Supplemental Digital Content is available in the text.
Keywords: ischemia, neuronal cell death, neuropharmacology, neuroprotection, stroke
Abstract
In vitro excitotoxic cell death experiments can be considered a screening model of stroke to evaluate the neuroprotective property of specific compounds. Survival of neurons following excitotoxicity is influenced by the neurotrophic factors (nerve growth factor and brain-derived neurotrophic factor). Here, a novel 12 amino-acid peptide [AYKSYVRALPLL (TUF1)] with a high level of evolutionary conservation was assessed for its neuroprotective property in an in vitro model of glutamate-induced N-methyl-d-aspartic acid receptor hyperactivation and excitotoxicity. This peptide shares 100% homology to the conserved motif (SYVRAL) of the neurotrophic factors, which is found in numerous US patents. Following exposure to toxic levels of glutamate (500 µM), cultured primary rat forebrain neurons treated with TUF1 showed a dose-dependent survival rate compared with untreated neurons. The neuroprotective effect was blocked by p75 neurotrophic receptor (p75NTR) inhibitor (MC192), but not by tyrosine kinase receptor inhibitor (K252a) or N-methyl-d-aspartic acid receptor antagonists (MK801 and d-amino-5-phosphonovaleric acid). Serine to alanine substitution that abolishes p75NTR interaction showed a loss of neuroprotective effect. Collectively, the findings showed that TUF1 can protect cultured primary cortical neurons from excitotoxic cell death through the p75NTR-dependent pathway. Given that TUF1 is derived from TMEM35 (NACHO), which is required for the assembly and expression of nicotinic acetylcholine receptors, mechanism of TUF1 action may involve organization of nicotinic acetylcholine receptor and p75 neurotrophin receptor to modulate neuronal responses, including Ca2+ signaling, to cytotoxic events. Unlike nerve growth factor, which requires a pre-insult exposure, TUF1 has neuroprotective properties even with post-insult administration, making it a potential target for therapeutic development in mitigating neuronal damage due to stroke and brain injury.
Introduction
Brain injuries associated with cerebrovascular stroke or ischemia can cause an extremely wide range of effects depending on the location and the severity of the damage. Neuronal cell death is a known sequala of ischemic brain injury 1,2. One mechanism for cell death following ischemic brain injury is excitotoxicity due to the excessive release of glutamate and other excitatory amino acids, leading to increased intracellular calcium influx 3,4. Antagonizing glutamate receptors [e.g. N-methyl-d-aspartic acid receptor (NMDAR)] by d-amino-5-phosphonovaleric acid (DAPV, competitive inhibitor) or dizocilpine (MK801, noncompetitive inhibitor) prevent neuronal cell death from excitotoxicity, indicating that activation of the NMDAR is crucial to excitotoxic neuronal cell death 5,6. However, in vivo and clinical trials of NMDAR antagonists have failed to produce clinical efficacy 7,8. This has left an urgent need for new treatments for stroke therapies as well as neuropathologies associated with aberrant excitotoxic cell death 9,10.
Neurotrophic factors promote neuronal survival under excitotoxic conditions, which is mediated by both the high [tropomysin receptor kinase (Trk)] and low [p75 neurotrophic receptor (p75NTR)] affinity receptors 11,12. Importantly, p75NTR has emerged as a pharmacological target for neuroprotection and repair against brain injury or neurodegenerative disorders 12,13. A synthetic 12-amino acid peptide (TUF1) has been shown to bind the p75NTR 14. This interaction is dependent on a conserved charged amino acid residue within the p75NTR binding motif of the neurotrophic factors 14,15. This peptide was derived from the hydrophilic region between the first and second hydrophobic transmembrane domains of an evolutionarily conserved neural-specific transmembrane 35 (TMEM35) protein. While the full-length TMEM35 has been shown as a putative endoplasmic reticulum protein required for assembly and expression of nicotinic acetylcholine receptors (nAChRs) 16,17, TUF1 may have an additional role given its potential extracellular localization and interaction with p75NTR 14. The present study assessed whether the TUF1 peptide has a neuroprotective property against glutamate-induced excitotoxic neuronal cell death.
