Abstract
Supplemental Digital Content is available in the text.
Preeclampsia is a relatively common complication of pregnancy with a prevalence of 3% to 5%.1 It is the leading cause of morbidity and mortality for pregnant women in the developed world and also has a significant economic burden on healthcare systems.2 Despite many decades of exhaustive research efforts, we have yet to reach a unifying theory explaining why and how preeclampsia occurs in women with no apparent risk factors. The placenta has been the major, long-standing focus of preeclampsia research—not surprising because placental lesions associated with preeclampsia were described as early as 1940.3 The triad of inadequate placentation, placental insufficiency, and vascular reactivity cascade is the most acknowledged explanation of the pathology underlying preeclampsia. However, clinical findings supporting this triad are only found in a minority of all preeclamptic pregnancies, and pathological placental lesions in support of this hypothesis may not be as specific as previously thought.4,5 The fact that our best hypothesis fails to explain the majority of preeclampsia cases led many researchers to take a broader view and look for other factors which may be associated with this serious and relatively common pregnancy-related disorder. We have known for some time that women with preeclampsia have poor long-term cardiovascular outcome, but the emerging evidence now suggests the impact of preeclampsia on maternal health is more immediate and profound than previously suspected.6–14 Moreover, preeclampsia and cardiovascular diseases share antecedents which were often thought to be a spurious association; however, many epidemiological studies now suggest that some cardiovascular risk factors also increase the risk for developing preeclampsia.15–18 Considering that cardiovascular problems associated with preeclampsia are observed both before and after the index pregnancy, it is reasonable to assume the cardiovascular system may not just be the victim of poor placentation in preeclampsia, but actually play a pivotal role in the pathogenesis of preeclampsia. More recent research has examined the association between the cardiovascular system and preeclampsia in an effort to build an overarching hypothesis to explain the pathophysiology of the disorder.19–22 These works have highlighted the fact that postpartum cardiovascular maternal health after preeclampsia is a largely neglected area of research and that women with preeclampsia may benefit from screening, follow-up, and intervention. In this review, we summarize some of the key evidence and clinical implications of the association of preeclampsia with the cardiovascular system.
Risk Factors for Developing Preeclampsia
Preeclampsia and cardiovascular diseases share genetic and nongenetic risk factors. In an umbrella review of published reviews, Giannakou et al16 suggested presence of obesity, smoking, psychological stress, chronic kidney disease, polycystic ovarian disease, and PAI-1polymorphism were consistently associated with preeclampsia. A recent genome-wide association study has implicated a locus near the fetal FLT1 region for the development of preeclampsia supporting the hypothesis that a placental isoform of sFlt-1 (soluble fms-like tyrosine kinase-1) is involved in the pathophysiology of the disease.23 A recent candidate gene association study in a Finnish cohort of preeclamptic mothers has also confirmed the involvement of the sFlt-1 gene in preeclampsia.24 Curiously, smoking is also paradoxically associated with an apparent reduction in the prevalence of mild preeclampsia at term.25 Although nicotine is associated with short-term vasoconstriction, carbon monoxide from smoking has been shown to lower the production of preeclampsia mediators (sFlt-1 and soluble endoglin) in endothelial cells and placental cultures.26 Carbon monoxide also has a more protracted hypotensive effect of 2 to 3 mm Hg, which would prevent some pregnancies from meeting the diastolic blood pressure threshold (90 mm Hg) for a diagnosis of preeclampsia.27 Even principally hormonal disorders, such as polycystic ovarian disease and premature ovarian failure (with ovum donation pregnancies), may affect increased preeclampsia risk by virtue that these disorders confer increased cardiovascular risk outside pregnancy.28
Several large cohort studies have also suggested triglyceride levels, cholesterol/HDL (high-density lipoprotein) ApoE concentrations, and ApoB/Apo A1 ratio were significantly different in preeclamptic pregnancies.17,18 Diabetes mellitus, prepregnancy weight, and maternal weight gain in pregnancy are independent risk factors for preeclampsia which may explain why metformin may be effective in reducing the prevalence of preeclampsia.29,30 Women with chronic hypertension, previous history of acute kidney injury, or a family history of myocardial infarction before the age of 60 years have an increased risk of preeclampsia.18,31 Previous preeclampsia is a risk factor for recurrence in a subsequent pregnancy, perhaps because of an inability of cardiovascular system to recover from preeclampsia as cardiovascular profiles in women with recurrent preeclampsia are poorer compared with those who have a normal pregnancy subsequently. Women with recurrent preeclampsia have increased carotid intima-media thickness and peak mitral filling early diastole/atrial contraction ratio, as well as lower cardiac output (CO) and left ventricular mass, compared with women with a normal follow-on pregnancy.31–35 In a landmark study, Romundstad et al15 assessed in a large epidemiological study whether the predisposition of preeclamptic women to increased risk for cardiovascular disease later in life can be attributed to pregnancy factors or to prepregnancy risk factors that are shared by both disorders. Their results suggested that the positive association of preeclampsia postpartum cardiovascular risk is due largely to shared prepregnancy risk factors rather than reflecting a direct influence of preeclamptic pregnancy on the maternal cardiovascular system. That all of these risk factors are also known to be correlated with cardiovascular morbidity in nonpregnant adults (Table 1) is consistent with the hypothesis that poor cardiovascular reserve predisposes to the placentally mediated disorder of preeclampsia (Figure 1).36–38
Table 1.
Risk Categories and Factors in Common for Both Preeclampsia and Cardiovascular Disease
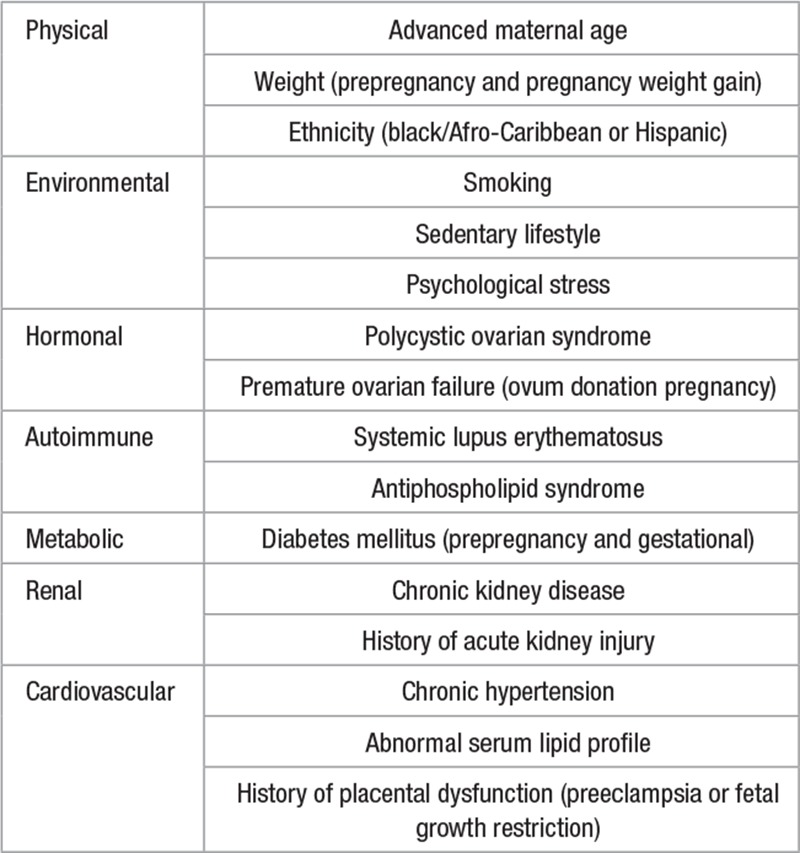
Figure 1.
Diagram illustrating the interaction between maternal cardiovascular function and placental function, maternal health, and fetal well-being. Placental oxidative stress or hypoxia is related to the relative balance of cardiovascular functional reserve and the cardiovascular volume/resistance load of pregnancy. The final common pathway that results in the signs and symptoms of preeclampsia involves the release of placental vasoactive substances. PIGF indicates placenta growth factor; and sFLT, soluble fms-like tyrosine kinase.
