Abstract
Background
Diabetes mellitus can occur after acute pancreatitis (AP), but there are currently no tools for evaluating the risk of developing diabetes after an attack of AP. The aim of the study was to develop a nomogram for prediction of new-onset diabetes mellitus after the first attack of AP.
Patients and methods
We enrolled 616 patients with first-attack AP. We collected and statistically analyzed demographic data (age, BMI, and duration of hospitalization) and laboratory data (glucose, low-density lipoprotein cholesterol, triglyceride, and cholesterol).
Results
Univariate analysis suggested duration of hospitalization (P=0.0003), BMI (P=0.0059), cholesterol (P=0.0005), triglyceride (P=0.0005), hemoglobin (P=0.0229), and glucose (P<0.001) at admission were significantly associated with newly developed diabetes after the first-attack AP. Multivariate analysis showed that age [odds ratio (OR)=1.01; 95% confidence interval (CI): 1.00–1.03; P=0.045], BMI (OR=1.06; 95% CI: 1.01–1.12; P=0.018), glucose (OR=1.07; 95% CI: 1.02–1.12; P=0.008), triglyceride (OR=1.03; 95% CI: 1.00–1.06; P=0.035), and low-density lipoprotein-cholesterol (OR=1.18; 95% CI: 1.00–1.38; P=0.044) at admission were important predictors.
Conclusion
The nomogram is a potentially clinically useful tool for predicting new-onset diabetes, which is currently clinically unprecedented. This finding is not confined to the patients with severe AP but is also for patients who have recovered from mild AP. The nomogram must to be validated externally.
Keywords: acute pancreatitis, diabetes mellitus, nomogram
Introduction
Acute pancreatitis (AP) is a common clinical condition of varying severity 1. Although AP has classically been considered a self-limiting, reversible disease, there is an expanding body of evidence that suggests the significance of new disturbances in glucose metabolism following AP 2. A recent comprehensive meta-analysis showed that prediabetes and/or diabetes mellitus (DM) was observed in nearly 40% of the patients following AP 3. This result challenged the widely accepted view that pancreatic injury is temporary, and that endocrine pancreatic function recovers fully after AP regardless of severity 4–6. DM can occur after AP 3,7–9, but there remains no consensus regarding the extent of the risk following AP.
DM contributes significantly to all-cause worldwide mortality, attributable to one in 12 adult deaths 10. DM owing to pancreatic disease, classified as type 3c according to the current classification system, accounts for 5–10% of the western diabetic population 11. The complication rate with DM was high in patients following pancreatic surgery (28–100%) 12,13 and chronic pancreatitis (CP) (12–80%) 14,15. However, 70–80% of patients with acquired impaired glucose metabolism following AP did not have CP or pancreatic surgery 8,16. The risk of diabetes increases two-fold after AP. Therefore, we believe a long-term screening method is needed to evaluate diabetes risk after an attack of AP, regardless of severity 7.
A previous study suggested that pancreatic endocrine insufficiency after AP may be an underestimated problem 17, and risk factors for DM after AP remain elusive 18.
The primary aim of the study was to develop a nomogram for prediction of new-onset DM within 3 months of a first attack of AP without CP or pancreatic surgery. The secondary aim was to identify associated risk factors for DM following a first episode of AP. Our study was better than other research studies whose aim was merely to obtain risk factors for hyperglycemia or DM after AP 3,8,16,19.
Patients and methods
Patients population, data collection, and ethics
This study was a cross-sectional follow-up study including all patients consecutively admitted to the First Affiliated Hospital of Wenzhou Medical University with AP between January 2013 and December 2015. Inclusion criteria were as follows: diagnosis of AP based on international guidelines 20, at least 18 years of age, at least 3-month follow-up, and informed consent.
