR-16-2322. [DOI] [PubMed] [Google Scholar]
- 367.Arcangeli A, Crociani O, Crescioli S, Sette A. Anti-hERG1 antibodies. International Patent Application PCT/EP2015/068178. February 11, 2016. [Google Scholar]
- 368.Ganapathi SB, Kester M, Elmslie KS. State-dependent block of HERG potassium channels by R-roscovitine: implications for cancer therapy. Am J Physiol Cell Physiol. 2009;296:C701–10. doi: 10.1152/ajpcell.00633.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Spadavecchia J, Movia D, Moore C, Maguire CM, Moustaoui H, Casale S, Volkov Y, Prina-Mello A. Targeted polyethylene glycol gold nanoparticles for the treatment of pancreatic cancer: from synthesis to proof-of-concept in vitro studies. Int J Nanomedicine. 2016;11:791–822. doi: 10.2147/IJN.S97476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Emery EC, Luiz AP, Wood JN. Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin Ther Targets. 2016;20:975–983. doi: 10.1517/14728222.2016.1162295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lee JH, Park CK, Chen G, Han Q, Xie RG, Liu T, Ji RR, Lee SY. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell. 2014;157:1393–1404. doi: 10.1016/j.cell.2014.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 372.Liu D, Tseng M, Epstein LF, Green L, Chan B, Soriano B, Lim D, Pan O, Murawsky CM, King CT, et al. Evaluation of recombinant monoclonal antibody SVmab1 binding to NaV1.7 target sequences and block of human NaV1.7 currents. F1000Res. 2016;5:2764. doi: 10.12688/f1000research.9918.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Bang S, Yoo J, Gong X, Liu D, Han Q, Luo X, Chang W, Chen G, Im ST, Kim YH, et al. Differential inhibition of Nav1.7 and neuropathic pain by hybridoma-produced and recombinant monoclonal antibodies that target Nav1.7: differential activities of Nav1.7-targeting monoclonal antibodies. Neurosci Bull. 2018;34:22–41. doi: 10.1007/s12264-018-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Martz L. Nav-i-gating antibodies for pain. 2014. June 12 https://www.biocentury.com/bc-innovations/cover-story/2014-06-12/nav-i-gating-antibodies-pain
- 375.McCafferty J. Iontas presentation: scaffolds within scaffold: generating ion channel blocking antibodies by fusing cysteine knot mini-proteins into peripheral CDR loops. CHI Discovery on Target; 2017. September 25–29; Boston, USA. [Google Scholar]
- 376.Biswas K, Nixey TE, Murray JK, Falsey JR, Yin L, Liu H, Gingras J, Hall BE, Herberich B, Holder JR, et al. Engineering antibody reactivity for efficient derivatization to generate NaV1.7 inhibitory GpTx-1 peptide-antibody conjugates. ACS Chem Biol. 2017;12:2427–2435. doi: 10.1021/acschembio.7b00542. [DOI] [PubMed] [Google Scholar]
- 377.Robinson L. Visterra presentation: structure-based strategy to develop therapeutic antibodies against Nav1.7 for pain. CHI Discovery on Target; 2017. September 25–29; Boston, USA. [Google Scholar]
- 378.Lee KJ, Wang W, Padaki R, Bi V, Plewa CA, Gavva NR. Mouse monoclonal antibodies to transient receptor potential ankyrin 1 act as antagonists of multiple modes of channel activation. J Pharmacol Exp Ther. 2014;350:223–231. doi: 10.1124/jpet.114.215574. [DOI] [PubMed] [Google Scholar]
- 379.Huang X, Shaffer PL, Ayube S, Bregman H, Chen H, Lehto SG, Luther JA, Matson DJ, McDonough SI, Michelsen K, et al. Crystal structures of human glycine receptor α3 bound to a novel class of analgesic potentiators. Nat Struct Mol Biol. 2017;24:108–113. doi: 10.1038/nsmb.3329. [DOI] [PubMed] [Google Scholar]
- 380.Buckley D. Regeneron presentation: membrane proteins as targets for therapeutic antibody discovery. CHI 14th Annual Discovery on Target; 2016. September 19–22; Boston, USA. [Google Scholar]
- 381.Shi E, Fury W, Li W, Mikulka W, Aldrich T, Rafique A, Chen G, Hoffenberg S, Daly TJ, Radziejewski C. Monoclonal antibody classification based on epitope-binding using differential antigen disruption. J Immunol Methods. 2006;314:9–20. doi: 10.1016/j.jim.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 382.MacDonald L, Gao M, Morra MR, Alessandri-Haber NM, LaCroix-Fralish ML. Anti-ASIC1 antibodies and uses thereof. Patent Publication 20140056907. February 27, 2014. [Google Scholar]
- 383.Qiang M, Dong X, Zha Z, Zuo XK, Song XL, Zhao L, Yuan C, Huang C, Tao P, Hu Q, et al. Selection of an ASIC1a-blocking combinatorial antibody that protects cells from ischemic death. Proc Natl Acad Sci U S A. 2018;115:E7469–E7477. doi: 10.1073/pnas.1807233115. [DOI] [PMC free article] [PubMed] [Google Scholar]