Early Pregnancy Cardiovascular Changes Related to Preeclampsia
Endothelium-derived vasoconstrictors are core components of preeclampsia pathophysiology, with studies demonstrating that derangement in Ang II (angiotensin-II), endothelin-1, and thromboxane A2 physiology occur long before onset of signs and symptoms of preeclampsia.39–46 Some of the biological consequences of these processes—higher blood pressure and peripheral arterial waveform resistance—are also observed long before the onset of preeclampsia. Both of these parameters (maternal mean arterial blood pressure and uterine artery resistance) are the most influential first trimester predictive biomarkers for preeclampsia.47 Importantly, other maternal peripheral arteries (ie, ophthalmic artery, brachial artery) also show signs of impaired function in early pregnancy reflecting abnormal generalized vascular physiology in preeclampsia rather than localized a vascular defect in the uteroplacental circulation as initially presumed.48–51 Recently, Foo et al52 demonstrated that women who subsequently developed preeclampsia have decreased CO and increased peripheral resistance even before conception when compared with healthy pregnancies. Similar findings together with cardiac remodeling and hypertrophy are reported for normal women at midgestation or women with chronic hypertension who later develop preeclampsia.53,54 These findings not only support the hypothesis for a shared vascular predisposition to preeclampsia and cardiovascular morbidity in the nonpregnant population but also open up the possibility that investigating cardiovascular function may help further elucidate the pathophysiology and clinical consequences of preeclampsia.38,55,56 In support of a shared cardiovascular predisposition to preeclampsia and cardiovascular disease, current prophylaxis and pharmacological management of preeclampsia involves principally compounds familiar to the field of cardiology—aspirin, statins, metformin, nitric oxide donors, and antihypertensive agents. Interestingly, statins have widespread use for the primary and secondary prevention of coronary disease and are also associated with reduced levels of circulating preeclampsia biomarkers in animal studies.57 A preliminary study suggested pravastatin use is safe during pregnancy and a larger trial with dose escalation may be feasible to test whether it is effective in prevention/treatment of preeclampsia.58 Use of nitric oxide donors are associated with reduction in total vascular resistance and reduced rate of adverse outcome in hypertensive pregnancies.59
Cardiovascular System in Pregnancy and Preeclampsia
Hemodynamic changes during pregnancy include a progressive increase in CO and a decrease in the systemic vascular resistance leading to a high-volume, low-resistance circulation. These changes peak in the midthird trimester before CO falls, and systemic vascular resistance increases towards 40 weeks’ gestation.60,61 The alteration in late pregnancy hemodynamics is biologically paradoxical when considering that the respiratory and metabolic demands of the maternal-fetal unit increases exponentially with advancing gestation.62 Echocardiographic studies of uncomplicated normal pregnancies have demonstrated an excessive increase in the left ventricular mass and remodeling with associated diastolic dysfunction in a small but significant proportion of women at term—all of which revert to normal postpartum.63,64 For this reason, pregnancy has been described as a stress test which unmasks women who have poor cardiovascular reserve or dysfunction.65
Maternal echocardiography studies in preeclampsia have demonstrated significant cardiac dysfunction both before and at clinical onset of preeclampsia. Valensise et al66 first demonstrated that CO was significantly lower in early-onset (<34 weeks) preeclampsia compared with late-onset (≥34 weeks) preeclampsia. Their findings were later confirmed and expanded on with the work of Melchiorre et al53 who showed that preeclampsia was also associated with abnormal cardiac geometry and diastolic dysfunction in the majority of women who developed preeclampsia. A recent systematic review summarized 36 studies of maternal cardiovascular function involving 815 women with preeclampsia, demonstrating that increased vascular resistance and left ventricular mass were the most consistent findings in preeclampsia (Table 2).67 Differentiating features from normal pregnancy were left ventricular wall thickness of ≥1.0 cm, exaggerated reduction in early diastole/atrial contraction, and lateral e′ of <14 cm/s which are the markers of diastolic dysfunction. Reduced stroke volume, diastolic dysfunction, and left ventricular remodeling are most marked in severe and early-onset preeclampsia and are associated with adverse maternal and fetal outcomes—irrespective of the conventional classification of preeclampsia based on clinical severity or gestation of onset.67–73 These findings demonstrate that even apparently normal pregnancy presents a significant strain on the maternal cardiovascular system and that in women with evidence of worsening cardiovascular maladaptation, preeclampsia is the recognized clinical phenotype (Figure 2).
Table 2.
Summary of Left-Sided Cardiovascular Findings at Presentation With Preeclampsia
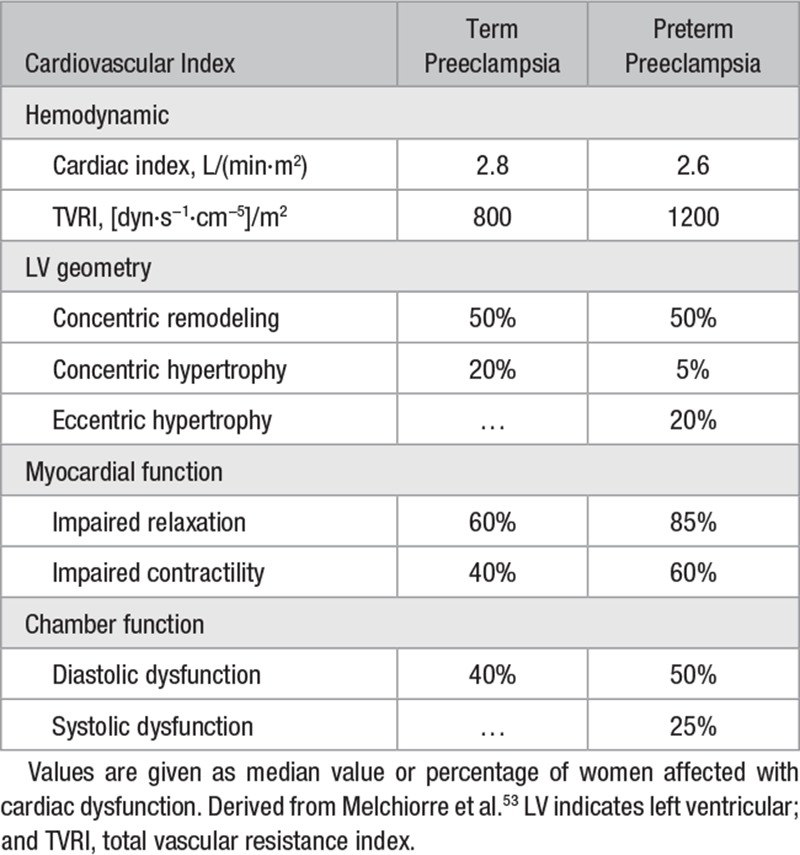
Figure 2.
Diagrammatic representation of the consequences of a relative imbalance of cardiovascular functional reserve and cardiovascular volume/resistance load of pregnancy. Cardiovascular adaptation in normal pregnancy (green dotted line) results in subclinical cardiac dysfunction in a small but significant proportion of women at term. Poor cardiovascular adaptation to pregnancy (red dotted line) is more likely to occur with advanced maternal age, obesity, and other risk factors. Depending on the cardiovascular load of pregnancy (normal load: green solid line, excessive load: red solid line), different preeclampsia (PE) phenotypes will manifest, such as early or late PE.