Exclusion criteria were as follows: more than 72 h after onset of symptoms, recurrent pancreatitis, previous diagnosis of diabetes, history of impaired fasting glucose or impaired glucose tolerance, CP, post-ERCP pancreatitis, malignant gastrointestinal tumor, liver disease, chronic kidney disease, pregnancy, intoxication, and death during admission.
Data collected included personal characteristics (age, sex, BMI, etiology, and alcohol intake), laboratory studies (blood biochemical indicators and complete blood counts), and abdominal imaging.
All patients provided full informed consent before clinical procedures to which they were subjected after study inclusion. This study protocol was approved by the Ethic Committee of the First Affiliated Hospital of Wenzhou Medical University.
Definitions
The diagnosis and the severity of AP were defined based on the 2012 revision of the Atlanta classification and definitions by international consensus 20, where patients must meet two of the following three criteria: (a) abdominal pain, (b) level of serum lipase or amylase at least three times greater than the upper limit of normal, and (c) findings on cross-sectional abdominal imaging consistent with AP. The severity of AP is further categorized into three levels: mild acute pancreatitis, moderately severe AP, and severe acute pancreatitis (SAP). SAP is defined as the presence of persistent organ failure (≥48 h). Organ failure is defined as a Marshall score of at least 2 for at least one of three involved organ systems: cardiovascular, respiratory, and renal 21.
DM is defined by persistent hyperglycemia. Pancreatic diabetes arising from pancreatic disease is now categorized as type 3c diabetes 22. There are no universally accepted diagnostic criteria for this type. Conceptually, the diagnosis of type 3c diabetes requires the following characteristics: diagnostic criteria for diabetes, disease of the exocrine pancreas (including AP), and diabetes reasonably certain to be secondary to exocrine pancreatic disease 23. According to the 1999 WHO classification, DM was defined as a fasting blood glucose of at least 7.0 mmol/l (126 mg/dl) or 2 h after oral glucose tolerance test of at least 11.1 mmol/l (200 mg/dl) 24. These definitions accorded with those defined by a meta-analysis of 24 prospective clinical studies 3.
BMI (kg/m2) was determined using a digital medical scale with stadiometer. For height measurement (cm), study participants were asked to remove their shoes and head attire. For weight measurement (kg), patients were asked to remove shoes, jacket, belt, and watches and to empty their pockets 25.
Statistical analysis
A Shapiro–Wilk test was used to evaluate whether the continuous data showed a normal distribution. According to its results, continuous values were expressed by median and interquartile range and were compared using one-way analysis of variance or the Kruskal–Wallis nonparametric test. Categorical values were described by count and proportions and compared by the χ2-test or Fisher’s exact test.
All variables were included as eligible factors in a forward-conditional stepwise logistic regression analysis. For this analysis, the conditional probabilities for stepwise entry and removal of a factor were 0.05 and 0.10, respectively 26. Odds ratios (ORs) were calculated, with 95% confidence interval (CI) 27. We generated a nomogram based on the multiple logistic regression analysis. We evaluated model calibration, representing the link between predicted and observed risk, by plotting the predicted-versus-observed deciles of predicted risk, and checked the result by Hosmer–Lemeshow goodness-of-fit test 28,29. A Hosmer–Lemeshow test with greater than 0.05 indicates no significant differences between observed data and predicted data.
Differences were considered to be statistically significant if the two-tailed P value was less than 0.05.
