Abstract
Thymic stromal lymphopoietin (TSLP) is an epithelial cell-derived cytokine expressed in the skin, gut, lungs, and thymus. TSLP triggers dendritic cell-mediated T helper 2 inflammatory responses by formation of a ternary complex consisting of a heterodimer of interleukin-7 (IL-7) receptor α chain (IL-7Rα), TSLP, and the TSLP receptor chain (TSLPR). The present study aimed to investigate the expression of this ternary complex and its interaction with signal transducer and activator of transcription 5 (STAT5) in a ischemic stroke model using middle cerebral artery occlusion. Using immunofluorescence staining, we found that TSLPR was expressed widely in neurons and gliocytes. Using immunoprecipitation analysis, we detected an increased interaction between STAT5 and the ternary complex in the cortex of stroke rats. Moreover, using western blots, we found that expressions of the ternary complex and STAT5 were markedly increased in the cortex of stroke rats compared with the control and sham rats. These results suggest that the formation of the ternary TSLPR : TSLP : IL-7Rα complex may activate STAT5 or a STAT5-related signaling pathway to mediate neuroinflammation in ischemic stroke.
Keywords: interleukin-7 receptor α chain, ischemic stroke, neuroinflammation, signal transducer and activator of transcription 5, thymic stromal lymphopoietin
Introduction
Ischemic stroke, the most common subtype of stroke, has been singled out by the WHO as an upcoming epidemic of the 21st century that results in neuronal death and consequent behavioral and motor symptoms and/or sudden bleeding 1. Although the underlying molecular mechanism is extremely complex, neuroinflammation plays an important role in the occurrence and development of ischemic stroke 2.
Thymic stromal lymphopoietin (TSLP), a pleiotropic cytokine originally isolated from a murine thymic stromal cell line, is an interleukin (IL)-7-like cytokine expressed at barrier surfaces of the skin, gut, nose, lung, and the maternal/fetal interphase 3. In contrast to the circumscribed expression of TSLP, the TSLP receptor (TSLPR) is distributed more widely. The formation of the ternary complex TSLPR : TSLP : IL-7Rα activates the signal transducer and activator of transcription 3 (STAT3) in humans and STAT5 in mice and humans in a Janus kinase (JAK)-independent manner to induce the expression of target genes 4. In the central nervous system (CNS), microglial cells can differentiate into noninflammatory tissue-resident dendritic cells (DCs), and Kitic et al. 5,6 reported that TSLP is produced by choroid plexus epithelial cells and astrocytes in the spinal cord. However, to the best of our knowledge, no study has been published on the expression of the TSLPR : TSLP : IL-7Rα ternary complex in the brain tissue of patients or animal models with ischemic stroke. Considering the specific effect of the ternary complex on inflammation, we hypothesized that altered expression of this complex may be involved in neuroinflammation and the pathogenesis of ischemic stroke. To verify our hypothesis, we used middle cerebral artery occlusion (MCAO) to produce an ischemic stroke model in rats and performed western blot, immunofluorescence, and immunoprecipitation (IP) experiments to detect the expression and interaction of the ternary complex and STAT5.
Material and methods
Middle cerebral artery occlusion and grouping
A total of 65 male Sprague–Dawley rats, weighing between 250 and 300 g, were purchased from the Animal Experiment Center of Institute of Radiation Medicine of the Chinese Academy of Medical Sciences for use in our experiments. All rats were housed in a controlled environment under a 12 h light/dark cycle and humidity of 55±5% and were provided with food and water ad libitum. Rats were divided randomly into three groups: control (normal control, n=20) group, sham (sham-operated, n=20) group, and stroke group (n=25) in which rats were subjected to MCAO surgery. MCAO surgery was performed as described previously 7.
Neurological deficit score
After the rats had recovered completely from MCAO surgery, a single observer who was blinded to the experimental groups assessed their neurological deficit scores according to previous studies 7. Briefly, neurological deficits were scored as follows: 0, no deficits; 1, unable to fully extend the contralateral forelimb; 2, cannot extend the contralateral forelimb; 3, mild circling to the contralateral side; 4, severe circling; and 5, falling to the contralateral side.
Tissue processing
To evaluate infarct volume, rat brains (n=10/group) were collected rapidly after the anesthesia with 10% chloral hydrate (250 mg/kg) by an intraperitoneal injection and sliced into five coronal sections for 2,3,5-triphenyltetrazolium chloride staining. To detect the location of TSLPR in the brains, brains of stroke rats (n=5) were removed and 10-µm frozen sections were prepared using a freezing microtome (Leica, Wetzlar, Germany) and stored at −80°C for immunofluorescence. To investigate changes in TSLP, TSLPR, IL-7Rα, and STAT5 expressions in the various groups (n=10/group), we collected rat cortices from the control, sham, and stroke groups under anesthesia and the separated cortices were stored at −80°C for western blotting and IP.