Materials and methods
Tissue culture
Cultured forebrain neurons from 1-day-old rat pups (Harlan, Indianapolis, Indiana, USA) were prepared as described previously 18. Briefly, the forebrain was dissociated in L-15 media containing 3 mg/ml papain (Sigma, St. Louis, Missouri, USA) and 3 mg/ml BSA (Sigma). After trituration in growth media [MEM without glutamine, 27.75 mM glucose, 10% NuSerum (Collaborative Research, Bedford, Massachusetts, USA), 50 U/ml penicillin, 50 μg/ml streptomycin], the cell suspension was layered on L-15 containing 100 mg/ml BSA and centrifuged at 500 rpm for 5 min. The pellet was resuspended in growth media. Overall, 200 k cells were seeded onto polylysine-coated 24-well plates. Fluorodeoxyuridine (15 μg/ml) and uridine (35 μg/ml) were added at 24 h after plating to restrict glial cell overgrowth. Cultures were maintained (37°C, 5% CO2) for 7–10 days before use.
Toxicity induction and cell death assessment
After neurons had developed a network of processes, two healthy fields with evenly distributed neurons per well were preselected. The growth medium was replaced by 1.5 ml of MEM containing (in mM) 27.75 glucose, 35 sucrose, 0.01 glycine, 10 Na-HEPES, and 500 µM glutamate (Glu). After 10 min, cultures were rinsed twice with Earle’s Balanced Salt Solution (EBSS+) containing (in mM) 116 NaCl, 5.4 KCl, 1.8 CaCl2, 0.1 MgSO4, 0.9 NaH2PO4, 26.2 NaHCO3, 27.75 glucose, 35 sucrose, and 0.01 glycine. After 24-h incubation, trypan blue dye was added (final concentration 0.12 mg/ml) and cells containing (dead) and excluding (live) dye were counted in the preselected fields.
Administration of TUF1 peptides and inhibitors
After second rinse with EBSS+ following Glu exposure, the following were added to the cell cultured wells: In-house synthesized TUF1 (AYKSYVRALPLL, >97% purity) and TUF1ΔS46A (AYKAYVRALPLL, >97% purity), MK801 (Sigma), DAPV (Sigma), K252a (Sigma), or MC192 (Abcam, Cambridge, Massachusetts, USA).
Statistical analysis
Data were analyzed by a one-way analysis of variance followed by post-hoc Bonferroni corrected t-test for pairwise comparison using GraphPad Prism, GraphPad Prism, La Jolla, California, USA. Mean differences between groups were considered significant if P values are less than 0.05.
Results and discussion
TUF1 promotes neuronal survival in a dose-dependent manner
TUF1 peptide composition was derived from TMEM35 (aka NACHO) based on the principle of evolutionary conservation that important protein motifs are highly conserved across evolution (Fig. 1a; Supplementary Fig., Supplemental digital content 1, http://links.lww.com/WNR/A499). Interestingly, the central six amino acids (SYVRAL) are a highly patented peptide sequence (data not shown). A range of TUF1 doses was tested to determine the efficacy of TUF1 in reducing cell death induced by a toxic level of glutamate. Treatment with glutamate produced a significant level of cell death comparable to previous observations (Fig. 1b) 18. The survival rate observed across TUF1 doses indicates a dose-dependent effect (Fig. 1b) and supports a role in neuroprotection against glutamate toxicity. The optimal effect was observed between nanomolar to micromolar range, which is far higher than the picomolar range for nerve growth factor (NGF) and brain-derived neurotrophic factor 11,19. This effect is consistent with the low affinity of TUF1 for p75NTR compared with NGF 14. Thus, additional optimization of TUF1 peptide will be necessary to increase its efficiency, including the generation of small molecule mimetics. To further determine a time point at which TUF1 neuroprotective activity is most effective, combinations of the different timed applications were tested. TUF1 neuroprotection was effective at all tested time points relative to induction of glutamate toxicity (Fig. 1c; F=19.95, P<0.0001). Interestingly, TUF1 treatment 2 h after glutamate exposure increased cell survival by 15.7% compared with untreated neurons (P=0.047). Although modest, this post-insult efficacy confers an advantage of TUF1 over NGF or brain-derived neurotrophic factor 11,19 given the poor translational efficacy of the neurotrophic factors in clinical settings 20. Additional studies will be needed to further determine its efficacy beyond 2-h postexcitotoxic induction. The effectiveness of treatment after cytotoxicity is important, as it is impossible to predict when brain injurious events, including strokes, will occur. As such, neuroprotective interventions must be efficacious even after the insult has occurred.