Results
Patient characteristics
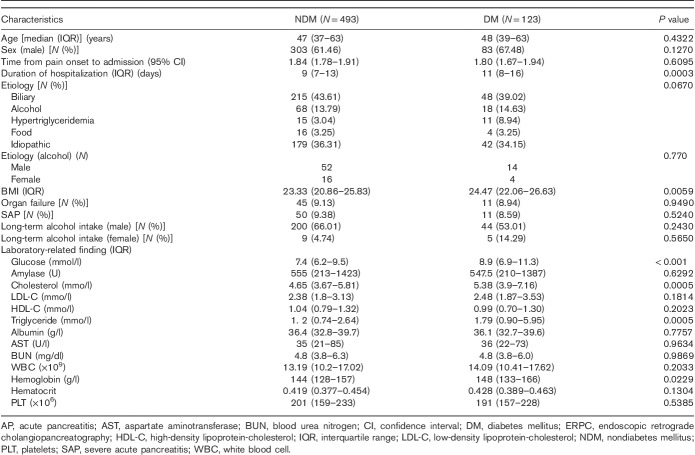
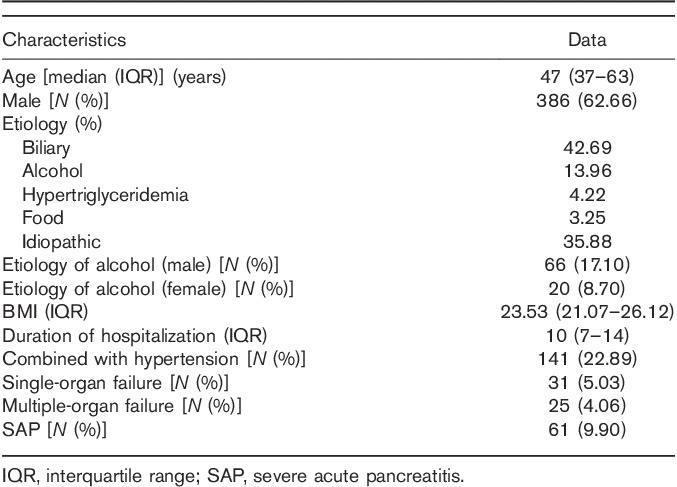
The median age of the 616 patients included in the study was 47 (37–63) years. Proportion of males was 62.7% (386). Demographic and clinical characteristics are displayed in Table 1. AP was most commonly biliary in nature. Degrees of AP were mild, 507 (76.70%); moderate/severe, 93 (14.07%); and severe, 61 (9.23%). Of the 31 (5.03%) patients who developed persistent organ failure, multiple-organ failure was noted in 25 (4.06%). The male and female drinking rates were 0.171 and 0.870, respectively. Eleven patients with who AP died following admission were excluded from our study. No patient died during follow-up.
Table 1.
Demographic and clinical characteristics of 616 patients with acute pancreatitis
Univariate and multivariate analyses
Using univariate and multivariate analyses, we tested 22 variables considered relevant to the presence of new-onset diabetes 3 months following the first episode of AP. Univariate analysis suggested that duration of hospitalization (P=0.0003), BMI (P=0.0059), cholesterol (P=0.0005), triglyceride (P=0.0005), hemoglobin (P=0.0229) and glucose (P<0.001) at admission were significantly associated with newly developed diabetes after the first-attack AP. The male and female drinking rates were inconsistent (P=0.004), so the stratification analysis was performed. There was no significant difference between alcoholic AP and nonalcoholic AP in male and female newly diabetics (P=0.7700). The factors on long-term alcohol intake of male and female patients were not statistically different (P=0.2430 and 0.5650; Table 2).
Table 2.
Univariate analysis of predictive factors of acute pancreatitis in 616 patients
Logistic regression
Multivariate analysis by logistic regression identified the following five independent variables as predictors of persistent new-onset diabetes after the first-attack of AP: age (OR=1.01; 95% CI: 1.00–1.03; P=0.045), BMI (OR=1.06; 95% CI: 1.01–1.12; P=0.018), glucose (OR=1.07; 95% CI: 1.02–1.12; P=0.008), triglyceride (OR=1.03; 95% CI: 1.00–1.06; P=0.035), and low-density lipoprotein-cholesterol (LDL-C) (OR=1.18; 95% CI: 1.00–1.38; P=0.044).
Logistic regression and Hosmer–Lemeshow test
The accuracy of the logistic regression was evaluated by producing a calibration plot.