Evaluation of infarction volume
The brain infarction volume following MCAO was detected with 5-triphenyltetrazolium chloride staining according to previous studies 8. The digital images were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, Maryland, USA). The corrected infarct volume was calculated as 9: corrected infarct volume (%)={[contralateral hemisphere area−(ipsilateral hemisphere area−measured infarct area)]/contralateral hemisphere area}×100%.
Immunofluorescence
After incubation with normal goat serum (Dingguo, Beijing, China) for 1 h, the sections were then incubated with rabbit anti-TSLPR (1 : 50; Millipore, Burlington, Massachusetts, USA), mouse anti-NeuN (1 : 50; Millipore), mouse anti-GFAP (1 : 50; Millipore), or mouse anti-Iba1 (1 : 100; Millipore) overnight at 4°C. On the following day, the sections were washed with PBS and incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 555-conjugated goat anti-mouse IgG (1 : 50; Beyotime, Beijing, China) in the dark for 1 h at 37°C. The sections were washed again in PBS and the nuclei were stained with diamidine phenylindole. Finally, images were captured using confocal laser scanning microscopy (Leica).
Immunoprecipitation
Total protein was extracted from the brains of sham and stroke animals using cell lysis buffer for western blotting and IP (Beyotime) according to the manufacturer’s instructions.
Western blot
Total protein from the brains of normal, sham, and stroke animals was extracted as described above. Protein concentrations were measured using a BCA Protein Assay Kit (Dingguo, Beijing, China). A total of 50 µg of protein from each sample was loaded into each lane of SDS-PAGE gels. Gel electrophoresis was performed, followed by transfer of the proteins onto a 0.45-μm polyvinylidene difluoride membrane (Millipore). The membrane was blocked in nonfat milk and probed with the primary and secondary antibodies. The following antibodies were used: rabbit anti-TSLPR (1 : 1000; Millipore), rabbit anti-TSLP (1 : 1000; Abcam), rabbit anti-IL-7R (1 : 1000; Abcam), rabbit anti-STAT5 (1 : 1000; Abcam), rabbit anti-GAPDH (1 : 1000; Proteintech, Chicago, Illinois, USA), and HRP-conjugated goat anti-rabbit IgG (1 : 1000, Proteintech). The blots were washed in TBST and the bands were visualized using ECL reagent (Thermo, New Tork City, New York, USA) and a Fusion FX5 image analysis system (Vilber Lourmat, Collégien, France). Relative protein expression levels were normalized to the GAPDH signal.
Statistical analysis
All data are presented as mean±SD and analyzed using Student’s t-test and one-way analysis of variance, followed by post-hoc Tukey’s test, to determine the levels of significance. P values less than 0.05 were considered statistically significant.
Results
TSLPR was expressed widely in neurons and gliocytes
We used NeuN, GFAP, and Iba1 to label neurons, astrocytes, and microglia, respectively. As shown in Fig. 1, TSLPR was expressed mainly on the cell membrane and in the cytoplasm of neurons, astrocytes, and microglia, which indicated that TSLPR was expressed widely in neurons and gliocytes.
Fig. 1.
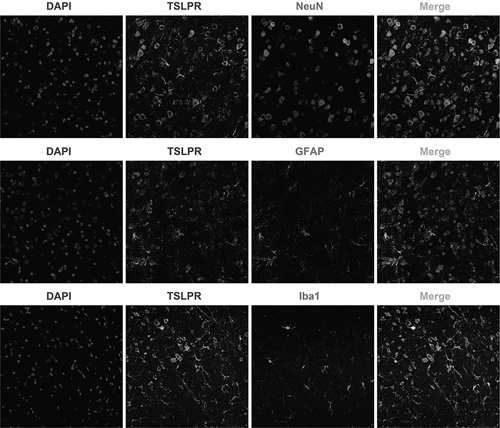
Immunofluorescent labeling of TSLPR in the rat cortex. TSLPR co-expressed with NeuN, GFAP, and Iba1 in the cortex (magnification: ×200). DAPI, diamidine phenylindole; TSLPR, thymic stromal lymphopoietin receptor.