Fig. 1.
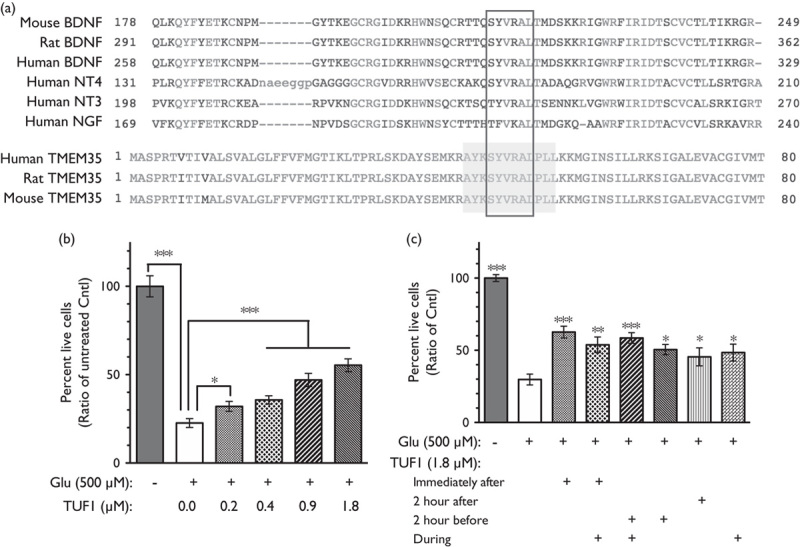
TUF1 dose and time responses. (a) Evolutionary conservation of the p75NTR binding motif between the classic neurotrophic factor and TMEM35 (line box). The shaded box indicates TUF1 peptide, which is flanked by potential proconvertase sites (KR and KK). (b) Survival of neurons 24 h after exposure to the toxic level of glutamate (Glu) for 10 min, and immediately treated with TUF1 at various concentrations. Values are mean±SEM, n=11–12/treatment, analysis of variance (ANOVA), ***P<0.001. (c) Cell survival response to the timing of TUF1 treatment. Live neurons 24 h after exposure to glutamate (Glu). Values are mean±SEM, n=9/treatment, ANOVA, ***P<0.001, **P<0.01, *P<0.05.
Neuroprotective activity of TUF1 is dependent on p75NTR receptor
Given that p75NTR can mediate neuroprotection against glutamate cytotoxicity 11,21, TUF1ΔS46A, which does not bind p75NTR 14, was tested and showed a loss of neuroprotective effect (Fig. 2a), suggesting a p75NTR-dependent mechanism analogous to the action of NGF 11,21. To further validate this notion, an inhibitor of p75NTR (MC192) was used together with TUF1. The insufficiency of MC192 alone or in combination with TUF1 to produce a significant cell survival following glutamate exposure (Fig. 2a) indicates a requirement for p75NTR activation. Conversely, inhibition of Trk receptors by K252a 22 did not significantly reduce the neuroprotection of TUF1 (Fig. 2a, P<0.01), suggesting a Trk-independent pathway. To further determine if the survival produced by TUF1 peptide was associated with NMDAR activation, inhibitors of NMDAR (MK801 or DAPV) were used in conjunction with TUF1. TUF1, MK801, or DAPV alone elicited a significant increase in the survival of neurons exposed to glutamate (Fig. 2b, P<0.001). There was no additive effect in survival rates when TUF1 was used together with either antagonist, suggesting that TUF1 acts in an NMDAR-independent mechanism.