Calibration plots indicate adequate predicted probabilities against observed proportions of incidence of new-onset diabetes after the first-attack pancreatitis (Fig. 1). The Hosmer–Lemeshow test of 0.3555 was greater than 0.05, indicating that the logistic regression fits the data better.
Fig. 1.
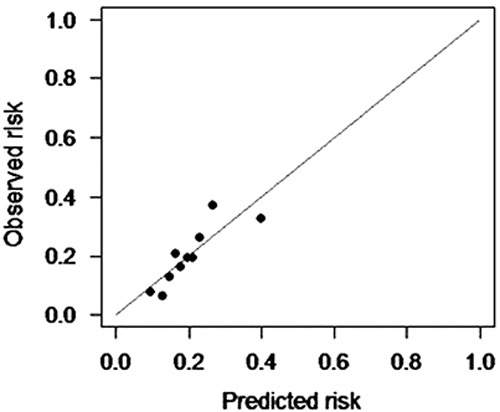
Calibration plot. LR model calibration plot. Patients were ranked by their predicted probability and divided into 10 equal groups. LR model, logistic regression model.
Nomogram
A predictive nomogram based on the logistic regression model was developed to predict probability of new-onset diabetes after the first-attack of pancreatitis (Fig. 2). Age, BMI, glucose, triglyceride, and LDL-C at admission were included in the nomogram, with probability of new-onset diabetes as the predicted outcome. It is a graphical version of the statistical model that reveals the relationship between the high-risk predictors and new-onset diabetes in proportional scale. In addition, the nomogram had a C-index of 0.686 (approximate to 0.7; Fig. 2). Nomogram C-index of 0.7 can verify the accuracy of the nomogram.
Fig. 2.
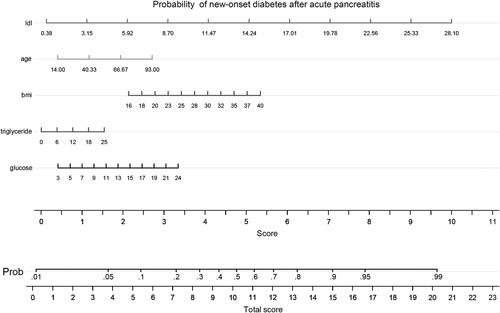
Nomogram indicating the probability of developing DM within 3 months of the first-attack of AP. The logistic regression-based nomogram is based on five input variables: age, BMI, glucose, triglyceride, and LDL-C at admission. Instructions: Locate patient values at each axis. Draw a vertical line to the ‘Point’ axis to determine how many points are attributed for each variable value. Sum the points for all variables. Locate the sum on the ‘total points’ line to be able to assess individual probability of new-onset diabetes after AP. AP, acute pancreatitis; DM, diabetes mellitus; LDL-C, low-density lipoprotein-cholesterol.
Discussion
To our knowledge, this is the first study to develop a nomogram for predicting new-onset DM within 3 months after a first-attack of AP. By logistic regression analysis and nomogram, BMI, age, glucose, triglycerides, and LDL-C at admission were significantly associated with increased risk of DM. The Hosmer–Lemeshow test (P=0.3555) suggests the logistic regression was credible.
Nomograms visually display the results of multivariate modeling, unlike complex statistical models 30. The verification method is convenient, and we can calculate the risk of diabetes in 1 min. Having age of 40 years, BMI of 23, triglyceride of 1 mmo/l, glucose of 11 mmol/l, and LDL-C of 3.15 mmo/l, a patient after AP has probability on DM of 2.5% by nomogram. With a total score of more than 15, the risk of DM significantly increases. In addition, the C-index verified the accuracy of nomogram.