MCAO surgery induced ischemic stroke
There was no statistical significance between the sham group and the control group in infarct volume; however, infarct volume in the stroke rats was significantly higher than that in the sham and control groups [Fig. 2a and b; F (2, 27)=466.7, P<0.0001, P>0.05 control vs. sham, P<0.0001 stroke vs. control, P<0.0001 stroke vs. sham]. There was no statistical significance between the sham group and the control group in neurological scores; however, neurological scores in the stroke rats were significantly higher than those in the sham and control groups [Fig. 2c; F(2, 27)=287.4, P<0.0001, P>0.05 control vs. sham, P<0.0001 stroke vs. control, P<0.0001 stroke vs. sham].
Fig. 2.
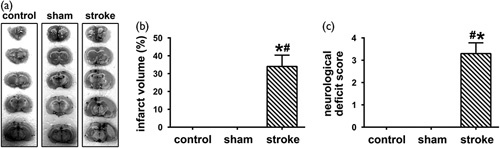
MCAO surgery induced ischemic stroke. (a) 2,3,5-Triphenyltetrazolium chloride staining showed that the control and sham groups had uniform dark staining, whereas large white areas of infarction can be observed in the right cerebral hemispheres of the stroke group. (b) An obvious increase in infarction volume was detected in the right cerebral hemisphere of stroke rats who underwent MCAO surgery compared with the control and sham groups (mean±SD, n=10/group, one-way ANOVA). (c) Prominent neurological deficits were observed in the stroke rats compared with the control and sham groups (mean±SD, n=10/group, one-way ANOVA). ANOVA, analysis of variance; MCAO, middle cerebral artery occlusion. *P<0.05 versus the control group, #P<0.05 versus the sham group.
Increased expression of TSLPR, TSLP, and IL-7R in rats with MCAO-induced ischemic stroke
No statistical significance was detected between the sham group and the control group in the TSLPR expression and TSLPR levels were higher in the stroke group compared with the control and sham groups [Fig. 3, F(2, 27)=68.32, P<0.0001, P>0.05 control vs. sham, P<0.0001 stroke vs. control, P<0.0001 stroke vs. sham]. No statistical significance was detected between the sham group and the control group in the TSLP expression and TSLP levels were higher in the stroke group compared with the control and sham groups [Fig. 3, F(2, 27)=54.04, P<0.0001, P>0.05 control vs. sham, P<0.0001 stroke vs. control, P<0.0001 stroke vs. sham]. No statistical significance was detected between the sham group and the control group in the IL-7R expression and IL-7R levels were higher in the stroke group compared with the control and sham groups [Fig. 3, F(2, 27)=36.76, P<0.0001, P>0.05 control vs. sham, P<0.0001 stroke vs. control, P<0.0001 stroke vs. sham].
Fig. 3.
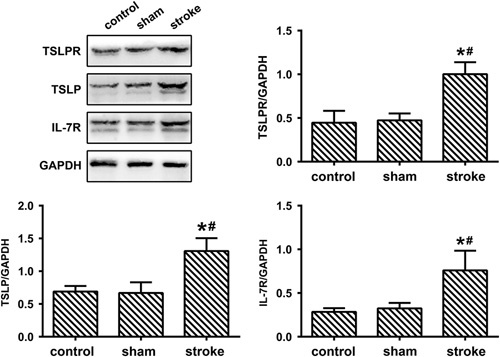
Increased expression of TSLPR, TSLP, and IL-7R in rats with MCAO-induced ischemic stroke. The levels of TSLPR, TSLP, and IL-7R in the cortex of control, sham, and stroke animals were detected by western blots (mean±SD, n=10/group, one-way analysis of variance). GAPDH, glyceraldehude-3-phosphate dehydrogenase; IL-7R, interleukin-7 receptor; MCAO, middle cerebral artery occlusion; TSLP, thymic stromal lymphopoietin; TSLPR, TSLP receptor. *P<0.05 versus the control group, #P<0.05 versus the sham group.
Increased expression of STAT5 in rats with MCAO-induced ischemic stroke
We next detected the expression of STAT5 in rats with MCAO-induced ischemic stroke using western blots. As shown in Fig. 4, no statistical significance was detected between the sham group and the control group in the STAT5 expression and STAT5 levels were higher in the stroke group compared with the control and sham groups [F(2, 27)=58.20, P<0.0001, P>0.05 control vs. sham, P<0.0001 stroke vs. control, P<0.0001 stroke vs. sham].
Fig. 4.