Putative Roles for Cardiovascular Assessment in the Management of Preeclampsia
The evaluation and control of hypertension is established in preeclampsia management. The potential impact of routine echocardiography in high-risk pregnancy remains to be established mainly because of lack of access and practicalities of undertaking these investigations in the emergency obstetric setting. However, noninvasive CO monitoring (such as with NICOM bioreactance and USCOM Doppler monitors) present alternative methods for monitoring of maternal hemodynamic parameters.74 Noninvasive monitors hold a significant edge over echocardiography by being more practical and requiring little training to operate competently. Although noninvasive cardiac monitors are most often used in an intensive care unit setting, recently, they have been assessed and validated in pregnancy. Noninvasive monitors show good agreement with transthoracic echocardiography for the assessment of CO but only in the third trimester.74 At earlier gestations or postnatally, the levels of agreement were poor indicating that indices derived from noninvasive monitors cannot be used interchangeably with those obtained by echocardiography. However, the difference in agreement between various techniques may be overcome if technology-specific reference ranges are used.60 Initial studies using these monitors have suggested cardiac indices may be helpful in the management of hypertensive disorders of pregnancy.73,75
Placental biomarkers with cardiovascular effects, such as sFlt-1 and PIGF (placenta growth factor), are valuable in diagnosis of preeclampsia.76,77 Zeisler et al77 recently demonstrated that a maternal sFlt-1:PlGF ratio with a cutoff of <38 can exclude the development of preeclampsia within 1 week with a negative predictive value of 99%, 80% sensitivity, and 78% specificity. A prospective pilot study of normotensive and hypertensive pregnant women showed that the addition of biophysical cardiovascular indices to sFlt-1:PlGF significantly improved detection of hypertensive disorders of pregnancy.73 Interestingly, these biomarkers seem to be elevated long after birth and delivery of the placenta and are related to long-term adverse maternal cardiovascular outcome.78
Cardiac assessment may also prove useful for guiding antihypertensive therapy and improving outcomes for women with preeclampsia.79–82 The choice of antihypertensive agent varies between national guidelines despite the fact that drugs of choice have vastly different mechanisms of action and side effect profiles. For instance, labetalol is the first line drug for treatment of pregnancy hypertension in United Kingdom.83 Beta-blockers have negative inotropic and chronotropic effects, and any cardiologist would not usually choose such an agent for a hypertensive patient with low CO and increased vascular resistance—typical of early/severe preeclampsia.53,82 In a randomized study of nonpregnant patients, Taler et al84 demonstrate superior blood pressure control using a treatment algorithm and serial hemodynamic measurements compared with clinical judgment alone. It is difficult to imagine why these findings should not be applicable to women with hypertensive disorders of pregnancy. The use of diuretics in women with preeclampsia had been an abandoned practice until a recent trial of nifedipine versus nifedipine plus furosemide demonstrated that diuretic use reduced the need for additional antihypertensive medication in preeclampsia.85 Diuretic use is likely to have been most beneficial in women with features of volume overload and less significant vascular resistance—typical of late/mild preeclampsia.71 Cardiovascular profiling of hypertensive women may explain why similar drug comparisons yield variable results in different drug trials, as well as lack of consensus, on optimal antihypertensive management despite several randomized trials.81,82,86–90
Combining biochemical tests and biophysical markers of cardiovascular function may allow for improved prediction of preeclampsia onset as well as peripartum maternal morbidity and postpartum cardiovascular disease. The most widely studied model for predicting adverse maternal outcomes is the fullPIERS (Preeclampsia Integrated Estimate of Risk) risk prediction model, which has been validated for different preeclampsia subtypes and various resource setting.91,92 Although the fullPIERS model show modest prediction capabilities, the most influential variables used in the model are actually clinical features of cardiovascular decompensation such as chest pain, dyspnea, or low oxygen saturation. Assessment of cardiac function to better identify women who are at risk of pulmonary edema is not entirely without biological plausibility because recent evidence suggests women who develop pulmonary edema have impaired diastolic dysfunction.68,93 Major complications of preeclampsia, such as pulmonary edema, eclampsia, and cerebrovascular incidents, are fortunately rare but often have devastating maternal sequelae. These severe complications of preeclampsia are often preventable with adequate blood pressure control, appropriate fluid management, and magnesium sulfate prophylaxis. Although management and prevention strategies are relatively simple, it is still a challenge to identify patients under risk so that they may receive a closer observation and treatment, which may be avoided in women at low risk of these complications. Profiling of cardiac function in women with preeclampsia during the immediate postnatal period and investigating its associations with short- and long-term postpartum complications would be an important step for establishing the role of cardiac assessment in postpartum preeclampsia management.
Cardiovascular System in the Immediate Postpartum Period
The obstetric cure for preeclampsia has remained the same for several decades—scheduled iatrogenic birth. Immediately after birth, resolution of preeclampsia symptoms occurs concomitantly with reduction in stroke volume, CO, and mean arterial pressure.94 These symptoms and signs reach an equilibrium and return to healthy pregnancy ranges within 3 to 4 days, except for total vascular resistance and mean arterial pressure which remain significantly higher compared with controls, despite generally having systolic and diastolic blood pressure levels in the normal range—supporting the clinical paradigm that birth cures preeclampsia. However, longitudinal assessment of preeclampsia reveals that ≈50% of women have persistent hypertension and increased rates of nocturnal, ambulatory, and masked hypertension at 12 weeks postpartum.95,96 The significance of persistent postpartum hypertension was underlined in a recent large cohort study by Behrens et al10 which showed a high rate of antihypertensive medication use within a year of hypertensive pregnancy when compared with normotensive pregnancy (11% versus 0.5%, respectively). In the same cohort study, the cumulative incidence of hypertension within 10 years of delivery was significantly higher for young women (20–29 years) after preeclampsia when compared with older women (40–49 years) with a nonpreeclamptic pregnancy. Thus, a woman in her 20s with preeclampsia has a worse cardiovascular prognosis within 10 years of delivery compared with a woman twice her age. Notably, the highest risk for development of chronic hypertension is within the first few years after birth (Figure 3). This reinforces the relative importance of preeclampsia as a stronger risk factor for cardiovascular disease than even smoking.
Figure 3.
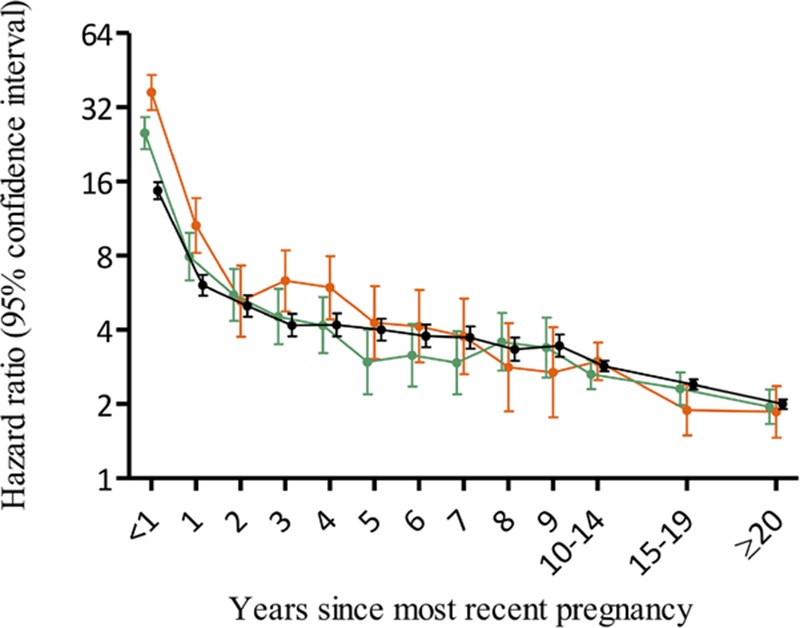
Hazard ratios for chronic hypertension by severity of preeclampsia and time since pregnancy according the national register cohort study by Behrens et al.10 Hazard ratios compare rates of chronic hypertension among women with hypertensive disorders of pregnancy and delivery <34 gestational wk (orange), delivery at 34–36 wk (green), and delivery ≥37 gestational wk (black).
The impact of preeclampsia on a woman’s life is far from just being a risk factor for heart disease. Up to 40% of women do not get pregnant again after early-onset preeclampsia pregnancy—presumably because of their experience of serious pregnancy morbidity.97 Increased rates of hospital readmission, poor mental health, increased fatigue, and impaired social functioning in the postpartum period—up to 3 years after the index pregnancy—are all associated with early/severe preeclampsia.98,99 For the mother, the immediate postpartum period is fraught with problems relating to care of new-born and factors such as low socioeconomic status, belonging to an ethnic minority, and having vaginal delivery are associated with nonattendance to postpartum visits.100 A possible solution to nonattendance would be empowering women to care for themselves. Home monitoring of blood pressure has been suggested as a safe, effective, and economical way of follow-up with women with hypertensive disorders of pregnancy.101,102 It is reasonable to assume similar efficacy during the postpartum period.103 However, blood pressure values and incidence of severe hypertension differ with home and clinic monitoring during pregnancy, and optimal cutoffs for action is subject to debate currently.104 Although preeclampsia should be regarded as an important risk factor for cardiovascular disease in women, it also presents a unique opportunity to identify and potentially intervene to ameliorate the adverse effects of preeclampsia on immediate postpartum maternal health.
Aging After Preeclampsia: The Long-Term Consequences
In addition to a globally increased risk of cardiovascular disease, a history of preeclampsia is also associated with 6 to 7× increased hazard of having recurrent ischemic attack within a year of developing acute coronary syndrome.105 Women with recurrent preeclampsia are characterized by a shorter life-span (48.9 versus 51.9 years), increased hazard of ischemic heart disease (hazard ratio, 3.30), and stroke (hazard ratio, 5.10).13 The risk estimates for cardiovascular diseases and mortality differ for women with various subtypes of hypertensive disorders (eclampsia, preeclampsia, gestational hypertension) with more severe forms corresponding to higher risk.106–108 A recent systematic review produced aggregate data to show that recurrent preeclampsia is associated with increased rates of hypertension, ischemic heart disease, heart failure, cerebrovascular accident, and hospitalization because of cardiovascular disease.107 The persistence and immediacy of findings, such as asymptomatic heart failure, remodeling, or masked hypertension, make them unique markers for identifying women at greatest risk.
So, is elective schedule birth really a cure for preeclampsia? Evidence from cohort studies suggest otherwise with an increased risk of heart failure with preserved ejection fraction and also present for chronic kidney disease.108–110 These long-term consequences of preeclampsia may potentially be explained by the fact that both organs are affected in the acute phase of the disease. However, recent studies suggest that preeclampsia may also increase the risk of dementia.111 Ciampa et al112 demonstrated vascular remodeling, inflammation, neuronal growth, and alterations in signaling proteins in the cerebrospinal fluid of women with preeclampsia (excluding eclampsia). Cerebral biomarkers of axonal injury and neuronal damage (such as the neurofilament light chain) not only predict preeclampsia with an accuracy similar to established angiogenic factors but are also elevated at 1-year postpartum.113–115 Persistent neuronal damage may be associated with vascular remodeling, potentially explaining white matter damage, increased risk of dementia, and vascular reactivity observed in elderly women with a history of preeclampsia.116–118 These recent studies investigating the association of preeclampsia with both cardiovascular dysfunction and vascular dementia create a strong argument against the notion preeclampsia is cured by delivering the placenta/birth.