We identified LDL-C as the largest predictor of new-onset diabetes after AP by the logistic analysis and nomogram. Kunutsor et al. 31 showed that the ratio of LDL-C/high-density lipoprotein-cholesterol was associated with higher prevalence of diabetes. There was hardly any literature study pointing out the direct intrinsic link between LDL-C and diabetes. However, we can establish an indirect correlation through obesity, alcohol intake, and abnormal glucose 32–34. With the increase of BMI, the risk of developing DM increased proportionally on our nomogram, as previous literature studies 24,35,36. Lipid metabolism (including LDL-C) had connection with alcohol consumption, and increased alcohol consumption (>200 g/week) was associated with increased LDL and triglycerides 34,37,38. Moreover, most studies reported that AP caused by the excessive consumption of alcohol are followed by persistent pancreatic dysfunction 36,39. However, the factors on long-term drinking after AP of male and female patients were no statistical difference in our study. The possible reasons for the inconsistency of drinking are as follows: daily average drinking in our study is low, and low drinking may have no significant correlation with DM. We also do not exclude the possibility of concealing the history of drinking.
Age proportionally increased the risk of developing DM after AP, according to our nomogram. Our result was similar to the studies of Huang et al. 9 and Pendharkar et al. 40. According to our nomogram, with the increase of the glucose, risk of developing DM increased proportionally. Higher plasma glucose may represent a physiological response to a more severe form of pancreatitis in these patients 31, leading to a higher incidence of DM 8,41.
The choice of appropriate follow-up time is a matter of debate. A meta-analysis of 24 prospective studies on patients with first-episode AP without pancreatic surgery demonstrated that the risk of diabetes increases by at least two-fold at 5 years, compared with the risk at 12 months 3. However, Shen et al. 7 showed that HR of DM after AP gradually decreased over time. Based on the feasibility of follow-up and sample size of our study increasing credibility, we chose 3 months as the follow-up interval for new-onset DM following AP.
To our knowledge, this is the first study to evaluate the possibility of DM after first AP using a predictive nomogram. The strength of this study included adequate sample size and excluded patients with recurrent AP, CP, and pancreatic surgery, which also led to endocrine insufficiency 7,14,15. Furthermore, our study avoided relying on diagnostic discharge codes for AP, the use of which results in at least 20% misclassification bias 40.
However, some limitations deserve comments. First, we did not test blood insulin, proinsulin, and C-peptide levels, which represent the true endocrine reserve of pancreas. Second, the factor on long-term drinking was qualitative data, so the analysis of this factor is not accurate enough.
Conclusion
We confirmed that AP is a major risk factor for diabetes (a rate of 20.0%). Age, BMI, glucose, triglyceride, and LDL-C at admission may predict development of DM following AP. This finding also holds for patients who have recovered from mild AP to SAP. Our nomogram has potential as a predictive tool for pancreatic diabetes, but it needs to be validated externally.
Acknowledgements
This work was supported by grants from the key construction academic subject (medical innovation) of Zhejiang Province, China (treatment of critical trauma discipline).