Fig. 2.
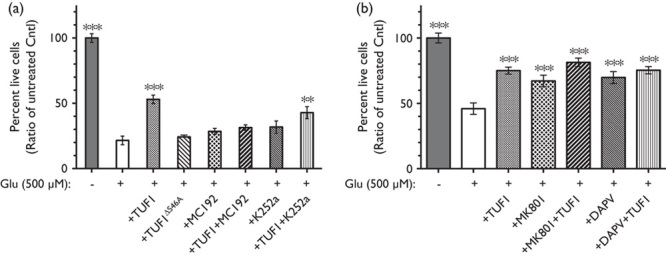
The p75NTR-dependent activity of TUF1 peptide. (a) Effects of co-application of TUF1 and antagonist of p75NTR or Trk. Survival of neurons 24 h after 10-min glutamate (Glu, 500 µM) exposure following by treatment with TUF1 (1.8 µM), TUF1ΔS46A (1.8 µM), MC192 (2.67 nM), K252a (200 nM), or a combination of TUF1 and specific antagonist. Values are mean±SEM, n=3/treatment, analysis of variance (ANOVA), ***P<0.001, **P<0.01. (b) Effects of co-application of TUF1 (1.8 µM) and NMDA receptor inhibitors [MK801 (20 µM), DAPV (50 µM)]. Values are mean±SEM, n=9–15/treatment, ANOVA, ***P<0.001.
The present study utilized the principle of evolutionary conservation to identify a potentially important protein motif (i.e. TUF1), which was subsequently showed to have a neuroprotective property in the context of glutamate-induced excitotoxic cell death of cultured rat forebrain neurons. Particularly, the ability of TUF1 to provide significant neuroprotection over an extended time period post-insult holds a potential for therapeutic development. A mechanism of action for TUF1 is likely involved the modulation of p75NTR activity. It is possible that TMEM35, from which TUF1 is derived, facilitates appropriate localization of nAChRs and p75NTR. This organization of receptors within and outside of the synapses may play a vital role in regulating neuronal responses, including Ca2+ signaling, to excitotoxicity 23–25. Clearly, additional studies are warranted to elucidate the precise role of this novel peptide.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.
Acknowledgements
The author thanks Dr Janet Dubinsky for invaluable advice, Mathew Janzen for experimental assistance, and Amanda Barks for editorial assistance. The author apologizes to colleagues whose works were not cited due to of space constraint.
This work was supported by the Viking’s Children Fund and Minnesota Medical Foundation awarded to P.V.T.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dreier JP, Lemale CL, Kola V, Friedman A, Schoknecht K. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology 2018; 134:189–207. [DOI] [PubMed] [Google Scholar]
- 2.Quillinan N, Herson PS, Traystman RJ. Neuropathophysiology of brain injury. Anesthesiol Clin 2016; 34:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol 1989; 36:106–112. [PubMed] [Google Scholar]
- 4.Pandya RS, Mao L, Zhou H, Zhou S, Zeng J, Popp AJ, Wang X. Central nervous system agents for ischemic stroke: neuroprotection mechanisms. Cent Nerv Syst Agents Med Chem 2011; 11:81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill R, Foster AC, Woodruff GN. Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. J Neurosci 1987; 7:3343–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippert K, Welsch M, Krieglstein J. Over-additive protective effect of dizocilpine and NBQX against neuronal damage. Eur J Pharmacol 1994; 253:207–213. [DOI] [PubMed] [Google Scholar]
- 7.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol 2002; 1:383–386. [DOI] [PubMed] [Google Scholar]
- 8.Lee TH, Yang JT, Ko YS, Kato H, Itoyama Y, Kogure K. Influence of ischemic preconditioning on levels of nerve growth factor, brain-derived neurotrophic factor and their high-affinity receptors in hippocampus following forebrain ischemia. Brain Res 2008; 1187:1–11. [DOI] [PubMed] [Google Scholar]