Left ventricular hypertrophy, coronary artery disease, heart failure, and stroke exhibit later presentation, more severe phenotypes, and worse prognosis in women compared with men. Depite this, there is a paucity of research focused on developing effective screening, follow-up, and intervention strategies for women after preeclampsia—despite presenting an unique opportunity for early intervention.119–123 The American Heart Association now recognizes that women with a history of preeclampsia face an increased risk of stroke, heart disease, and deep venous thrombosis in the 5 to 15 years after pregnancy.124 The American Heart Association recommends that at-risk individuals should educate themselves about cardiovascular disease risk reduction, such as smoking cessation, improved diet, and regular exercise. It is likely that more sophisticated assessment of cardiovascular function in the postnatal period may better identify women who are going to develop short and long-term cardiovascular morbidity.125 These studies are urgently required to facilitate the appropriate long-term cardiovascular follow-up and entry into therapeutic trials to ameliorate the outcome. One such study in progress is PHOEBE (In women with preterm pre-eclampsia does planned delivery improve postpartum maternal cardiac function through attenuation of myocardial ischaemia at time of disease?; PHOENIX-3 [Pre-eclampsia in Hospital: Early Induction or Expectant Management]), which is a randomized trial in severe preterm preeclampsia where women will all have a detailed cardiovascular assessment (including echocardiography and cardiac biomarker evaluation) at 6 months postpartum. It is envisaged that the PHOEBE trial should be able to identify the optimal biomarkers to screen and identify postpartum cardiovascular morbidity (https://ukctg.nihr.ac.uk/trials/trial-details/trial-details?trialNumber=ISRCTN01879376).
Conclusions
The risk factors for preeclampsia are cardiovascular in nature, cardiovascular signs and symptoms predominate in the clinical syndrome of preeclampsia, and cardiovascular morbidity persists for decades after preeclampsia. All of these make a strong case for the involvement of the maternal cardiovascular system in the pathogenesis of preeclampsia (Figure 4). The pathogenesis of preeclampsia has always known to be a consequence of placental damage secondary to oxidative stress or hypoxia resulting in the release in a maternal systemic antiangiogenic imbalance.126 Preeclampsia was originally recognized by the presence of eclamptic fits before knowledge of signs and symptoms of the disorder led to a clinical severity-based classification. The latter has now been superseded to a temporal classification according to the gestation of onset of preeclampsia—as early/late or preterm/term preeclampsia. In the future, cardiovascular phenotyping of preeclampsia is likely to prove more clinically useful. A better understanding of maternal cardiovascular function in pregnancy would allow improved prediction and diagnosis of preeclampsia, guide antihypertensive therapy, and improve clinical outcomes for women with preeclampsia. The magnitude of cardiovascular dysfunction in preeclampsia is better understood when it is evident that hypertensive disorders of pregnancy are a stronger factor for the postnatal development of cardiovascular and cerebrovascular disease than smoking alone. A strong focus on better postnatal cardiovascular assessment after preeclampsia is required so as not to waste a unique opportunity to alter disease trajectory and improve health inequalities in the cardiovascular and cerebrovascular health of women.
Figure 4.

Infographic outlining involvement of the maternal cardiovascular system in the pathogenesis and recovery from preeclampsia (PE). BP indicates blood pressure.
Sources of Funding
This writing of this article was supported by funds from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 765274 (www.iplacenta.eu).
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.118.11191.
References
- 1.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. doi: 10.1136/bmj.f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox A, McHugh S, Browne J, Kenny LC, Fitzgerald A, Khashan AS, Dempsey E, Fahy C, O’Neill C, Kearney PM. Estimating the cost of preeclampsia in the healthcare system: cross-sectional study using data from SCOPE study (Screening for Pregnancy End Points). Hypertension. 2017;70:1243–1249. doi: 10.1161/HYPERTENSIONAHA.117.09499. doi: 10.1161/HYPERTENSIONAHA.117.09499. [DOI] [PubMed] [Google Scholar]
- 3.Tenney B, Parker F. The placenta in toxemia of pregnancy. Am J Obstet Gynecol. 1940;39:1000–1005. [Google Scholar]
- 4.Melchiorre K, Wormald B, Leslie K, Bhide A, Thilaganathan B. First-trimester uterine artery Doppler indices in term and preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:133–137. doi: 10.1002/uog.5400. doi: 10.1002/uog.5400. [DOI] [PubMed] [Google Scholar]
- 5.Falco ML, Sivanathan J, Laoreti A, Thilaganathan B, Khalil A. Placental histopathology associated with pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:295–301. doi: 10.1002/uog.17494. doi: 10.1002/uog.17494. [DOI] [PubMed] [Google Scholar]
- 6.Chesley LC. Remote prognosis after eclampsia. Perspect Nephrol Hypertens. 1976;5:31–40. [PubMed] [Google Scholar]
- 7.Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, Mori TA, Newnham J, Beilin LJ, Leeson P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5:e008136. doi: 10.1136/bmjopen-2015-008136. doi: 10.1136/bmjopen-2015-008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. doi: 10.1542/peds.2011-3093. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 9.Maher GM, O’Keeffe GW, Kearney PM, Kenny LC, Dinan TG, Mattsson M, Khashan AS. Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:809–819. doi: 10.1001/jamapsychiatry.2018.0854. doi: 10.1001/jamapsychiatry.2018.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, Thilaganathan B, Boyd HA. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. doi: 10.1136/bmj.j3078. doi: 10.1136/bmj.j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930. doi: 10.1016/j.ahj.2008.06.042. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Scantlebury DC, Kattah AG, Weissgerber TL, Agarwal S, Mielke MM, Weaver AL, Vaughan LE, Henkin S, Zimmerman K, Miller VM, White WM, Hayes SN, Garovic VD. Impact of a history of hypertension in pregnancy on later diagnosis of atrial fibrillation. J Am Heart Assoc. 2018;7:e007584. doi: 10.1161/JAHA.117.007584. doi: 10.1161/JAHA.117.007584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol. 2018;219:107.e1–107.e6. doi: 10.1016/j.ajog.2018.04.002. doi: 10.1016/j.ajog.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension. 2017;70:798–803. doi: 10.1161/HYPERTENSIONAHA.117.09246. doi: 10.1161/HYPERTENSIONAHA.117.09246. [DOI] [PubMed] [Google Scholar]
- 15.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122:579–584. doi: 10.1161/CIRCULATIONAHA.110.943407. doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 16.Giannakou K, Evangelou E, Papatheodorou SI. Genetic and non-genetic risk factors for pre-eclampsia: umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet Gynecol. 2018;51:720–730. doi: 10.1002/uog.18959. doi: 10.1002/uog.18959. [DOI] [PubMed] [Google Scholar]
- 17.Serrano NC, Guio-Mahecha E, Quintero-Lesmes DC, Becerra-Bayona S, Paez MC, Beltran M, Herrera VM, Leon LJ, Williams D, Casas JP. Lipid profile, plasma apolipoproteins, and pre-eclampsia risk in the GenPE case-control study. Atherosclerosis. 2018;276:189–194. doi: 10.1016/j.atherosclerosis.2018.05.051. doi: 10.1016/j.atherosclerosis.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 18.Egeland GM, Klungsøyr K, Øyen N, Tell GS, Næss Ø, Skjærven R. Preconception cardiovascular risk factor differences between gestational hypertension and preeclampsia: cohort norway study. Hypertension. 2016;67:1173–1180. doi: 10.1161/HYPERTENSIONAHA.116.07099. doi: 10.1161/HYPERTENSIONAHA.116.07099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thilaganathan B. Pre-eclampsia and the cardiovascular-placental axis. Ultrasound Obstet Gynecol. 2018;51:714–717. doi: 10.1002/uog.19081. doi: 10.1002/uog.19081. [DOI] [PubMed] [Google Scholar]