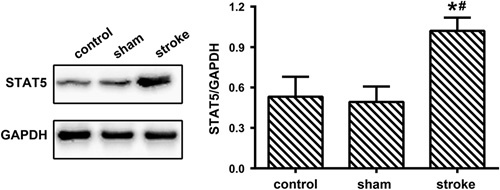
Increased expression of STAT5 in rats with middle cerebral artery occlusion-induced ischemic stroke. The level of STAT5 in the cortex of control, sham, and stroke animals was detected by western blots (mean±SD, n=10/group, one-way analysis of variance). GAPDH, glyceraldehude-3-phosphate dehydrogenase; STAT5, signal transducer and activator of transcription 5. *P<0.05 versus the control group, #P<0.05 versus the sham group.
Increased interaction between STAT5 and the ternary complex in rats with MCAO-induced ischemic stroke
To test whether the interaction between STAT5 and the ternary complex was increased in rats with MCAO-induced ischemic stroke, we carried out an IP analysis to detect protein–protein interactions. We used rabbit anti-STAT5 antibody to precipitate the proteins that bound to STAT5, and precipitated proteins were detected using western blots. As shown in Fig. 5, we found that STAT5 interacted with the ternary complex and that this interaction was significantly increased in the stroke group compared with the control and sham groups. These data suggested that increased interaction between the ternary complex and STAT5 could promote the expression of target genes.
Fig. 5.
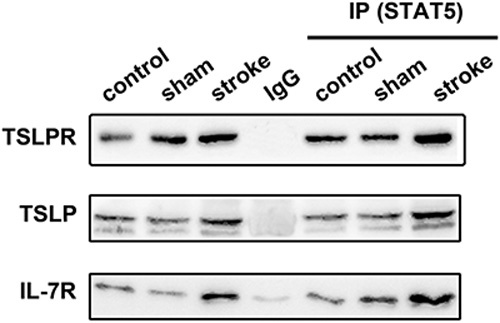
Increased interaction between STAT5 and the ternary complex in rats with MCAO-induced ischemic stroke. Cell lysates were subjected to IP with an anti-STAT5 antibody and immunoblotted with anti-TSLPR, anti-TSLP, or anti-IL-7R antibodies. Representative immunoblots from five independent experiments show the increased interaction between STAT5 and the ternary complex in rats with MCAO-induced ischemic stroke. IL-7R, interleukin-7 receptor; IP, immunoprecipitation; MCAO, middle cerebral artery occlusion; STAT5, signal transducer and activator of transcription 5; TSLP, thymic stromal lymphopoietin; TSLPR, TSLP receptor.
Discussion
Many studies have confirmed that the neuroinflammatory cascade is activated immediately after vessel occlusion has occurred and is necessary to protect the brain against insults, but it can also be an intrinsic component that promotes the development of ischemic stroke 10,11. Thus, blocking neuroinflammation might be a promising therapeutic strategy to ameliorate ischemic stroke. Previous studies have indicated that increased expression of inflammation-associated proteins is a critical step required to trigger the inflammatory cascade, which is mediated by STAT proteins 11,12. The STATs contain seven members (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6). After STATs are phosphorylated in a JAK-independent or JAK-dependent manner, they form homodimers or heterodimers and translocate into the nucleus to bind to specific DNA target sequences modulating gene transcription 13,14. Of the seven members in the STAT family, emerging evidence has shown that STAT5 is expressed in many tissues and plays an important role in inflammation. Meynard et al. 15 found that inflammation induced by IL-6 in a human hepatoma cell line or induced by lipopolysaccharide in mice suppressed STAT5 activation, accompanied by downregulation of the transmembrane serine protease matriptase-2 and unbalanced iron homeostasis that aggravated inflammatory damage. In addition, the activation of enterocyte STAT5 promoted intestinal mucosal wound healing through suppression of myosin light-chain kinase-mediated loss of barrier function and inflammation 16. In ischemic stroke, Sola et al. 17,18 discovered that STAT5 activation induced by erythropoietin had a neuroprotective effect and improved brain injury in a stroke model. Similarly, we found that the expression of STAT5 was higher in the cortex of stroke rats than in sham and control animals, which suggests that STAT5 activation in the early stage of ischemic stroke might limit or compensate for brain damage.