Jing-Ye Pan joined in the design of the study and carried out the studies. Ji-Hong Ma and You-Jun Yuan participated in data collection. Ji-Hong Ma conducted data analysis and drafted the manuscript. Su-Han Lin helped to finalize the manuscript. All the authors read and approved the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock 2007; 28:130–140. [DOI] [PubMed] [Google Scholar]
- 2.Gillies NA, Pendharkar SA, Singh RG, Asrani VM, Petrov MS. Lipid metabolism in patients with chronic hyperglycemia after an episode of acute pancreatitis. Diabetes Metab Syndr 2017; Suppl 1:S233–S241. [DOI] [PubMed] [Google Scholar]
- 3.Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut 2014; 63:818–831. [DOI] [PubMed] [Google Scholar]
- 4.Ibars EP, Sánchez de Rojas EA, Quereda LA, Ramis RF, Sanjuan VM, Peris RT. Pancreatic function aft er acute biliary pancreatitis: does it change? World J Surg 2002; 26:479–486. [DOI] [PubMed] [Google Scholar]
- 5.Uomo G, Gallucci F, Madrid E, Miraglia S, Manes G, Rabitti PG. Pancreatic functional impairment following acute necrotizing pancreatitis: long-term outcome of a non-surgically treated series. Dig Liver Dis 2010; 42:149–152. [DOI] [PubMed] [Google Scholar]
- 6.Singer MV, Gyr K, Sarles H. Revised classification of pancreatitis. Report of the Second International Symposium on the Classification of Pancreatitis in Marseille, France, March 28–30, 1984. Gastroenterology 1985; 89:683–685. [PubMed] [Google Scholar]
- 7.Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of diabetes mellitus after first-attack acute pancreatitis: a national population-based study. Am J Gastroenterol 2015; 110:1698–1706. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, et al. Long-term outcome of severe acute pancreatitis. J Hepatobiliary Pancreat Surg 2008; 15:397–402. [DOI] [PubMed] [Google Scholar]
- 9.Lee YK, Huang MY, Hsu CY, Su YC. Bidirectional relationship between diabetes and acute pancreatitis: a population-based cohort study in Taiwan. Medicine 2016; 95:2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IDF Diabetes Atlas Group. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: estimates for the year 2013. Diabetes Res Clin Pract 2015; 109:461–465. [DOI] [PubMed] [Google Scholar]
- 11.Teeling M. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33 (Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, et al. Early and late complications after pancreatic necrosectomy. Surgery 2005; 137:499–505. [DOI] [PubMed] [Google Scholar]
- 13.Halonen KI, Pettilä V, Leppäniemi AK, Kemppainen EA, Puolakkainen PA, Haapiainen RK. Long-term health-related quality of life in survivors of severe acute pancreatitis. Intensive Care Med 2003; 29:782–786. [DOI] [PubMed] [Google Scholar]
- 14.Thow J, Samad A, Alberti KGMM. Tiengo A, Alberti KGMM, DelPrato S, Vranic M. Epidemiology and general aspects of diabetes secondary to pancreatopathy. Diabetes secondary to pancreatopathy. Amsterdam: Excerpta Medica; 1988. 7–20. [Google Scholar]
- 15.Larsen S, Hilsted J, Tronier B, Worning H. Metabolic control and B cell function in patients with insulin-dependent diabetes mellitus secondary to chronic pancreatitis. Metabolism 1987; 36:964–967. [DOI] [PubMed] [Google Scholar]
- 16.Pelli H, Lappalainenlehto R, Piironen A, Järvinen S, Sand J, Nordback I. Pancreatic damage after the first episode of acute alcoholic pancreatitis and its association with the later recurrence rate. Pancreatology 2009; 9:245–251. [DOI] [PubMed] [Google Scholar]
- 17.Andersson B, Pendse ML, Andersson R. Pancreatic function, quality of life and costs at long-term follow-up after acute pancreatitis. World J Gastroenterol 2010; 16:4944–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appelros S, Lindgren S, Borgström A. Short and long term outcome of severe acute pancreatitis. Eur J Surg 2001; 167:281–286. [DOI] [PubMed] [Google Scholar]
- 19.Tu J, Yang Y, Zhang J, Yang Q, Lu G, Li B, et al. Effect of the disease severity on the risk of developing new-onset diabetes after acute pancreatitis. Medicine 2018; 97:e10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quiros JA, Marcin JP, Kuppermann N, Nasrollahzadeh F, Rewers A, DiCarlo J, et al. Elevated serum amylase and lipase in pediatric diabetic ketoacidosis. Pediatr Crit Care Med 2008; 9:418–422. [DOI] [PubMed] [Google Scholar]