- 20.Perry H, Khalil A, Thilaganathan B. Preeclampsia and the cardiovascular system: an update. Trends Cardiovasc Med. 2018;28:505–513. doi: 10.1016/j.tcm.2018.04.009. doi: 10.1016/j.tcm.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Kalafat E, Thilaganathan B. Cardiovascular origins of preeclampsia. Curr Opin Obstet Gynecol. 2017;29:383–389. doi: 10.1097/GCO.0000000000000419. doi: 10.1097/GCO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 22.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703–714. doi: 10.1161/CIRCULATIONAHA.113.003664. doi: 10.1161/CIRCULATIONAHA.113.003664. [DOI] [PubMed] [Google Scholar]
- 23.McGinnis R, Steinthorsdottir V, Williams NO, et al. FINNPEC Consortium; GOPEC Consortium. Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nat Genet. 2017;49:1255–1260. doi: 10.1038/ng.3895. doi: 10.1038/ng.3895. [DOI] [PubMed] [Google Scholar]
- 24.Lokki AI, Daly E, Triebwasser M, et al. Protective low-frequency variants for preeclampsia in the Fms related tyrosine kinase 1 gene in the Finnish population. Hypertension. 2017;70:365–371. doi: 10.1161/HYPERTENSIONAHA.117.09406. doi: 10.1161/HYPERTENSIONAHA.117.09406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei J, Liu CX, Gong TT, Wu QJ, Wu L. Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta-analysis of prospective studies. Oncotarget. 2015;6:43667–43678. doi: 10.18632/oncotarget.6190. doi: 10.18632/oncotarget.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 27.Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heida KY, Bots ML, de Groot CJ, van Dunné FM, Hammoud NM, Hoek A, Laven JS, Maas AH, Roeters van Lennep JE, Velthuis BK, Franx A. Cardiovascular risk management after reproductive and pregnancy-related disorders: a Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiol. 2016;23:1863–1879. doi: 10.1177/2047487316659573. doi: 10.1177/2047487316659573. [DOI] [PubMed] [Google Scholar]
- 29.Hutcheon JA, Stephansson O, Cnattingius S, Bodnar LM, Wikström AK, Johansson K. Pregnancy weight gain before diagnosis and risk of preeclampsia: a population-based cohort study in nulliparous women. Hypertension. 2018;72:433–441. doi: 10.1161/HYPERTENSIONAHA.118.10999. doi: 10.1161/HYPERTENSIONAHA.118.10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalafat E, Sukur YE, Abdi A, Thilaganathan B, Khalil A. Metformin for the prevention of hypertensive disorders of pregnancy in women with gestational diabetes and obesity: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;52:706–714. doi: 10.1002/uog.19084. doi:10.1002/uog.19084. [DOI] [PubMed] [Google Scholar]
- 31.Tangren JS, Powe CE, Ankers E, Ecker J, Bramham K, Hladunewich MA, Karumanchi SA, Thadhani R. Pregnancy outcomes after clinical recovery from AKI. J Am Soc Nephrol. 2017;28:1566–1574. doi: 10.1681/ASN.2016070806. doi: 10.1681/ASN.2016070806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stergiotou I, Bijnens B, Cruz-Lemini M, Figueras F, Gratacos E, Crispi F. Maternal subclinical vascular changes in fetal growth restriction with and without pre-eclampsia. Ultrasound Obstet Gynecol. 2015;46:706–712. doi: 10.1002/uog.14815. doi: 10.1002/uog.14815. [DOI] [PubMed] [Google Scholar]
- 33.Sep SJ, Schreurs MP, Bekkers SC, Kruse AJ, Smits LJ, Peeters LL. Early-pregnancy changes in cardiac diastolic function in women with recurrent pre-eclampsia and in previously pre-eclamptic women without recurrent disease. BJOG. 2011;118:1112–1119. doi: 10.1111/j.1471-0528.2011.02951.x. doi: 10.1111/j.1471-0528.2011.02951.x. [DOI] [PubMed] [Google Scholar]
- 34.Ghossein-Doha C, Spaanderman MEA, Doulah R Al, Van Kuijk SM, Peeters LLH. Maternal cardiac adaptation to subsequent pregnancy in formerly pre-eclamptic women according to recurrence of pre-eclampsia. Ultrasound Obstet Gynecol. 2016;47:96–103. doi: 10.1002/uog.15752. doi:10.1002/uog.15752. [DOI] [PubMed] [Google Scholar]
- 35.Milic NM, Milin-Lazovic J, Weissgerber TL, Trajkovic G, White WM, Garovic VD. Preclinical atherosclerosis at the time of pre-eclamptic pregnancy and up to 10 years postpartum: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;49:110–115. doi: 10.1002/uog.17367. doi:10.1002/uog.17367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song C, Burgess S, Eicher JD, O’Donnell CJ, Johnson AD. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J Am Heart Assoc. 2017;6:e004918. doi: 10.1161/JAHA.116.004918. doi:10.1161/jaha.116.004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, Hunn BH. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28:377–387. doi: 10.1681/ASN.2016010105. doi: 10.1681/ASN.2016010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedaghat S, van Sloten TT, Laurent S, London GM, Pannier B, Kavousi M, Mattace-Raso F, Franco OH, Boutouyrie P, Ikram MA, Stehouwer CDA. Common carotid artery diameter and risk of cardiovascular events and mortality: pooled analyses of Four cohort studies. Hypertension. 2018;72:85–92. doi: 10.1161/HYPERTENSIONAHA.118.11253. doi: 10.1161/HYPERTENSIONAHA.118.11253. [DOI] [PubMed] [Google Scholar]
- 39.Goulopoulou S. Maternal vascular physiology in preeclampsia. Hypertension. 2017;70:1066–1073. doi: 10.1161/HYPERTENSIONAHA.117.08821. doi: 10.1161/HYPERTENSIONAHA.117.08821. [DOI] [PubMed] [Google Scholar]
- 40.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Increased angiotensin II sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension. 2017;70:382–389. doi: 10.1161/HYPERTENSIONAHA.117.09386. doi: 10.1161/HYPERTENSIONAHA.117.09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham MW, Jr, Williams JM, Amaral L, Usry N, Wallukat G, Dechend R, LaMarca B. Agonistic autoantibodies to the angiotensin II type 1 receptor enhance angiotensin II-induced renal vascular sensitivity and reduce renal function during pregnancy. Hypertension. 2016;68:1308–1313. doi: 10.1161/HYPERTENSIONAHA.116.07971. doi: 10.1161/HYPERTENSIONAHA.116.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan HL, Butler E, Ritchie S, Herse F, Dechend R, Beattie E, McBride MW, Graham D. Modeling superimposed preeclampsia using Ang II (Angiotensin II) infusion in pregnant stroke-prone spontaneously hypertensive rats. Hypertension. 2018;72:208–218. doi: 10.1161/HYPERTENSIONAHA.118.10935. doi: 10.1161/HYPERTENSIONAHA.118.10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdonk K, Saleh L, Lankhorst S, Smilde JE, van Ingen MM, Garrelds IM, Friesema EC, Russcher H, van den Meiracker AH, Visser W, Danser AH. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension. 2015;65:1316–1323. doi: 10.1161/HYPERTENSIONAHA.115.05267. doi: 10.1161/HYPERTENSIONAHA.115.05267. [DOI] [PubMed] [Google Scholar]
- 44.Li F, Kakoki M, Smid M, Boggess K, Wilder J, Hiller S, Bounajim C, Parnell SE, Sulik KK, Smithies O, Maeda-Smithies N. Causative effects of genetically determined high maternal/fetal endothelin-1 on preeclampsia-like conditions in mice. Hypertension. 2018;71:894–903. doi: 10.1161/HYPERTENSIONAHA.117.10849. doi: 10.1161/HYPERTENSIONAHA.117.10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaartokallio T, Klemetti MM, Timonen A, Uotila J, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, Lakkisto P, Laivuori H. Microsatellite polymorphism in the heme oxygenase-1 promoter is associated with nonsevere and late-onset preeclampsia. Hypertension. 2014;64:172–177. doi: 10.1161/HYPERTENSIONAHA.114.03337. doi: 10.1161/HYPERTENSIONAHA.114.03337. [DOI] [PubMed] [Google Scholar]