- 9.Castillo J, Loza MI, Mirelman D, Brea J, Blanco M, Sobrino T, Campos F. A novel mechanism of neuroprotection: blood glutamate grabber. J Cereb Blood Flow Metab 2016; 36:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayuso MI, Montaner J. Advanced neuroprotection for brain ischemia: an alternative approach to minimize stroke damage. Expert Opin Investig Drugs 2015; 24:1137–1142. [DOI] [PubMed] [Google Scholar]
- 11.Kume T, Nishikawa H, Tomioka H, Katsuki H, Akaike A, Kaneko S, et al. p75-mediated neuroprotection by NGF against glutamate cytotoxicity in cortical cultures. Brain Res 2000; 852:279–289. [DOI] [PubMed] [Google Scholar]
- 12.Meeker RB, Williams KS. The p75 neurotrophin receptor: at the crossroad of neural repair and death. Neural Regen Res 2015; 10:721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons DA, Belichenko NP, Ford EC, Semaan S, Monbureau M, Aiyaswamy S, et al. A small molecule p75NTR ligand normalizes signalling and reduces Huntington’s disease phenotypes in R6/2 and BACHD mice. Hum Mol Genet 2016; 25:4920–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran PV, Georgieff MK, Engeland WC. Sodium depletion increases sympathetic neurite outgrowth and expression of a novel TMEM35 gene-derived protein (TUF1) in the rat adrenal zona glomerulosa. Endocrinology 2010; 151:4852–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiesmann C, Ultsch MH, Bass SH, de Vos AM. Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature 1999; 401:184–188. [DOI] [PubMed] [Google Scholar]
- 16.Gu S, Matta JA, Lord B, Harrington AW, Sutton SW, Davini WB, Bredt DS. Brain alpha7 nicotinic acetylcholine receptor assembly requires NACHO. Neuron 2016; 89:948–955. [DOI] [PubMed] [Google Scholar]
- 17.Matta JA, Gu S, Davini WB, Lord B, Siuda ER, Harrington AW, Bredt DS. NACHO mediates nicotinic acetylcholine receptor function throughout the brain. Cell Rep 2017; 19:688–696. [DOI] [PubMed] [Google Scholar]
- 18.Dubinsky JM. Intracellular calcium levels during the period of delayed excitotoxicity. J Neurosci 1993; 13:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kume T, Kouchiyama H, Kaneko S, Maeda T, Kaneko S, Akaike A, et al. BDNF prevents NO mediated glutamate cytotoxicity in cultured cortical neurons. Brain Res 1997; 756:200–204. [DOI] [PubMed] [Google Scholar]
- 20.Molina-Holgado F, Doherty P, Williams G. Tandem repeat peptide strategy for the design of neurotrophic factor mimetics. CNS Neurol Disord Drug Targets 2008; 7:110–119. [DOI] [PubMed] [Google Scholar]
- 21.Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, Krieglstein J. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience 2002; 115:1089–1108. [DOI] [PubMed] [Google Scholar]
- 22.Knusel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem 1992; 59:1987–1996. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Nai Q, Chen M, Dittus JD, Howard MJ, Margiotta JF. Brain-derived neurotrophic factor and trkB signaling in parasympathetic neurons: relevance to regulating alpha7-containing nicotinic receptors and synaptic function. J Neurosci 2004; 24:4340–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brumwell CL, Johnson JL, Jacob MH. Extrasynaptic alpha 7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity. J Neurosci 2002; 22:8101–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mrzljak L, Levey AI, Belcher S, Goldman-Rakic PS. Localization of the m2 muscarinic acetylcholine receptor protein and mRNA in cortical neurons of the normal and cholinergically deafferented rhesus monkey. J Comp Neurol 1998; 390:112–132. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.