TSLP, an IL-7-like cytokine, triggers DC-mediated T helper (Th) 2 inflammatory responses 19. Like other stimuli activated by DCs such as CD40L and Toll-like receptor ligands, TSLP strongly upregulates the expression of some proteins in DCs, including MHC class II, CD54, CD80, CD83, and CD86. However, unlike CD40L and Toll-like receptor ligands, TSLP does not regulate DCs to produce the Th1-polarizing cytokine IL-12 or the proinflammatory cytokines tumor necrosisi factor, IL-1β, and IL-6 19. TSLP induces inflammatory Th2 cells mainly in two ways. First, TSLP induces the maturation of DCs without help from IL-12. Second, TSLP triggers the differentiation of inflammatory Th2 cells directly by inducing the expression of OX40L on DCs 19. Although the detailed mechanism underlying the TSLP-induced Th2 phenotype is unclear, it is widely known that this unique Th2 phenotype involves STAT5 activation, which is independent of the classical nuclear factor-κB and myD88 signaling pathways 19. In the CNS, TSLP expression by choroid plexus epithelial cells and astrocytes was found to regulate the survival and the effector functions of ternary complex-expressing Th2 cells in the meningeal/perivascular DC network 5. In the present study, we observed higher levels of TSLP, TSLPR, and IL-7Rα in the cortex of rats with MCAO-induced ischemic stroke compared with the sham and control groups, which was accompanied by increased interaction between STAT5 and the ternary complex and activation of STAT5. Moreover, we also showed that TSLPR was expressed widely in neurons and gliocytes. These data suggest that in the early stage of ischemic stroke, the TSLP-induced Th2 phenotype may regulate neuroinflammatory processes to balance inflammatory and anti-inflammatory responses and thus protect against brain damage.
Conclusion
Our results are the first, to our knowledge, to show the expression of TSLP, TSLPR, and IL-7Rα in the cortex of rats with ischemic stroke. After MCAO-induced ischemic stroke, the expression of TSLP, TSLPR, and IL-7Rα increased markedly in stroke rats. In addition, the TSLPR was expressed widely in neurons and gliocytes. Furthermore, we found that the interaction of the ternary complex with STAT5 increased in stroke rats. Our findings may contribute toward our understanding of the TSLP-induced Th2 phenotype in the CNS. Although abnormal levels of TSLP, TSLPR, IL-7Rα, and STAT5 were detected in the cortex of stroke rats, further researches will be needed to determine the detailed mechanism underlying the role of the TSLP-induced Th2 phenotype in the occurrence and development of ischemic stroke.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sarikaya H, Ferro J, Arnold M. Stroke prevention: medical and lifestyle measures. Eur Neurol 2015; 73:150–157. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann J, Härtig W, Seidel A, Füldner C, Hobohm C, Grosche J, et al. Inflammatory cell recruitment after experimental thromboembolic stroke in rats. Neuroscience 2014; 279:139–154. [DOI] [PubMed] [Google Scholar]
- 3.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia 2001; 15:1286–1292. [DOI] [PubMed] [Google Scholar]
- 4.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol 1999; 163:5971–5977. [PubMed] [Google Scholar]
- 5.Kitic M, Wimmer I, Adzemovic M, Kögl N, Rudel A, Lassmann H, et al. Thymic stromal lymphopoietin is expressed in the intact central nervous system and upregulated in the myelin-degenerative central nervous system. Glia 2014; 62:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer HG, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol 2000; 164:4826–4834. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, Yan J, Wang L, Tian X, Zhang T, Guo L, et al. The Pyk2/MCU pathway in the rat middle cerebral artery occlusion model of ischemic stroke. Neurosci Res 2018; 131:52–62. [DOI] [PubMed] [Google Scholar]
- 8.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986; 17:1304–1308. [DOI] [PubMed] [Google Scholar]
- 9.Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo C, et al. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflammation 2014; 11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in stroke brain damage. Stroke 2016; 47:1165–1172. [DOI] [PubMed] [Google Scholar]
- 11.Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics 2016; 13:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, et al. The role of JAK-STAT signaling within the CNS. JAKSTAT 2013; 2:e22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994; 264:1415–1421. [DOI] [PubMed] [Google Scholar]
- 14.Schindler C. Cytokines and JAK-STAT signaling. Exp Cell Res 1999; 253:7–14. [DOI] [PubMed] [Google Scholar]
- 15.Meynard D, Sun CC, Wu Q, Chen W, Chen S, Nelson CN, et al. Inflammation regulates TMPRSS6 expression via STAT5. PLoS One 2013; 8:e82127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert S, Zhang R, Denson L, Moriggl R, Steinbrecher K, Shroyer N, et al. Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol Med 2012; 4:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sola A, Rogido M, Lee BH, Genetta T, Wen TC. Erythropoietin after focal cerebral ischemia activates the Janus kinase-signal transducer and activator of transcription signaling pathway and improves brain injury in postnatal day 7 rats. Pediatr Res 2005; 57:481–487. [DOI] [PubMed] [Google Scholar]
- 18.Gan Y, Xing J, Jing Z, Stetler RA, Zhang F, Luo Y, et al. Mutant erythropoietin without erythropoietic activity is neuroprotective against ischemic brain injury. Stroke 2012; 43:3071–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med 2006; 203:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]