- 46.Mousa AA, Strauss JF, III, Walsh SW. Reduced methylation of the thromboxane synthase gene is correlated with its increased vascular expression in preeclampsia. Hypertension. 2012;59:1249–1255. doi: 10.1161/HYPERTENSIONAHA.111.188730. doi: 10.1161/HYPERTENSIONAHA.111.188730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50:492–495. doi: 10.1002/uog.18816. doi: 10.1002/uog.18816. [DOI] [PubMed] [Google Scholar]
- 48.Kalafat E, Laoreti A, Khalil A, Da Silva Costa F, Thilaganathan B. Ophthalmic artery Doppler for prediction of pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51:731–737. doi: 10.1002/uog.19002. doi:10.1002/uog.19002. [DOI] [PubMed] [Google Scholar]
- 49.Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic VD. Impaired flow-mediated dilation before, during, and after preeclampsia: a systematic review and meta-analysis. Hypertension. 2016;67:415–423. doi: 10.1161/HYPERTENSIONAHA.115.06554. doi: 10.1161/HYPERTENSIONAHA.115.06554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porto LB, Brandão AHF, Leite HV, Cabral ACV. Longitudinal evaluation of uterine perfusion, endothelial function and central blood flow in early onset pre-eclampsia. Pregnancy Hypertens. 2017;10:161–164. doi: 10.1016/j.preghy.2017.08.005. doi: 10.1016/j.preghy.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Lopes van Balen VA, van Gansewinkel TAG, de Haas S, van Kuijk SMJ, van Drongelen J, Ghossein-Doha C, Spaanderman MEA. Physiological adaptation of endothelial function to pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:697–708. doi: 10.1002/uog.17431. doi: 10.1002/uog.17431. [DOI] [PubMed] [Google Scholar]
- 52.Foo FL, Mahendru AA, Masini G, Fraser A, Cacciatore S, MacIntyre DA, McEniery CM, Wilkinson IB, Bennett PR, Lees CC. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension. 2018;72:442–450. doi: 10.1161/HYPERTENSIONAHA.118.11092. doi: 10.1161/HYPERTENSIONAHA.118.11092. [DOI] [PubMed] [Google Scholar]
- 53.Melchiorre K, Sutherland G, Sharma R, Nanni M, Thilaganathan B. Mid-gestational maternal cardiovascular profile in preterm and term pre-eclampsia: a prospective study. BJOG. 2013;120:496–504. doi: 10.1111/1471-0528.12068. doi: 10.1111/1471-0528.12068. [DOI] [PubMed] [Google Scholar]
- 54.Ambia AM, Morgan JL, Wells CE, Roberts SW, Sanghavi M, Nelson DB, Cunningham FG. Perinatal outcomes associated with abnormal cardiac remodeling in women with treated chronic hypertension. Am J Obstet Gynecol. 2018;218:519.e1–519.e7. doi: 10.1016/j.ajog.2018.02.015. doi: 10.1016/j.ajog.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4:e002270. doi: 10.1161/JAHA.115.002270. doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myredal A, Gan LM, Osika W, Friberg P, Johansson M. Increased intima thickness of the radial artery in individuals with prehypertension and hypertension. Atherosclerosis. 2010;209:147–151. doi: 10.1016/j.atherosclerosis.2009.09.017. doi: 10.1016/j.atherosclerosis.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Costantine MM, Tamayo E, Lu F, Bytautiene E, Longo M, Hankins GD, Saade GR. Using pravastatin to improve the vascular reactivity in a mouse model of soluble fms-like tyrosine kinase-1-induced preeclampsia. Obstet Gynecol. 2010;116:114–120. doi: 10.1097/AOG.0b013e3181e10ebd. doi: 10.1097/AOG.0b013e3181e10ebd. [DOI] [PubMed] [Google Scholar]
- 58.Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren Z, Easterling TR, Haas DM, Haneline LS, Caritis SN, Venkataramanan R, West H, D’Alton M, Hankins G Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol. 2016;214:720.e1–720.e17. doi: 10.1016/j.ajog.2015.12.038. doi: 10.1016/j.ajog.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasapollo B, Novelli GP, Gagliardi G, Tiralongo GM, Pisani I, Manfellotto D, Giannini L, Valensise H. Medical treatment of early-onset mild gestational hypertension reduces total peripheral vascular resistance and influences maternal and fetal complications. Ultrasound Obstet Gynecol. 2012;40:325–331. doi: 10.1002/uog.11103. doi: 10.1002/uog.11103. [DOI] [PubMed] [Google Scholar]
- 60.Vinayagam D, Thilaganathan B, Stirrup O, Mantovani E, Khalil A. Maternal hemodynamics in normal pregnancy: reference ranges and role of maternal characteristics. Ultrasound Obstet Gynecol. 2018;51:665–671. doi: 10.1002/uog.17504. doi: 10.1002/uog.17504. [DOI] [PubMed] [Google Scholar]
- 61.Meah VL, Cockcroft JR, Backx K, Shave R, Stöhr EJ. Cardiac output and related haemodynamics during pregnancy: a series of meta-analyses. Heart. 2016;102:518–526. doi: 10.1136/heartjnl-2015-308476. doi: 10.1136/heartjnl-2015-308476. [DOI] [PubMed] [Google Scholar]
- 62.Dunsworth HM, Warrener AG, Deacon T, Ellison PT, Pontzer H. Metabolic hypothesis for human altriciality. Proc Natl Acad Sci USA. 2012;109:15212–15216. doi: 10.1073/pnas.1205282109. doi: 10.1073/pnas.1205282109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melchiorre K, Sharma R, Khalil A, Thilaganathan B. Maternal cardiovascular function in normal pregnancy: evidence of maladaptation to chronic volume overload. Hypertension. 2016;67:754–762. doi: 10.1161/HYPERTENSIONAHA.115.06667. doi: 10.1161/HYPERTENSIONAHA.115.06667. [DOI] [PubMed] [Google Scholar]
- 64.Savu O, Jurcuţ R, Giuşcă S, van Mieghem T, Gussi I, Popescu BA, Ginghină C, Rademakers F, Deprest J, Voigt JU. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging. 2012;5:289–297. doi: 10.1161/CIRCIMAGING.111.970012. doi: 10.1161/CIRCIMAGING.111.970012. [DOI] [PubMed] [Google Scholar]
- 65.Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Ther Adv Cardiovasc Dis. 2008;2:249–259. doi: 10.1177/1753944708094227. doi: 10.1177/1753944708094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873–880. doi: 10.1161/HYPERTENSIONAHA.108.117358. doi: 10.1161/HYPERTENSIONAHA.108.117358. [DOI] [PubMed] [Google Scholar]
- 67.Castleman JS, Ganapathy R, Taki F, Lip GY, Steeds RP, Kotecha D. Echocardiographic structure and function in hypertensive disorders of pregnancy: a systematic review. Circ Cardiovasc Imaging. 2016;9:e004888. doi: 10.1161/CIRCIMAGING.116.004888. Doi: 10.1161/CIRCIMAGING.116.004888. [DOI] [PubMed] [Google Scholar]
- 68.Vaught AJ, Kovell LC, Szymanski LM, Mayer SA, Seifert SM, Vaidya D, Murphy JD, Argani C, O’Kelly A, York S, Ouyang P, Mukherjee M, Zakaria S. Acute cardiac effects of severe pre-eclampsia. J Am Coll Cardiol. 2018;72:1–11. doi: 10.1016/j.jacc.2018.04.048. doi: 10.1016/j.jacc.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy. 2012;31:454–471. doi: 10.3109/10641955.2012.697951. doi: 10.3109/10641955.2012.697951. [DOI] [PubMed] [Google Scholar]
- 70.Ferrazzi E, Stampalija T, Monasta L, Di Martino D, Vonck S, Gyselaers W. Maternal hemodynamics: a method to classify hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2018;218:124.e1–124.e11. doi: 10.1016/j.ajog.2017.10.226. doi: 10.1016/j.ajog.2017.10.226. [DOI] [PubMed] [Google Scholar]
- 71.Borges VTM, Zanati SG, Peraçoli MTS, Poiati JR, Romão-Veiga M, Peraçoli JC, Thilaganathan B. Maternal left ventricular hypertrophy and diastolic dysfunction and brain natriuretic peptide concentration in early- and late-onset pre-eclampsia. Ultrasound Obstet Gynecol. 2018;51:519–523. doi: 10.1002/uog.17495. doi: 10.1002/uog.17495. [DOI] [PubMed] [Google Scholar]
- 72.Hieda M, Yoo JK, Sun DD, Okada Y, Parker RS, Roberts-Reeves MA, Adams-Huet B, Nelson DB, Levine BD, Fu Q. Time course of changes in maternal left ventricular function during subsequent pregnancy in women with a history of gestational hypertensive disorders. Am J Physiol Regul Integr Comp Physiol. 2018;315:R587–R594. doi: 10.1152/ajpregu.00040.2018. doi: 10.1152/ajpregu.00040.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verlohren S, Perschel FH, Thilaganathan B, Dröge LA, Henrich W, Busjahn A, Khalil A. Angiogenic markers and cardiovascular indices in the prediction of hypertensive disorders of pregnancy. Hypertension. 2017;69:1192–1197. doi: 10.1161/HYPERTENSIONAHA.117.09256. doi: 10.1161/HYPERTENSIONAHA.117.09256. [DOI] [PubMed] [Google Scholar]