- 21.Ueda T, Takeyama Y, Yasuda T, Matsumura N, Sawa H, Nakajima T, et al. Simple scoring system for the prediction of the prognosis of severe acute pancreatitis. Surgery 2007; 141:51–58. [DOI] [PubMed] [Google Scholar]
- 22.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1998; 21:5–19. [PubMed] [Google Scholar]
- 23.Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016; 1:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1999; 15:539–553. [DOI] [PubMed] [Google Scholar]
- 25.Pelli H, Lappalainenlehto R, Piironen A, Sand J, Nordback I. Risk factors for recurrent acute alcohol-associated pancreatitis: a prospective analysis. Scand J Gastroenterol 2008; 43:614–621. [DOI] [PubMed] [Google Scholar]
- 26.Whiting PF, Sterne JA, Westwood ME, Bachmann LM, Harbord R, Egger M, et al. Graphical presentation of diagnostic information. BMC Med Res Methodol 2008; 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 2014; 348:7450. [DOI] [PubMed] [Google Scholar]
- 28.Hong W, Wu J, Chen X. Receiver operating characteristic curve and odds ratio should be used with caution. Hepat Mon 2011; 11:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, et al. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol 2012; 10:84–90. [DOI] [PubMed] [Google Scholar]
- 30.Bevan MG, Asrani VM, Pendharkar SA, Goodger RL, Windsor JA, Petrov MS. Nomogram for predicting oral feeding intolerance in patients with acute pancreatitis. Nutrition 2017; 36:41–45. [DOI] [PubMed] [Google Scholar]
- 31.Kunutsor SK, Zaccardi F, Karppi J, Kurl S, Laukkanen JA. Is high serum LDL/HDL cholesterol ratio an emerging risk factor for sudden cardiac death? Findings from the KIHD Study. J Atheroscler Thromb 2017; 24:600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard WJ, Russell M, Fleg JL, Mete M, Ali T, Devereux RB, et al. Prevention of atherosclerosis with low-density lipoprotein cholesterol lowering-lipoprotein changes and interactions: the SANDS study. J Clin Lipidol 2009; 3:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popa-Wagner A, Furczyk K, Richter J, Irmisch G, Thome J. Neurotrophin levels at admission did not change significantly upon alcohol deprivation and were positively correlated with the BMI and LDL levels. J Clin Lipidol 2010; 5:455–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Würtz P, Cook S, Wang Q, Tiainen M, Tynkkynen T, Kangas AJ, et al. Metabolic profiling of alcohol consumption in 9778 young adults. Int J Epidemiol 2016; 45:1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eriksson J, Doepel M, Widén E, Halme L, Ekstrand A, Groop L, et al. Pancreatic surgery, not pancreatitis, is the primary cause of diabetes after acute fulminant pancreatitis. Gut 1992; 33:843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YK, Huang MY, Hsu CY, Su YC. Bidirectional relationship between diabetes and acute pancreatitis: a population-based cohort study in Taiwan. Medicine 2016; 95:e2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Au Yeung SL, Jiang C, Cheng KK, Cowling BJ, Liu B, Zhang W, et al. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PLoS One 2013; 8:e68054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakabayashi I. Associations of alcohol drinking and cigarette smoking with serum lipid levels in healthy middle-aged men. Alcohol Alcohol 2008; 43:274–280. [DOI] [PubMed] [Google Scholar]
- 39.Ho TW, Wu JM, Kuo TC, Yang CY, Lai HS, Hsieh SH, et al. Change of both endocrine and exocrine insufficiencies after acute pancreatitis in non-diabetic patients: a nationwide population-based study. Medicine 2015; 94:1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pendharkar SA, Asrani VM, Xiao AY, Yoon HD, Murphy R, Windsor JA, Petrov MS. Relationship between pancreatic hormones and glucose metabolism: a cross-sectional study in patients after acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2016; 311:G50–G80. [DOI] [PubMed] [Google Scholar]
- 41.Wang SH, Chou YC, Shangkuan WC, Wei KY, Pan YH, Lin HC. Relationship between plasma triglyceride level and severity of hypertriglyceridemic pancreatitis. PLoS One 2016; 11:0163984. [DOI] [PMC free article] [PubMed] [Google Scholar]