- 74.Vinayagam D, Patey O, Thilaganathan B, Khalil A. Cardiac output assessment in pregnancy: comparison of two automated monitors with echocardiography. Ultrasound Obstet Gynecol. 2017;49:32–38. doi: 10.1002/uog.15915. doi: 10.1002/uog.15915. [DOI] [PubMed] [Google Scholar]
- 75.Vinayagam D, Gutierrez J, Binder J, Mantovani E, Thilaganathan B, Khalil A. Impaired maternal hemodynamics in morbidly obese women: a case-control study. Ultrasound Obstet Gynecol. 2017;50:761–765. doi: 10.1002/uog.17428. doi: 10.1002/uog.17428. [DOI] [PubMed] [Google Scholar]
- 76.Ukah UV, Hutcheon JA, Payne B, Haslam MD, Vatish M, Ansermino JM, Brown H, Magee LA, von Dadelszen P. Placental growth factor as a prognostic tool in women with hypertensive disorders of pregnancy: a systematic review. Hypertension. 2017;70:1228–1237. doi: 10.1161/HYPERTENSIONAHA.117.10150. doi: 10.1161/HYPERTENSIONAHA.117.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennstrom M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. Doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 78.Wolf M, Hubel CA, Lam C, Sampson M, Ecker JL, Ness RB, Rajakumar A, Daftary A, Shakir AS, Seely EW, Roberts JM, Sukhatme VP, Karumanchi SA, Thadhani R. Preeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89:6239–6243. doi: 10.1210/jc.2004-0548. doi: 10.1210/jc.2004-0548. [DOI] [PubMed] [Google Scholar]
- 79.Giannubilo SR, Pasculli A, Tidu E, Biagini A, Boscarato V, Ciavattini A. Relationship between maternal hemodynamics and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia and fetal growth restriction. J Perinatol. 2017;37:484–487. doi: 10.1038/jp.2016.264. doi: 10.1038/jp.2016.264. [DOI] [PubMed] [Google Scholar]
- 80.Stott D, Bolten M, Paraschiv D, Papastefanou I, Chambers JB, Kametas NA. Longitudinal hemodynamics in acute phase of treatment with labetalol in hypertensive pregnant women to predict need for vasodilatory therapy. Ultrasound Obstet Gynecol. 2017;49:85–94. doi: 10.1002/uog.17335. doi:10.1002/uog.17335. [DOI] [PubMed] [Google Scholar]
- 81.Stott D, Papastefanou I, Paraschiv D, Clark K, Kametas NA. Serial hemodynamic monitoring to guide treatment of maternal hypertension leads to reduction in severe hypertension. Ultrasound Obstet Gynecol. 2017;49:95–103. doi: 10.1002/uog.17341. doi:10.1002/uog.17341. [DOI] [PubMed] [Google Scholar]
- 82.McLaughlin K, Scholten RR, Kingdom JC, Floras JS, Parker JD. Should maternal hemodynamics guide antihypertensive therapy in preeclampsia? Hypertension. 2018;71:550–556. doi: 10.1161/HYPERTENSIONAHA.117.10606. doi: 10.1161/HYPERTENSIONAHA.117.10606. [DOI] [PubMed] [Google Scholar]
- 83.National Institute for Health and Care Excellence. Hypertension in Pregnancy: Diagnosis and Management (CG107). https://www.nice.org.uk/guidance/cg107. Accessed July 2017. [PubMed]
- 84.Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002;39:982–988. doi: 10.1161/01.hyp.0000016176.16042.2f. [DOI] [PubMed] [Google Scholar]
- 85.Veena P, Perivela L, Raghavan SS. Furosemide in postpartum management of severe preeclampsia: a randomized controlled trial. Hypertens Pregnancy. 2017;36:84–89. doi: 10.1080/10641955.2016.1239735. doi: 10.1080/10641955.2016.1239735. [DOI] [PubMed] [Google Scholar]
- 86.Webster LM, Myers JE, Nelson-Piercy C, Harding K, Cruickshank JK, Watt-Coote I, Khalil A, Wiesender C, Seed PT, Chappell LC. Labetalol versus nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: a randomized controlled trial. Hypertension. 2017;70:915–922. doi: 10.1161/HYPERTENSIONAHA.117.09972. doi: 10.1161/HYPERTENSIONAHA.117.09972. [DOI] [PubMed] [Google Scholar]
- 87.Sharma KJ, Greene N, Kilpatrick SJ. Oral labetalol compared to oral nifedipine for postpartum hypertension: a randomized controlled trial. Hypertens Pregnancy. 2017;36:44–47. doi: 10.1080/10641955.2016.1231317. doi: 10.1080/10641955.2016.1231317. [DOI] [PubMed] [Google Scholar]
- 88.Magee LA, von Dadelszen P, Singer J, et al. CHIPS Study Group. Do labetalol and methyldopa have different effects on pregnancy outcome? Analysis of data from the Control of Hypertension In Pregnancy Study (CHIPS) trial. BJOG. 2016;123:1143–1151. doi: 10.1111/1471-0528.13569. doi: 10.1111/1471-0528.13569. [DOI] [PubMed] [Google Scholar]
- 89.Cairns AE, Pealing L, Duffy JMN, Roberts N, Tucker KL, Leeson P, MacKillop LH, McManus RJ. Postpartum management of hypertensive disorders of pregnancy: a systematic review. BMJ Open. 2017;7:e018696. doi: 10.1136/bmjopen-2017-018696. doi: 10.1136/bmjopen-2017-018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lees C, Ferrazzi E. Relevance of haemodynamics in treating pre-eclampsia. Curr Hypertens Rep. 2017;19:76. doi: 10.1007/s11906-017-0766-6. doi: 10.1007/s11906-017-0766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ukah UV, Payne B, Hutcheon JA, Ansermino JM, Ganzevoort W, Thangaratinam S, Magee LA, von Dadelszen P. Assessment of the fullPIERS risk Prediction Model in Women With Early-Onset Preeclampsia. Hypertension. 2018;71:659–665. doi: 10.1161/HYPERTENSIONAHA.117.10318. doi: 10.1161/HYPERTENSIONAHA.117.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ukah UV, Payne B, Lee T, Magee LA, von Dadelszen P fullPIERS and miniPIERS Working Groups. External validation of the fullPIERS model for predicting adverse maternal outcomes in pregnancy hypertension in low- and middle-income Countries. Hypertension. 2017;69:705–711. doi: 10.1161/HYPERTENSIONAHA.116.08706. doi: 10.1161/HYPERTENSIONAHA.116.08706. [DOI] [PubMed] [Google Scholar]
- 93.Bhorat I, Naidoo DP, Moodley J. Maternal cardiac haemodynamics in severe pre-eclampsia complicated by acute pulmonary oedema: a review. J Matern Fetal Neonatal Med. 2017;30:2769–2777. doi: 10.1080/14767058.2016.1262842. doi: 10.1080/14767058.2016.1262842. [DOI] [PubMed] [Google Scholar]
- 94.Lavie A, Ram M, Lev S, Blecher Y, Amikam U, Shulman Y, Avnon T, Weiner E, Many A. Maternal cardiovascular hemodynamics in normotensive versus preeclamptic pregnancies: a prospective longitudinal study using a noninvasive cardiac system (NICaS™). BMC Pregnancy Childbirth. 2018;18:229. doi: 10.1186/s12884-018-1861-7. doi: 10.1186/s12884-018-1861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goel A, Maski MR, Bajracharya S, Wenger JB, Zhang D, Salahuddin S, Shahul SS, Thadhani R, Seely EW, Karumanchi SA, Rana S. Epidemiology and mechanisms of De Novo and persistent hypertension in the postpartum period. Circulation. 2015;132:1726–1733. doi: 10.1161/CIRCULATIONAHA.115.015721. doi: 10.1161/CIRCULATIONAHA.115.015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ditisheim A, Wuerzner G, Ponte B, Vial Y, Irion O, Burnier M, Boulvain M, Pechère-Bertschi A. Prevalence of hypertensive phenotypes after preeclampsia: a prospective cohort study. Hypertension. 2018;71:103–109. doi: 10.1161/HYPERTENSIONAHA.117.09799. doi: 10.1161/HYPERTENSIONAHA.117.09799. [DOI] [PubMed] [Google Scholar]
- 97.Seeho SK, Algert CS, Roberts CL, Ford JB. Early-onset preeclampsia appears to discourage subsequent pregnancy but the risks may be overestimated. Am J Obstet Gynecol. 2016;215:785.e1–785.e8. doi: 10.1016/j.ajog.2016.07.038. doi: 10.1016/j.ajog.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 98.Brussé IA, Duvekot JJ, Meester I, Jansen G, Rizopoulos D, Steegers EA, Visser GH. Electroencephalography in normotensive and hypertensive pregnancies and subsequent quality of life. PLoS One. 2016;11:e0155299. doi: 10.1371/journal.pone.0155299. doi: 10.1371/journal.pone.0155299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mogos MF, Salemi JL, Spooner KK, McFarlin BL, Salihu HH. Hypertensive disorders of pregnancy and postpartum readmission in the United States: national surveillance of the revolving door. J Hypertens. 2018;36:608–618. doi: 10.1097/HJH.0000000000001594. doi: 10.1097/HJH.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 100.Wilcox A, Levi EE, Garrett JM. Predictors of non-attendance to the postpartum follow-up visit. Matern Child Health J. 2016;20(suppl 1):22–27. doi: 10.1007/s10995-016-2184-9. doi: 10.1007/s10995-016-2184-9. [DOI] [PubMed] [Google Scholar]
- 101.Xydopoulos G, Perry H, Sheehan E, Thilaganathan B, Fordham R, Khalil A. Home blood-pressure monitoring in a hypertensive pregnant population: cost minimisation study [published online March 8, 2018]. Ultrasound Obstet Gynecol. doi: 10.1002/uog.19041. doi: 10.1002/uog.19041. https://obgyn.onlinelibrary.wiley.com/doi/abs/10.1002/uog.19041. [DOI] [PubMed] [Google Scholar]
- 102.Perry H, Sheehan E, Thilaganathan B, Khalil A. Home blood-pressure monitoring in a hypertensive pregnant population. Ultrasound Obstet Gynecol. 2018;51:524–530. doi: 10.1002/uog.19023. doi: 10.1002/uog.19023. [DOI] [PubMed] [Google Scholar]
- 103.Cairns AE, Tucker KL, Leeson P, Mackillop LH, Santos M, Velardo C, Salvi D, Mort S, Mollison J, Tarassenko L, McManus RJ SNAP-HT Investigators. Self-management of postnatal hypertension: the SNAP-HT trial. Hypertension. 2018;72:425–432. doi: 10.1161/HYPERTENSIONAHA.118.10911. doi: 10.1161/HYPERTENSIONAHA.118.10911. [DOI] [PubMed] [Google Scholar]
- 104.Kalafat E, Mir I, Perry H, Thilaganathan B, Khalil A. Is home blood-pressure monitoring in hypertensive disorders of pregnancy consistent with clinic recordings? Ultrasound Obstet Gynecol. 2018;52:515–521. doi: 10.1002/uog.19094. doi: 10.1002/uog.19094. [DOI] [PubMed] [Google Scholar]
- 105.Grand’Maison S, Pilote L, Schlosser K, Stewart DJ, Okano M, Dayan N. Clinical features and outcomes of acute coronary syndrome in women with previous pregnancy complications. Can J Cardiol. 2017;33:1683–1692. doi: 10.1016/j.cjca.2017.08.025. doi: 10.1016/j.cjca.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 106.Theilen LH, Fraser A, Hollingshaus MS, Schliep KC, Varner MW, Smith KR, Esplin MS. All-cause and cause-specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128:238–244. doi: 10.1097/AOG.0000000000001534. doi: 10.1097/AOG.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, van Rijn BB. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. 2018;125:1642–1654. doi: 10.1111/1471-0528.15394. doi: 10.1111/1471-0528.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghossein-Doha C, van Neer J, Wissink B, Breetveld NM, de Windt LJ, van Dijk AP, van der Vlugt MJ, Janssen MC, Heidema WM, Scholten RR, Spaanderman ME. Pre-eclampsia: an important risk factor for asymptomatic heart failure. Ultrasound Obstet Gynecol. 2017;49:143–149. doi: 10.1002/uog.17343. doi: 10.1002/uog.17343. [DOI] [PubMed] [Google Scholar]
- 109.Breetveld NM, Ghossein-Doha C, van Neer J, Sengers MJJM, Geerts L, van Kuijk SMJ, van Dijk AP, van der Vlugt MJ, Heidema WM, Brunner-La Rocca HP, Scholten RR, Spaanderman MEA. Decreased endothelial function and increased subclinical heart failure in women several years after pre-eclampsia. Ultrasound Obstet Gynecol. 2018;52:196–204. doi: 10.1002/uog.17534. doi: 10.1002/uog.17534. [DOI] [PubMed] [Google Scholar]
- 110.Orabona R, Sciatti E, Prefumo F, Vizzardi E, Bonadei I, Valcamonico A, Metra M, Frusca T. Pre-eclampsia and heart failure: a close relationship. Ultrasound Obstet Gynecol. 2018;52:297–301. doi: 10.1002/uog.18987. doi: 10.1002/uog.18987. [DOI] [PubMed] [Google Scholar]
- 111.Elharram M, Dayan N, Kaur A, Landry T, Pilote L. Long-term cognitive impairment after preeclampsia: a systematic review and meta-analysis. Obstet Gynecol. 2018;132:355–364. doi: 10.1097/AOG.0000000000002686. doi: 10.1097/AOG.0000000000002686. [DOI] [PubMed] [Google Scholar]
- 112.Ciampa E, Li Y, Dillon S, Lecarpentier E, Sorabella L, Libermann TA, Karumanchi SA, Hess PE. Cerebrospinal fluid protein changes in preeclampsia. Hypertension. 2018;72:219–226. doi: 10.1161/HYPERTENSIONAHA.118.11153. doi: 10.1161/HYPERTENSIONAHA.118.11153. [DOI] [PubMed] [Google Scholar]
- 113.Evers KS, Atkinson A, Barro C, Fisch U, Pfister M, Huhn EA, Lapaire O, Kuhle J, Wellmann S. Neurofilament as neuronal injury blood marker in preeclampsia. Hypertension. 2018;71:1178–1184. doi: 10.1161/HYPERTENSIONAHA.117.10314. doi: 10.1161/HYPERTENSIONAHA.117.10314. [DOI] [PubMed] [Google Scholar]
- 114.Bergman L, Zetterberg H, Kaihola H, Hagberg H, Blennow K, Åkerud H. Blood-based cerebral biomarkers in preeclampsia: plasma concentrations of NfL, tau, S100B and NSE during pregnancy in women who later develop preeclampsia - A nested case control study. PLoS One. 2018;13:e0196025. doi: 10.1371/journal.pone.0196025. doi: 10.1371/journal.pone.0196025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bergman L, Åkerud H, Wikström AK, Larsson M, Naessen T, Akhter T. Cerebral biomarkers in women with preeclampsia are still elevated 1 year postpartum. Am J Hypertens. 2016;29:1374–1379. doi: 10.1093/ajh/hpw097. doi: 10.1093/ajh/hpw097. [DOI] [PubMed] [Google Scholar]
- 116.Barnes JN, Harvey RE, Miller KB, Jayachandran M, Malterer KR, Lahr BD, Bailey KR, Joyner MJ, Miller VM. Cerebrovascular reactivity and vascular activation in postmenopausal women with histories of preeclampsia. Hypertension. 2018;71:110–117. doi: 10.1161/HYPERTENSIONAHA.117.10248. doi: 10.1161/HYPERTENSIONAHA.117.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Siepmann T, Boardman H, Bilderbeck A, Griffanti L, Kenworthy Y, Zwager C, McKean D, Francis J, Neubauer S, Yu GZ, Lewandowski AJ, Sverrisdottir YB, Leeson P. Long-term cerebral white and gray matter changes after preeclampsia. Neurology. 2017;88:1256–1264. doi: 10.1212/WNL.0000000000003765. doi: 10.1212/WNL.0000000000003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018;363:k4109. doi: 10.1136/bmj.k4109. doi: 10.1136/bmj.k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 120.Varela-Roman A, Gonzalez-Juanatey JR, Basante P, Trillo R, Garcia-Seara J, Martinez-Sande JL, Gude F. Clinical characteristics and prognosis of hospitalised inpatients with heart failure and preserved or reduced left ventricular ejection fraction. Heart. 2002;88:249–254. doi: 10.1136/heart.88.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O’Meara E, Clayton T, McEntegart MB, McMurray JJ, Piña IL, Granger CB, Ostergren J, Michelson EL, Solomon SD, Pocock S, Yusuf S, Swedberg K, Pfeffer MA CHARM Investigators. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–3120. doi: 10.1161/CIRCULATIONAHA.106.673442. doi: 10.1161/CIRCULATIONAHA.106.673442. [DOI] [PubMed] [Google Scholar]
- 122.Simon T, Mary-Krause M, Funck-Brentano C, Jaillon P. Sex differences in the prognosis of congestive heart failure: results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II). Circulation. 2001;103:375–380. doi: 10.1161/01.cir.103.3.375. [DOI] [PubMed] [Google Scholar]
- 123.Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart. 2015;101:1182–1189. doi: 10.1136/heartjnl-2015-307516. doi: 10.1136/heartjnl-2015-307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bushnell C, McCullough LD, Awad IA, et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council for High Blood Pressure Research. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valensise H, Lo Presti D, Gagliardi G, Tiralongo GM, Pisani I, Novelli GP, Vasapollo B. Persistent maternal cardiac dysfunction after preeclampsia identifies patients at risk for recurrent preeclampsia. Hypertension. 2016;67:748–753. doi: 10.1161/HYPERTENSIONAHA.115.06674. doi: 10.1161/HYPERTENSIONAHA.115.06674. [DOI] [PubMed] [Google Scholar]
- 126.Turanov AA, Lo A, Hassler MR, et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat Biotechnol. 2018;36:1164–1173. doi: 10.1038/nbt.4297. doi: 10.1038/nbt.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]


